Summary
Tenofovir disoproxil fumarate (TDF), prodrug of tenofovir (TFV), is one of the most widely used nucleotide reverse transcriptase inhibitors (NRTIs) for the treatment of HIV infection in resource-rich and resource-limited settings with proven efficacy and safety, and also for the treatment of hepatitis B infections. However, TDF can cause renal proximal tubular dysfunction and also reduces estimated glomerular filtration rate (eGFR) more than other NRTIs. To date, TDF-associated renal dysfunction is generally regarded as mild and tolerable. However, it is notable that low body weight is one of the risk factors for TFV nephrotoxicity and that Asians are generally of smaller body stature and can be susceptible to such nephrotoxicity, as shown in several cohort studies. Until tenofovir alafenamide (TAF), another prodrug of TFV with minimal renal toxicity, becomes widely accessible for people living with HIV and replaces TDF, it is warranted that physicians who prescribe TDF have a good understanding of TFV nephrotoxicity. This paper reviews recent literature on TFV nephrotoxicity among people living with HIV especially focusing on Asians who might be susceptible to TFV nephrotoxicity due to their lower body weight and discusses implications for clinical care and future directions.
Keywords: Tenofovir, tenofovir disoproxil fumarate, tenofovir alafenamide, nephrotoxicity, HIV infection, Asians
Introduction
The advent and evolution of antiretroviral therapy (ART) substantially improved the prognosis of people living with HIV (PLHIV) (1). As life expectancy of PLHIV increases and patients age, the importance of the management of non-communicable diseases (NCDs) has increased (1,2). Both chronic kidney disease (CKD) and end-stage renal disease (ESRD) are important NCDs that affect morbidity and mortality (3,4). Maintaining renal function is particularly important among PLHIV, as HIV infection is currently not curable and patients need lifelong ART. Tenofovir disoproxil fumarate (TDF), prodrug of tenofovir (TFV), is one of the most widely used nucleotide reverse transcriptase inhibitors (NRTIs) for the treatment of HIV infection in resource-rich and resource-limited settings (5,6) with proven efficacy and safety (7-9), and also for the treatment of hepatitis B infections (5,6).
However, TDF can cause renal proximal tubular dysfunction (10-13) and also reduces estimated glomerular filtration rate (eGFR) more than other NRTIs (14-16). To date, TDF-associated renal dysfunction is generally regarded as mild and tolerable (17,18), and one meta-analysis published in 2010 recommended that TDF use should not be restricted even when regular monitoring of renal function and serum phosphate levels is impractical (17). But it is notable that low body weight is one of the risk factors for TFV nephrotoxicity and that Asians are generally of smaller body stature and have a lower median body weight than Whites and Blacks (19,20), who mostly comprise the cohorts of studies published to date.
This report reviews recent literature on TFV nephrotoxicity among PLHIV especially focusing on Asians who might be susceptible to TFV nephrotoxicity due to their smaller body stature and discusses implications for clinical care and future directions. Although tenofovir alafenamide (TAF), a new prodrug of TFV, which is safer for kidney than TDF, has been licenced and is available in some resource rich countries (21), the main focus of this review will be on TDF-associated nephrotoxicity, since TDF has been and will be used by the vast majority of PLHIV especially in low and middle income countries including many Asian countries.
Tenofovir nephrotoxicity: its mechanism and history
Compared with abacavir (ABC) or other NRTIs, TDF is highly potent with a high genetic barrier (22). TDF was first licensed for use in 2001 (23), and soon after, a series of cases which developed tubulopathy such as Fanconi syndrome or acute tubular necrosis, or acute renal failure have been reported (10-13). TFV, a metabolite of TDF, is excreted through glomerular filtration and via active tubular secretion at the proximal tubules of the kidney (24). TDF-associated tubulopathy is considered to be a result of accumulation of TFV, which causes mitochondria toxicity in tubular cells through inhibition of mitochondrial DNA polymerase-γ (25-27) (Figure 1). Renal biopsy of cases, which presented with TFV tubulopathy showed mitochondrial enlargement, depletion, and dysmorphic changes in proximal tubular cells (28). The use of TDF is also associated with increased bone turnover and bone demineralization, and although the mechanism is not fully understood, renal phosphate loss due to proximal tubulopathy is considered to be a primary cause (29,30).
Figure 1.
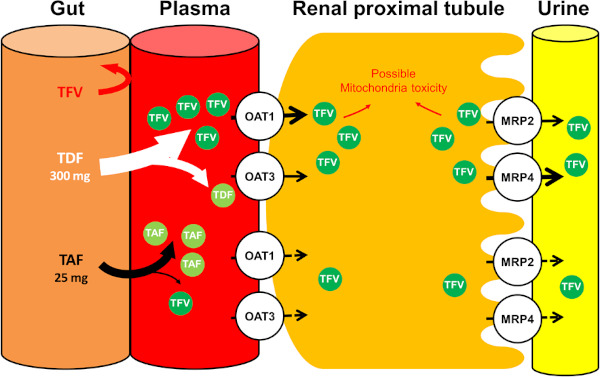
Excretion of tenofovir at the proximal tubular cells of the kidney and mechanism of tenofovir nephrotoxicity. Tenofovir (TFV), which is a metabolite from tenofovir disoproxil fumarate (TDF) and tenofovir alafenamide (TAF), is excreted through glomerular filtration and enters kidney tubular cells through the basolateral membrane and is transported mainly by organic anion transporter (OAT) 1 and, to a lesser extent, OAT 3 (60). TFV is excreted into the urine at the apical membrane by 2 transporters on the luminal membrane; multidrug resistance protein (MRP) 4 and MRP 2 (61,62). TFV cannot be absorbed from the gut. TDF is rapidly metabolized to TFV in the plasma, whereas TAF is stable in the plasma and largely metabolized to TFV within target cells, resulting in lower plasma TFV levels (21,63). Accumulation of TFV within proximal tubular cells leads to mitochondrial injury and tissue hypoxia, but with TAF, likelihood of tubular injury is less (25-27,63). TAF itself is not a substrate for OAT- 1 or OAT-3.
A post-marketing report for Australia, Europe and US in 2007 showed that cases, which developed tubulopathy or acute renal failure were rare; among 10,343 patients, acute and chronic renal failure was reported in 0.3% and Fanconi syndrome in < 0.1%. Also other renal events, such as nephrogenic diabetes insipidus, nephritis, and proteinuria were reported in ≤ 0.1% of patients (8). In tenofovir-induced nephrotoxicity, tubulopathy is considered to precede the decline in GFR (31,32). In 2010, a meta-analysis, which analyzed 17 randomized trials and cohort studies on renal safety of TDF in PLHIV (17) was published and it concluded that, although TDF use was associated with a statistically significant loss of renal function (mean difference compared with control subjects in calculated creatinine clearance, 3.92 mL/ min, 95% CI: 2.13-5.70 mL/min), the clinical magnitude of this effect was modest and they do not support the need to restrict TDF use in jurisdictions where regular monitoring of renal function and serum phosphate levels is impractical. However, it is notable that only one study from Asia (33) was included in this meta-analysis and that this study from Japan showed largest decrement in eGFR in TDF users compared to other NRTI users among 17 studies (mean difference: -17 mL/min (95% CI: -31.35, -2.65)) (17).
Low body weight as one of the risk factors for TFV nephrotoxicity
Many risk factors for TFV nephrotoxicity have been identified; including HIV specific factors, such as concomitant use of didanosine or ritonavir-boosted protease inhibitors (PI/r), advanced HIV infection, and classic risk factors for renal dysfunction, such as older age, impaired baseline renal function, diabetes mellitus, hypertension, coinfection with hepatitis C virus, and concurrent use of nephrotoxic medication (8,34). Low body weight is one of the risk factors for TFV nephrotoxicity; the animal model study reported that tenofovir tubulopathy occurs dose-dependently (35) and also a post-marketing report showed association between low body weight and renal dysfunction (8). Patients with low body weight are potentially at higher risk for larger drug exposure and, thus, more severe toxicity (19,20,36). However, most evidence on tenofovir nephrotoxicity has been from Europe and US, where body stature is generally larger than that of Asians. For example, whereas the mean body weight for the Japanese male 30-39 years old is 71 kg (37), that for US male 30-39 is 90.2kg (38); the mean body weight for American males is approximately 20kg heavier than that of the Japanese male. It is notable that the body weight of PLHIV is even lighter; median 60 kg in West India (39), median 56.5 kg in Thailand (19), and mean 55 kg for Vietnam (40).
Tenofovir nephrotoxicity for Asians living with HIV infection
The reported degree of TDF-associated renal function decrement in Asians is not negligible. Among treatment-experienced 405 Thai patients with median baseline body weight of 56.5 kg who initiated TDF, 19.3% experienced a 25% decrement in GFR with an incidence rate of 16.2 per 100 person-years (19). Also among 495 treatment-naïve Japanese patients with median weight of 63kg who initiated TDF-containing ART, 19.6% developed ≥ 25% decrement in eGFR (36).
Among the 792 treatment-naïve Japanese patients with a median weight of 63kg who either initiated TDF- or ABC-containing ART, those who initiated TDF-containing ART were twice as likely to develop > 10 mL/min/1.73 m2 decrement in eGFR and ≥ 25% decrement in eGFR than those who initiated ABC-containing ART (20). Among patients with body weight of < 70 kg, the effect of TDF use on the risk of > 10 mL/min/1.73m2 decrement in eGFR was more evident (adjusted OR: 2.5, 95% CI: 1.55-4.00, p < 0.001) than that among the entire study population (adjusted OR: 2.1, 95% CI: 1.45-3.14, p < 0.001) (20).
Although evidence is still limited to draw any firm conclusions, it is interesting that two observational studies from Asia showed that the loss in eGFR among the patients who started TDF-containing ART relative to that in those who started non-TDF-containing ART continued to increase over time (20,41), whereas one cohort study from Canada did not show such finding and concluded that most of the loss in eGFR was acquired during the first year of exposure and stabilized after that (15) (Figure 2). In the Japanese cohort study, the loss in eGFR in the TDF group relative to the ABC group continuously increased over time, and reached -10.3 mL/min/1.73 m2 at 5 years of TDF exposure (20) (Figure 2). Another multi-country observational study from East and South-East Asia reported that among 2547 patients with median body weight of 56 kg (703 on TDF-containing ART, 1844 on non-TDF-containing ART), the loss in eGFR in the TDF group relative to the non-TDF group increased over time and reached -7.1 mL/min/1.73 m2 at 5 years of TDF exposure (41). It is notable that whereas in the Japanese study 85% of the study patients were on PI/r, one of the risk factors for TFV nephrotoxicity, it was only 18.7% for the multi-country study and it still showed the increasing loss in eGFR in the TDF group compared to the non-TDF group (41). On the other hand, a single-center study from Montreal, Canada reported that among 1043 patients mostly comprised of Whites, there was no trend between the years of exposure and the loss in eGFR in the TDF group relative to non-TDF group (15). Body weight for this study was not available.
Figure 2.
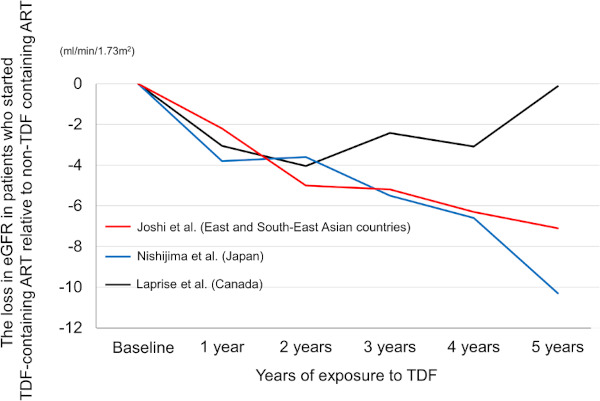
The loss in eGFR in patients who started TDF-containing ART relative to non-TDF containing ART: results from two Asian studies and one study from Canada. Whereas two studies from Asia (Joshi et al. and Nishijima et al.) showed that the loss in eGFR among the patients who started TDF containing ART relative to those who started non-TDF containing ART continued to increase over time, the study from Canada (Laprise et al.) showed that most of the loss in eGFR was acquired during the first year of exposure and stabilized after that (15,20,41). eGFR, estimated glomerular filtration rate; ART, antiretroviral therapy; TDF, tenofovir disoproxil fumarate.
Utility of renal tubular markers for prediction of tenofovir nephrotoxicity
Because tenofovir tubulopathy precedes actual decrement in GFR, renal tubular markers are considered to be more sensitive than creatinine based eGFR (31,42). Among the renal tubular markers, urinary β2 microglobulin (β2M) has been most studied (31,42-46). β2M has been shown to be a sensitive marker for TFV nephrotoxicity (31), and can predict TDF-related GFR decrement in PLHIV who initiate TDF containing antiretroviral therapy (42). Whether new tubular markers, such as kidney injury molecule 1 (KIM-1), liver type fatty acid binding protein (L-FABP), and neutrophil gelatinase-associated lipocalin (NGAL) are useful in diagnosing or predicting TDF-related GFR decrement remains to be elucidated.
Many antiretroviral agents increase serum creatinine by inhibiting excretion of creatinine at the renal tubule, which can complicate interpretation of eGFR decrement shortly after initiation of TDF containing ART (Figure 3). Dolutegravir, an integrase inhibitor, which is a component of preferred ART regimen in many treatment guidelines including the WHO guidelines (5,47,48), is one such agent (49). Measurement of urinary tubular markers, such as β2M, might help distinguishing causes of eGFR decrement, which is due to inhibition of creatinine by antiretroviral agents or due to TFV nephrotoxicity, although evidence is limited.
Figure 3.
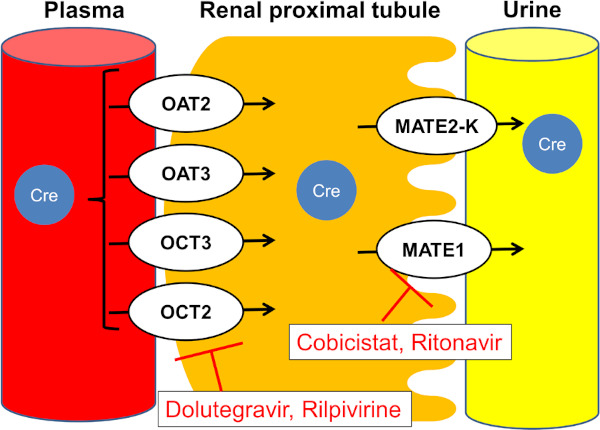
Mechanism for serum creatinine elevation by several antiretroviral drugs and pharmacoenhancers. Creatinine is transported through tubular cells on the basolateral side by organic anion transporters (OAT) 2 and 3, and organic cation transporters (OCT) 2 and 3. Creatinine is secreted via multidrug and toxin extrusion transporter 1 (MATE1) and MATE2-K on the apical side. Ritonavir and cobicistat inhibit MATE1 and inhibit creatinine efflux to urine. Dolutegravir and rilpivirine inhibit OCT2 and inhibit creatinine entry into the tubular cell (21,49,64,65).
Tenofovir alafenamide
Tenofovir alafenamide (TAF), a new prodrug of TFV is stable within plasma and metabolized to TFV mostly within target cells, enabling a small amount of dosing (25mg), which results in low plasma TFV levels and is thus safer to the kidney (50) (Figure 1). High efficacy and tolerability, especially minimal renal toxicity, of TAF have been shown in phase 3 trials and other studies including those which examined treatment-naïve patients, treatment-experienced patients, and patients with renal impairment (51-54). The phase 3 study, which randomly compared the efficacy and tolerability of elvitegravir/cobicistat/emtricitabine/TAF and elvitegravir/cobicistat/emtricitabine/TDF among treatment-naïve patients showed that a median change from baseline in creatinine clearance was significantly lower with TAF (-1.6 mL/min) than TDF (-7.7 mL/min) at week 144 (55). Furthermore, a recent pooled analysis of 26 trials showed the renal safety of TAF over TDF by examining a total of 12,519 person-years of exposure to TAF; there were no cases of proximal renal tubulopathy or Fanconi syndrome, and significantly fewer discontinuations due to renal adverse events in the TAF group than the TDF group (51). A sub-analysis of phase 3 clinical trials, which investigated efficacy and safety of elvitegravir/cobicistat/emcticitabine/TAF extracted the data of Asians and showed comparable efficacy and safety data between Asians and non-Asians (56). TAF is included as one of the components of the preferred ART regimens in the treatment guidelines in many high income countries (5,48,57,58).
Tenofovir nephrotoxicity in the future
TDF has been one of the most widely used NRTIs for the treatment of HIV infection with proven efficacy and safety (7-9) and will remain as the main NRTI especially in resource-limited settings (5,6). It will take time for TAF, another prodrug of TFV with minimal renal toxicity, to be widely accessible for people living with HIV to replace TDF. In the meantime, it is warranted that physicians who prescribe TDF have a good understanding of TFV nephrotoxicity.
Prior to initiating TDF, it is suggested that renal function is monitored with use of at least serum creatinine and a urine dipstick test, and they should be regularly monitored. Risk factors for renal dysfunction or chronic kidney diseases, such as diabetes mellitus, hypertension, hepatitis B or C infection, should be also screened. If eGFR is < 50 mL/min/1.73m2 or there is persistent proteinuria, TDF should be switched to TAF or if TAF is not available, the dose of TDF should be adjusted or TDF should be switched to abacavir or zidovudine, if available. Measurement of renal tubular markers, such as β2M, is useful to diagnose TDF-associated tubulopathy (59).
To date, TFV nephrotoxicity is generally regarded as mild and tolerable (17,18); severe tubulopathy such as Fanconi syndrome or acute tubular necrosis is rare (8), and a TDF-related eGFR decrement is generally modest (17). However, it is notable that low body weight is one of the risk factors for TFV nephrotoxicity and that Asians are generally of smaller body stature and can be susceptible to such nephrotoxicity, as shown in several cohort studies (19,20,41).
References
- 1. Ghosn J, Taiwo B, Seedat S, Autran B, Katlama C. HIV. Lancet. 2018; 392:685-697. [DOI] [PubMed] [Google Scholar]
- 2. Smit M, Brinkman K, Geerlings S, Smit C, Thyagarajan K, Sighem A, de Wolf F, Hallett TB, cohort Ao. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis. 2015; 15:7:810-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brennan A, Evans D, Maskew M, Naicker S, Ive P, Sanne I, Maotoe T, Fox M. Relationship between renal dysfunction, nephrotoxicity and death among HIV adults on tenofovir. AIDS. 2011; 25:1603-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Choi AI, Rodriguez RA, Bacchetti P, Bertenthal D, Volberding PA, O'Hare AM. The impact of HIV on chronic kidney disease outcomes. Kidney Int. 2007; 72:1380-1387. [DOI] [PubMed] [Google Scholar]
- 5. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services. Available at. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf (Accessed May 13, 2019).
- 6. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach - Second edition. https://www.who.int/hiv/pub/arv/arv-2016/en/ (accessed May 13, 2019).
- 7. Arribas JR, Pozniak AL, Gallant JE, Dejesus E, Gazzard B, Campo RE, Chen SS, McColl D, Holmes CB, Enejosa J, Toole JJ, Cheng AK. Tenofovir disoproxil fumarate, emtricitabine, and efavirenz compared with zidovudine/ lamivudine and efavirenz in treatment-naive patients: 144-week analysis. J Acquir Immune Defic Syndr. 2008; 47:1:74-78. [DOI] [PubMed] [Google Scholar]
- 8. Nelson MR, Katlama C, Montaner JS, Cooper DA, Gazzard B, Clotet B, Lazzarin A, Schewe K, Lange J, Wyatt C, Curtis S, Chen SS, Smith S, Bischofberger N, Rooney JF. The safety of tenofovir disoproxil fumarate for the treatment of HIV infection in adults: the first 4 years. AIDS. 2007; 21:10:1273-1281. [DOI] [PubMed] [Google Scholar]
- 9. Post FA, Moyle GJ, Stellbrink HJ, Domingo P, Podzamczer D, Fisher M, Norden AG, Cavassini M, Rieger A, Khuong-Josses MA, Branco T, Pearce HC, Givens N, Vavro C, Lim ML. Randomized comparison of renal effects, efficacy, and safety with once-daily abacavir/lamivudine versus tenofovir/emtricitabine, administered with efavirenz, in antiretroviral-naive, HIV- 1-infected adults: 48-week results from the ASSERT study. J Acquir Immune Defic Syndr. 2010; 55:1:49-57. [DOI] [PubMed] [Google Scholar]
- 10. Peyriere H, Reynes J, Rouanet I, Daniel N, de Boever CM, Mauboussin JM, Leray H, Moachon L, Vincent D, Salmon-Ceron D. Renal tubular dysfunction associated with tenofovir therapy: report of 7 cases. J Acquir Immune Defic Syndr. 2004; 35:269-273. [DOI] [PubMed] [Google Scholar]
- 11. Rollot F, Nazal EM, Chauvelot-Moachon L, Kelaidi C, Daniel N, Saba M, Abad S, Blanche P. Tenofovir-related Fanconi syndrome with nephrogenic diabetes insipidus in a patient with acquired immunodeficiency syndrome: the role of lopinavir-ritonavir-didanosine. Clin Infect Dis. 2003; 37:e174-176. [DOI] [PubMed] [Google Scholar]
- 12. Schaaf B, Aries SP, Kramme E, Steinhoff J, Dalhoff K. Acute renal failure associated with tenofovir treatment in a patient with acquired immunodeficiency syndrome. Clin Infect Dis. 2003; 37:e41-43. [DOI] [PubMed] [Google Scholar]
- 13. Verhelst D, Monge M, Meynard JL, Fouqueray B, Mougenot B, Girard PM, Ronco P, Rossert J. Fanconi syndrome and renal failure induced by tenofovir: a first case report. Am J Kidney Dis. 2002; 40:1331-1333. [DOI] [PubMed] [Google Scholar]
- 14. Gallant JE, Winston JA, DeJesus E, Pozniak AL, Chen SS, Cheng AK, Enejosa JV. The 3-year renal safety of a tenofovir disoproxil fumarate vs. a thymidine analogue-containing regimen in antiretroviral-naive patients. AIDS. 2008; 22:16:2155-2163 [DOI] [PubMed] [Google Scholar]
- 15. Laprise C, Baril JG, Dufresne S, Trottier H. Association between tenofovir exposure and reduced kidney function in a cohort of HIV-positive patients: results from 10 years of follow-up. Clin Infect Dis. 2013; 56:567-575. [DOI] [PubMed] [Google Scholar]
- 16. Nishijima T, Gatanaga H, Komatsu H, Tsukada K, Shimbo T, Aoki T, Watanabe K, Kinai E, Honda H, Tanuma J, Yazaki H, Honda M, Teruya K, Kikuchi Y, Oka S. Renal function declines more in tenofovir- than abacavir-based antiretroviral therapy in low-body weight treatment-naive patients with HIV infection. PLoS One. 2012; 7:e29977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cooper RD, Wiebe N, Smith N, Keiser P, Naicker S, Tonelli M. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis. 2010; 51:496-505. [DOI] [PubMed] [Google Scholar]
- 18. Gallant JE, Moore RD. Renal function with use of a tenofovir-containing initial antiretroviral regimen. AIDS. 2009; 23:1971-1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chaisiri K, Bowonwatanuwong C, Kasettratat N, Kiertiburanakul S. Incidence and risk factors for tenofovir-associated renal function decline among Thai HIV-infected patients with low-body weight. Curr HIV Res. 2010; 8:7:504-509. [DOI] [PubMed] [Google Scholar]
- 20. Nishijima T, Kawasaki Y, Tanaka N, Mizushima D, Aoki T, Watanabe K, Kinai E, Honda H, Yazaki H, Tanuma J, Tsukada K, Teruya K, Kikuchi Y, Gatanaga H, Oka S. Long-term exposure to tenofovir continuously decrease renal function in HIV-1-infected patients with low body weight. AIDS. 2014; 28:13:1903-1910. [DOI] [PubMed] [Google Scholar]
- 21. Jotwani V, Atta MG, Estrella MM. Kidney Disease in HIV: Moving beyond HIV-Associated Nephropathy. J Am Soc Nephrol. 2017; 28:3142-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Louie M, Hogan C, Hurley A, Simon V, Chung C, Padte N, Lamy P, Flaherty J, Coakley D, Di Mascio M, Perelson AS, Markowitz M. Determining the antiviral activity of tenofovir disoproxil fumarate in treatment-naive chronically HIV-1-infected individuals. AIDS. 2003; 17:1151-1156. [DOI] [PubMed] [Google Scholar]
- 23. Pozniak A. Tenofovir: what have over 1 million years of patient experience taught us? Int J Clin Pract. 2008; 62:1285-1293. [DOI] [PubMed] [Google Scholar]
- 24. Barditch-Crovo P, Deeks SG, Collier A, Safrin S, Coakley DF, Miller M, Kearney BP, Coleman RL, Lamy PD, Kahn JO, McGowan I, Lietman PS. Phase i/ ii trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob Agents Chemother. 2001; 45:2733-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kohler JJ, Hosseini SH. Subcellular renal proximal tubular mitochondrial toxicity with tenofovir treatment. Methods Mol Biol. 2011; 755:267-277. [DOI] [PubMed] [Google Scholar]
- 26. Lee H, Hanes J, Johnson KA. Toxicity of nucleoside analogues used to treat AIDS and the selectivity of the mitochondrial DNA polymerase. Biochemistry. 2003; 42:14711-14719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kohler JJ, Hosseini SH, Hoying-Brandt A, Green E, Johnson DM, Russ R, Tran D, Raper CM, Santoianni R, Lewis W. Tenofovir renal toxicity targets mitochondria of renal proximal tubules. Lab Invest. 2009; 89:513-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Herlitz LC, Mohan S, Stokes MB, Radhakrishnan J, D'Agati VD, Markowitz GS. Tenofovir nephrotoxicity: acute tubular necrosis with distinctive clinical, pathological, and mitochondrial abnormalities. Kidney Int. 2010; 78:1171-1177. [DOI] [PubMed] [Google Scholar]
- 29. Bedimo R, Rosenblatt L, Myers J. Systematic review of renal and bone safety of the antiretroviral regimen efavirenz, emtricitabine, and tenofovir disoproxil fumarate in patients with HIV infection. HIV Clin Trials. 2016; 17:246-266. [DOI] [PubMed] [Google Scholar]
- 30. Bedimo R, Maalouf NM, Zhang S, Drechsler H, Tebas P. Osteoporotic fracture risk associated with cumulative exposure to tenofovir and other antiretroviral agents. AIDS. 2012; 26:825-831. [DOI] [PubMed] [Google Scholar]
- 31. Gatanaga H, Tachikawa N, Kikuchi Y, Teruya K, Genka I, Honda M, Tanuma J, Yazaki H, Ueda A, Kimura S, Oka S. Urinary beta2-microglobulin as a possible sensitive marker for renal injury caused by tenofovir disoproxil fumarate. AIDS Res Hum Retroviruses. 2006; 22:744-748. [DOI] [PubMed] [Google Scholar]
- 32. Papaleo A, Warszawski J, Salomon R, Jullien V, Veber F, Dechaux M, Blanche S. Increased beta-2 microglobulinuria in human immunodeficiency virus-1- infected children and adolescents treated with tenofovir. Pediatr Infect Dis J. 2007; 26:949-951. [DOI] [PubMed] [Google Scholar]
- 33. Kinai E, Hanabusa H. Progressive renal tubular dysfunction associated with long-term use of tenofovir DF. AIDS Res Hum Retroviruses. 2009; 25:387-394. [DOI] [PubMed] [Google Scholar]
- 34. Goicoechea M, Liu S, Best B, Sun S, Jain S, Kemper C, Witt M, Diamond C, Haubrich R, Louie S; California Collaborative Treatment Group 578 Team. Greater tenofovir-associated renal function decline with protease inhibitor-based versus nonnucleoside reverse-transcriptase inhibitor-based therapy. J Infect Dis. 2008; 197:102-108. [DOI] [PubMed] [Google Scholar]
- 35. Van Rompay KK, Brignolo LL, Meyer DJ, e t al. Biological effects of short-term or prolonged administration of 9-[2-(phosphonomethoxy)propyl] adenine (tenofovir) to newborn and infant rhesus macaques. Antimicrob Agents Chemother. 2004; 48:1469-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nishijima T, Komatsu H, Gatanaga H, Aoki T, Watanabe K, Kinai E, Honda H, Tanuma J, Yazaki H, Tsukada K, Honda M, Teruya K, Kikuchi Y, Oka S. Impact of small body weight on tenofovir-associated renal dysfunction in HIV-infected patients: a retrospective cohort study of Japanese patients. PLoS One. 2011; 6:e22661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Japanese Ministry of Health, Labour and Welfare. National Health and Nutrition Survey 2017. https://www.mhlw.go.jp/bunya/kenkou/kenkou_eiyou_chousa.html (accessed May 19, 2019) (in Japanese).
- 38. Fryar CD, Gu Q, Ogden CL, Flegal KM. Anthropometric Reference Data for Children and Adults: United States, 2011-2014. Vital Health Stat 3. 2016; 39:1-46. [PubMed] [Google Scholar]
- 39. Patel KK, Patel AK, Ranjan RR, Patel AR, Patel JK. Tenofovir-associated renal dysfunction in clinical practice: An observational cohort from western India. Indian J Sex Transm Dis. 2010; 31:1:30-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mizushima D, Tanuma J, Kanaya F, Nishijima T, Gatanaga H, Lam NT, Dung NT, Kinh NV, Kikuchi Y, Oka S. WHO antiretroviral therapy guidelines 2010 and impact of tenofovir on chronic kidney disease in Vietnamese HIV-infected patients. PLoS One. 2013; 8:e79885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Joshi K, Boettiger D, Kerr S, et al. Changes in renal function with long-term exposure to antiretroviral therapy in HIV-infected adults in Asia. Pharmacoepidemiol Drug Saf. 2018; 27:1209-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nishijima T, Kurosawa T, Tanaka N, Kawasaki Y, Kikuchi Y, Oka S, Gatanaga H. Urinary beta2 microglobulin can predict tenofovir disoproxil fumarate-related renal dysfunction in HIV-1-infected patients who initiate tenofovir disoproxil fumarate-containing antiretroviral therapy. AIDS. 2016; 30:1563-1571. [DOI] [PubMed] [Google Scholar]
- 43. Nishijima T, Mutoh Y, Kawasaki Y, Tomonari K, Kikuchi Y, Gatanaga H, Oka S, Team ACCS. Cumulative exposure of TDF is associated with kidney tubulopathy whether it is currently used or discontinued. AIDS. 2018; 32:179-188. [DOI] [PubMed] [Google Scholar]
- 44. Ascher SB, Scherzer R, Estrella MM, et al. Association of urinary biomarkers of kidney injury with estimated GFR decline in HIV-infected individuals following tenofovir disoproxil fumarate initiation. Clin J Am Soc Nephrol. 2018; 13:1321-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nishijima T, Komatsu H, Higasa K, Takano M, Tsuchiya K, Hayashida T, Oka S, Gatanaga H. Single nucleotide polymorphisms in ABCC2 associate with tenofovir-induced kidney tubular dysfunction in Japanese patients with HIV-1 infection: a pharmacogenetic study. Clin Infect Dis. 2012; 55:1558-1567. [DOI] [PubMed] [Google Scholar]
- 46. Nishijima T, Shimbo T, Komatsu H, Takano M, Tanuma J, Tsukada K, Teruya K, Gatanaga H, Kikuchi Y, Oka S. Urinary beta-2 microglobulin and alpha-1 microglobulin are useful screening markers for tenofovir-induced kidney tubulopathy in patients with HIV-1 infection: a diagnostic accuracy study. J Infect Chemother. 2013; 19:5:850-857. [DOI] [PubMed] [Google Scholar]
- 47. World Health Organization. Policy brief: Update of recommendations on first- and second-line antiretroviral regimens. 2019. https://apps.who.int/iris/bitstream/handle/10665/325892/WHO-CDS-HIV-19.15-eng.pdf (accessed May 13 2019).
- 48. The Guidelines for the Treatment of HIV Infection, March 2019 version. The Japanese Ministry of Health, Labour and Welfare. https://www.haart-support.jp/guideline.htm (accessed July 21, 2019) (in Japanese).
- 49. Lepist EI, Zhang X, Hao J, Huang J, Kosaka A, Birkus G, Murray BP, Bannister R, Cihlar T, Huang Y, Ray AS. Contribution of the organic anion transporter OAT2 to the renal active tubular secretion of creatinine and mechanism for serum creatinine elevations caused by cobicistat. Kidney Int. 2014; 86:350-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gotham D, Hill A, Pozniak AL. Candidates for inclusion in a universal antiretroviral regimen: tenofovir alafenamide. Curr Opin HIV AIDS. 2017; 12:324-333. [DOI] [PubMed] [Google Scholar]
- 51. Gupta SK, Post FA, Arribas JR, et al. Renal safety of tenofovir alafenamide vs. tenofovir disoproxil fumarate: a pooled analysis of 26 clinical trials AIDS. 2019; 33:1455-1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mills A, Arribas JR, Andrade-Villanueva J, et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect Dis. 2016; 16:43-52. [DOI] [PubMed] [Google Scholar]
- 53. Pozniak A, Arribas JR, Gathe J, et al. Switching to tenofovir alafenamide, coformulated with elvitegravir, cobicistat, and emtricitabine, in HIV-infected patients with renal impairment: 48-week results from a single-arm, multicenter, open-label phase 3 study. J Acquir Immune Defic Syndr. 2016; 71:530-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015; 385:2606-2615. [DOI] [PubMed] [Google Scholar]
- 55. Arribas JR, Thompson M, Sax PE, Haas B, McDonald C, Wohl DA, DeJesus E, Clarke AE, Guo S, Wang H, Callebaut C, Plummer A, Cheng A, Das M, McCallister S. Brief report: randomized, double-blind comparison of tenofovir alafenamide (TAF) vs tenofovir disoproxil fumarate (TDF), each coformulated with elvitegravir, cobicistat, and emtricitabine (E/C/F) for initial HIV- 1 treatment: week 144 results. J Acquir Immune Defic Syndr. 2017; 75:211-218. [DOI] [PubMed] [Google Scholar]
- 56. Kim YS, Oka S, Chetchotisakd P, et al. Efficacy and safety of elvitegravir/cobicistat/emtricitabine/ tenofovir alafenamide in Asian participants with human immunodeficiency virus 1 infection: A sub-analysis of phase 3 clinical trials. HIV Res Clin Pract. 2019:1-9. [DOI] [PubMed] [Google Scholar]
- 57. European AIDS Clinical Society. European AIDS Clinical Society Guidelines Version 9. 1 October 2018. http://www.eacsociety.org/guidelines/eacs-guidelines/eacs-guidelines.html (accessed May 13, 2019).
- 58. British HIV Association. British HIV Association guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2015 (2016 interim update). https://www.bhiva.org/HIV-1-treatment-guidelines (accessed May 13, 2019). [DOI] [PubMed]
- 59. Gatanaga H, Nishijima T, Tsukada K, Kikuchi Y, Oka S. Clinical importance of hyper-beta-2-microglobulinuria in patients with HIV-1 infection on tenofovir-containing antiretroviral therapy. J Acquir Immune Defic Syndr. 2014; 65:e155-157. [DOI] [PubMed] [Google Scholar]
- 60. Uwai Y, Ida H, Tsuji Y, Katsura T, Inui K. Renal transport of adefovir, cidofovir, and tenofovir by SLC22A family members (hOAT1, hOAT3, and hOCT2). Pharm Res. 2007; 24:811-815. [DOI] [PubMed] [Google Scholar]
- 61. Imaoka T, Kusuhara H, Adachi M, Schuetz JD, Takeuchi K, Sugiyama Y. Functional involvement of multidrug resistance-associated protein 4 (MRP4/ABCC4) in the renal elimination of the antiviral drugs adefovir and tenofovir. Mol Pharmacol. 2007; 71:619-627. [DOI] [PubMed] [Google Scholar]
- 62. Mallants R, Van Oosterwyck K, Van Vaeck L, Mols R, De Clercq E, Augustijns P. Multidrug resistance-associated protein 2 (MRP2) affects hepatobiliary elimination but not the intestinal disposition of tenofovir disoproxil fumarate and its metabolites. Xenobiotica. 2005; 35:1055-1066. [DOI] [PubMed] [Google Scholar]
- 63. Ruane PJ, DeJesus E, Berger D, Markowitz M, Bredeek UF, Callebaut C, Zhong L, Ramanathan S, Rhee MS, Fordyce MW, Yale K. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1-positive adults. J Acquir Immune Defic Syndr. 2013; 63:449-455. [DOI] [PubMed] [Google Scholar]
- 64. Cohen CJ, Andrade-Villanueva J, Clotet B, Fourie J, Johnson MA, Ruxrungtham K, Wu H, Zorrilla C, Crauwels H, Rimsky LT, Vanveggel S, Boven K, THRIVE study group. Rilpivirine versus efavirenz with two background nucleoside or nucleotide reverse transcriptase inhibitors in treatment-naive adults infected with HIV-1 (THRIVE): a phase 3, randomised, non-inferiority trial. Lancet. 2011; 378:229-237. [DOI] [PubMed] [Google Scholar]
- 65. Koteff J, Borland J, Chen S, Song I, Peppercorn A, Koshiba T, Cannon C, Muster H, Piscitelli SC. A phase 1 study to evaluate the effect of dolutegravir on renal function via measurement of iohexol and para-aminohippurate clearance in healthy subjects. Br J Clin Pharmacol. 2013; 75:990-996. [DOI] [PMC free article] [PubMed] [Google Scholar]


