Summary
Following a lot of reports of coronavirus disease 2019 (COVID-19) CT images, the feature of FDG-PET/ CT imaging of COVID-19 was reported in several articles. Since FDG accumulates in activated inflammatory cells, FDG-PET/CT has huge potential for diagnosing and monitoring of inflammatory disease. However, FDG-PET/CT cannot be routinely used in an emergency setting and is not generally recommended as a first choice for diagnosis of infectious diseases. In this review, we demonstrate FDG-PET/CT imaging features of COVID-19, including our experience and current knowledge, and discuss the value of FDG-PET/CT in terms of estimating the pathologic mechanism.
Keywords: COVID-19, FDG, PET/CT, diagnosis
Introduction
The coronavirus disease 2019 (COVID-19) outbreak that originated in Wuhan, China, spread across the world within a few months from the first report identified as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in January 2020 (1-3). Clinical manifestations and nucleic acid testing (RT-PCR) are essential in the diagnosis of COVID-19 (4). The common features of chest CT in patients with COVID-19 are multifocal patchy shadows and ground-glass opacities (5,6) (Figure 1). However, various diseases can mimic these features, including other viral pneumonias (7), and chest CT is therefore not currently recommended as an initial screening tool (8). Following numerous reports regarding the CT appearance of COVID-19, several groups have reported [18F]-2-fluoro-2-deoxy-D-glucose (FDG) - positron emission tomography/computed tomography (PET/CT) imaging findings of COVID-19 (9-16). We experienced two measurements of FDG-PET/CT in a COVID-19 infected patient, one was 4 weeks from symptoms onset and the other was 4 weeks after the first FDG-PET/CT scan (Figure 2).
Figure 1.

Chest CT images of COVID-19. Chest CT images at 14 days after symptom onset showed peripheral grand glass opacity with crazy-paving appearance in both lungs, which were typical findings of COVID-19 pneumonia.
Figure 2.
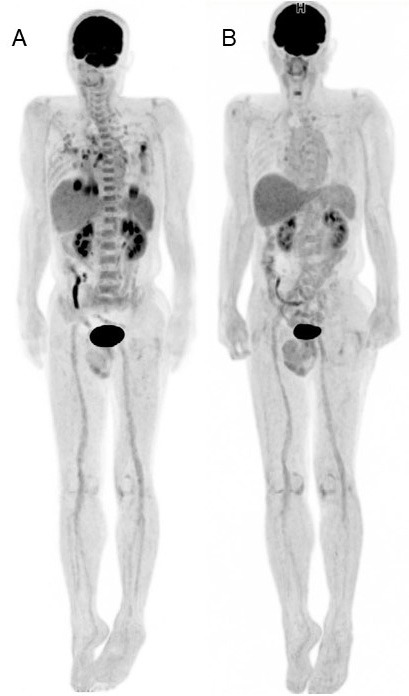
Whole-body FDG-PET images of COVID-19. (A): Whole body FDG-PET image of the patient with COVID-19 pneumonia (4 weeks after symptom onset and 3 weeks after negative RT-PCRs). (B): Whole-body FDG-PET image of the patient with COVID-19 pneumonia (4 weeks from previous FDG-PET scan). Intense FDG uptake was confirmed in lung lesion and mediastinal lymph node in the first image. In addition, increased FDG was seen in bone marrow and spleen. FDG uptake in lung lesion and mediastinal lymph node disappeared, and uptake in bone marrow and spleen were decreased as physiological uptake level.
Here, we review the features of FDG-PET/CT, including our experience and the latest knowledge of COVID-19, and discuss the imaging findings to approach the pathological mechanism.
FDG-PET/CT
The glucose analog FDG is a molecular imaging probe used to evaluate tissue glucose utilization and glucose metabolism. A PET/CT test can provide metabolic and anatomic information of lesions simultaneously. FDG-PET/CT has utility in the staging, restaging, and assessment of therapeutic effects in malignancy, and is used in the management of patients with malignancy (17). Because FDG accumulates in activated inflammatory cells including neutrophils and macrophages, FDG-PET/ CT has huge potential for diagnosing and monitoring inflammatory disease (18).
COVID-19 as an incidental finding
Patients with cancer and cardiovascular disease have a greater risk for worse clinical outcomes of COVID-19 infections (19). It is particularly noteworthy that the incidence of positive CT findings specific to COVID-19 was high among those who were asymptomatic but tested positive (20). Thus, departments treating patients with cancer and cardiovascular disease encountered high-risk patients with COVID-19, and imaging departments that possessed a CT scanner have a relatively high incidence of encountering highly suspicious findings of COVID-19 infection (21). In fact, some reports demonstrate incidental detection of COVID-19 infection in FDG-PET/CT examination in patients with malignancy (11,21,22). With regard to FDG-PET/CT, which is used in the management of patients with cancer, abnormal findings on chest CT and abnormal FDG uptake related to COVID-19 should be surveyed carefully, and we should be alerted immediately if COVID-19 infection is suspected.
As an advanced preparation, the nuclear medicine department should have established effective procedures for patients and staff flow when facing known, suspected, and incidentally detected COVID-19 patients, and should control transmission of the virus while continuing to provide essential and critical services (23).
FDG-PET/CT requires a wait of at least 60 min after injection and approximately 20 min (depending on the machine) for scanning one patient. Therefore, patients with COVID-19 undergoing FDG-PET/CT would stay longer in the PET/CT department than in the CT scan room. The long-term care of COVID-19 patients in a small and closed space with limited equipment will be a burden for any staff in a PET/CT department. For this reason, FDG-PET/CT is not used routinely in an emergency setting and would not be the first choice for diagnosis of infectious diseases.
FDG-PET/CT imaging findings in COVID-19
Pneumonia
The most remarkable features of FDG-PET/CT in patients with COVID-19 are increased FDG uptake in lung lesions that form segmental ground-glass densities and plaques (10,16,23), which are typical CT findings in the early-stage of the disease. In non-small cell lung cancer (NSCLC), FDG uptake in the lesion correlates with tumor cell density and cell proliferation, thus early stage NSCLC featuring ground-glass opacities generally shows low FDG uptake (24). Therefore, FDG uptake of lung lesions in COVID-19 has an atypical appearance in terms of density-based considerations. Similar features have been confirmed in active interstitial pneumonia, in which FDG uptake reflects activity of the lesion (25).
SARS-CoV-2 infects cells expressing the surface receptors angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine 2 (TMPRSS2). The active replication and release of the virus lead the host cell to undergo pyroptosis and release damage-associated molecular patterns. These patterns are recognized by neighboring epithelial cells, endothelial cells and alveolar macrophages, and trigger the generation of pro-inflammatory cytokines and chemokines (26). Thus, FDG uptake in segmental ground-glass density lesions suggests a high level of inflammatory related processes occurred in the lesion and looks like an early stage of COVID-19 in CT.
As a clinical progression of COVID-19, CT images reveal inflammatory exudation, consolidation, and increased density, accompanied by thickening of pulmonary vascular shadowing, bronchus sign, paving-stone sign, interlobular septal thickening, and pleural effusion (12). In most reports, FDG uptake was confirmed in progressed lung lesions in patients with common COVID-19 manifestation (10,11,13-16). It is well known that in pneumonia, intense FDG uptake appears in the active stage and during progression. Das et al. observed significant FDG uptake in progressed lesions such as lung nodules and cavities in patients with Middle East respiratory syndrome coronavirus (MERS-CoV) infection (27).
At the time of our first FDG-PET/CT scan, the respiratory symptoms of the patient were improved, and chest CT showed a reduction in the size of the segmental ground-glass opacities and plaques, decreasing lesion density, formulating liner opacity and trabecular shadowing were the same as the previous report (28). It is noteworthy that there was still intense FDG uptake in these CT features indicating the recovery stage of COVID-19 (Figure 3). In general, metabolic changes precede morphological changes; therefore, functional imaging using PET is a useful early predictor of the therapeutic response in inflammation and cancer lesions. Intense FDG uptake in lung lesions indicates a high level of inflammatory change persists even in the recovery stage. However, it is still unclear whether the inflammatory change is caused by the remaining COVID-19 itself, the immunotherapeutic response, or angiovascular damage. In our experience, FDG uptake in lung lesions was decreased at 4 weeks after the first FDG-PET/CT scan (Figure 3). Therefore, we should note that FDG uptake in the recovery stage will not always forecast disease progression of COVID-19. Considering this finding, it is of interest whether FDG uptake can predict potential damage of lung tissue.
Figure 3.
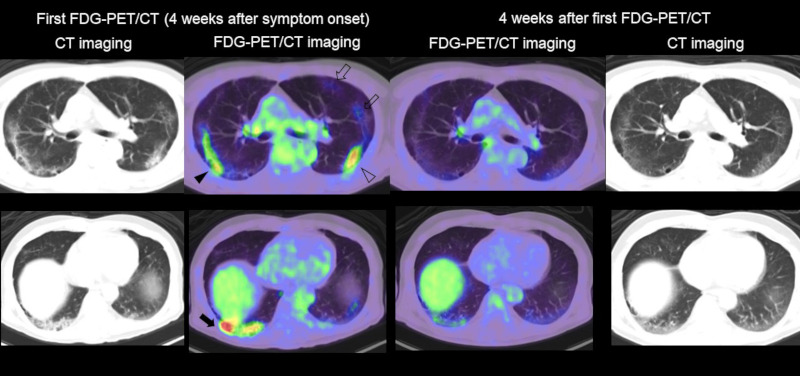
FDG-PET/CT imaging of lung lesion in COVID-19. Left side: CT and fused FDG-PET/CT image of the chest in the first examination (4 weeks after symptom onset). Right side: CT and fused FDG-PET/CT image of the chest (4 weeks after the first FDG-PET/CT examination). Intense FDG uptake was seen in liner opacity (black arrowhead), reticular opacity with consolidation (black arrow), and grand glass opacity with consolidation (open arrowhead) in the lung. Moderate FDG uptake was confirmed in grand glass opacity (open arrows) at the left upper lobe. All the FDG uptake in the first examination was significantly decreased in the second PET/CT scan.
Lymph nodes
FDG uptake in lymph nodes is frequently seen in patients with COVID-19 (Figure 4). The FDG uptake in lymph nodes is thought to reflect immunoreactions activated by inflammatory cells such as neutrophils, monocytes, and effector T cells by the release of local chemokines. In the immune response to viral infections, the number of monocytes in lymphoid tissue increases, thus leading to increased FDG uptake (12,29).
Figure 4.
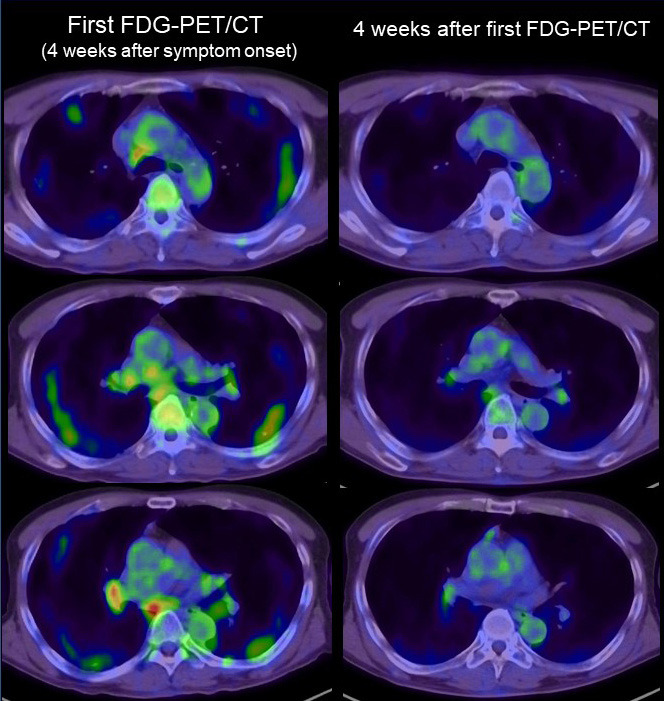
FDG-PET/CT imaging of lymph nodes in COVID-19. Left side: FDG-PET/CT image of the chest in the first examination (4 weeks after symptom onset). Right side: FDG-PET/CT image of the chest (4 weeks after the first FDG-PET/CT examination). Intense FDG uptake was seen in mediastinal and hilar lymph nodes. All the FDG uptake in the first examination was significantly decreased in the second FDG-PET/CT scan.
A previous study reported that in COVID-19, lymph node enlargement is a rare finding on CT, which present in < 1% of patients (30). The size and the shape of these lymph nodes showing intense FDG uptake was not clear in some reports, but it is generally small, nonspecific, and regular in shape as we identified in our case (Figure 5). In our experience, FDG uptake was confirmed in mediastinal lymph nodes without significant enlargement, and the uptake decreased during 4 weeks of observation (Figure 4). The CT image showed little change in size of a lymph node during the clinical course (Figure 5), but CT may be less sensitive to host reactions compared with FDG-PET/CT, and therefore the actual percentage of lymph node involvement may be higher than seen on CT.
Figure 5.
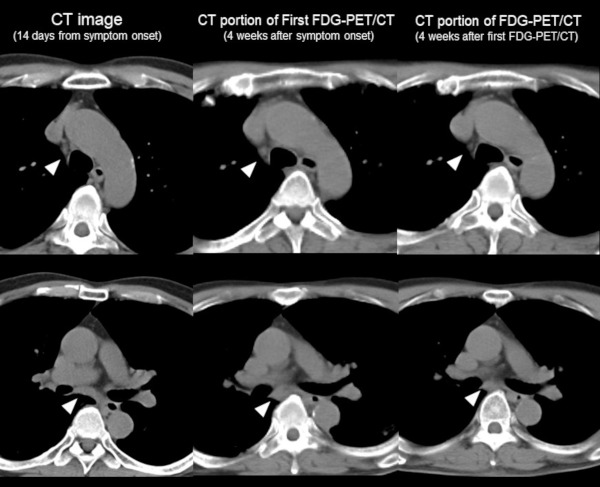
Change of CT feature of lymph node over time. Left side: CT image (14 days from symptom onset), Middle: CT portion of FDG-PET/CT (4 weeks after symptom onset), Right side: CT portion of second FDG-PET/CT (4 weeks after first FDG-PET/CT examination). CT image (14 days from symptom onset) showed no evidence of mediastinal lymph node swelling (arrowhead). Although the size of the lymph node is not significant, it was increased compared to 2 weeks from CT imaging and decreased within 4 weeks after the first FDG-PET/CT examination.
In contrast, lymph node swelling has been manifested in pneumonia caused by parainfluenza virus and adenovirus (30). FDG uptake in small axillary lymph nodes is a common feature just after influenza vaccination (31). In COVID-19, however, several studies have reported negative FDG uptake in these lymph nodes, which may occur in the minimally invasive and early stages of the disease (32). Therefore, the immune response is weak or almost absent in the early stage and becomes more active over time. Moreover, reduction of FDG uptake in lymph nodes may indicate normalization of hyperactive immune response in the body, but further investigation is necessary to confirm this hypothesis.
Possible identification of lesions related to COVID-19
Patients with COVID-19 show various symptoms that can cause damage to the gastrointestinal tract, kidneys, heart, bone marrow, and other organs (33). Small vessel vasculitis causing skin disease (34) and symptoms similar to those of Kawasaki disease (35) have been reported as related to COVID-19. However, FDG-PET/ CT is limited in its ability to diagnose small or middle vessel aortitis and medium-to-large vessel aortitis. In the case that vasculitis causes organ damage, the abnormal FDG-PET/CT findings on organs may indirectly indicate the existence of small-sized or middle-sized vessel aortitis (36).
Damage to endothelial tissue is considered to be the underlying mechanism of cardiovascular complications in COVID-19 (37). No report has described FDG uptake by the vascular wall that would suggest endothelial tissue damage. FDG can visualize metabolically active atherosclerosis because FDG is taken up by macrophages within atherosclerotic plaques (38,39). However, considering that complications of COVID-19 tend to occur in the elderly, it is questionable whether FDG can distinguish the uptake of FDG caused by atherosclerosis. Further investigation of the relationship between endothelial tissue damage and FDG uptake in the arterial wall in COVID-19 is required.
Because active thrombosis can be depicted as intense FDG uptake, a survey of FDG uptake when thrombosis is suspected may be of additional value in patients with COVID-19 (40).
Increased FDG uptake in bone marrow may be an additional imaging feature in COVID-19 as it was confirmed in another report (9,13,15,16). Chefer et al. reported high uptake by bone marrow over a long period of time in a MERS-CoV animal model (41). SARS-CoV-2 invades host cells via two receptors: angiotensin-converting enzyme 2 (ACE2) and CD147 (42). CD147 is expressed by mesenchymal stem cells of human cord blood and bone marrow origin (43), and CD147 expression is induced by high glucose concentration in monocytes (44). Based on this mechanism, FDG uptake by bone marrow may be an additional feature of COVID-19. In a COVID-19 patient, more neutrophils and scattered plasma cell infiltration are frequently found in the spleen. The author suggested that pathological changes of the spleen might be related to the direct attack of virus and the attack of immune cells (45). Similar to our case, slight to moderate FDG uptake in the spleen is confirmed in some reports (10,13), however the significance of this feature is still unknown. In another report, FDG-PET/CT imaging revealed hypoactivity of the orbitofrontal cortex in COVID-19 patients with anosmia (46).
Conclusions
We review the FDG-PET/CT imaging features of COVID-19, including our experience and current knowledge. FDG-PET/CT may have potential to increase our understanding of the mechanism of COVID-19. Further investigation is required to confirm the substantial value of FDG-PET/CT in patients with COVID-19.
Acknowledgements
We thank all the staff in National Center for Global Health and Medicine who struggled with COVID-19. We also thank Kaori Saito, Daisuke Horikawa, Tomoya Takeuchi, Hisayoshi Mizunuma, Yui Yamada, Satsuki Hironaka, Kazuhiko Nakajima, Hironori Kajiwara, and Futoshi Matsunaga for contributing to the PET/CT examination.
References
- 1. Zhu N, Zhang D, Wang W, et al. China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. . 2020; 382:727-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. 2019 Novel coronavirus, Wuhan, China: 2019-nCoV situation summary. https://stacks.cdc.gov/view/cdc/84621 (accessed June 10, 2020).
- 3. Yamayoshi S, Kawaoka Y. Emergence of SARS-CoV-2 and its outlook. Global Health & Medicine. 2020; 2:1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Youyao Xu, Yizhen Chen, Xiaoyan Tang. Guidelines for the diagnosis and treatment of coronavirus disease 2019 (COVID-19) in China. Global Health & Medicine. 2020; 2:66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, Fan Y, Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020; 20:425-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X, Cui J, Xu W, Yang Y, Fayad ZA, Jacobi A, Li K, Li S, Shan H. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV). Radiology. 2020; 295:202-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hani C, Trieu NH, Saab I, Dangeard S, Bennani S, Chassagnon G, Revel MP. COVID-19 pneumonia: A review of typical CT findings and differential diagnosis. Diagn Interv Imaging. 2020; 101:263-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. ACR Recommendations for the use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection. https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection (accessed June 10, 2020).
- 9. Zou S, Zhu X. FDG PET/CT of COVID-19. Radiology. 2020:200770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Qin C, Liu F, Yen TC, Lan X. 18F-FDG PET/CT findings of COVID-19: a series of four highly suspected cases. Eur J Nucl Med Mol Imaging. 2020; 47:1281-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Polverari G, Arena V, Ceci F, Pelosi E, Ianniello A, Poli E, Sandri A, Penna D. 18F-Fluorodeoxyglucose uptake in patient with asymptomatic severe acute respiratory syndrome coronavirus 2 (Coronavirus Disease 2019) Referred to Positron Emission Tomography/Computed Tomography for NSCLC Restaging. J Thorac Oncol. 2020; 15:1078-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deng Y, Lei L, Chen Y, Zhang W. The potential added value of FDG PET/CT for COVID-19 pneumonia. Eur J Nucl Med Mol Imaging. 2020; 47:1634-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Amini H, Divband G, Montahaei Z, Dehghani T, Kaviani H, Adinehpour Z, Akbarian Aghdam R, Rezaee A, Vali R. A case of COVID-19 lung infection first detected by [18F]FDG PET-CT. Eur J Nucl Med Mol Imaging. 2020; 47:1771-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Setti L, Kirienko M, Dalto SC, Bonacina M, Bombardieri E. FDG-PET/CT findings highly suspicious for COVID-19 in an Italian case series of asymptomatic patients. Eur J Nucl Med Mol Imaging. 2020; 47:1649-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu C, Zhou J, Xia L, Cheng X, Lu D. 18F-FDG PET/CT and Serial Chest CT Findings in a COVID-19 Patient With Dynamic Clinical Characteristics in Different Period. Clin Nucl Med. 2020; 45:495-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kamani CH, Jreige M, Pappon M, Fischbacher A, Borens O, Monney P, Nicod Lalonde M, Schaefer N, Prior JO. Added value of 18F-FDG PET/CT in a SARS-CoV-2- infected complex case with persistent fever. Eur J Nucl Med Mol Imaging. 2020; 16:1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wahl RL. Principles and practice of PET and PET/CT. 2nd ed. Lippincott Williams & Wilkins 2008. [Google Scholar]
- 18. Kubota K, Ogawa M, Ji B. Basic science of PET imaging for inflammatory diseases. In Toyama H. et al. ed. "PET/ CT for inflammatory diseases". Springer, 2020, pp1-42. [Google Scholar]
- 19. Dai M, Liu D, Liu M, et al. Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak. Cancer Discov. 2020; 10:783-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Inui S, Fujikawa A, Jitsu M, Kunishima N, Watanabe S, Suzuki Y, Umeda S, Uwabe Y. Chest CT findings in cases from the cruise ship "Diamond Princess" with coronavirus disease 2019 (COVID-19). Radiology: Cardiothoracic Imaging. 2020; 2:e200155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Albano D, Bertagna F, Bertoli M, Bosio G, Lucchini S, Motta F, Panarotto MB, Peli A, Camoni L, Bengel FM, Giubbini R. Incidental Findings Suggestive of COVID-19 in Asymptomatic Patients Undergoing Nuclear Medicine Procedures in a High-Prevalence Region. J Nucl Med. 2020; 61:632-636. [DOI] [PubMed] [Google Scholar]
- 22. Zanoni L, Mosconi C, Cervati V, Diegoli M, Monteduro F, Golfieri R, Fanti S. [18F]-FDG PET/CT for suspected lymphoma relapse in a patient with concomitant pneumococcal pneumonia during COVID-19 outbreak: unexpected SARS-Cov-2 co-infection despite double RT-PCR negativity. Eur J Nucl Med Mol Imaging. 2020; 19:1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paez D, Gnanasegaran G, Fanti S, et al. COVID-19 Pandemic: Guidance for Nuclear Medicine Departments. Eur J Nucl Med Mol Imaging. 2020; 47:1615-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dooms C, van Baardwijk A, Verbeken E, van Suylen RJ, Stroobants S, De Ruysscher D, Vansteenkiste J. Association between 18F-fluoro-2-deoxy-D-glucose uptake values and tumor vitality: prognostic value of positron emission tomography in early-stage non-small cell lung cancer. J Thorac Oncol. 2009; 4:822-828. [DOI] [PubMed] [Google Scholar]
- 25. Win T, Screaton NJ, Porter JC, et al. Pulmonary 18F-FDG uptake helps refine current risk stratification in idiopathic pulmonary fibrosis (IPF). Eur J Nucl Med Mol Imaging. 2018; 45:806-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020; 20:363-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Das KM, Lee EY, Langer RD, Larsson SG. Middle East Respiratory Syndrome Coronavirus: What Does a Radiologist Need to Know? AJR Am J Roentgenol. 2016 ; 206:1193-1201. [DOI] [PubMed] [Google Scholar]
- 28. Pan F, Ye T, Sun P, Gui S, Liang B, Li L, Zheng D, Wang J, Hesketh RL, Yang L, Zheng C. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology. 2020; 295:715-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jones HA, Marino PS, Shakur BH, Morrell NW. In vivo assessment of lung inflammatory cell activity in patients with COPD and asthma. Eur Respir J. 2003; 21:567-573. [DOI] [PubMed] [Google Scholar]
- 30. Xu X, Yu C, Qu J, et al. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. 2020; 47:1275-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Panagiotidis E, Exarhos D, Housianakou I, Bournazos A, Datseris I. FDG uptake in axillary lymph nodes after vaccination against pandemic (H1N1). Eur Radiol. 2010; 20:1251-1253. [DOI] [PubMed] [Google Scholar]
- 32. Kirienko M, Padovano B, Serafini G, Marchianò A, Gronchi A, Seregni E, Alessi A. [18F]FDG-PET/CT and clinical findings before and during early Covid-19 onset in a patient affected by vascular tumour. Eur J Nucl Med Mol Imaging. 2020; 47:1769-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gao QY, Chen YX, Fang JY. 2019 novel coronavirus infection and gastrointestinal tract. J Dig Dis. 2020; 21:125-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Castelnovo L, Capelli F, Tamburello A, Faggioli PM, Mazzone A. Symmetric cutaneous vasculitis in COVID-19 pneumonia. J Eur Acad Dermatol Venereol. 2020; doi:10.1111/jdv.16589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Viner RM, Whittaker E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet. 2020; 395:1741-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Farrah TE, Basu N, Dweck M, Calcagno C, Fayad ZA, Dhaun N. Advances in Therapies and Imaging for Systemic Vasculitis. Arterioscler Thromb Vasc Biol. 2019; 39:1520-1541. [DOI] [PubMed] [Google Scholar]
- 37. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020; 395:1417-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rosenbaum D, Millon A, Fayad ZA. Molecular imaging in atherosclerosis: FDG PET. Curr Atheroscler Rep. 2012; 14:429-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Cury RC, Yates D, LaMuraglia GM, Furie K, Houser S, Gewirtz H, Muller JE, Brady TJ, Fischman AJ. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006; 48:1818-1824. [DOI] [PubMed] [Google Scholar]
- 40. Rondina MT, Lam UT, Pendleton RC, Kraiss LW, Wanner N, Zimmerman GA, Hoffman JM, Hanrahan C, Boucher K, Christian PE, Butterfield RI, Morton KA. 18F-FDG PET in the evaluation of acuity of deep vein thrombosis. Clin Nucl Med. 2012; 37:1139-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chefer S, Thomasson D, Seidel J, Reba RC, Bohannon JK, Lackemeyer MG, Bartos C, Sayre PJ, Bollinger L, Hensley LE, Jahrling PB, Johnson RF. Modeling [18F]- FDG lymphoid tissue kinetics to characterize nonhuman primate immune response to Middle East respiratory syndrome-coronavirus aerosol challenge. EJNMMI Res. 2015; 5:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020; 367:1444-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Amati E, Perbellini O, Rotta G, Bernardi M, Chieregato K, Sella S, Rodeghiero F, Ruggeri M, Astori G. High-throughput immunophenotypic characterization of bone marrow- and cord blood-derived mesenchymal stromal cells reveals common and differentially expressed markers: identification of angiotensin-converting enzyme (CD143) as a marker differentially expressed between adult and perinatal tissue sources. Stem Cell Res Ther. 2018; 9:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bao W, Min D, Twigg SM, Shackel NA, Warner FJ, Yue DK, McLennan SV. Monocyte CD147 is induced by advanced glycation end products and high glucose concentration: possible role in diabetic complications. Am J Physiol Cell Physiol. 2010; 299:C1212-1219. [DOI] [PubMed] [Google Scholar]
- 45. Xu X, Chang XN, Pan HX, et al. Pathological changes of the spleen in ten patients with coronavirus disease 2019(COVID-19) by postmortem needle autopsy. Zhonghua Bing Li Xue Za Zhi. 2020; 49:576-582. (in Chinese). [DOI] [PubMed] [Google Scholar]
- 46. Karimi-Galougahi M, Yousefi-Koma A, Bakhshayeshkaram M, Raad N, Haseli S. 18FDG PET/CT Scan Reveals Hypoactive Orbitofrontal Cortex in Anosmia of COVID-19. Acad Radiol. 2020; 27:1042-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]


