Abstract
Purpose of Review
To describe the current state of research on interactions between inflammation and female sexual function.
Recent findings
Inflammation may interfere with female sexual desire and arousal via direct (neural) and indirect (endocrine, vascular, social/behavioral) pathways. There are significant sex differences in the effect of inflammation on sexual function, arising from different evolutionary selection pressures on regulation of reproduction. A variety of inflammation-related conditions are associated with risk of female sexual dysfunction, including cardiovascular disease, metabolic syndrome, and chronic pain.
Summary
Clinical implications include the need for routine assessment for sexual dysfunction in patients with inflammation-related conditions, the potential for anti-inflammatory diets to improve sexual desire and arousal function, and consideration of chronic inflammation as moderator of sexual effects of hormonal treatments. Although the evidence points to a role for inflammation in the development and maintenance of female sexual dysfunction, the precise nature of these associations remains unclear.
Keywords: inflammation, sexual desire, sexual arousal, C-reactive protein, cytokines, Interleukin-6
Introduction
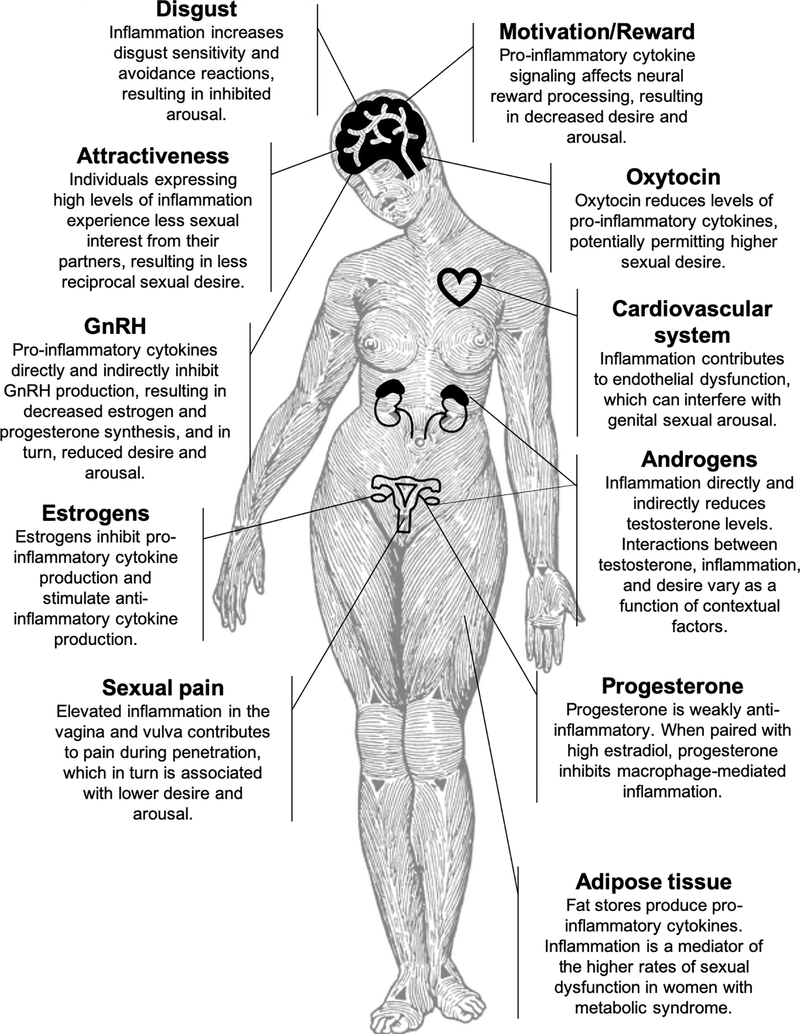
Despite much research on interactions between immunity and female reproductive health [1], there is much less known about the effect of immunity on women’s sexual wellbeing. The present review examines one important aspect of immune response – inflammation – and its potential effects on women’s sexual desire and arousal (see Figure 1 for visual summary). While there is much indirect evidence for important interactions between inflammation and female sexual function, causal studies are lacking; thus, the broader purpose of this review is to encourage future research.
Figure 1.
Summary of research on inflammation and female sexual desire and arousal function.
Image design credit: Anneliis Sartin-Tarm.
Definition of desire and arousal
Sexual desire is a motivational state, reflecting the interest in or receptiveness to sexual activity either with or without a partner [2]. As such, it has been characterized as either an acute response to sexual cues [3] (including sexual arousal [4, 5]) or as an ongoing orientation towards sexual stimuli [2, 6]. Sexual arousal, on the other hand, is typically defined as a temporary state that occurs in response to sexual stimulation. Female sexual arousal involves thoughts and emotions as well as physiologic responses including both sympathetic nervous system activation (e.g., increased heart rate, blood pressure, and breathing rate) and arousal of the sex organs (e.g., engorgement of the vulva and vaginal lubrication) [7]. There are common psychological and physiologic foundations for both arousal and desire, including some shared neural networks [8, 9]; moreover, in healthy females, desire can lead to arousal, and vice versa, in a cyclical feedback loop [5, 10]. Insofar as inflammation influences sexual desire, it will likely also impact arousal.
Primer on inflammation
Inflammation is a suite of immune processes that respond to pathogens and toxins, identify and clear out damaged cells, and stimulate and direct the adaptive immune system. In the acute phase, stimulated immune cells exert a variety of inflammatory actions: increasing blood flow and permeability of blood vessels to permit diffusion of plasma into the tissues; phagocytosis of pathogens and cellular waste; release of substances such as histamines that damage foreign cells; and generation of acute-phase products such as coagulation factors and complement proteins. The acute inflammatory response generally resolves rapidly, as it relies on the presence of stimulating factors which degrade rapidly as the harmful agents are removed. However, if these stimulating factors remain, the inflammatory response evolves to a chronic phase that can result in lasting tissue damage, and contributes to future immune hypersensitivity and autoimmunity [11, 12] as well as a number of chronic diseases such as metabolic and mood disorders [13].
In addition to its critical role in immune defense, inflammation contributes to somatic maintenance. For example, many of the tasks faced by the female reproductive system (e.g., menstrual cycling [14], ovulation [15], implantation [16], and parturition [17]) rely on inflammation processes that assist with tissue repair, vascular reorganization, and coordination with endocrine and nervous systems. One of the negative effects of chronic inflammation on reproduction is disruption of the normal activity of acute inflammation and subsequent loss of these important functions.
Much research on inflammation indexes the level of cytokines, proteins secreted both by immune cells (e.g., macrophages) and other tissues (e.g., endothelial cells). Like hormones, these proteins act on receptors to produce an effect, including regulating inflammation processes; notably, cytokines can also act on cells outside the immune system such as the nervous system (e.g., neurons and microglia [18]) and endocrine system (e.g., gonads [19]). Cytokines may exert either a pro- or anti-inflammatory effect, depending on contextual factors such as where they are secreted, sender and receiver cell type, and activity of other cytokines, hormones, or neurotransmitters. However, there are a few cytokines that are typically indexed for their pro- or anti-inflammatory action. The triad of IL-6, IL-1β, and tumor necrosis factor-α (TNFα) are thought to have a predominantly pro-inflammatory role [20, 21], as each encourages the inflammatory action of macrophages and stimulates the production of acute phase proteins and prostaglandins (see below). The action of these cytokines on the nervous system are also critical for the psychological and behavioral aspects of sickness [22]. In contrast, IL-4 and IL-10 are thought to be anti-inflammatory as they inhibit the action of pro-inflammatory cytokine signaling [23] and encourage development of Th2 cells, which regulate acute inflammation and moderate tissue damage [24, 25].
Another commonly indexed marker of inflammation is C-reactive protein (CRP). Acute-phase proteins like CRP aid in disposal of dead cells and pathogens by binding to their surface and attracting other immune actors to dispose of them. The liver produces CRP in response to cytokine signaling from across the body, making it a useful index of system-level inflammation.
Finally, prostaglandins are compounds that exert a hormone-like effect to moderate inflammation processes [26]. Like cytokines, prostaglandins can exert direct action on receptors in the nervous system, resulting in pain [27]. Of note, prostaglandin action in the central nervous system is an important mediator of anorexia (loss of appetite) associated with sickness [28]; there is speculation that prostaglandins may moderate other motivation systems as well including, potentially, female sexual desire [29–31]. Outside the brain, prostaglandins can serve a pro-sexual role by promoting vasodilation, including that associated with genital arousal in both males and females [32].
Mechanisms by which inflammation may influence female sexual desire and arousal Central nervous system
Cytokines can exert a powerful effect on the central nervous system, contributing to behavioral responses, potentially including sexual response. Areas of the brain that have been shown to be relevant for coordination of sexual desire and arousal, such as the mesolimbic reward system, cingulate cortex, and thalamus [8, 33], have been shown to respond to cytokine signaling, both directly on neuronal receptors [34, 35] and indirectly though interactions with dopamine [36] and other neurotransmitters [37, 38]. Several studies have found that, even at levels too low to trigger other symptoms such as fever, pro-inflammatory cytokines can interfere with neural processing associated with motivation and reward [39, 40].
A number of animal studies suggest that pro-inflammatory cytokines can lower interest in sexual behavior, particularly among females. When given a dose of IL-1β or lipopolysaccharide (LPS, a substance that induces robust inflammatory responses), female rats had significantly reduced interest in sexual activity. At an equivalent dose, male rats showed no decrease in sexual interest (and, at high doses, even showed increased sexual interest) [41, 42]. The desire-suppressing effect of inflammation appears to be the result of interactions between multiple cytokine systems, including IL-1β and TNF-α [42, 43], as well as increased prostaglandin synthesis and activity in the hypothalamus [31].
These findings have not yet been thoroughly replicated in humans, but preliminary evidence suggests convergence. In a small exploratory study of premenopausal women, higher levels of CRP and IL-6 predicted lower levels of self-reported sexual desire, arousal and pleasure during sexual activity [44]. In contrast, a small study of men found significant positive correlations between high sexual desire and TNF-α (but not CRP) [45]. In a separate study of 11 healthy men, Haake et al. found sexual arousal was associated with increases in IL-6 and TNF-α, albeit non-significantly [46]. Together, these findings echo the sex differences noted in animal models, with markers of inflammation associated with lower desire and arousal in females, but either no effect or higher desire/arousal in males.
Why would the nervous system respond to inflammation cues, and why would there be sex differences in the effect of inflammation on sexual desire? There is a suite of behavioral and psychological effects induced by increased inflammation, such as fatigue and anhedonia. These “sickness behaviors” may have evolved to focus resources on fighting infections by lowering activity levels and reducing exposure to new sources of infection (such as other animals) [47]. Signals of inflammation may thus reduce interest in all social interactions, including partnered sexual activity [48]. Reproduction requires significant resources, more so for females than for males [49]. As such, modulation of sexual desire may be a means of timing female reproductive investment in accordance with resource availability (or likely future availability) [50]. Inflammation processes are energetically costly, precluding long-term investment in non-essential processes such as reproduction [51]. Thus, signals of inflammation may exert a more powerful effect on female sexual desire in order to delay reproductive investment to healthier times [52, 53].
The behavioral sequelae of inflammation are not limited to lower female sexual desire; there is reason to believe inflammation may inhibit arousal via increased disgust sensitivity. Disgust is a form of “behavioral immunity”, reducing the chance of exposure to infection by producing an avoidance/withdrawal reaction to disease cues [54, 55]. When inflammation levels rise, disgust sensitivity and avoidance reactions become more pronounced [55–57]. Disgust can interfere with approach-related emotions, including sexual arousal [58, 59]. Experimentally-induced disgust strongly inhibits female sexual arousal [60–62]. At a trait level, disgust is associated with higher rates of female sexual desire and arousal dysfunction [63]. The reciprocal inhibition between disgust and sexual arousal may be particularly evident in females, as the female body faces higher reproductive burden of sexually transmitted infection relative to males [58, 64, 65].
Taken together, these findings suggest that inflammation may act on the central nervous system to contribute to female sexual desire and arousal dysfunction via interference with reward processing (decreasing motivation for sex) and increased disgust (increasing avoidance and lowering arousal [65]).
Endocrine effects
In addition to the direct effects of cytokines on neural processing, inflammation can influence endocrine function. As noted above, there are significant interactions between female reproductive and immune systems that are thought to have evolved to balance tradeoffs between investing in reproduction vs. somatic maintenance [66]. Alongside direct neural effects, these tradeoffs are often negotiated via immune interactions with sex steroid hormones (such as estrogens and androgens) and other hormones such as leptin [67, 68] and kisspeptin [69]. A decidedly mixed literature has revealed that the normal function of these hormones is a necessary, but not sufficient factor in human sexual behavior: while their presence does not guarantee sexual function, their absence almost always impairs it.
Estrogens and progesterone
A large body of clinical and basic science research has documented the effects of estrogens and progesterone on inflammation. Notably, the action of each hormone depends the level expressed, and the presence of the other [70–72]. At high levels (e.g., during pregnancy) estrogens typically have an anti-inflammatory action, by inhibiting production of pro-inflammatory cytokines [73] such as IL-6 and TNF-α [74–77], stimulating production of anti-inflammatory cytokines such as IL-4 and IL-10 [78], and accelerating the resolution of ongoing inflammation [79]. However, at lower levels estrogens can be pro-inflammatory as they stimulate production of IL-1β [80, 81] and increase the inflammatory action of neutrophils and monocytes [82, 83]. Progesterone is typically weakly anti-inflammatory [84], but in the presence of high levels of estradiol, can exert powerful inhibitory effects on macrophage-mediated inflammation processes [85, 86].
In turn, inflammatory processes suppress production of sex steroid hormones and can modulate the action of their receptors. Inflammation processes inhibit hypothalamic release of gonadotropin releasing hormone (GnRH) [87, 88], which in turn reduces production of gonadal hormones. Concurrently, pro-inflammatory cytokines activate the hypothalamic-pituitary-adrenal (HPA) axis via increased production of adrenocorticotropin releasing hormone [89], which further antagonizes production of GnRH [90]. More directly, TNF-α and IL-1β inhibit production of progesterone and estradiol by ovaries [91–93], uterus [94], and breast tissue [95]. Women who have high circulating levels of IL-6 and IL-1β show significantly lower GnRH receptor sensitivity [96]. In animal models, inflammation is associated with reduction in the number and sensitivity of the estrogen receptor-α (ERα) [97]. In sum, inflammation can interfere with synthesis and activity of estrogen and progesterone, reducing their potentially stimulatory effect on female sexual desire and arousal.
It should be noted that effect of estrogens and progesterone on inflammation appear to differ if these hormones are endogenous (i.e., those produced by the body) vs. exogenous (administered). Whereas endogenous estrogens can have an anti-inflammatory effect, use of exogenous estrogens are associated with increased levels of inflammation, particularly when administered orally [70, 98–100]. There is no consensus as to the effect of hormonal treatments (such as oral contraceptives or hormone replacement therapies) on sexual desire and arousal in healthy women [101, 102]. However, a number of studies have documented that at least a subset of patients report either decreased sexual desire [103–105] or lack of improvement in sexual desire [106, 107] while taking these medications. Given the strong associations between endocrine and immune systems, it is likely that inflammatory effects partially mediate some of the negative effects of estrogen and progesterone treatments on women’s sexual desire and arousal; clearly, this is an area needing further research.
Testosterone
Early work characterized testosterone and other androgens as universally immunosuppressant, as males typically have lower immune response (and lower rates of autoimmune disorders) than do females [108]. It is now clear that, like estrogens, endogenous androgens can have either pro- or anti-inflammatory actions in females, depending on menstrual cycle phase [109], menopausal status [110–114], and even level of sexual activity [115]. Treatment with exogenous testosterone similarly can have either a pro- or anti-inflammatory effect, again depending on menstrual cycle phase and/or menopausal status [109, 116, 117]. Studies of androgen treatment in hypogonadal women have found no significant effect of testosterone on CRP [118, 119] or other markers of inflammation [120]. In the other direction, inflammation is known to reduce gonadal production of testosterone [121, 122] (except in the case of polycystic ovarian syndrome [123]). Moreover, high levels of IL-6 and TNF-α stimulate aromatase activity [124, 125], which converts testosterone to estrogen, further decreasing bioavailable testosterone.
There have been similarly inconsistent reports of the effects of testosterone on female sexual desire and arousal function: some studies have found significant positive associations [126–128], others negative [129, 130], and others no association [131, 132]. Of note, women who are regularly sexually active with a partner have lower endogenous testosterone than single (and sexually inactive) women [133, 134]. Systematic reviews suggest that while testosterone treatment for low libido can be effective in postmenopausal women [135, 136], it is not significantly better than placebo for premenopausal women with normal gonadal function [137, 138]. The inconsistency across both lines of literature makes it difficult to draw definite conclusions regarding the interactions among testosterone, inflammation and female sexual desire; however, it is clear that these interactions will be sensitive to contextual factors such as menopausal status, menstrual cycle phase, and sexual partnership.
Oxytocin
Finally, oxytocin plays an important role in female sexual function, as it is released during sexual arousal and orgasm [139], and contributes to the sense of intimacy and pleasure associated with sexual activity [140, 141]. Women with low scores on the FSFI arousal and lubrication subscales have lower levels of oxytocin than women who are satisfied with their sexual function [142]. In addition to its role in coordinating sociosexual behavior, oxytocin reduces the level of pro-inflammatory cytokines produced by macrophages [143, 144] and microglial cells of the nervous system [145]. Thus, it is possible that the high levels of oxytocin associated with sexual activity (and particularly with orgasm) may provide a permissive environment for sexual desire by reducing cytokine signaling in the nervous system.
Other mechanisms
Endothelial dysfunction
One of the ways in which inflammation serves immune defense is by encouraging local blood vessels to dilate and increase permeability; this allows defensive and wound-healing factors from plasma to perfuse the inflamed tissue [146]. However, chronically high inflammation can interfere with nitric-oxide mediated vasodilation, which is necessary for genital arousal [147]. In males, high levels of TNF-α and IL-6 are associated with erectile dysfunction [148–150], even when controlling for a variety of other factors such as age, metabolic syndrome and blood pressure [151]. Specifically, TNF-α has been demonstrated to reduce synthesis of nitric oxide in erectile tissue [152], reducing the capacity to support genital arousal. To date, these effects have not been replicated in females; however, given similar mechanisms underlying erectile function in the penis and the clitoris [153], it is very likely that high levels of TNF-α would interfere with genital arousal function in females.
Sexual pain
One of the key clinical signs of inflammation is pain (dolor), and thus it is not surprising that women who report significant pain with penetration also have elevated markers of inflammation, both locally in the vulva and vagina [154] and systemically [155, 156]. Women with sexual pain disorders report significantly lower sexual desire and arousal than women without such disorders [157]. Thus, it is reasonable to expect that to the degree that inflammation contributes to pain during sex, it will also contribute to lower sexual desire.
Attractiveness
In rodent models, males are significantly less likely to initiate or be receptive to sexual activity with a female who has been treated with IL-1β [158] or LPS [159], suggesting that the attractiveness of potential sexual partners was reduced when they were expressing higher levels of inflammation. From an evolutionary perspective, mating with an unhealthy female can be both risky (in terms of potential exposure to infection) and counterproductive (in terms of likelihood of spontaneous abortion). Humans are similarly able to detect experimentally-induced increases in inflammation via olfactory and visual cues [160, 161]. In one study, the faces of participants who had been injected with LPS were rated significantly less sexually attractive than the faces of the same participants following saline injection [162]. Insofar as sexual desire can be responsive to partner initiation [163], these findings suggest that individuals who are expressing higher levels of inflammation may experience less interest from their partners, and in turn, less desire themselves.
Sexual desire and arousal function in populations with inflammation-related conditions
Further evidence of the negative impact of chronic inflammation on female sexual desire and arousal function comes from clinical observations in populations with inflammation-related conditions. In each case, sexual desire appears to be impacted more severely than other aspects of sexual function such as orgasm.
Cardiovascular disease (CVD)
There is a well-known connection between CVD and erectile dysfunction in men [164], which is mediated via chronic inflammation and subsequent endothelial dysfunction [165–167]. Although less well documented, the associations among inflammation, CVD, and sexual arousal dysfunction have also been observed in women [168–170]. Similarly, hypertensive women report lower sexual desire and arousal function than normotensive women [171], although it should also be noted that anti-hypertensive medications are known to have iatrogenic effects on sexual function [171, 172].
Metabolic syndrome
Chronic low-grade inflammation is also associated with obesity and metabolic syndrome, as adipose tissue generates pro-inflammatory cytokines which in turn impair insulin sensitivity [173]. Across numerous studies, metabolic syndrome is associated with significantly higher levels of sexual desire dysfunction among women [174–176]. In studies that include both pre- and post-menopausal women, there are higher rates of sexual desire dysfunction among women with metabolic syndrome than in women without; however, arousal and orgasm function are similar across groups [177, 178]. In one study of women with and without metabolic syndrome, scores on a validated index of sexual function were inversely related to levels of serum CRP [179], further evidence that inflammation may partially mediate the higher rates of sexual dysfunction in women with metabolic syndrome.
Chronic pain
Although the etiology of chronic pain is complex, there are many pain conditions for which inflammation plays a significant role [180]. There are higher rates of sexual desire and arousal dysfunction among women with inflammatory bowel diseases [181, 182], rheumatoid arthritis [183], fibromyalgia [184], and chronic pelvic pain [185]. Among women with rheumatoid arthritis, sexual desire and arousal dysfunction is predicted by higher levels of CRP [186].
Effects of anti-inflammatory treatments on female sexual function
As noted above, inflammation-related conditions can have a deeply negative effect on female sexual function. However, the effect of treatments for these conditions have mixed effects on sexual function: while anti-inflammatory diets and lifestyle interventions appear to have a positive effect, anti-inflammatory medications can have a negative effect. In considering this paradox, it should be noted that cytokines and prostaglandins have a variety of homeostatic functions not limited to inflammation per se [187]; external manipulation of these systems may have iatrogenic effects that environmental or behavioral interventions lack.
Non-steroidal anti-inflammatories (NSAIDS)
In men, long-term use of NSAIDs has been associated with erectile dysfunction, both in exaggeration of pre-existing sexual dysfunction as well as new onset cases. Two studies found the association attenuated when considering patient’s underlying medical conditions [188, 189]; however, others have found increased risk even when controlling for indication and age [190, 191]. NSAIDs have their therapeutic action via inhibition of cyclooxygenase (COX) synthesis, which in turn reduces the activity of prostaglandins [192]. However, this in turn may interfere with prostaglandin-mediated vasodilation of erectile tissue [193]. The effect of NSAIDs on female sexual arousal is unknown. Given similar mechanisms of clitoral and penile erection [153], it is likely that NSAIDs would interfere with female sexual arousal as they do in males. However, in animal models, prostaglandins strongly suppressed female but not male sexual desire [31], suggesting potentially different effects across domains of female sexual function.
Anti-inflammatory diets
The Mediterranean diet – emphasizing whole grains, fresh fruits and vegetables, fish and poly-unsaturated fats – has been demonstrated to reduce markers of inflammation in both healthy weight [194, 195] and overweight women [196]. A Mediterranean diet has also shown benefits for sexual function for women with metabolic syndrome, particularly in sexual desire and arousal function [197–200]; these gains are mediated by decreased levels of CRP [197] (but see also [198]). Similarly, a calorie-restricted diet (also shown to reduce chronic inflammation [201]) has been shown to increase sexual desire and arousal in overweight women [202].
Clinical implications
Taken together, the above research strongly suggests that inflammation may play a significant role in female sexual desire and arousal function – although the precise nature of that role is still unknown. Thus, the following clinical implications should be considered speculative but worthy of future research.
Reducing chronic exposure to systemic inflammation is associated with improvements across a variety of domains of health and wellbeing; it is reasonable to suspect benefits for sexual function as well. As noted above, preliminary data strongly suggest that anti-inflammatory diets improve sexual desire and arousal, particularly among overweight women; given their high potential benefit and low risk of harm, these diets may be recommended as a preventive first-line treatment or adjunctive to treatments for sexual desire or arousal concerns. It is also reasonable to consider chronic inflammation as a possible contributing factor when assessing sexual desire and arousal concerns, particularly in women with immune conditions and in overweight women. Given interactions between immune and endocrine systems, it may be worthwhile to monitor changes in circulating markers of inflammation when introducing hormone regimens intended to treat sexual dysfunction; if a hormonal treatment stimulates desire through central mechanisms, but also increases systemic inflammation, the final result may be less dramatic than hoped. Finally, it is commonly accepted that, in men, sexual dysfunction may be an early warning sign of inflammation-related conditions such as CVD [203–205]; although much further research is needed, the evidence points to the same being true of women [206], suggesting benefits for routine assessment of female sexual desire and arousal function in the context of preventative care.
Conclusions
Chronic inflammation can contribute to dysfunction across metabolic, cardiovascular, endocrine, and neurological systems. Thus, it is not surprising that inflammation may impact female sexual function. However, women’s sexual desire and arousal may be particularly sensitive to the effects of inflammation, owing to the important interactions between female reproductive and immune systems. The aggregate evidence suggests that inflammation may interfere with female sexual desire and arousal by both direct (neural) and indirect (endocrine, endothelial, social/behavioral) mechanisms. However, this evidence comes mostly from animal studies, studies in males, and observations of higher rates of sexual dysfunction among clinical populations characterized by chronic inflammation. Direct evidence of the effects of inflammation on female sexual function is lacking. Future research elucidating the interactions between sexuality and immune function will likely be quite fruitful in uncovering new avenues for treatment for female sexual dysfunction
Acknowledgements
This review was prepared with support from UNL Department of Psychology, the Nebraska Tobacco Settlement Biomedical Research Development Fund, and the UNL Office of Research and Economic Development. Particular thanks to Anneliis Sartin-Tarm, who designed the visual summary.
Footnotes
Conflict of Interest
Tierney Lorenz declares no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Jabbour HN, Sales KJ, Catalano RD, Norman JE. Inflammatory pathways in female reproductive health and disease. Reproduction. 2009;138(6):903–19. [DOI] [PubMed] [Google Scholar]
- 2.Spector IP, Carey MP, Steinberg L. The sexual desire inventory: Development, factor structure, and evidence of reliability. J Sex Marital Ther. 1996;22(3):175–90. [DOI] [PubMed] [Google Scholar]
- 3.Basson R The female sexual response: A different model. Journal of Sex &Marital Therapy. 2000;26(1):51–65. [DOI] [PubMed] [Google Scholar]
- 4.Laan E, Both S. What makes women experience desire? Fem Psychol. 2008;18(4):505–14. [Google Scholar]
- 5.Both S, Everaerd W, Laan E. Desire emerges from excitement: A psychophysiological perspective on sexual motivation. The psychophysiology of sex. 2007:327–39. [Google Scholar]
- 6.Singer B, Toates FM. Sexual motivation. The Journal of Sex Research. 1987;23(4):481–501. [Google Scholar]
- 7.Heiman JR, Pfaff D. Sexual arousal and related concepts: An introduction. Horm Behav. 2011;59(5):613–5. [DOI] [PubMed] [Google Scholar]
- 8.Georgiadis JR, Kringelbach ML, Pfaus JG. Sex for fun: a synthesis of human and animal neurobiology. Nat Rev Urol. 2012;9(9):486–98. [DOI] [PubMed] [Google Scholar]
- 9.Farmer M, Huang L, Apkarian A. 005 Neural Networks Underlying Variants of Female Sexual Dysfunction. The Journal of Sexual Medicine. 2017;14(6):e352. [Google Scholar]
- 10.Basson R The Female Sexual Response: A Different Model. J Sex Marital Ther. 2000;26(1):51–65. [DOI] [PubMed] [Google Scholar]
- 11.Neurath MF, Finotto S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev. 2011;22(2):83–9. [DOI] [PubMed] [Google Scholar]
- 12.Holmdahl R, Malmström V, Burkhardt H. Autoimmune priming, tissue attack and chronic inflammation — The three stages of rheumatoid arthritis. Eur J Immunol. 2014;44(6):1593–9. [DOI] [PubMed] [Google Scholar]
- 13.Rosenblat JD, Cha DS, Mansur RB, McIntyre RS. Inflamed moods: a review of the interactions between inflammation and mood disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2014;53:23–34. [DOI] [PubMed] [Google Scholar]
- 14.Greaves E, Cousins FL, Murray A, Esnal-Zufiaurre A, Fassbender A, Horne AW, et al. A Novel Mouse Model of Endometriosis Mimics Human Phenotype and Reveals Insights into the Inflammatory Contribution of Shed Endometrium. The American Journal of Pathology. 2014;184(7):1930–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boots CE, Jungheim ES, editors. Inflammation and human ovarian follicular dynamics Semin Reprod Med; 2015: Thieme Medical Publishers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gnainsky Y, Granot I, Aldo P, Barash A, Or Y, Mor G, et al. Biopsy-induced inflammatory conditions improve endometrial receptivity: the mechanism of action. Reproduction. 2015;149(1):75–85. [DOI] [PubMed] [Google Scholar]
- 17.Brubaker D, Barbaro A, Chance MR, Mesiano S. A dynamical systems model of progesterone receptor interactions with inflammation in human parturition. BMC Syst Biol. 2016;10(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sawada M, Itoh Y, Suzumura A, Marunouchi T. Expression of cytokine receptors in cultured neuronal and glial cells. Neurosci Lett. 1993;160(2):131–4. [DOI] [PubMed] [Google Scholar]
- 19.Spangelo BL, Judd AM, Call GB, Zumwalt J, Gorospe WC. Role of the cytokines in the hypothalamic-pituitary-adrenal and gonadal axes. Neuroimmunomodulation. 1995;2(5):299–312. [DOI] [PubMed] [Google Scholar]
- 20.Michaud M, Balardy L, Moulis G, Gaudin C, Peyrot C, Vellas B, et al. Proinflammatory Cytokines, Aging, and Age-Related Diseases. J Am Med Dir Assoc. 2013;14(12):877–82. [DOI] [PubMed] [Google Scholar]
- 21.Akira S, Hirano T, Taga T, Kishimoto T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF). The FASEB Journal. 1990;4(11):2860–7. [PubMed] [Google Scholar]
- 22.Kelley KW, Bluthé R-M, Dantzer R, Zhou J-H, Shen W-H, Johnson RW, et al. Cytokine-induced sickness behavior. Brain, Behav, Immun. 2003;17(1):112–8. [DOI] [PubMed] [Google Scholar]
- 23.Schuerwegh AJ, Dombrecht EJ, Stevens WJ, Van Offel JF, Bridts CH, De Clerck LS. Influence of pro-inflammatory (IL-1α, IL-6, TNF-α, IFN-γ) and anti-inflammatory (IL-4) cytokines on chondrocyte function. Osteoarthritis Cartilage. 2003;11(9):681–7. [DOI] [PubMed] [Google Scholar]
- 24.Chatterjee P, Chiasson VL, Bounds KR, Mitchell BM. Regulation of the anti-inflammatory cytokines interleukin-4 and interleukin-10 during pregnancy. Front Immunol. 2014;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allen JE, Wynn TA. Evolution of Th2 Immunity: A Rapid Repair Response to Tissue Destructive Pathogens. PLoS Pathog. 2011;7(5):e1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao C, Sakata D, Esaki Y, Li Y, Matsuoka T, Kuroiwa K, et al. Prostaglandin E2-EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nat Med. 2009;15(6):633–40. [DOI] [PubMed] [Google Scholar]
- 27.Ricciotti E, FitzGerald GA. Prostaglandins and Inflammation. Atertio Thromb Vasc Biol. 2011;31(5):986–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poon DC-H, Ho Y-S, Chiu K, Wong H-L, Chang RC-C. Sickness: From the focus on cytokines, prostaglandins, and complement factors to the perspectives of neurons. Neurosci Biobehav Rev. 2015;57:30–45. [DOI] [PubMed] [Google Scholar]
- 29.Beyer C, González‐Flores O, González‐Mariscal G. Progesterone receptor participates in the stimulatory effect of LHRH, prostaglandin E2, and cyclic AMP on lordosis and proceptive behaviours in rats. J Neuroendocrinol. 1997;9(8):609–14. [DOI] [PubMed] [Google Scholar]
- 30.McCarthy MM, Nugent BM, Lenz KM. Neuroimmunology and neuroepigenetics in the establishment of sex differences in the brain. Nat Rev Neurosci. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Avitsur R, Weidenfeld J, Yirmiya R. Cytokines inhibit sexual behavior in female rats: II. Prostaglandins mediate the suppressive effects of interleukin-1β. Brain, Behav, Immun 1999;13(1):33–45. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein SW, Gonzalez JR, Gagnon C, Goldstein I. Peripheral Female Genital Arousal as Assessed by Thermography Following Topical Genital Application of Alprostadil vs Placebo Arousal Gel: A Proof-of-Principle Study Without Visual Sexual Stimulation. Sexual Medicine. 2016;4(3):e166–e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng JC, Secondary J, Burke WH, Fedoroff JP, Dwyer RG. Neuroimaging and Sexual Behavior: Identification of Regional and Functional Differences. Curr Psychiatry Rep. 2015;17(7):55. [DOI] [PubMed] [Google Scholar]
- 34.Patel A, Zhu Y, Kuzhikandathil EV, Banks WA, Siegel A, Zalcman SS. Soluble interleukin-6 receptor induces motor stereotypies and co-localizes with gp130 in regions linked to cortico-striato-thalamo-cortical circuits. PLoS One. 2012;7(7):e41623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rothaug M, Becker-Pauly C, Rose-John S. The role of interleukin-6 signaling in nervous tissue. Biochim Biophys Acta. 2016;1863(6):1218–27. [DOI] [PubMed] [Google Scholar]
- 36.Felger JC, Miller AH. Cytokine effects on the basal ganglia and dopamine function: The subcortical source of inflammatory malaise. Front Neuroendocrinol. 2012;33(3):315–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ye JH, Tao L, Zalcman SS. Interleukin-2 modulates N-methyl-d-aspartate receptors of native mesolimbic neurons. Brain Res. 2001;894(2):241–8. [DOI] [PubMed] [Google Scholar]
- 38.Prossin AR, Zalcman SS, Heitzeg MM, Koch AE, Campbell PL, Phan KL, et al. Dynamic Interactions Between Plasma IL-1 Family Cytokines and Central Endogenous Opioid Neurotransmitter Function in Humans. Neuropsychopharmacology. 2015;40(3):554–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yohn SE, Arif Y, Haley A, Tripodi G, Baqi Y, Müller CE, et al. Effort-related motivational effects of the pro-inflammatory cytokine interleukin-6: pharmacological and neurochemical characterization. Psychopharmacology (Berl). 2016;233(19–20):3575–86. [DOI] [PubMed] [Google Scholar]
- 40.Pollmächer T, Haack M, Schuld A, Reichenberg A, Yirmiya R. Low levels of circulating inflammatory cytokines—Do they affect human brain functions? Brain, Behav, Immun 2002;16(5):525–32. [DOI] [PubMed] [Google Scholar]
- 41.Yirmiya R, Avitsur R, Donchin O, Cohen E. Interleukin-1 Inhibits Sexual Behavior in Female but Not in Male Rats. Brain, Behav, Immun 1995;9(3):220–33. [DOI] [PubMed] [Google Scholar]
- 42.Avitsur R, Pollak Y, Yirmiya R. Different receptor mechanisms mediate the effects of endotoxin and interleukin-1 on female sexual behavior. Brain Res. 1997;773(1):149–61. [DOI] [PubMed] [Google Scholar]
- 43.Avitsur R, Yirmiya R. Cytokines Inhibit Sexual Behavior in Female Rats: I. Synergistic Effects of Tumor Necrosis Factor α and Interleukin-1. Brain, Behav, Immun 1999;13(1):14–32.•• This classic article outlines a series of studies showing the sex differences in the effect of inflammatory cyotkines on sexual behavior and sexual reward processing.
- 44.Lorenz T, Heiman J. Inflammation may alter perception of sexual arousal and pleasure in healthy women International Academy of Sex Research; July; Charleston, SC2017. [Google Scholar]
- 45.Jokinen J, Chatzittofis A, Nordström P, Arver S. The role of neuroinflammation in the pathophysiology of hypersexual disorder. Psychoneuroendocrinology. 2016;71:55. [DOI] [PubMed] [Google Scholar]
- 46.Haake P, Krueger TH, Goebel MU, Heberling KM, Hartmann U, Schedlowski M. Effects of sexual arousal on lymphocyte subset circulation and cytokine production in man. Neuroimmunomodulation. 2004;11(5):293–8. [DOI] [PubMed] [Google Scholar]
- 47.Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12(2):123–37. [DOI] [PubMed] [Google Scholar]
- 48.Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21(2):153–60.•• A classic review of the research on the effects of immune activation on the nervous system and behavior, including sexual behavior.
- 49.McKean KA, Nunney L. Bateman’s principle and immunity: phenotypically plastic reproductive strategies predict changes in immunological sex differences. Evolution. 2005;59(7):1510–7. [PubMed] [Google Scholar]
- 50.Chisholm JS, Ellison PT, Evans J, Lee P, Lieberman LS, Pavlik Z, et al. Death, hope, and sex: life-history theory and the development of reproductive strategies [and comments and reply]. Curr Anthrop. 1993;34(1):1–24. [Google Scholar]
- 51.Lochmiller RL, Deerenberg C. Trade‐offs in evolutionary immunology: Just what is the cost of immunity? Oikos. 2000;88(1):87–98. [Google Scholar]
- 52.Lorenz T, Worthman C, Vitzthum VJ. Links between inflammation, sexual activity and ovulation: Evolutionary trade-offs and clinical implications. Evolution, Medicine, and Public Health. 2015;1:304–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vitzthum VJ. The ecology and evolutionary endocrinology of reproduction in the human female. Am J Phys Anthropol. 2009;140(S49):95–136. [DOI] [PubMed] [Google Scholar]
- 54.Curtis V, Aunger R, Rabie T. Evidence that disgust evolved to protect from risk of disease. Proc R Soc Lond B Biol Sci. 2004;271(Suppl 4):S131–S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller SL, Maner JK. Sick Body, Vigilant Mind. Psychol Sci. 2011;22(12):1467–71. [DOI] [PubMed] [Google Scholar]
- 56.Stevenson RJ, Hodgson D, Oaten MJ, Sominsky L, Mahmut M, Case TI. Oral Immune Activation by Disgust and Disease-Related Pictures. Journal of Psychophysiology. 2015;29(3):119–29. [Google Scholar]
- 57.Oaten MJ, Stevenson RJ, Case TI. Compensatory up-regulation of behavioral disease avoidance in immuno-compromised people with rheumatoid arthritis. Evolution and Human Behavior. 2017;38(3):350–6. [Google Scholar]
- 58.de Jong PJ, van Overveld M, Borg C. Giving In to Arousal or Staying Stuck in Disgust? Disgust-Based Mechanisms in Sex and Sexual Dysfunction. The Journal of Sex Research. 2013;50(3–4):247–62. [DOI] [PubMed] [Google Scholar]
- 59.Zsok F, Fleischman DS, Borg C, Morrison E. Disgust Trumps Lust: Women’s Disgust and Attraction Towards Men Is Unaffected by Sexual Arousal. Evolutionary Psychological Science. 2017. [Google Scholar]
- 60.Fleischman DS, Hamilton LD, Fessler DMT, Meston CM. Disgust versus Lust: Exploring the Interactions of Disgust and Fear with Sexual Arousal in Women. PLoS One. 2015;10(6):e0118151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andrews AR, Crone T, Cholka CB, Cooper TV, Bridges AJ. Correlational and experimental analyses of the relation between disgust and sexual arousal. Motiv Emotion. 2015;39(5):766–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee EM, Ambler JK, Sagarin BJ. Effects of Subjective Sexual Arousal on Sexual, Pathogen, and Moral Disgust Sensitivity in Women and Men. Arch Sex Behav. 2014;43(6):1115–21. [DOI] [PubMed] [Google Scholar]
- 63.Grauvogl A, de Jong P, Peters M, Evers S, van Overveld M, van Lankveld J. Disgust and Sexual Arousal in Young Adult Men and Women. Arch Sex Behav. 2015;44(6):1515–25. [DOI] [PubMed] [Google Scholar]
- 64.Fleischman DS. Women’s Disgust Adaptations In: Weekes-Shackelford VA, Shackelford TK, editors. Evolutionary Perspectives on Human Sexual Psychology and Behavior. New York, NY: Springer New York; 2014. p. 277–96. [Google Scholar]
- 65.Borg C, de Jong PJ, Elgersma H. Sexual aversion and the DSM-5: An excluded disorder with unabated relevance as a trans-diagnostic symptom. Arch Sex Behav. 2014;43(7):1219–23. [DOI] [PubMed] [Google Scholar]
- 66.Segner H, Verburg-van Kemenade BML, Chadzinska M. The immunomodulatory role of the hypothalamus-pituitary-gonad axis: Proximate mechanism for reproduction-immune trade offs? Dev Comp Immunol. 2017;66:43–60.• This review summarizes the state of the literature on the co-regulation of inflammation and gonadal hormones as related to reproductive and sexual function.
- 67.Molvarec A, Szarka A, Walentin S, Bekő G, Karádi I, Prohászka Z, et al. Serum leptin levels in relation to circulating cytokines, chemokines, adhesion molecules and angiogenic factors in normal pregnancy and preeclampsia. Reprod Biol Endocrinol. 2011;9(1):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sylvia KE, Lorenz TK, Heiman JR, Demas GE. Physiological predictors of leptin vary during menses and ovulation in healthy women. Reprod Biol. 2018;18(1):132–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iwasa T, Matsuzaki T, Murakami M, Shimizu F, Kuwahara A, Yasui T, et al. Decreased expression of kisspeptin mediates acute immune/inflammatory stress-induced suppression of gonadotropin secretion in female rat. J Endocrinol Invest. 2008;31(7):656–9. [DOI] [PubMed] [Google Scholar]
- 70.Straub RH. The Complex Role of Estrogens in Inflammation. Endocr Rev. 2007;28(5):521–74. [DOI] [PubMed] [Google Scholar]
- 71.Calabrese EJ. Estrogen and related compounds: biphasic dose responses. Crit Rev Toxicol. 2001;31(4–5):503–15. [DOI] [PubMed] [Google Scholar]
- 72.Chakrabarti S, Lekontseva O, Davidge ST. Estrogen is a modulator of vascular inflammation. IUBMB Life. 2008;60(6):376–82. [DOI] [PubMed] [Google Scholar]
- 73.Puder JJ, Freda PU, Goland RS, Wardlaw SL. Estrogen Modulates the Hypothalamic-Pituitary-Adrenal and Inflammatory Cytokine Responses to Endotoxin in Women1. The Journal of Clinical Endocrinology & Metabolism. 2001;86(6):2403–8. [DOI] [PubMed] [Google Scholar]
- 74.Rogers A, Eastell R. The effect of 17β-estradiol on production of cytokines in cultures of peripheral blood. Bone. 2001;29(1):30–4. [DOI] [PubMed] [Google Scholar]
- 75.Kim M-S, Chae H-J, Shin T-Y, Kim H-M, Kim H-R. Estrogen regulates cytokine release in human mast cells. Immunopharmacol Immunotoxicol. 2001;23(4):495–504. [DOI] [PubMed] [Google Scholar]
- 76.Crane-Godreau MA, Wira CR. Effects of estradiol on lipopolysaccharide and Pam3Cys stimulation of CCL20/macrophage inflammatory protein 3 alpha and tumor necrosis factor alpha production by uterine epithelial cells in culture. Infect Immun. 2005;73(7):4231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ray P, Ghosh SK, Zhang D-H, Ray A. Repression of interleukin‐6 gene expression by 17β‐estradiol. FEBS Lett. 1997;409(1):79–85. [DOI] [PubMed] [Google Scholar]
- 78.Shivers K-Y, Amador N, Abrams L, Hunter D, Jenab S, Quiñones-Jenab V. Estrogen alters baseline and inflammatory-induced cytokine levels independent from hypothalamic–pituitary–adrenal axis activity. Cytokine. 2015;72(2):121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Villa A, Rizzi N, Vegeto E, Ciana P, Maggi A. Estrogen accelerates the resolution of inflammation in macrophagic cells. Sci Rep. 2015;5:15224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.STOCK JL, CODERRE JA, McDONALD B, ROSENWASSER LJ. Effects of estrogen in vivo and in vitro on spontaneous interleukin-1 release by monocytes from postmenopausal women. The Journal of Clinical Endocrinology & Metabolism. 1989;68(2):364–8. [DOI] [PubMed] [Google Scholar]
- 81.Shanker G, Sorci-Thomas M, Register T, Adams M. The inducible expression of THP-1 cell interleukin-1 mRNA: effects of estrogen on differential response to phorbol ester and lipopolysaccharide. Lymphokine Cytokine Res. 1994;13(1):1–7. [PubMed] [Google Scholar]
- 82.POLAN ML, LOUKIDES J, NELSON P, CARDING S, DIAMOND M, WALSH A, et al. Progesterone and estradiol modulate interleukin-1 β messenger ribonucleic acid levels in cultured human peripheral monocytes. The Journal of Clinical Endocrinology & Metabolism. 1989;69(6):1200–6. [DOI] [PubMed] [Google Scholar]
- 83.Polan ML, Daniele A, Kuo A. Gonadal steroids modulate human monocyte interleukin-1 (IL-1) activity. Fertil Steril. 1988;49(6):964–8. [PubMed] [Google Scholar]
- 84.Bamberger C, Schulte H. Molecular mechanisms of dissociative glucocorticoid activity. Eur J Clin Invest. 2000;30(s3):6–9. [DOI] [PubMed] [Google Scholar]
- 85.Tibbetts TA, Conneely OM, O’Malley BW. Progesterone via Its Receptor Antagonizes the Pro-Inflammatory Activity of Estrogen in the Mouse Uterus1. Biol Reprod. 1999;60(5):1158–65. [DOI] [PubMed] [Google Scholar]
- 86.Huang H, He J, Yuan Y, Aoyagi E, Takenaka H, Itagaki T, et al. Opposing effects of estradiol and progesterone on the oxidative stress-induced production of chemokine and proinflammatory cytokines in murine peritoneal macrophages. The Journal of Medical Investigation. 2008;55(1,2):133–41. [DOI] [PubMed] [Google Scholar]
- 87.Battaglia DF, Bowen JM, Krasa HB, Thrun LA, Viguié C, Karsch FJ. Endotoxin Inhibits the Reproductive Neuroendocrine Axis While Stimulating Adrenal Steroids: A Simultaneous View from Hypophyseal Portal and Peripheral Blood. Endocrinology. 1997;138(10):4273–81. [DOI] [PubMed] [Google Scholar]
- 88.Lainez N, Coss D. Obesity-Induced Cytokines Diminish Gonadotropin-Releasing Hormone Gene Expression. Kisspeptin-GnRH-Gonadotrope Axis: Endocrine Society; 2015. p. PP16–1-PP-1. [Google Scholar]
- 89.Song C, Wang H. Cytokines mediated inflammation and decreased neurogenesis in animal models of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(3):760–8. [DOI] [PubMed] [Google Scholar]
- 90.Camille Melón L, Maguire J. GABAergic regulation of the HPA and HPG axes and the impact of stress on reproductive function. The Journal of Steroid Biochemistry and Molecular Biology. 2016;160:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.SAKUMOTO R, SHIBAYA M, OKUDA K. Tumor Necrosis Factor-α (TNF α) Inhibits Progesterone and Estradiol-17β Production from Cultured Granulosa Cells: Presence of TNFα Receptors in Bovine Granulosa and Theca Cells. J Reprod Dev. 2003;49(6):441–9. [DOI] [PubMed] [Google Scholar]
- 92.Williams EJ, Sibley K, Miller AN, Lane EA, Fishwick J, Nash DM, et al. ORIGINAL ARTICLE: The Effect of Escherichia coli Lipopolysaccharide and Tumour Necrosis Factor Alpha on Ovarian Function. Am J Reprod Immunol. 2008;60(5):462–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Terranova PF, Rice VM. Review: Cytokine Involvement in Ovarian Processes. Am J Reprod Immunol. 1997;37(1):50–63. [DOI] [PubMed] [Google Scholar]
- 94.Elovitz M, Wang Z. Medroxyprogesterone acetate, but not progesterone, protects against inflammation-induced parturition and intrauterine fetal demise. Am J Obstet Gynecol. 2004;190(3):693–701. [DOI] [PubMed] [Google Scholar]
- 95.Purohit A, Newman SP, Reed MJ. The role of cytokines in regulating estrogen synthesis: implications for the etiology of breast cancer. Breast Cancer Res. 2002;4(2):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Al-Safi ZA, Chosich J, Berenbaum K, Bradford A, Santoro N, Polotsky AJ. Reduction of pro-inflammatory cytokines is associated with improvement of gonadotropin sensitivity in obese women. Fertil Steril.102(3):e3.24907914 [Google Scholar]
- 97.Puri J, Hutchins B, Bellinger LL, Kramer PR. Estrogen and inflammation modulate estrogen receptor alpha expression in specific tissues of the temporomandibular joint. Reproductive Biology and Endocrinology : RB&E. 2009;7:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Goodman MP. Are All Estrogens Created Equal? A Review of Oral vs. Transdermal Therapy. J Womens Health. 2011;21(2):161–9. [DOI] [PubMed] [Google Scholar]
- 99.Störk S, von Schacky C, Angerer P. The effect of 17β-estradiol on endothelial and inflammatory markers in postmenopausal women: a randomized, controlled trial. Atherosclerosis. 2002;165(2):301–7. [DOI] [PubMed] [Google Scholar]
- 100.Shifren JL, Rifai N, Desindes S, McIlwain M, Doros G, Mazer NA. A Comparison of the Short-Term Effects of Oral Conjugated Equine Estrogens Versus Transdermal Estradiol on C-Reactive Protein, Other Serum Markers of Inflammation, and Other Hepatic Proteins in Naturally Menopausal Women. The Journal of Clinical Endocrinology & Metabolism. 2008;93(5):1702–10. [DOI] [PubMed] [Google Scholar]
- 101.Pastor Z, Holla K, Chmel R. The influence of combined oral contraceptives on female sexual desire: A systematic review. The European Journal of Contraception & Reproductive Health Care. 2013;18(1):27–43. [DOI] [PubMed] [Google Scholar]
- 102.Alexander JL, Kotz K, Dennerstein L, Kutner SJ, Wallen K, Notelovitz M. The effects of postmenopausal hormone therapies on female sexual functioning: a review of double-blind, randomized controlled trials. Menopause. 2004;11(6):749–65. [DOI] [PubMed] [Google Scholar]
- 103.Sanders SA, Graham CA, Bass JL, Bancroft J. A prospective study of the effects of oral contraceptives on sexuality and well-being and their relationship to discontinuation. Contraception. 2001;64(1):51–8. [DOI] [PubMed] [Google Scholar]
- 104.Wallwiener M, Wallwiener L-M, Seeger H, Mueck AO, Zipfel S, Bitzer J, et al. Effects of sex hormones in oral contraceptives on the female sexual function score: a study in German female medical students. Contraception. 2010;82(2):155–9. [DOI] [PubMed] [Google Scholar]
- 105.Zethraeus N, Dreber A, Ranehill E, Blomberg L, Labrie F, von Schoultz B, et al. Combined Oral Contraceptives and Sexual Function in Women—a Double-Blind, Randomized, Placebo-Controlled Trial. The Journal of Clinical Endocrinology & Metabolism. 2016;101(11):4046–53. [DOI] [PubMed] [Google Scholar]
- 106.González M, Viáfara G, Caba F, Molina E. Sexual function, menopause and hormone replacement therapy (HRT). Maturitas. 2004;48(4):411–20. [DOI] [PubMed] [Google Scholar]
- 107.Nappi RE, Polatti F. Continuing Medical Education: The Use of Estrogen Therapy in Women’s Sexual Functioning (CME). The Journal of Sexual Medicine. 2009;6(3):603–16. [DOI] [PubMed] [Google Scholar]
- 108.Klein SL. The effects of hormones on sex differences in infection: from genes to behavior. Neurosci Biobehav Rev. 2000;24(6):627–38. [DOI] [PubMed] [Google Scholar]
- 109.Konecna L, Yan MS, Miller LE, Schölmerich J, Falk W, Straub RH. Modulation of IL-6 production during the menstrual cycle in vivo and in vitro. Brain, Behav, Immun 2000;14(1):49–61. [DOI] [PubMed] [Google Scholar]
- 110.Maggio M, Ceda GP, Lauretani F, Bandinelli S, Corsi AM, Giallauria F, et al. SHBG, sex hormones, and inflammatory markers in older women. The Journal of Clinical Endocrinology & Metabolism. 2011;96(4):1053–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maggio M, Basaria S, Ble A, Lauretani F, Bandinelli S, Ceda GP, et al. Correlation between testosterone and the inflammatory marker soluble interleukin-6 receptor in older men. The Journal of Clinical Endocrinology & Metabolism. 2006;91(1):345–7. [DOI] [PubMed] [Google Scholar]
- 112.Joffe HV, Ridker PM, Manson JE, Cook NR, Buring JE, Rexrode KM. Sex hormone-binding globulin and serum testosterone are inversely associated with C-reactive protein levels in postmenopausal women at high risk for cardiovascular disease. Ann Epidemiol. 2006;16(2):105–12. [DOI] [PubMed] [Google Scholar]
- 113.Maturana MA, Breda V, Lhullier F, Spritzer PM. Relationship between endogenous testosterone and cardiovascular risk in early postmenopausal women. Metabolism. 2008;57(7):961–5. [DOI] [PubMed] [Google Scholar]
- 114.Prasad A, Mumford SL, Buck Louis GM, Ahrens KA, Sjaarda LA, Schliep KC, et al. Sexual activity, endogenous reproductive hormones and ovulation in premenopausal women. Horm Behav. 2014;66(2):330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lorenz T, Heiman JR, Demas GE. Testosterone and immune-reproductive tradeoffs in healthy women. Horm Behav. 2017;88:122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Posma E, Moes H, Heineman MJ, Faas MM. The Effect of Testosterone on Cytokine Production in the Specific and Non‐specific Immune Response. Am J Reprod Immunol. 2004;52(4):237–43. [DOI] [PubMed] [Google Scholar]
- 117.Corcoran MP, Meydani M, Lichtenstein AH, Schaefer EJ, Dillard A, Lamon-Fava S. Sex hormone modulation of proinflammatory cytokine and C-reactive protein expression in macrophages from older men and postmenopausal women. J Endocrinol. 2010;206(2):217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Huang G, Tang E, Aakil A, Anderson S, Jara H, Davda M, et al. Testosterone dose-response relationships with cardiovascular risk markers in androgen-deficient women: a randomized, placebo-controlled trial. The Journal of Clinical Endocrinology & Metabolism. 2014;99(7):E1287–E93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Herbst KL, Calof OM, Hsia SH, Sinha-Hikim I, Woodhouse LJ, Buchanan TA, et al. Effects of transdermal testosterone administration on insulin sensitivity, fat mass and distribution, and markers of inflammation and thrombolysis in human immunodeficiency virus–infected women with mild to moderate weight loss. Fertil Steril. 2006;85(6):1794–802. [DOI] [PubMed] [Google Scholar]
- 120.Stephenson K, Neuenschwander PF, Kurdowska AK. The effects of compounded bioidentical transdermal hormone therapy on hemostatic, inflammatory, immune factors; cardiovascular biomarkers; quality-of-life measures; and health outcomes in perimenopausal and postmenopausal women. International journal of pharmaceutical compounding. 2013;17(1):74–85. [PubMed] [Google Scholar]
- 121.Tsigos C, Papanicolaou DA, Kyrou I, Raptis SA, Chrousos GP. Dose-dependent effects of recombinant human interleukin-6 on the pituitary-testicular axis. J Interferon Cytokine Res. 1999;19(11):1271–6. [DOI] [PubMed] [Google Scholar]
- 122.van der Poll T, Romijn JA, Endert E, Sauerwein HP. Effects of tumor necrosis factor on the hypothalamic-pituitary-testicular axis in healthy men. Metabolism. 1993;42(3):303–7. [DOI] [PubMed] [Google Scholar]
- 123.Benson S, Janssen O, Hahn S, Tan S, Dietz T, Mann K, et al. Obesity, depression, and chronic low-grade inflammation in women with polycystic ovary syndrome. Brain, Behav, Immun 2008;22(2):177–84. [DOI] [PubMed] [Google Scholar]
- 124.Purohit A, Ghilchik MW, Duncan L, Wang DY, Singh A, Walker MM, et al. Aromatase activity and interleukin-6 production by normal and malignant breast tissues. The Journal of Clinical Endocrinology & Metabolism. 1995;80(10):3052–8. [DOI] [PubMed] [Google Scholar]
- 125.Macdiarmid F, Wang D, Duncan LJ, Purohit A, Ghilchik MW, Reed MJ. Stimulation of aromatase activity in breast fibroblasts by tumor necrosis factor. Mol Cell Endocrinol. 1994;106(1):17–21. [DOI] [PubMed] [Google Scholar]
- 126.Wåhlin-Jacobsen S, Pedersen AT, Kristensen E, Læssøe NC, Lundqvist M, Cohen AS, et al. Is There a Correlation Between Androgens and Sexual Desire in Women? The Journal of Sexual Medicine. 2015;12(2):358–73. [DOI] [PubMed] [Google Scholar]
- 127.Leiblum S, Bachmann G, Kemmann E, Colburn D, Swartzman L. Vaginal atrophy in the postmenopausal woman: the importance of sexual activity and hormones. JAMA. 1983;249(16):2195–8. [PubMed] [Google Scholar]
- 128.Goldey KL, van Anders SM. Sexy thoughts: Effects of sexual cognitions on testosterone, cortisol, and arousal in women. Horm Behav. 2011;59(5):754–64. [DOI] [PubMed] [Google Scholar]
- 129.van Anders S Testosterone and Sexual Desire in Healthy Women and Men. Arch Sex Behav. 2012;41(6):1471–84. [DOI] [PubMed] [Google Scholar]
- 130.Goldey KL, van Anders S. Sexy thoughts: Effects of sexual cognitions on testosterone, cortisol, and arousal in women. Horm Behav. 2011;59(5):754–64. [DOI] [PubMed] [Google Scholar]
- 131.Roney JR, Simmons ZL. Hormonal predictors of sexual motivation in natural menstrual cycles. Horm Behav. 2013;63(4):636–45. [DOI] [PubMed] [Google Scholar]
- 132.Van Anders S, Brotto L, Farrell J, Yule M. Associations Among Physiological and Subjective Sexual Response, Sexual Desire, and Salivary Steroid Hormones in Healthy Premenopausal Women. The Journal of Sexual Medicine. 2009;6(3):739–51. [DOI] [PubMed] [Google Scholar]
- 133.van Anders S, Watson NV. Testosterone levels in women and men who are single, in long-distance relationships, or same-city relationships. Horm Behav. 2007;51(2):286–91. [DOI] [PubMed] [Google Scholar]
- 134.Van Anders S, Goldey KL. Testosterone and partnering are linked via relationship status for women and ‘relationship orientation’for men. Horm Behav. 2010;58(5):820–6. [DOI] [PubMed] [Google Scholar]
- 135.Elraiyah T, Sonbol MB, Wang Z, Khairalseed T, Asi N, Undavalli C, et al. The Benefits and Harms of Systemic Testosterone Therapy in Postmenopausal Women With Normal Adrenal Function: A Systematic Review and Meta-analysis. The Journal of Clinical Endocrinology & Metabolism. 2014;99(10):3543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Achilli C, Pundir J, Ramanathan P, Sabatini L, Hamoda H, Panay N. Efficacy and safety of transdermal testosterone in postmenopausal women with hypoactive sexual desire disorder: a systematic review and meta-analysis. Fertil Steril. 2017;107(2):475–82.e15. [DOI] [PubMed] [Google Scholar]
- 137.Reed BG, Bou Nemer L, Carr BR. Has testosterone passed the test in premenopausal women with low libido? A systematic review. International Journal of Women’s Health. 2016;8:599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cappelletti M, Wallen K. Increasing women’s sexual desire: The comparative effectiveness of estrogens and androgens. Horm Behav. 2016;78:178–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Blaicher W, Gruber D, Bieglmayer C, Blaicher AM, Knogler W, Huber JC. The Role of Oxytocin in Relation to Female Sexual Arousal. Gynecol Obstet Invest. 1999;47(2):125–6. [DOI] [PubMed] [Google Scholar]
- 140.Carmichael MS, Warburton VL, Dixen J, Davidson JM. Relationships among cardiovascular, muscular, and oxytocin responses during human sexual activity. Arch Sex Behav. 1994;23(1):59–79. [DOI] [PubMed] [Google Scholar]
- 141.Carter CS. Oxytocin and sexual behavior. Neurosci Biobehav Rev. 1992;16(2):131–44. [DOI] [PubMed] [Google Scholar]
- 142.Caruso S, Mauro D, Scalia G, Palermo CI, Rapisarda AMC, Cianci A. Oxytocin plasma levels in orgasmic and anorgasmic women. Gynecol Endocrinol. 2017:1–4. [DOI] [PubMed] [Google Scholar]
- 143.Clodi M, Vila G, Geyeregger R, Riedl M, Stulnig TM, Struck J, et al. Oxytocin alleviates the neuroendocrine and cytokine response to bacterial endotoxin in healthy men. American Journal of Physiology-Endocrinology and Metabolism. 2008;295(3):E686–E91. [DOI] [PubMed] [Google Scholar]
- 144.Szeto A, Sun-Suslow N, Mendez AJ, Hernandez RI, Wagner KV, McCabe PM. Regulation of the macrophage oxytocin receptor in response to inflammation. Am J Physiol Endocrinol Metab. 2017;312(3):E183–E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yuan L, Liu S, Bai X, Gao Y, Liu G, Wang X, et al. Oxytocin inhibits lipopolysaccharide-induced inflammation in microglial cells and attenuates microglial activation in lipopolysaccharide-treated mice. J Neuroinflammation. 2016;13(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sprague AH, Khalil RA. Inflammatory Cytokines in Vascular Dysfunction and Vascular Disease. Biochem Pharmacol. 2009;78(6):539–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Huang AL, Vita JA. Effects of Systemic Inflammation on Endothelium-Dependent Vasodilation. Trends Cardiovasc Med. 2006;16(1):15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bouloukaki I, Papadimitriou V, Sofras F, Mermigkis C, Moniaki V, Siafakas NM, et al. Abnormal Cytokine Profile in Patients with Obstructive Sleep Apnea-Hypopnea Syndrome and Erectile Dysfunction. Mediators Inflamm. 2014;2014:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Carneiro FS, Webb RC, Tostes RC. Emerging Role for TNF‐α in Erectile Dysfunction. The journal of sexual medicine. 2010;7(12):3823–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Carneiro FS, Zemse S, Giachini FR, Carneiro ZN, Lima VV, Webb RC, et al. TNF‐α infusion impairs corpora cavernosa reactivity. The journal of sexual medicine. 2009;6(S3):311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Matos G, Hirotsu C, Alvarenga TA, Cintra F, Bittencourt L, Tufik S, et al. The association between TNF-α and erectile dysfunction complaints. Andrology. 2013;1(6):872–8. [DOI] [PubMed] [Google Scholar]
- 152.Carneiro FS, Sturgis LC, Giachini FRC, Carneiro ZN, Lima VV, Wynne BM, et al. TNF-α Knockout Mice Have Increased Corpora Cavernosa Relaxation. The Journal of Sexual Medicine. 2009;6(1):115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Munarriz R, Kim SW, Kim NN, Traish A, Goldstein I. A Review of the Physiology and Pharmacology of Peripheral (Vaginal and Clitoral) Female Genital Arousal in the Animal Model. The Journal of Urology. 2003;170(2):S40–S5. [DOI] [PubMed] [Google Scholar]
- 154.Wesselmann U, Bonham A, Foster D. Vulvodynia: Current state of the biological science. Pain. 2014;155(9):1696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pukall CF, Cahill CM. New developments in the pathophysiology of genital pain: Role of central sensitization. Current Sexual Health Reports. 2014;6(1):11–9. [Google Scholar]
- 156.Fugl‐Meyer KS, Bohm‐Starke N, Damsted Petersen C, Fugl‐Meyer A, Parish S, Giraldi A. Standard operating procedures for female genital sexual pain. The journal of sexual medicine. 2013;10(1):83–93. [DOI] [PubMed] [Google Scholar]
- 157.Masheb RM, Lozano-Blanco C, Kohorn EI, Minkin MJ, Kerns RD. Assessing Sexual Function and Dyspareunia with the Female Sexual Function Index (FSFI) in Women with Vulvodynia. J Sex Marital Ther. 2004;30(5):315–24. [DOI] [PubMed] [Google Scholar]
- 158.Avitsur R, Cohen E, Yirmiya R. Effects of Interleukin-1 on Sexual Attractivity in a Model of Sickness Behavior. Physiol Behav. 1997;63(1):25–30. [DOI] [PubMed] [Google Scholar]
- 159.Klein SL, Nelson RJ. Activation of the immune–endocrine system with lipopolysaccharide reduces affiliative behaviors in voles. Behav Neurosci. 1999;113(5):1042–8. [DOI] [PubMed] [Google Scholar]
- 160.Olsson MJ, Lundström JN, Kimball BA, Gordon AR, Karshikoff B, Hosseini N, et al. The scent of disease: human body odor contains an early chemosensory cue of sickness. Psychol Sci. 2014;25(3):817–23. [DOI] [PubMed] [Google Scholar]
- 161.Shirasu M, Touhara K. The scent of disease: volatile organic compounds of the human body related to disease and disorder. The Journal of Biochemistry. 2011;150(3):257–66. [DOI] [PubMed] [Google Scholar]
- 162.Regenbogen C, Axelsson J, Lasselin J, Porada DK, Sundelin T, Peter MG, et al. Behavioral and neural correlates to multisensory detection of sick humans. Proceedings of the National Academy of Sciences. 2017;114(24):6400–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Baumeister RF. Gender differences in erotic plasticity: the female sex drive as socially flexible and responsive. Psychol Bull. 2000;126(3):347. [DOI] [PubMed] [Google Scholar]
- 164.Dong J-Y, Zhang Y-H, Qin L-Q. Erectile dysfunction and risk of cardiovascular disease: meta-analysis of prospective cohort studies. J Am Coll Cardiol. 2011;58(13):1378–85. [DOI] [PubMed] [Google Scholar]
- 165.Vlachopoulos C, Aznaouridis K, Ioakeimidis N, Rokkas K, Vasiliadou C, Alexopoulos N, et al. Unfavourable endothelial and inflammatory state in erectile dysfunction patients with or without coronary artery disease. Eur Heart J. 2006;27(22):2640–8. [DOI] [PubMed] [Google Scholar]
- 166.Giugliano F, Esposito K, Di Palo C, Ciotola M, Giugliano G, Marfella R, et al. Erectile dysfunction associates with endothelial dysfunction and raised proinflammatory cytokine levels in obese men. J Endocrinol Invest. 2004;27(7):665–9. [DOI] [PubMed] [Google Scholar]
- 167.Luciano Rodrigues F, Sobrano Fais R, C Tostes R, S Carneiro F. There is a link between erectile dysfunction and heart failure: it could be inflammation. Curr Drug Targets. 2015;16(5):442–50. [DOI] [PubMed] [Google Scholar]
- 168.Drory Y, Kravetz S, Weingarten M, Infarction ISGoFAM. Comparison of sexual activity of women and men after a first acute myocardial infarction. The American journal of cardiology. 2000;85(11):1283–7. [DOI] [PubMed] [Google Scholar]
- 169.Steinke EE. Sexual Dysfunction in Women With Cardiovascular Disease: What Do We Know? J Cardiovasc Nurs. 2010;25(2):151–8. [DOI] [PubMed] [Google Scholar]
- 170.Oren A, Megiddo E, Banai S, Justo D. Sexual dysfunction, cardiovascular risk factors, and inflammatory biomarkers in women undergoing coronary angiography. J Women Aging. 2016;28(3):203–10. [DOI] [PubMed] [Google Scholar]
- 171.Doumas M, Tsiodras S, Tsakiris A, Douma S, Chounta A, Papadopoulos A, et al. Female sexual dysfunction in essential hypertension: a common problem being uncovered. J Hypertens. 2006;24(12):2387–92. [DOI] [PubMed] [Google Scholar]
- 172.Thomas HN, Evans GW, Berlowtiz DR, Chertow GM, Conroy MB, Foy CG, et al. Antihypertensive medications and sexual function in women: Baseline data from the Systolic Blood Pressure Intervention Trial (SPRINT). J Hypertens. 2016;34(6):1224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105(2):141–50. [DOI] [PubMed] [Google Scholar]
- 174.Martelli V, Valisella S, Moscatiello S, Matteucci C, Lantadilla C, Costantino A, et al. Prevalence of Sexual Dysfunction among Postmenopausal Women with and without Metabolic Syndrome. The Journal of Sexual Medicine. 2012;9(2):434–41. [DOI] [PubMed] [Google Scholar]
- 175.Trompeter SE, Bettencourt R, Barrett-Connor E. Metabolic Syndrome and Sexual Function in Postmenopausal Women. The American Journal of Medicine. 2016;129(12):1270–7.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Esposito K, Maiorino MI, Bellastella G, Giugliano F, Romano M, Giugliano D. Determinants of female sexual dysfunction in type 2 diabetes. Int J Impot Res. 2010;22(3):179–84. [DOI] [PubMed] [Google Scholar]
- 177.Otunctemur A, Dursun M, Ozbek E, Sahin S, Besiroglu H, Koklu I, et al. Effect of Metabolic Syndrome on Sexual Function in Pre- and Postmenopausal Women. J Sex Marital Ther. 2015;41(4):440–9. [DOI] [PubMed] [Google Scholar]
- 178.Ponholzer A, Temml C, Rauchenwald M, Marszalek M, Madersbacher S. Is the metabolic syndrome a risk factor for female sexual dysfunction in sexually active women? Int J Impot Res. 2008;20(1):100. [DOI] [PubMed] [Google Scholar]
- 179.Esposito K, Ciotola M, Marfella R, Di Tommaso D, Cobellis L, Giugliano D. The metabolic syndrome: a cause of sexual dysfunction in women. Int J Impot Res. 2005;17(3):224. [DOI] [PubMed] [Google Scholar]
- 180.Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005;6(7):521. [DOI] [PubMed] [Google Scholar]
- 181.Timmer A, Bauer A, Dignass A, Rogler G. Sexual function in persons with inflammatory bowel disease: a survey with matched controls. Clin Gastroenterol Hepatol. 2007;5. [DOI] [PubMed] [Google Scholar]
- 182.Timmer A, Kemptner D, Bauer A, Takses A, Ott C, Fürst A. Determinants of female sexual function in inflammatory bowel disease: a survey based cross-sectional analysis. BMC Gastroenterol. 2008;8(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhang Q, Zhou C, Chen H, Zhao Q, Li L, Cui Y, et al. Rheumatoid arthritis is associated with negatively variable impacts on domains of female sexual function: evidence from a systematic review and meta-analysis. Psychol Health Med. 2017:1–12. [DOI] [PubMed] [Google Scholar]
- 184.Aydin G, Başar MM, Keleş I, Ergün G, Orkun Sm, Batislam E. Relationship between sexual dysfunction and psychiatric status in premenopausal women with fibromyalgia. Urology. 2006;67(1):156–61. [DOI] [PubMed] [Google Scholar]
- 185.Reed BD, Haefner HK, Punch MR, Roth RS, Gorenflo DW, Gillespie BW. Psychosocial and sexual functioning in women with vulvodynia and chronic pelvic pain. A comparative evaluation. The Journal of reproductive medicine. 2000;45(8):624–32. [PubMed] [Google Scholar]
- 186.Lin M-C, Lu M-C, Livneh H, Lai N-S, Guo H-R, Tsai T-Y. Factors associated with sexual dysfunction in Taiwanese females with rheumatoid arthritis. BMC Womens Health. 2017;17(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Turner MD, Nedjai B, Hurst T, Pennington DJ. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta. 2014;1843(11):2563–82. [DOI] [PubMed] [Google Scholar]
- 188.Patel DP, Schenk JM, Darke A, Myers JB, Brant WO, Hotaling JM. Non-steroidal anti-inflammatory drug (NSAID) use is not associated with erectile dysfunction risk: results from the Prostate Cancer Prevention Trial. BJU Int. 2016;117(3):500–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kupelian V, Hall SA, McKinlay JB. Common prescription medication use and erectile dysfunction: results from the Boston Area Community Health (BACH) survey. BJU Int. 2013;112(8):1178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shiri R, Koskimäki J, Häkkinen J, Tammela TLJ, Auvinen A, Hakama M. Effect of Nonsteroidal Anti-Inflammatory Drug Use on the Incidence of Erectile Dysfunction. The Journal of Urology. 2006;175(5):1812–6. [DOI] [PubMed] [Google Scholar]
- 191.Gleason JM, Slezak JM, Jung H, Reynolds K, Van Den Eeden SK, Haque R, et al. Regular Nonsteroidal Anti-Inflammatory Drug Use and Erectile Dysfunction. The Journal of Urology. 2011;185(4):1388–93. [DOI] [PubMed] [Google Scholar]
- 192.Rainsford KD. Anti-inflammatory drugs in the 21st century. Subcell Biochem. 2007;42:3–27. [DOI] [PubMed] [Google Scholar]
- 193.Senbel A, El Din NN, Norel X, El Din MM. On the potential effects of prostaglandin modulators and nonsteroidal antinflammoatory drugs on erectile process, an in vitro study in rabbit corpus cavernosum. Maturitas. 2015;81(1):209. [Google Scholar]
- 194.Chrysohoou C, Panagiotakos DB, Pitsavos C, Das UN, Stefanadis C. Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults. J Am Coll Cardiol. 2004;44(1):152–8. [DOI] [PubMed] [Google Scholar]
- 195.Urpi-Sarda M, Casas R, Chiva-Blanch G, Romero-Mamani ES, Valderas-Martínez P, Arranz S, et al. Virgin olive oil and nuts as key foods of the Mediterranean diet effects on inflammatory biomarkers related to atherosclerosis. Pharmacol Res. 2012;65(6):577–83. [DOI] [PubMed] [Google Scholar]
- 196.Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292(12):1440–6. [DOI] [PubMed] [Google Scholar]
- 197.Esposito K, Ciotola M, Giugliano F, Schisano B, Autorino R, Iuliano S, et al. Mediterranean diet improves sexual function in women with the metabolic syndrome. Int J Impot Res. 2007;19(5):486–91. [DOI] [PubMed] [Google Scholar]
- 198.Maiorino MI, Bellastella G, Caputo M, Castaldo F, Improta MR, Giugliano D, et al. Effects of Mediterranean diet on sexual function in people with newly diagnosed type 2 diabetes: The MÈDITA trial. J Diabetes Complications. 2016;30(8):1519–24. [DOI] [PubMed] [Google Scholar]
- 199.Giugliano F, Maiorino MI, Di Palo C, Autorino R, De Sio M, Giugliano D, et al. ORIGINAL RESEARCH—WOMEN’S SEXUAL HEALTH: Adherence to Mediterranean Diet and Sexual Function in Women with Type 2 Diabetes. The Journal of Sexual Medicine. 2010;7(5):1883–90. [DOI] [PubMed] [Google Scholar]
- 200.Maiorino MI, Bellastella G, Chiodini P, Romano O, Scappaticcio L, Giugliano D, et al. Primary Prevention of Sexual Dysfunction With Mediterranean Diet in Type 2 Diabetes: The MÈDITA Randomized Trial. Diabetes Care. 2016;39(9):e143–e4.•• This article describes a successful clinical trial of anti-inflammatory diets to improve sexual function in diabetic men and women.
- 201.González OA, Tobia C, Ebersole JL, Novak MJ. Caloric Restriction and Chronic Inflammatory Diseases. Oral Dis. 2012;18(1):16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Aversa A, Bruzziches R, Francomano D, Greco EA, Violi F, Lenzi A, et al. Weight Loss by Multidisciplinary Intervention Improves Endothelial and Sexual Function in Obese Fertile Women. The Journal of Sexual Medicine. 2013;10(4):1024–33. [DOI] [PubMed] [Google Scholar]
- 203.Jackson G, Rosen RC, Kloner RA, Kostis JB. REPORT: The Second Princeton Consensus on Sexual Dysfunction and Cardiac Risk: new guidelines for sexual medicine. The journal of sexual medicine. 2006;3(1):28–36. [DOI] [PubMed] [Google Scholar]
- 204.Gandaglia G, Briganti A, Jackson G, Kloner RA, Montorsi F, Montorsi P, et al. A Systematic Review of the Association Between Erectile Dysfunction and Cardiovascular Disease. Eur Urol. 2014;65(5):968–78. [DOI] [PubMed] [Google Scholar]
- 205.Vlachopoulos C, Rokkas K, Ioakeimidis N, Stefanadis C. Inflammation, Metabolic Syndrome, Erectile Dysfunction, and Coronary Artery Disease: Common Links. Eur Urol. 2007;52(6):1590–600. [DOI] [PubMed] [Google Scholar]
- 206.Maseroli E, Fanni E, Cipriani S, Scavello I, Pampaloni F, Battaglia C, et al. Cardiometabolic Risk and Female Sexuality: Focus on Clitoral Vascular Resistance . The Journal of Sexual Medicine. 2016;13(11):1651–61.• This article outlines the possible role of inflammation in reducing clitoral blood flow and subsequently impairing female sexual arousal function.