Summary
The ongoing spread of coronavirus disease (COVID-19) is a worldwide crisis. Hokkaido Prefecture in Japan promptly declared a state of emergency following the rapid increase of COVID-19 cases, and the policy became an example to mitigate the spread of COVID-19. We herein report 15 cases of COVID-19 including 3 cases requiring mechanical ventilation. Based on review of our cases, among patients over 50 years of age with underlying diseases such as hypertension and diabetes mellitus, and those who required oxygen administration tended to deteriorate. These cases highlight the importance of understanding the background and clinical course of severe cases to predict prognosis.
Keywords: COVID-19, SARS-CoV-2, emergency declaration, mechanical ventilation, risk factor
Introduction
Starting in Wuhan, China in December 2019, the number of coronavirus disease (COVID-19) has been increasing worldwide especially since the World Health Organization (WHO) declared a pandemic on March 11. As of April 12, 2020, there were a total of 6,748 laboratory confirmed cases in Japan (excluding the cruise ship) and 267 cases in Hokkaido where the first outbreak occurred in Japan. The Mayor of Hokkaido independently declared a state of emergency on February 28 that was 5 weeks earlier than the Prime Minister of Japan did. In this report, we reviewed our first consecutive 15 cases of COVID-19 in which three cases required mechanical ventilation.
Patients and Methods
Sapporo City General Hospital
Our hospital is designated as the Category I Infectious Diseases Medical Facility in Hokkaido. In addition to two private rooms for patients with Category I Infectious Diseases, there are three rooms for two patients with Category II Infectious Diseases. Then, we can treat the 8 patients at the same time period. As of April 4, our hospital has treated a total of 31 adult cases of COVID-19. In this study, we presented the first consecutive 15 cases. Among them, three cases required mechanical ventilation.
Three cases required mechanical ventilation
Case 1: Man in his 60's
Chief complaint: general fatigue, fever.
Past medical history: diabetes mellitus, hypertension, atrial fibrillation.
History of present illness: On Day X (Hereinafter, date of symptom onset is defined as X in each patient), patient presented to medical institution A (1st visit) with fever of 37.5°C. First influenza antigen test was negative. He was sent home with symptomatic treatment. On X + 1 day, he visited the same medical institution A (2nd visit) for persistent fever of 37 to 38°C. The second influenza antigen test was negative. He was sent home with symptomatic treatment. On X + 5 days, he visited the same medical institution A (3rd visit) for persistent fever of 38°C and higher. The third influenza antigen test was negative. He denied shortness of breath and SpO2 was 95% in room air. He was sent home with cefcapene. He started to have a higher fever of 40°C, cough, and rhinorrhea after returning home. On X + 6 days, he visited the same medical institution A (4th visit), then he was referred to our hospital by the Sapporo City Public Health Center for suspected COVID-19. Nasopharyngeal specimen was obtained for SARS-CoV-2 PCR. Vital signs at initial consultation was temperature 37.7°C, heart rate 70 beats per minute, respiratory rate 14 breaths per minute, and SpO2 97% (room air). The patient was in good physical condition without any respiratory distress. Chest CT showed ground glass opacity bilaterally. Clinical course and imaging study were considered to be consistent with COVID-19 (Figure 1, A1, A2). He was sent home until the PCR result available. On X + 8 days, nasopharyngeal SARS-CoV-2 PCR was confirmed positive, and he was admitted to our hospital.
Figure 1.
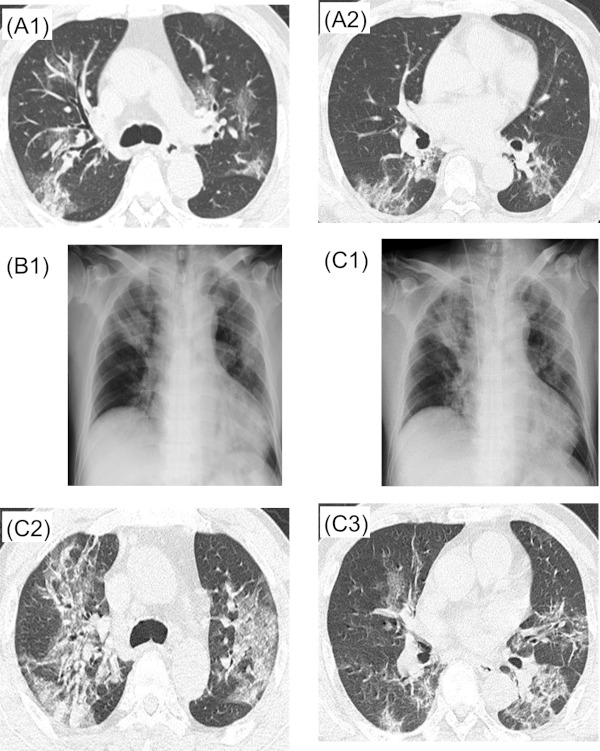
Case 1. A1, A2: X + 6 days, B1: X + 9 days, C1, C2, C3: X + 10 days.
Review of systems: Positive for fever, general fatigue, cough, rhinorrhea. Negative for headache, nausea, sore throat, shortness of breath, abdominal pain, diarrhea, arthralgia, rash.
Physical examination on admission: Alert and oriented. Temperature 39.3°C, heart rate 82 beats per minute, blood pressure 141/62 mmHg, respiratory rate 12 breaths per min, SpO2 97% (room air). A fine crackle was heard on bilateral lower lobe. Abdomen soft and flat, non-tender.
Blood test on admission: WBC 2,900/μL (Seg 72%, Lym 21%), CRP 11.6 mg/dL, PCT 0.08 ng/mL, AST 44 U/L, ALT 25 U/L.
Culture test: Blood culture 2 sets negative on X + 8 days, sputum culture negative on X + 8 days.
Hospital course: On admission (X + 8 days), fever around 39°C and cough were noted. He denied shortness of breath and no hypoxia was observed. Due to drug interactions with rivaroxaban and other medication, lopinavir/ritonavir was not initiated. On X + 9 days, although his symptoms remained relatively mild, his oxygenation level decreased, and 3 L/min of oxygen was started. Chest X-ray on the same day showed bilateral upper lobe infiltration (Figure 1, B1). Ampicillin sulbactam 3 g every 8 hours was added for possible concomitant bacterial pneumonia. On X + 10 days, chest X-ray and chest CT showed worsening of bilateral pneumonia (Figure 1, C1-C3). Blood pressure dropped the same night, antibiotics was changed to meropenem 1 g every 8 hours for possible septic shock. On X + 11 days, oxygenation status became worse and nasal high flow therapy was initiated, however, his SpO2 remained around 90% with FiO2 of 1.0. Because of the rapid deterioration of oxygenation, favipiravir was started after accelerated approval by hospital ethics committee regarding its off-label and compassionate use. Favipiravir was given 1,800 mg twice every 12 hours on day 1, then 800 mg once every 12 hours from day 2. In addition, ciclesonide 200 μg inhalation four times a day was also started on the same day. On X + 12 days, respiratory condition became worse, and mechanical ventilation was started after intubation. On X + 13 days, due to lack of improvement, he was transferred to another hospital for ECMO management.
Case 2: Man in his 70's
Chief complaint: fever.
Past medical history: hypertension, emphysema, benign prostatic hyperplasia.
History of present illness: On Day X, patient started to have fever of 37.6°C, then fever of 37 to 38°C persisted thereafter. On X + 2 days, he visited medical institution B (1st visit). He was sent home with garenoxacin. On X + 5 days, he visited the same medical institution B due to persistent fever (2nd visit). Chest X-ray did not show any obvious pneumonia. On X + 8 days, he visited the same medical institution B (third visit). Chest X-ray showed pneumonia (Figure 2, A1), and he was referred to medical institution C. Vital signs at that time showed temperature 38.8°C, SpO2 87% (room air). He was admitted with 2 L/min oxygen. Chest CT showed diffuse ground-glass opacities bilaterally (Figure 2, A2-A4). For possible COVID-19, sputum and throat swab specimens were obtained for SARS-CoV-2 PCR testing. Respiratory condition worsened, endotracheal intubation was performed and mechanical ventilation was started. Levofloxacin and ciclesonide were started. On X + 9 days, SARS-CoV-2 PCR was found to be positive. On X + 10 days, patient was transferred to our hospital.
Figure 2.
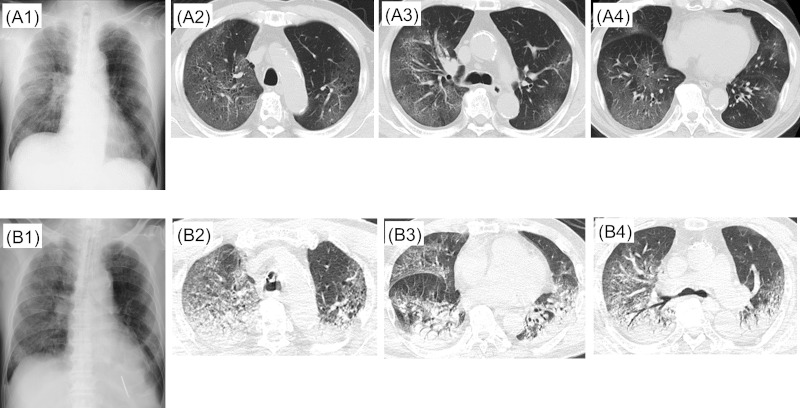
Case 2. A1, A2, A3, A4: X + 8 days, B1, B2, B3, B4: X + 10 days.
Vital signs on admission: temperature 37.7°C, heart rate 71 beats per minutes, blood pressure 83/54 mmHg, respiratory rate 20 breaths per minute, SpO2 90%. settings on admission: SIMV + PS, FiO2 1.0, PEEP12, PC15, PS8.
Blood test on admission: WBC 8,600/μL (Seg 87%, Lym 5%), CRP 10.68 mg/dL, PCT 0.08 ng/mL, AST 53 U/L, ALT 25U/L.
Culture test: Blood culture 1 set negative on X + 10 days, urine culture negative on X + 10 days, endotracheal aspiration sputum culture positive only with normal flora on X + 10 days.
Hospital course: Patient was in severe respiratory failure on admission to our hospital transfer (X + 10 days) and he was transferred to the ICU. Chest X-ray and chest CT on the same day showed rapid progression of bilateral ground-glass opacities (Figure 2, B1-B4). Ciclesonide was increased to 400 μg three times a day for severe COVID-19, and lopinavir/ritonavir 5 mL (400 mg/100 mg) twice daily was started. Since no improvement of pneumonia was confirmed, ceftriaxone 2 g daily was added for possible complication with bacterial pneumonia. On the same day, right chest tube was inserted for pneumothorax. Medical condition did not improve, and he died on day X + 15 days due to COVID-19 associated respiratory failure.
Case 3: Man in his 50's
Chief complaint: fever, general fatigue, cough.
Past medical history: diabetes mellitus, hypertension, dyslipidemia, hyperuricemia, sleep apnea syndrome.
History of present illness: On Day X, patient started to have fever, malaise, and cough. On X + 3 days, he visited medical institution D (1st visit), and first influenza antigen test for influenza was negative. On Day X + 4 days, he visited the same medical institution D (2nd visit), and the 2nd influenza antigen test was negative. Chest X-ray showed pneumonia in upper right lung field (Figure 3, A1). He was referred to medical institution E where chest CT was obtained (Figure 3, A2,A3). He was sent home for pneumonia after ceftriaxone administration and azithromycin prescription. On X + 7 days, he visited the same medical institution E (3rd visit). Ceftriaxone was given and he was sent home with garenoxacin. On X + 11 days, he visited medical institution F due to persistent fever and shortness of breath. His vital signs were temperature 38.7°C and SpO2 around 80% (room air). Chest X-ray and CT showed worsening pneumonia bilaterally (Figure 3, B1-B3). After admission, nasal high flow oxygen therapy, steroid pulse therapy for ARDS, and meropenem and minocycline for possible concomitant bacterial pneumonia were started. On X + 12 days, since respiratory condition did not improve, he was intubated, and mechanical ventilation was started in the ICU. On X + 14 days, although respiratory status improved, cause of severe pneumonia was unknown, SARS CoV-2 PCR test was performed on sputum and throat swab specimens, which came back positive. On X + 15 days, he was transferred to our hospital. Vital signs and physical examination on admission: temperature 37.4°C, heart rate 60 beats per minute, blood pressure 170/80 mmHg, respiratory rate 15 per minute, SpO2 98%. Fine crackles were noted on bilateral posterior lower lobe diffusely.
Figure 3.
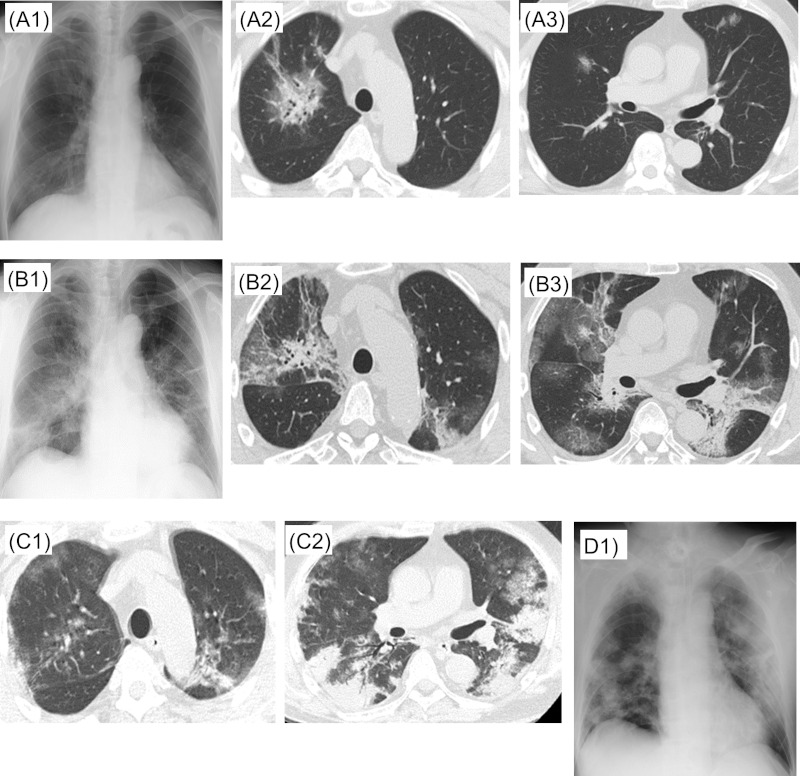
Case 3. A1, A2, A3: X + 4 days, B1, B2, B3: X + 11 days, C1, C2: X + 22 days, D1: X + 23 days.
Ventilator settings on admission: SIMV + PS, FiO2 0.6, PEEP10, PS10, RR15.
Blood test at admission: WBC 8,600/μL (Seg 90%, Lym 3%), CRP 2.6mg/dL, PCT 0.11ng/mL, AST 185 U/L, ALT 442 U/L.
Culture test: sputum culture Klebsiella oxytoca on X + 11 days, endotracheal aspiration sputum with normal flora on X + 15 days. Blood culture 2 sets negative on X + 17 days.
Hospital course: We did not give systemic steroid based on available recommendations for COVID-19 management (1). There was no established evidence of antiviral effectiveness at that time. Of clinical trials conducted in China on lopinavir/ritonavir, inclusion criteria were within 7 days of onset (NCT04261907), or within 72 hours after confirmation of abnormal chest X-Ray or symptom onset (NCT04251871). Since patient was transferred to our hospital 15 days after symptom onset, we initially did not give lopinavir/ ritonavir. We discontinued minocycline due to elevated liver function tests, and continued meropenem 1 g every 8 hours for possible concomittant bacterial pneumonia. After admission to our hospital, fever above in 39°C continued every day, and respiratory condition did not improve. Although there was no clear evidence of benefit at that time, we started lopinavir/ ritonavir (400/100 mg twice daily) from X + 18 days after accelerated approval by hospital ethics committee regarding its off-label and compassionate use. On X + 19 days, the intubation tube was blocked due to clogged sputum, it was emergently exhanged. After this event, respiratory status worsened and there was a continuing high risk of re-occlusion of endotracheal tube due to viscous sputum, emergency tracheostomy was performed on the same day. Thereafter, his respiratory condition gradually improved to the point he could participate in a rehabilitation program in the ICU. Meropenem was discontinued on X + 21 days. On X + 22 days, chest CT showed organizing process of ground-glass infiltration bilaterally (Figure 3, C1,C2). On X + 23 days, chest X-ray showed bilateral consolidation process possibly due to effect of scarring (Figure 3, D1). After X + 25 days, fever resolved, and a total of two SARS-CoV-2 PCR were negative on X + 25 days and X + 26 days. On X + 27, he was transferred to the High Care Unit. Lopinavir/ritonavir was discontinued on X + 27 days.
Results and Discussion
Declaration of a state of emergency in Hokkaido on Feb 28
The number of COVID-19 had increased in mid- February in Hokkaido (Figure 4). This was the first outbreak of COVID-19 in Japan. According to the bad situation, the Mayor of Hokkaido independently declared a state of emergency on February 28 when the number of COVID-19 was 141 cases. At that time, the number was the largest in Japan. After the declaration, the number of new cases has decreased and the outbreak was successfully mitigated, although social distancing policy in Hokkaido was not as strict as lockdowns conducted in France, Italy, and the United States. Strong political commitment has been clearly demonstrated in Hokkaido. The Prime Minister of Japan declared a state of emergency at Tokyo, Kanagawa, Saitama, Chiba, Osaka, Hyogo, and Fukuoka prefectures on April 7 for one month, due to the steep increase of COVID-19 in these areas. The number of COVID-19 was over 3,000 cases around Tokyo area, and over 1,000 cases around Osaka area. There are some arguments that the declaration of Japan might be delayed. We need to watch and observe the trend of new cases during April 7 to May 6. In contrast, the number in Hokkaido was still stable at around 250 cases with some new cases in mid- April. The declaration of Hokkaido has been successful so far. However, further and intense social distancing is urgently needed to prevent a surge of patients.
Figure 4.
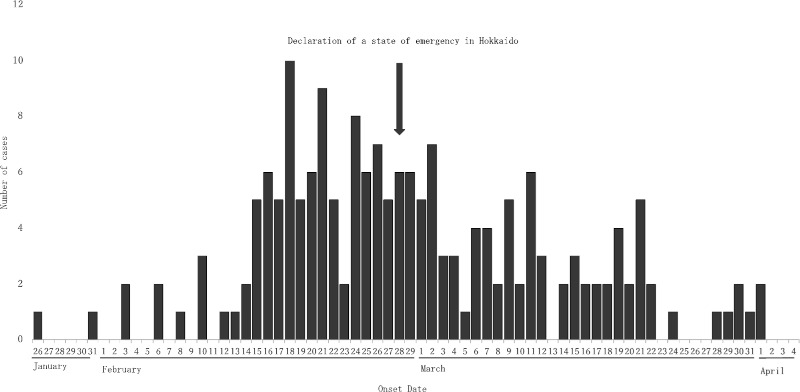
COVID-19 epidemic curve in Hokkaido. (Based on information from the Hokkaido Government website as of 12:00 on April 4, 2020.)
The first consecutive 15 cases
We treated 15 cases until March 11. A summary of the cases is listed in Table 1 (https://www.globalhealthmedicine.com/site/supplementaldata.html?ID=3). As described in case reports, 3 cases of COVID-19 who required endotracheal intubation and mechanical ventilation intubation experienced relatively mild symptom until just before admission. Eight to 11 days after the onset, oxygen therapy was started, and endotracheal intubation was required 1-3 days afterwards. As previously reported (2), our patients also rapidly deteriorated after oxygen administration became necessary. Risk factors for severe disease are elderly, underlying diseases (hypertension, diabetes mellitus, cerebrovascular diseases, etc.), lymphopenia, and increased inflammatory markers (3,4). In our hospital, all intubated patients were over 50 years of age, and had risk factors for severe disease such as hypertension or diabetes. In all three cases, lymphopenia and elevated inflammatory markers were seen. Based on clinical course of 15 cases in our hospital, among patients over 50 years of age, those who required oxygen administration tended to deteriorate.
At the time of this writing, antiviral drugs and ciclesonide are treatment options in Japan. In our cases, with the approval of the hospital ethics committee, and consent from patients or patients' family, antiviral drugs lopinavir/ritonavir and favipiravir were given based on guideline from The Japanese Association for Infectious Diseases and reports from China (5,6) until a subsequent study from China which revealed lopinavir/ ritonavir might not be effective for COVID-19 (7). According to the guideline, antiviral therapy is indicated mainly for those who are older than 50 years of age and who require oxygen. Since patients who require mechanical ventilation usually progress rapidly, timing to give antivirals for patients with risk factors for severe disease should be further discussed. In addition, it has been reported that ciclesonide is also effective (8). We will use ciclesonide in patients with risk factors such as diabetes mellitus until further study results are available. According to package insert, combination of ciclesonide and lopinavir/ritonavir is not recommended due to drug interactions. Care must be taken when choosing drug combination therapy. Further studies are needed in safety, efficacy, and timing of those treatments.
Acknowledgements
We gratefully acknowledge the work of members of our hospital and Public Health Center.
References
- 1. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020; 395:473-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sano S, Nishioka H, Yamazaki M, Miyamoto I, Adachi T. Progress report of 4 cases of coronavirus disease 2019 (COVID-19) requiring oxygen administration. 2020; http://www.kansensho.or.jp/uploads/files/topics/2019ncov/covid19casereport_200225.pdf (accessed April 4, 2020). (in Japanese) .
- 3. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, china. JAMA. 2020. February 7 doi: 0.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kunhua L, Jiong W, Faqi W, Dajing G, Linli C, Zheng F, Chuanming L. The clinical and chest CT features associated with severe and critical covid-19 pneumonia. Invest Radiol. 2020. February 29 doi: 10.1097/RLI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. The Japanese Association for Infectious Disease. Concept of antiviral treatment for COVID-19. 2020; http://www.kansensho.or.jp/uploads/files/topics/2019ncov/covid19_antiviral_drug_200227.pdf (accessed April 11, 2020). (in Japanese) .
- 6. Chen J, Ling Y, Xi X, Liu P, Li F, Shang Z, Wang M, Shen Y, Lu H. Efficacies of lopinavir/ritonavir and abidol in the treatment of novel coronavirus pneumonia. Chin J Infect Dis. 2020; doi: 10.3760/cma.j.cn311365-20200210-00050 (in Chinese). [Google Scholar]
- 7. Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N Engl J Med. 2020; doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Iwabuchi K, Yoshie K, Kurakami Y, Takahashi K, Kato Y, Morishima T. Therapeutic potential of ciclesonide inahalation for COVID-19 pneumonia: Report of three cases. J Infect Chemother. 2020; DOI: https://doi.org/10.1016/j.jiac.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


