Summary
Nearly 30% of Japanese hemophiliacs were infected with HIV-1 in the early 1980s. They have unique characteristics compared to HIV-1-infected individuals through other routes, including date of infection of 1986 or earlier, mean age of nearly 50 years, and common co-infection with hepatitis C, but rarely with other sexually transmitted diseases. Antiretroviral therapy (ART) was introduced in Japan in 1997. The clinical courses before and after 1997 were quite different. Careful analysis of the pre-1997 clinical data allowed expansion of our knowledge about the natural course and pathogenesis of the disease. Switching to the second receptor agents proved critical in subsequent disease progression. HIV-1 continued to escape immune pressure, pushing disease progression faster. In contrast, ART was effective enough to overcome the natural course. Prognosis improved dramatically and cause of death changed from AIDS-related opportunistic infections and malignancies before 1997, to hepatitis C virus-related cirrhosis and hepatocellular carcinoma (HCC) around 2010, and again to non-AIDS defining malignancies recently. In most cases, hepatitis C was cured with direct acting antiviral therapy. However, HCV progressed to cirrhosis in some cases and risk of HCC is still high among these patients. Together with improvement in anticoagulants and aging of the patients, risk of myocardial infarction has increased recently. In addition, the numbers of patients with life-style related co-morbidities, such as diabetes mellitus, hypertension, and chronic kidney disease have been also increasing. Finally, stigma is still an important barrier to a better life in HIV-1-positive individuals.
Keywords: natural course, escape from immune pressure, antiretroviral therapy, cause of death, HCV co-infection, co-morbidity
Introduction
The AIDS Clinical Center (ACC) was established at the National Center for Global Health and Medicine (NCGM) in April 1997 based on the out-of-court settlement among the HIV-1-infected Japanese hemophiliacs, the Japanese Government, and various pharmaceutical firms that supplied the HIV-1- contaminated blood products imported from the United States of America. Therefore, the main mission for the newly established ACC was to treat and care for the infected hemophiliacs. Subsequently, the role of the ACC was expanded to include the same treatment and care for all HIV-1-infected patients without any reference to the route of infection.
AIDS was first reported in homosexual men in 1981 (1-5). In the next year, AIDS cases were also identified in hemophiliacs (6). Seroprevalence was surveyed in Japanese hemophiliacs in the early 1980s, which showed that 29% of the tested hemophiliacs were positive for HIV-1 antibody (7). Based on the study, the time of HIV-1 infection of Japanese hemophiliacs was estimated to be 1983. Approval for the use of heat-treated blood products was granted in 1985, however, the use of contagious, untreated blood products was not prohibited before the end of 1986. Accordingly, Japanese hemophiliacs were thus exposed to HIV-1 and developed AIDS at that time. Based on the nationwide survey of coagulation disorders in HIV-1-infection, a total of 1,432 individuals had been infected with HIV- 1 through contaminated blood products (8). At the time of writing this review, 33 years has passed since these patients were infected with HIV-1. Among them, 722 patients (50.4%) were still alive as of the end of 2017 (8). Their prognosis changed considerably in 1997 when the combination antiretroviral therapy (ART) was introduced in Japan. The causes of death were accordingly changed from AIDS-related before 1997 to other diseases thereafter.
We review here the natural course and pathogenesis of HIV-1 infection in Japanese hemophiliacs before 1997 and, then summarize the current and future clinical issues among these patients.
Differences between infected and uninfected hemophiliacs
As described, nearly 30% of Japanese hemophiliacs were infected with HIV-1 (7). In other words, the other 70% were not infected although they must have been exposed repeatedly to HIV-1 before 1987. HIV- 1 can enter CD4+ T lymphocyte cells (CD4 cells) with second receptors of CCR5 and/or CXCR4 (9). Other studies confirmed that the delta-32 mutant of the CCR5 allele plays an important role in HIV-1 transmission and disease progression (10). Persons homozygous for delta-32 are well known to be resistant to HIV-1 infection. However, there are no Japanese homozygous for delta-32. In this regard, an international genome-wide study of resistance to HIV-1 infection in highly exposed uninfected hemophiliacs could not find any specific nucleoside polymorphisms related to the resistance (11). We do not have an accurate answer so far as to why some hemophiliacs were infected with HIV-1 but others were not at the host genetic level.
Viral tropism, pathogenesis, and disease progression
HIV-1 exists in the host as quasispecies, i.e., wide-range heterogeneity, in which the sequences of each virus have high similarities but are not identical to each other. HIV-1 quasispecies evolve over time throughout the clinical course. Isolation of HIV-1 with peripheral blood mononuclear cells revealed two distinct in vitro biological features of HIV-1; one isolate grows rapidly and yields high reverse transcriptase (RT) activity in the culture supernatant, and the other grows slowly with low RT activity (12). Accordingly, the rapid/ high virus was termed the syncytium-inducing (SI) phenotype/T-cell line tropic virus and the slow/low virus was termed the non-syncytium-inducing (NSI) phenotype/macrophage-tropic virus. NSI variants are widely distributed in the body and are the predominant population throughout the asymptomatic to advanced stages. In contrast, the SI variants are often isolated from at least some patients with advanced disease (13) but are usually not isolated from chronic slow progressors. When isolated in the presence of high CD4 count, the CD4 count subsequently falls rapidly, with accelerated disease progression. Therefore, the emergence of the SI variants was thought to be a sign of disease deterioration. However, there was still a heated debate on whether the emergence of the SI variants was the cause or result of immunodeficiency. In a series of molecular studies from our laboratories, we found that a naturally occurring single amino acid substitution in the envelope variable 3 (V3) region alters the phenotype from NSI to SI (14) and that the basic amino acid arginine substitution at position 11 of the V3 region confers the SI phenotype (15). Using this molecular information, we investigated four hemophiliac patients (the clinical courses of two shifted to rapid progression while the other two remained slow progressors) with evolutional sequence analysis. The SI genotypes were only detected in the two rapid progressors just before CD4 depletion and thereafter, while the NSI genotypes were found in all patients throughout the clinical course. Interestingly, using phylogenetic analysis, we also demonstrated in another study that the SI genotypes were under stronger elimination pressure, compared with the NSI genotypes (16). Another study from our group confirmed the slow turnover of NSI virus at cell levels (17).
After identifying the second receptors (9), the SI genotype was found to be a CXCR-4 tropic virus (X4 virus) while the NSI genotype was a CCR-5 tropic virus (R5 virus) (15). In order to determine the pathogenesis of X4 and R5 viruses, we applied the deep sequencing method to investigate five slow progressor hemophiliacs who were ART-naïve for over 20 years (18). Among the five patients, two exhibited rapid decline in CD4 count during the clinical course and received ART, while the other three were untreated and remained ART naïve after completion of the study. Interestingly, in two patients with CD4 decline, X4 virus emerged before CD4 depletion. In the first case, the X4 virus was detected first in July 2006 when the CD4 count was 619/mm3 and plasma RNA viral load (pVL) was 18,000/mL. The mean proportion of the X4 virus was only 0.9% at that time. However, in November 2007 (16 months later), the CD4 count decreased to 88/mm3, pVL increased to 58,000/mL and the proportion of X4 virus increased to 17.4%. Then, ART was initiated in this patient. In the second case, the X4 virus was detected first (90.5%) in January 2009 when the CD4 count was 221/mm3 and pVL was 530/mL. In November 2011, the CD4 count decreased to 44/mm3, pVL increased to 11,000/mL, and the proportion of X4 virus decreased to 16%. Then, ART was initiated. Although the V3 sequences of X4 virus in both cases were quite unique and similar to each other, phylogenetic tree analysis showed that each X4 virus evolved from R5 virus in each patient independently. Our results suggest that the emergence of X4 virus preceded disease progression.
Immune pressure, escape mutations and disease progression
The natural course of HIV-1 infection has been well described in large cohorts before the ART era (19). There is a consensus that the asymptomatic period usually lasts around 10 years before development of AIDS. However, disease progression varies widely among patients and depends on a variety of factors, such as the viral factors described above. Another important factor is human leukocyte antigen (HLA). There is strong evidence that HLA-B*57/5801 and HLA-B*27 are associated with slow disease progression (20). However, both alleles are very rare in Japanese. After extensive studies on the association between HLA and disease progression in Japanese hemophiliacs, Takiguchi and coworkers (21) concluded that HLA-B*5101 was a protective allele. They showed that HIV-1-specific cytotoxic T cells (CTL) restricted by HLA-B*5101 can strongly suppress HIV-1 replication in vitro and concluded that the presence of HLA-B*5101 allele in hemophiliac patients was significantly associated with slow disease progression. However, they also found that HLA-B*5101-restricted immunodominant CTL epitope Pol283 selected mutations at position 8 (position 135 of reverse transcriptase). Four amino acid mutations at position 135 (I135R, I135T, I135L, and I135V) were identified. Among them, the virus with I135V had a high fitness cost, but the others had the same replication capacity compared with the wild type virus. A proportion of slow progressors among the Japanese hemophiliacs had I135V virus and their pVL was extremely low probably due to the slow replicative capacity of the virus (21,22). In contrast, when the I135X (T, R, or L) emerged in patients carrying HLA-B*5101 and was then transmitted to their partners who did not carry HLA-B*5101, it existed in the new host in the absence of HLA-B*5101 selective pressure. This means accumulation of the mutant I135X in the peripheral circulation of HIV-1 in Japan (22). Actually, a significantly rapid disease progression was recorded in 59 acutely infected patients (infected with HIV-1 after 1997), relative to that of hemophiliacs (infected with HIV-1 before 1986) (23), probably due to lack of protection offered by HLA-B*5101 against disease progression in the recently infected Japanese (22). In this regard, adaptation of HIV-1 to escape HLA class I has deteriorated over time worldwide (22). If the highly active ART had not been established, the natural course of the disease could have probably accelerated, and some patients would have developed AIDS faster than before (23).
Anti-retroviral therapy before and after 1997
HIV-1 was first isolated by Barré-Sinoussi in 1983 (24) and the strong in vitro inhibitory effect of the first anti-retroviral agent, azidothymidine (AZT), against HIV-1 was demonstrated by Mitsuya in 1985 (25). A double-blind, placebo-controlled trial of 1,500 mg/day of AZT was conducted in the United States and the successful results of the 8-24 week treatment was published in July 1987 (26). In Japan, AZT was immediately approved without any local clinical trials and was made available for clinical use under the Japanese National Health Insurance at the end of 1987. However, the use of AZT at the recommended dose of 1,500 mg/day by our Japanese hemophiliacs was associated with severe bone marrow suppression. Similarly, the same severe side effect was also reported in some patients in a clinical trial in the United States (27). Prior to the availability of AZT in Japan, we conducted a small pilot trial of interferon-α (IFN-α) in 1987 in the early stage HIV- 1 infection. The trial involved treatment of HIV-1- infected hemophiliacs with IFN-α at 3 × 106 units, three times a week. While side effects of IFN-α were limited, the treatment was unsuccessful (28,29). In contrast, daily treatment with IFN-α using a much higher dose of 35 × 106 units of successfully controlled plasma HIV-1 P24 (P24) antigen, in a randomized placebo-controlled trial in the United States (30), although severe side effects, such as high fever and general fatigue, were noted. At that time, no method was available to measure pVL and P24 antigen was the only virologic surrogate marker used to monitor clinical efficacy. Considered together, the high dose IFN-α was effective but toxic, whereas the low dose was ineffective.
Subsequent research led to a complete shift in treatment from the use of IFN-α to anti-retroviral agents. As described, although 1,500 mg /day of AZT was effective, it was associated with substantial toxicity (27). Therefore, a lower dose of AZT (600 mg/day) was tried in a randomized clinical study in the United States and the results showed the same efficacy but with less toxicity (31). In Japan, we also conducted a randomized clinical trial using a lower dose (400 vs. 800 mg/day) of AZT and the results of 400 mg/day showed less toxicity and more beneficial effects (32). In that trial, 79% of the study participants were Japanese hemophiliacs. In late 1980s and early 1990s, several nucleoside reverse transcriptase inhibitors (NRTI) were developed (33-36) and used as monotherapies in the clinical management of hemophiliacs. The combination of two NRTIs was also used for some of them. However, the clinical efficacy of NRTIs was very limited unless they were administered for about one year. For this reason, most of the Japanese hemophiliacs underwent long-term mono- or dual-NRTIs and experienced treatment failures until 1997.
Mortality of HIV-1-infected hemophiliacs
A total of 249 patients (including 245 HIV-1-infected Japanese hemophiliacs and 4 patients with von Willebrand disease) were registered at the Department of Infectious Diseases, the Institute of Medical Science, University of Tokyo from April 1986 through March 1997 and at ACC, NCGM from April 1997 and thereafter. Among them, 90 patients died as of August 2019. Thus, the mortality rate in this group is 36.1%, which is far better than that of the entire population of Japanese hemophiliacs (8). The survival curves are shown in Figure 1. As described above, ART was introduced in Japan in early 1997. According to the Kaplan-Meier curve, the mortality rate improved after 2002 (Figure 1A), although the year 1997 saw a sharp fall in the number of AIDS-related deaths (Figure 1B). However, the number of HCV-related deaths has been gradually increasing since 2000 (Figure 1C).
Figure 1.
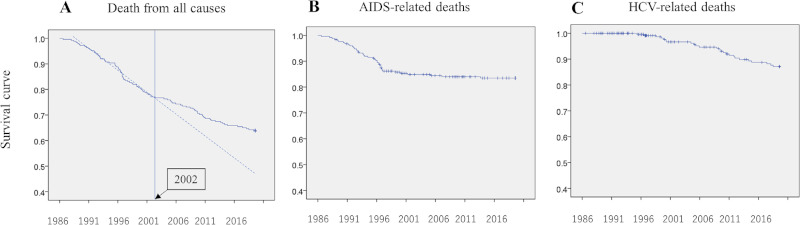
Kaplan-Meier survival curves. (A), Deaths from all causes (the dotted line is the approximate survival curve before 2002, the vertical line represents 2002); (B), AIDS-related deaths; (C), HCV-related deaths.
Causes of death
Figure 2A provides details about the causes of death in our 90 patients. During the last three decades, the two major causes of death were AIDS (43.3%) and HCV (24.4%). However, the trend changed dramatically when the observation period was divided into before and after 1997. Before 1997 (Figure 2B), the majority of deaths (83%) were AIDS-related, whereas after 1997 (Figure 2C), these were largely (40.8%) HCV-related deaths. The second cause of death was intracranial bleeding (18.4%), which might be related to the increased bleeding tendency associated with the use of PI-based regimens. To further dissect the causes of deaths, we analyzed the number and causes of deaths using 5-year bins (Figure 2D). The results showed bleeding-related deaths were the main cause between 1996 and 2000 when the first-generation PI-based regimen was mainly selected during that time period since no other options of effective HAART existed. Direct active antiviral (DAA) drugs against HCV were introduced in 2016 and almost all hemophiliacs with HCV infection achieved complete cure thereafter. The rate of HCV-related deaths gradually decreased after 2016, although some were still encountered probably because HCV hepatitis had already advanced to cirrhosis, at least in some patients. After 2001, although the number of deaths decreased (Figure 2D); suicide (6.1%) was the main cause in relation to poor mental health, followed by aging-related problems of non- AIDS defining malignancies (NADM) (4.1%) and chronic kidney diseases (CKD) (6.1%) (Figure 2C).
Figure 2.
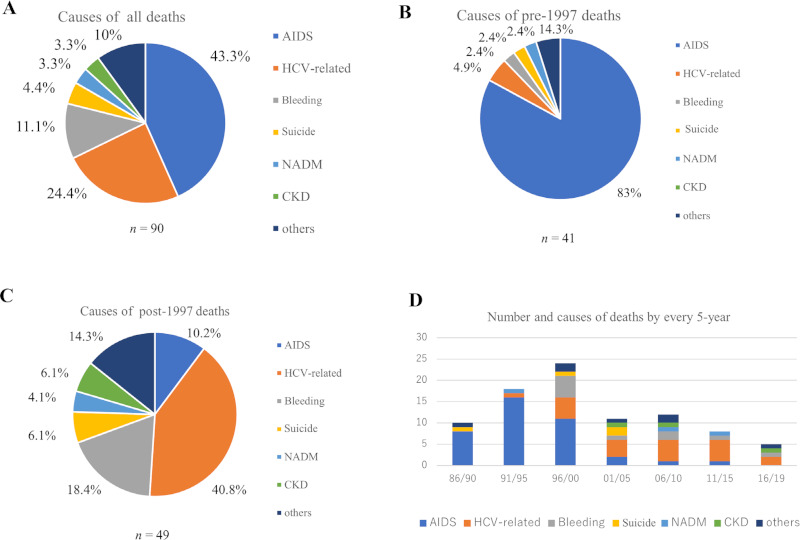
Causes of death in HIV-1-infected hemophiliacs. (A), Total number of deaths = 90; (B), Causes of pre-1997 deaths (n = 41); (C), Causes of post-1997 deaths (n = 49); (D), Number and causes of death by every 5-year (Ordinate: number of deaths, abscissa: calendar years). Bleeding, intracranial bleeding; NADM, non-AIDS defining malignancies. CKD; chronic kidney diseases.
Impact of HCV co-infection
Almost all Japanese hemophiliacs were infected with HCV (46). It is well known that HIV-1/HCV co-infection accelerates HCV disease progression (47). Therefore, hepatitis C progressed to cirrhosis in some HIV-1-infected patients before they reached 40 years of age, which is otherwise unusual in the general population (47). Some of these HIV-1/HCV co-infected hemophiliacs who had already advanced to late-stage cirrhosis underwent living donor liver transplantation between 2001 and 2004 and the majority of the donors were their mothers less than 60 years old (48).
In the Japanese HCV-infected general population, HCV 1b is the main genotype followed by HCV 2a (49). However, in the Japanese hemophiliacs, the genotype pattern varied and corresponded to that described for the US, and included HCV 3, 4, and 6 (personal information). Therefore, for treatment of hepatitis C with genotype specific DAA, we faced difficulties related to the combination of these agents (50). However, we managed to achieve HCV cure in almost all HIV-1-infected hemophiliacs. As described above, the main cause of death was HCV-related after 1997 in the hemophiliacs. However, since almost all co-infected hemophiliacs had already been cured of HCV infection with DAA, HCV-related deaths have been decreasing in recent years (Figure 2D)
Non-AIDS defining malignancies (NADM) in the patients
Along with the ART-induced improvement prognosis of patients with HIV-1 infection and cure of HCV with DAA in the hemophiliacs, there has been a shift in the cause of death in recent years (Figure 2D). When we examined the causes of death in the past three years among our HIV-1-infected patients, one of the most important causes was non-AIDS defining malignancies (NADM) (Figure 3). The number of NADM has been increasing recently in our cohort (51). Although the precise reason for the steady increase in NADM is unknown, despite the decrease in other opportunistic infections, it can be speculated that recovery of immunosuppression is still incomplete (52,53). The importance of NADM was also confirmed in another large cohort study (54). Then, we conducted a double-cancer screening clinical trial between 2017 through 2019 in 69 Japanese hemophiliacs using 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET), chest CT, gastric endoscopy, occult stool blood and cancer biomarkers (55). In the first screening, we found 4 cases of malignancies (3 cases of thyroid cancer and one neuroendocrine tumor in the pancreas) with a mean age of 48.9 years. Thus, the prevalence of NADM was 5.8%. In the second screening, we identified two more new cases (one each of pancreatic cancer and hepatocellular carcinoma) within 1.2 years (68.2 person-years). Thus, the overall estimated incidence of NADM in the hemophiliacs was 2.99/100 person-years (55). Both the prevalence and incidence were unexpectedly high. In Japan, the number of hemophiliacs living with HIV-1 in 2017 is 718 (8). Therefore, we can predict that the annual number of new cases with undiagnosed cancer is 40 and that 20 new hemophiliacs per year will develop cancer. These results highlight the importance of cancer screening of HIV-1-infected hemophiliacs across Japan.
Figure 3.
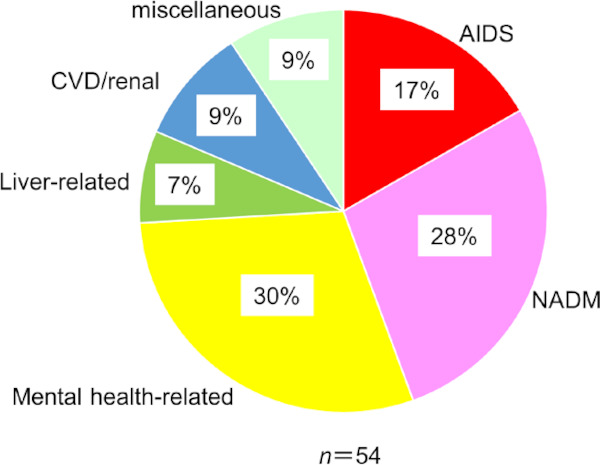
Causes of death in HIV-1-infected patients between 2016 through 2018. The data include all HIV-1- infected patients rather than hemophiliacs only. NADM, non- AIDS defining malignancies; CVD, cardiovascular diseases.
Neurocognitive impairment
The report of the CHARTER study of stable well controlled HIV-1 infected patients, concluded that HIV- 1-associated neurocognitive disorders (HAND) have a strong and negative impact on prognosis (56). The same study found high prevalence of HAND (47%) among patients confirmed with a neuropsychological (NP) test battery. We also conducted a nationwide surveillance of HAND in Japanese patients (J-HAND study) and found a prevalence of 25.3% (57). Detailed analysis of the results showed a specific decrease in neurocognitive domains during the aging process (58). However, the J-HAND study did not include any hemophiliac patients as they were excluded by the study design. In another study, we co-screened neurocognitive impairments including psychiatric dementia using FDG-PET (together with the study of cancer screening (55)), brain magnetic resonance imaging (MRI), and the NP test battery that was also used in the J-HAND study. Based on the J-HAND study criteria, the prevalence of neurocognitive impairment was 44.3%, which was nearly twice as high as that in HIV-1- infected patients (57). However, the impairment was significantly associated with MRI findings of scars of faint intracranial bleedings during their childhood (χ2 analysis; p < 0.05) (Table 1). These results suggested that the prevalence was influenced by the combination of HAND and sequelae of intracranial bleeding. Interestingly, the FDG-PET findings did not correlate with neurocognitive impairment in this study.
Table 1. Radiological findings and neurocognitive impairments in HIV-1-infected hemophiliacs.
| Items | Neurocognitive impairments | p | |
|---|---|---|---|
| + | - | ||
| MRI findings | |||
| + | 7 | 2 | p < 0.05 |
| - | 20 | 29 | |
| FDG-PET abnormalities | |||
| + | 15 | 22 | n.s. |
| - | 12 | 9 | |
A total of 58 HIV-1-infected hemophiliacs underwent the neuropsychological (NP) test battery, brain MRI, and FDG-PET. The correlations between radiological findings and neurocognitive impairments were examined by the χ2 analysis. Neurocognitive impairments were examined by the 14 NP tests that assessed 8 cognitive domains used in the J-HAND study (58). Positive MRI findings: scars of faint intracranial bleeding. MRI, magnetic resonance imaging; FDG-PET, 18F-fluorodeoxyglucose-positron emission tomography; n.s., not significant.
Stigma and future clinical issues
More than three decades have passed since our Japanese hemophiliacs were infected with HIV-1. Their mean age is approaching 50 years. Life-style related or age-related co-morbidities, such as chronic kidney diseases, hypertension, and diabetes mellitus, are the main clinical issues, rather than HIV-1 infection, in our hemophiliac patients, similar to other patients infected with HIV- 1 (59). Recent years have witnessed improvement in anti-coagulation therapy and lessening of bleedings in hemophiliac patients. In contrast, cardiovascular diseases such as myocardial infarction have also increased recently. Furthermore, these patients have suffered stigma and discrimination against not only HIV-1 but also hemophilia itself over a long period of time and this will continue in the future. Persistent psychiatric pressures over many years have induced mental health problems. We must pay attention to the relatively high suicide rate of 6.1% recorded after 1997 (Figure 2C). Comprehensive treatment including mental support is especially important and indispensable for HIV-1- infected hemophiliacs for better quality of life. More importantly, we have to achieve zero stigma in our society for people living with HIV-1.
Acknowledgements
The authors thank all past and current staff members of the ACC, NCGM and the Habataki-Welfare Agency. We also thank Prof. Takiguchi and his colleagues at the Center for AIDS Research, Kumamoto University, for the extensive study of Japanese hemophiliacs coinfected with HIV-1.
This work was supported in part by Research on HIV/AIDS, Health, Labour and Welfare Science Research Grants and Health, Labour and Welfare Policy Research Grants. The authors are also indebted to Ms. Akiko Nakano for the logistic work in the transaction of the research grants and to Prof. FG Issa for editing this manuscript.
References
- 1. Centers for Disease Control (CDC) Kaposi's sarcoma and Pneumocystis pneumonia among homosexual men- -New York City and California. MMWR Morb Mortal Wkly Rep. 1981; 30:305-308. [PubMed] [Google Scholar]
- 2. Hymes KB, Cheung T, Greene JB, Prose NS, Marcus A, Ballard H, William DC, Laubenstein LJ. Kaposi's sarcoma in homosexual men-a report of eight cases. Lancet. 1981; 2:598-600. [DOI] [PubMed] [Google Scholar]
- 3. Gottlieb MS, Schroff R, Schanker HM, Weisman JD, Fan PT, Wolf RA, Saxon A. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N Engl J Med. 1981; 305:1425-1431. [DOI] [PubMed] [Google Scholar]
- 4. Masur H, Michelis MA, Greene JB, Onorato I, Stouwe RA, Holzman RS, Wormser G, Brettman L, Lange M, Murray HW, Cunningham-Rundles S. An outbreak of community-acquired Pneumocystis carinii pneumonia: initial manifestation of cellular immune dysfunction. N Engl J Med. 1981; 305:1431-1438. [DOI] [PubMed] [Google Scholar]
- 5. Siegal FP, Lopez C, Hammer GS, et al. Severe acquired immunodeficiency in male homosexuals, manifested by chronic perianal ulcerative herpes simplex lesions. N Engl J Med. 1981; 305:1439-1444. [DOI] [PubMed] [Google Scholar]
- 6. Essex M, McLane MF, Lee TH, Tachibana N, Mullins JI, Kreiss J, Kasper CK, Poon MC, Landay A, Stein SF, Francis DP, Cabradilla C, Lawrence DN, Evatt BL. Antibodies to human T-cell leukemia virus membrane antigens (HTLV-MA) in hemophiliacs. Science. 1983; 221:1061-1064. [DOI] [PubMed] [Google Scholar]
- 7. Tsuchie H, Kurimura T, and Hinuma Y. Survey of the prevalence of AIDS-associated virus (LAV) infection in Japan. J Infect. 1985; 10:272-276. [DOI] [PubMed] [Google Scholar]
- 8. Project entrusted by Ministry of Health, Labour And Welfare. Nationwide Survey on Coagulation Disorders 2018. http://api-net.jfap.or.jp/library/alliedEnt/02/index.html (accessed August 14, 2019). (in Japanese) .
- 9. Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci U S A. 1997; 94:1925-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang Y, Paxton WA, Wolinsky SM, et al. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med. 1996; 2:1240-1243. [DOI] [PubMed] [Google Scholar]
- 11. Lane J, McLaren PJ, Dorrell L, et al. A genome-wide association study of resistance to HIV infection in highly exposed uninfected individuals with hemophilia A. Hum Mol Genet. 2013; 22:1903-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fenyö EM, Morfeldt-Månson L, Chiodi F, Lind B, von Gegerfelt A, Albert J, Olausson E, Asjö B. Distinct replicative and cytopathic characteristics of human immunodeficiency virus isolates. J Virol. 1988; 62:4414-4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saag MS, Hammer SM, Lange JM. Pathogenicity and diversity of HIV and implications for clinical management: a review. J Acquir Immune Defic Syndr. 1994; 7 Suppl 2:S2-S10; discussion S10-S11. [PubMed] [Google Scholar]
- 14. Shioda T, Oka S, Ida S, Nokihara K, Toriyoshi H, Mori S, Takebe Y, Kimura S, Shimada K, Nagai Y. A naturally occurring single basic acid substitution in the V3 region of HIV-1 ENV protein alters the viral cellular host range and antigenic structure. J Virol. 1994; 68:7689-7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tsuchiya K, Ode H, Hayashida T, Kakizawa J, Sato H, Oka S, Gatanaga H. Arginine insertion at position 11 and loss of N-linked glycosylation site in HIV-1 Env V3 region confer CXCR4-tropism. Sci Rep. 2013; 3:2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ida S, Gatanaga H, Shioda T, Nagai Y, Kobayashi N, Shimada K, Kimura S, Iwamoto A, Oka S. HIV-1 V3 variation dynamics in vivo. Long-term persistence of NSI genotype and transient presence of SI genotype during the course of progressive AIDS. AIDS Res Hum Retrovirus. 1997; 13:1597-1609. [DOI] [PubMed] [Google Scholar]
- 17. Suzuki Y, Tachikawa N, Gatanaga H, Oka S. Slow turnover of HIV-1 receptors on quiescent CD4+ T cells causes prolonged surface retention of gp120 immune complexes in vivo. PLOS One. 2014; 9:e86479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hayashida T, Tsuchiya K, Kikuchi Y, Oka S, Gatanaga H. Emergence of CXCR4-tropic HIV-1 variant followed by rapid disease progression in hemophiliac slow progressors. PLOS One. 2017; 12:e0177033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Collaborating Group on AIDS Incubation and HIV Survival. Time from HIV-1 seroconversion to AIDS and death before widespread use of highly active antiretroviral therapy: a collaborative re-analysis. Lancet. 2000; 355:1131-1137. [PubMed] [Google Scholar]
- 20. Kaslow RA, Carrington M, Apple R, Park L, Muñoz A, Saah AJ, Goedert JJ, Winkler C, O'Brien SJ, Rinaldo C, Detels R, Blattner W, Phair J, Erlich H, Mann DL. Influence of combinations of human major histocompatibility complex genes on the course of HIV- 1 infection. Nat Med. 1996; 2:405-411. [DOI] [PubMed] [Google Scholar]
- 21. Kawashima Y, Kuse N, Gatanaga H, Naruto T, Fujiwara M, Dohki S, Maenaka K, Goulder P, Oka S, Takiguchi M. Long-term control of HIV-1 by HIV-1 pol-specific CTLs in hemophiliacs carrying slow-progressing allele HLA-B*5101. J Virol. 2010; 84:7151-7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kawashima Y, Pfafferott K, Duda A, et al. Adaptation of HIV-1 to HLA I. Nature. 2009; 458:641-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nakamura H, Teruya K, Takano M, Tsukada K, Tanuma J, Yazaki H, Honda H, Honda M, Gatanaga H, Kikuchi Y, Oka S. Clinical symptoms and courses of primary HIV- 1 infection in recent years in Japan. Intern Med. 2011; 50:95-101. [DOI] [PubMed] [Google Scholar]
- 24. Barré-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vézinet- Brun F, Rouzioux C, Rozenbaum W, Montagnier L. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983; 220:868-871. [DOI] [PubMed] [Google Scholar]
- 25. Mitsuya H, Weinhold KJ, Furman PA, St Clair MH, Lehrman SN, Gallo RC, Bolognesi D, Barry DW, Broder S. 3'-Azido-3'-deoxythymidine (BW A509U): an antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc Natl Acad Sci U S A. 1985; 82:7096-7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fischl MA, Richman DD, Grieco MH, et al. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987; 317:185- 191. [DOI] [PubMed] [Google Scholar]
- 27. Richman DD, Fischl MA, Grieco MH, et al. The toxicity of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987; 317:192- 197. [DOI] [PubMed] [Google Scholar]
- 28. Oka S, Hirabayashi Y, Mohri H, Sakurada S, Goto H, Ohnishi K, Kimura S, Mitamura K, Shimada K. β-interferon and early stage HIV infection. J Acquir Immune Defic Syndr. 1989; 2:125-128. [PubMed] [Google Scholar]
- 29. Oka S, Urayama K, Hirabayashi Y, Kimura S, Mitamura K, and Shimada K. Human immunodeficiency virus DNA copies as a virologic marker in a clinical trial with β-interferon. J Acquir Immune Defic Syndr. 1992; 5:707-711. [PubMed] [Google Scholar]
- 30. Lane HC, Davey V, Kovacs JA, et al. Interferon-alpha in patients with asymptomatic human immunodeficiency virus (HIV) infection. A randomized, placebo-controlled trial. Ann Intern Med. 1990; 112:805-811. [DOI] [PubMed] [Google Scholar]
- 31. Fischl MA, Parker CB, Pettinelli C, et al. A randomized controlled trial of a reduced daily dose of zidovudine in patients with the acquired immunodeficiency syndrome. The AIDS Clinical Trials Group. N Engl J Med. 1990; 323:1009-1014. [DOI] [PubMed] [Google Scholar]
- 32. Kimura S, Oka S, Toyoshima T, Hirabayashi Y, Kikuchi Y, Mitamura K, Shimada K. A randomized trial of reduced doses of azidothymidine in Japanese patients with human immunodeficiency virus type 1 infection. Intern Med. 1992; 31:871-876. [DOI] [PubMed] [Google Scholar]
- 33. Yarchoan R, Mitsuya H, Thomas RV, Pluda JM, Hartman NR, Perno CF, Marczyk KS, Allain JP, Johns DG, Broder S. In vivo activity against HIV and favorable toxicity profile of 2',3'-dideoxyinosine. Science. 1989; 245:412-415. [DOI] [PubMed] [Google Scholar]
- 34. Kim CH, Marquez VE, Broder S, Mitsuya H, Driscoll JS. Potential anti-AIDS drugs. 2',3'-Dideoxycytidine analogues. J Med Chem. 1987; 30:862-866. [DOI] [PubMed] [Google Scholar]
- 35. Coates JA, Cammack N, Jenkinson HJ, Jowett AJ, Jowett MI, Pearson BA, Penn CR, Rouse PL, Viner KC, Cameron JM. (-)-2'-deoxy-3'-thiacytidine is a potent, highly selective inhibitor of human immunodeficiency virus type 1 and type 2 replication in vitro. Antimicrob Agents Chemother. 1992; 36:733-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mansuri MM, Starrett JE Jr, Ghazzouli I, et al. 1-(2,3-Dideoxy-beta-D-glycero-pent-2-enofuranosyl) thymine. A highly potent and selective anti-HIV agent. J Med Chem. 1989; 32:461-466. [DOI] [PubMed] [Google Scholar]
- 37. Collier AC, Coombs RW, Schoenfeld DA, Bassett RL, Timpone J, Baruch A, Jones M, Facey K, Whitacre C, McAuliffe VJ, Friedman HM, Merigan TC, Reichman RC, Hooper C, Corey L. Treatment of human immunodeficiency virus infection with saquinavir, zidovudine, and zalcitabine. AIDS Clinical Trials Group. N Engl J Med. 1996; 334:1011-1017. [DOI] [PubMed] [Google Scholar]
- 38. Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, Currier JS, Eron JJ Jr, Feinberg JE, Balfour HH Jr, Deyton LR, Chodakewitz JA, Fischl MA. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997. 337:725-733. [DOI] [PubMed] [Google Scholar]
- 39. Lohse N, Hansen AB, Pedersen G, Kronborg G, Gerstoft J, Sørensen HT, Vaeth M, Obel N. Survival of persons with and without HIV infection in Denmark, 1995-2005. Ann Intern Med. 2007; 146:87-95. [DOI] [PubMed] [Google Scholar]
- 40. Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008; 372:293-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. May MT, Gompels M, Delpech V, et al. Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS. 2014; 28:1193-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Antiretroviral Therapy Cohort Collaboration. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV. 2017; 4:e349-e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kawado M, Hashimoto S, Yamaguchi T, Oka S, Yoshizaki K, Kimura S, Fukutake K, Higasa S, Shirasaka T. Progression to AIDS by CD4 cell count, plasma HIV-RNA level and use of antiretroviral therapy among HIV patients infected through blood products in Japan. J Epidemiol. 2006; 16:101-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stanworth SJ, Bolton MJ, Hay CR, Shiach CR. Increased bleeding in HIV-positive haemophiliacs treated with antiretroviral protease inhibitors. Haemophilia. 1998; 4:109-114. [DOI] [PubMed] [Google Scholar]
- 45. Kawado M, Hashimoto S, Oka S, Fukutake K, Higasa S, Yatsuhashi H, Ogane M, Okamoto M and Shirasak T. Clinical improvement by switching to an integrase strand transfer inhibitor in hemophiliac patients with HIV: the Japan cohort study of HIV patients infected through blood products. Open AIDS J. 2017; 11:18-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yotsuyanagi H, Kikuchi Y, Tsukada K, Nishida K, Kato M, Sakai H, Takamatsu J, Hige S, Chayama K, Moriya K, Koike K. Chronic hepatitis C in patients co-infected with human immunodeficiency virus in Japan: a retrospective multicenter analysis. Hepatol Res. 2009; 39:657-63. [DOI] [PubMed] [Google Scholar]
- 47. Miuma S, Hidaka M, Takatsuki M, Natsuda K, Soyama A, Miyaaki H, Kanda Y, Tamada Y, Shibata H, Ozawa E, Taura N, Eguchi S, Nakao K. Current characteristics of hemophilia patients co-infected with HIV/HCV in Japan. Exp Ther Med. 2018; 15:2148-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tsukada K, Sugawara Y, Kaneko J, Tamura S, Tachikawa N, Morisawa Y, Okugawa S, Kikuchi Y, Oka S, Kimura S, Yatomi Y, Makuuchi M, Kokudo N, Koike K. Living donor liver transplantations in HIV- and hepatitis C virus- coinfected hemophiliacs: Experience in a Single Center. Transplantation. 2011; 91:1261-1264. [DOI] [PubMed] [Google Scholar]
- 49. Ishida Y, Hayashida T, Sugiyama M, Tsuchiya K, Kikuchi Y, Mizokami M, Oka S, and Gatanaga H. Full-genome analysis of hepatitis C virus in Japanese and non-Japanese patients coinfected with HIV-1 in Tokyo. JAIDS J Acquir Immune Defic Syndr. 2019; 80:350-357. [DOI] [PubMed] [Google Scholar]
- 50. Uemura H, Tsukada K, Mizushima D, Aoki T, Watanabe K, Kinai E, Teruya K, Gatanaga H, Kikuchi Y, Sugiyama M, Mizokami M, Oka S. Interferon-free therapy with direct acting antivirals for HCV/HIV-1 co-infected Japanese patients with inherited bleeding disorders. PLoS One. 2017; 12:e0186255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nagata N, Nishijima T, Niiura R, Yokoyama T, Matsushita Y, Watanabe K, Teruya K, Kikuchi Y, Akiyama J, Yanase M, Uemura N, Oka S, Gatanaga H. Increased risk of non-AIDS-defining cancers and mortality in Asian HIV-infected patients: a long-term cohort study. BMC Cancer. 2018; 18:1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mutoh Y, Nishijima T, Inaba Y, Tanaka N, Kikuchi Y, Gatanaga H, Oka S. Incomplete recovery of CD4 cell count, CD4 percentage, and CD4/CD8 ratio in patients with human immunodeficiency virus infection and suppressed viremia during long-term antiretroviral therapy. Clin Infect Dis. 2018; 67:927-933. [DOI] [PubMed] [Google Scholar]
- 53. Shiels MS, Althoff KN, Pfeiffer RM, et al. HIV Infection, immunosuppression, and age at diagnosis of non-AIDS-defining cancers. Clin Infect Dis. 2017; 64:468-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Smith CJ, Ryom L, Weber R, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): A multicohort collaboration. Lancet. 2014; 384:241-248. [DOI] [PubMed] [Google Scholar]
- 55. Oka S, Ogata M, Takano M, Minamimoto R, Hotta M, Tajima T, Nagata N, Tsukada K, Teruya K, Kikuchi Y, Gatanaga H, the Cancer Screening in Hemophiliac/HIV Patient Study Group. Non-AIDS-defining malignancies in Japanese hemophiliacs with HIV-1 infection. Global Health & Medicine. 2019; 1:49-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Heaton RK, Clifford DB, Franklin DR Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010; 75:2087-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kinai E, Komatsu K, Sakamoto M, Taniguchi T, Nakao A, Igari H, Takada K, Watanabe A, Takahashi-Nakazato A, Takano M, Kikuchi Y, Oka S; for HIV-associated neurocognitive disorders in Japanese (J-HAND study group) Association of age and time of disease with HIV-associated neurocognitive disorders: a Japanese nationwide multicenter study. J Neurovirol. 2017; 23:864-874. [DOI] [PubMed] [Google Scholar]
- 58. Komatsu K, Kinai E, Sakamoto M, Taniguchi T, Nakao A, Sakata T, Iizuka A, Koyama T, Ogata T, Inui A, Oka S; HIV-Associated Neurocognitive Disorders in Japanese (J-HAND) Study Group (The J-HAND Study Group) Various associations of aging and long-term HIV infection with different neurocognitive functions: detailed analysis of a Japanese nationwide multicenter study. J Neurovirol. 2019; 25:208-220. [DOI] [PubMed] [Google Scholar]
- 59. Nishijima T, Kawasaki Y, Mutoh Y, Tomonari K, Tsukada K, Kikuchi Y, Gatanaga H, Oka S. Prevalence and factors associated with chronic kidney disease and end-stage renal disease in HIV-1-infected Asian patients in Tokyo. Sci Rep. 2017; 7:14565. [DOI] [PMC free article] [PubMed] [Google Scholar]


