Abstract
Background
Filamentous basidiomycetes are mainly considered to be respiratory tract colonizers but the clinical significance of their isolation in a specimen is debatable. Hormographiella aspergillata was first reported as a human pathogen in 1971. We discuss the role of this mold as a pathogen or colonizer and give an update on diagnostic tools and in vitro antifungal susceptibility.
Case presentation
We identified three cases of H. aspergillata with respiratory symptoms in a short period of time. One invasive infection and two colonizations were diagnosed. Culture supernatants showed that H. aspergillata can produce galactomannan and β-D-glucan but not glucuronoxylomannan. For the first time, isavuconazole susceptibility was determined and high minimum inhibitory concentrations (MICs) were found. Liposomal amphotericin B and voriconazole have the lowest MICs.
Conclusion
To date, 22 invasive infections involving H. aspergillata have been reported. On isolation of H. aspergillata, its pathogenic potential in clinical settings can be tricky. Molecular identification and antifungal susceptibility testing are essential considering high resistance against several antifungal therapies.
Keywords: Hormographiella aspergillata, Coprinus cinereus, Mould, Antifungal susceptibility, Fungal colonization, Basidiomycete
Background
Filamentous basidiomycetes are mainly considered to be respiratory tract colonizers but increasingly these molds are being documented in invasive infections [1]. Hence, the clinical significance of their isolation in a specimen is debatable. Hormographiella aspergillata is a filamentous basidiomycete growing on horse dung. It was found in numerous environmental substrates and first reported as a human pathogen in 1971 [2–4]. Since, a few infections were reported all over the world with various clinical outcomes, essentially pulmonary but also disseminated or located to the eye or the skin [2, 5–22]. Thus, data are sparse for the diagnosis and management of such infections. Here, we report a new case of human infection involving H. aspergillata and two cases of colonization. We then review all previously published cases and discuss diagnostic strategy and clinical management.
Case presentation
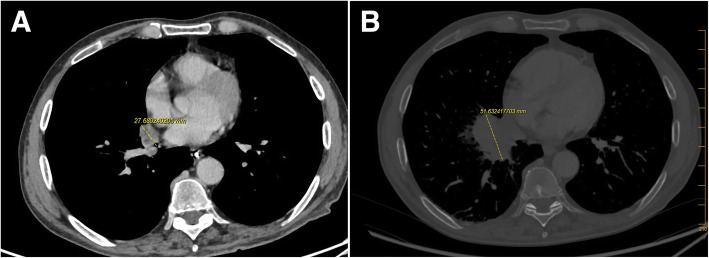
The first case (HA1) was an 70-year-old man admitted to the hematology department for prolonged febrile neutropenia and anorexia. He had a history of acute myeloid leukemia (AML) and hematopoietic stem cell transplantation (HSCT). His C-reactive protein (CRP, positivity threshold value: 3 mg/L) was 135 mg/L and empirical antibiotic therapy (ceftriaxone) was started at day 210 (D210, 7th month) post-HSCT. Chest computed tomography (CT) scan showed right lower lobe opacification (Fig. 1a) that had increased 1 week later (Fig. 1b). Invasive fungal infection (IFI) was suspected, and liposomal amphotericin B (lAmB 5 mg/kg/day) was started on D232 (7th month). Microscopic examination of a bronchoalveolar lavage (BAL) sampled at D237 (7th month) showed septate hyphae (Fig. 2) but cultures on Sabouraud media incubated at 25 °C and 35 °C were sterile after 7 days. H. aspergillata was identified by sequencing the internal transcribed spacer (ITS) region of fungi directly from the BAL. Interestingly, serum galactomannan monitoring was negative (< 0.1 on repeated samples; Platelia® Aspergillus assay, Bio-Rad; positivity threshold index: > 0.5) and β-D-glucan (Fungitell®, Cape Cod; positivity threshold value: 80 pg/mL) was weakly positive on D237 (7th month; 98 pg/mL) but negative on D248 (8th month; 46 pg/mL). In accordance with the 2008 European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) criteria, the patient was classified as having probable IFI [23]. His condition worsened following pulmonary Stenotrophomonas maltophilia infection and so it was decided to initiate palliative care. lAmB was stopped on D253 (8th month), 3 weeks after its introduction. The patient died on D298 (9th month).
Fig. 1.
Chest computed tomography scan of HA1 patient showing a right lower lobe opacification and b increase in the lesion size 1 week later
Fig. 2.
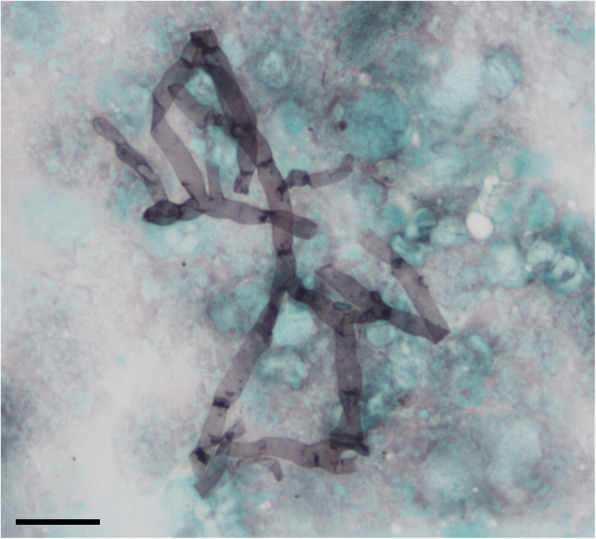
Microscopy examination of bronchoalveolar lavage from patient HA1 by Gomori-Grocott staining showing the presence of septate hyphae. Scale-bar: 10 μm
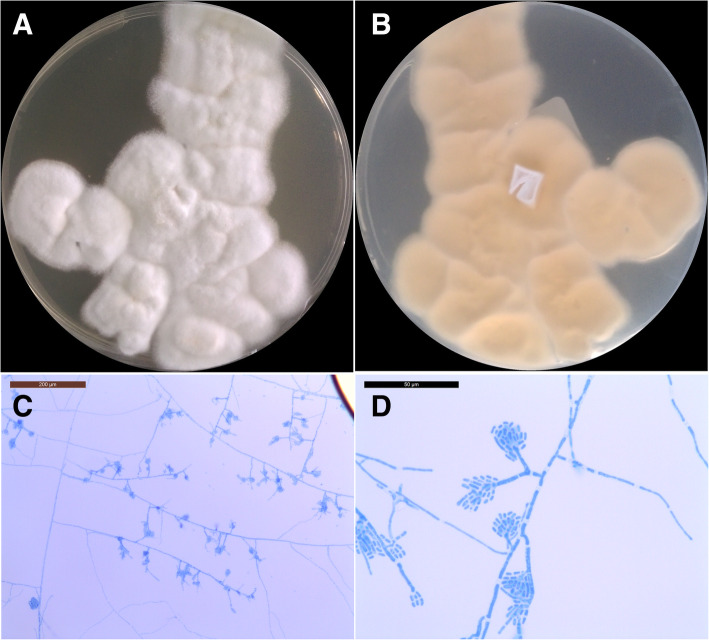
The second patient (HA2) was a 49-year-old man admitted to the intensive care unit for pneumopathy with acute respiratory failure. He had a history of psychiatric disorders, diabetes mellitus, asthma, smoking and middle cerebral artery stroke with persistent sequelae. CRP was negative on admission. The following day, it was positive at 108.0 mg/L but procalcitonin remained negative. Mechanical ventilation and empirical antibiotic therapy (ceftazidime) were initiated. A mucous plug containing purulent secretions in the left lung was removed by fibroscopy and transmitted to Bacteriology and Mycology Laboratories. Microscopy examinations of samples were negative but cultures identified oropharyngeal microbiota associated with a white mold on Sabouraud media at 25 °C and 35 °C after 7 days. Subcultures of mold grew with white to slightly cream-colored velvety colonies (Fig. 3a and b) on potato dextrose agar media. Microscopy examination of cultures showed hyaline septate hyphae with conidiophores producing cylindrical arthroconidia (Fig. 3c and d). H. aspergillata identification was confirmed by sequencing the ITS region. In vitro antifungal susceptibility testing was performed via broth microdilution technique according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines [24]. Minimum inhibitory concentrations (MICs) are given in Table 1. The chest CT scan was unremarkable and there was no risk factor for IFI and so no antifungal therapy was initiated. The inflammatory syndrome decreased rapidly 3 days later, and the patient’s condition improved. A putative diagnosis of bacterial aspiration pneumonia with fungal colonization was established.
Fig. 3.
Macroscopic and microscopic morphology of Hormographiella aspergillata on potato dextrose agar (PDA) subculture after 3 days of incubation at 25 °C. a White to cream colored velvety colonies with irregular margin on the recto side. b Verso side of the colonies showing light yellow color. c, d Slide culture of Hormographiella aspergillata showing hyaline septate hyphae with conidiophores and cylindrical arthroconidia without clamp connection, scale-bar: 200 μm (c) and 50 μm (d)
Table 1.
Antifungal susceptibility testing of Hormographiella aspergillata from the literature and our cases
| MIC (mg/L) for single isolates | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| References | Year | Method | AmB | 5-FC | FCZ | ITZ | VRZ | PSZ | ISA | CSF | MCF |
| Speller and MacIver [2]. | 1971 | Dilution method on Yeast Morphology Agar | 0.25 | > 250 | / | / | / | / | / | / | / |
| Verweij et al. [6] | 1997 | Broth macrodilution method with RPMI-1640 | 0.5 | / | > 64 | 8 | / | / | / | / | / |
| Lagrou et al. [8] | 2005 | E-test® | 0.5 | / | / | 2 | 0.25 | / | / | / | / |
| Abuali et al. [10] | 2009 | Broth microdilution method according to CLSI M38-A2 | 4 | / | / | / | 0.25 | 0.5 | / | > 2 | > 4 |
| Conen et al. [11] | 2011 | E-test® | 0.5 | / | 256 | / | 0.125 | / | / | 32 | / |
| Conen et al. [11] | 2011 | E-test® | 0.5 | / | / | / | 0.125 | 2 | / | / | / |
| Conen et al. [11] | 2011 | E-test® | 0.5 | / | > 256 | / | 0.25 | / | / | > 32 | / |
| Suarez et al. [12] | 2011 | Broth microdilution method according to EUCAST | 2 | > 64 | 64 | > 8 | 1 | / | / | 2 | / |
| Bojic et al. [14] | 2013 | E-test® | 0.094 | > 32 | > 256 | / | 0.125 | 0.064 | / | / | / |
| Nanno et al. [17] | 2016 | Not available | 0.125 | > 64 | 4 | 0.25 | 0.015 | / | / | / | > 16 |
| Koncan et al. [18] | 2016 | Sensititre YeastOne | 0.12 | / | 16 | / | 0.03 | 0.125 | / | / | / |
| Jain et al. [21] | 2019 | Not available | 0.3 | / | / | 4 | 0.5 | / | / | 4 | 4 |
|
Our report HA2 |
2019 | Broth microdilution method according to EUCAST | 0.125 | / | / | > 8 | 2 | 4 | 4 | / | / |
|
Our report HA3 |
2019 | Broth microdilution method according to EUCAST | 0.125 | / | / | > 8 | 8 | > 8 | 16 | / | / |
*AMB Amphotericin B, 5-FC Flucytosine, FCZ Fluconazole, ITZ Itraconazole, VRZ Voriconazole, PSZ Posaconazole, ISA Isavuconazole. CSF Caspofungin, MCF Micafungin
The third patient (HA3) was a 28-year-old woman admitted for investigation of an inflammatory disease affecting the central nervous system treated by methylprednisolone for 3 days (1 g/day). Bronchial fibroscopy was performed along with other investigations. Initial microscopy examination of the sample was negative but H. aspergillata grew after 3 weeks on Lowenstein-Jensen medium at 35 °C because of mycobacterial suspicion (identification confirmed by ITS sequencing). Antifungal susceptibility testing was performed as described above (Table 1). The patient was asymptomatic and her chest CT scan normal, suggesting colonization, and so no antifungal treatment was initiated.
Literature review
We reviewed the literature since 1971 to date using the terms “Hormographiella aspergillata” or “Coprinus cinereus” and “infection” in MEDLINE database (Tables 1 and 2). For each strain, antifungals MIC with the method used were reported in Table 1 when available. According to the 2008 EORTC/MSG criteria, all probable or proven IFI due to H. aspergillata were reported in Table 2 with significant clinical details.
Table 2.
Literature review of Hormographiella aspergillata infections in humans published since 1971
| References | Country | Year | Infection site | EORTC/MSG classification | Underlying disease | Diagnosis Samples - Methods |
Antifungal treatment | Surgery | Outcome | |
|---|---|---|---|---|---|---|---|---|---|---|
| Speller and MacIver [2]. | England | 1971 | Heart | Proven | Prosthetic Valve | Autopsy | Histology + culture | None | Yes | Died |
| Nenoff et al. [5] | Germany | 1997 | Lung | Proven | ALL | Autopsy | Histology + culture | AmB | No | Died |
| Verweij et al. [6] | Netherlands | 1997 | Lung | Proven | ALL | Autopsy | Histology + culture + RFLP | AmB ➔ ITZ | No | Died |
| Surmont et al. [7] | Belgium | 2002 | Lung | Proven | Lymphoma | Transthoracic puncture | DE + culture | AmB | No | Alive |
| Lagrou et al. [8] | Belgium | 2005 | Lung | Probable | AML | BAL | DE + culture | CSF | No | Died |
| Greer et al. [9] | USA | 2008 | Heart | Proven | Valve prosthesis | Resected valve | Histology + culture | lAmB | Yes | Alive |
| Abuali et al. [10] | USA | 2009 | Skin | Proven | AML | Skin biopsy | Culture | VRZ ➔ PSZ + CSF ➔ lAmB + CSF | No | Died |
| Conen et al. [11] | Switzerland | 2011 | Lung, eye, CNS, blood | Proven | AML | Autopsy | Histology + culture | VRZ ➔ PSZ ➔ CSF | No | Died |
| Lung | Proven | AML | Lung biopsy | Histology + culture | VRZ ➔ PSZ ➔ lAmB ➔ VRZ | Yes | Died | |||
| Lung | Proven | AML | Lung biopsy | Histology + culture | VRZ ➔ lAmB ➔ VRZ | Yes | Died | |||
| Suarez et al. [12] | France | 2011 | Lung | Proven | BAL | Lung biopsy | DE + culture | CSF ➔ VRZ ➔ lAmB | No | Alive |
| Lung | Proven | X-ALD | Autopsy | Histology + culture + PF-PCR | CSF ➔ lAmB | No | Died | |||
| Pang et al. [13] | France | 2012 | Lung | Proven | ALL | Lung biopsy | Culture | CSF ➔ VRZ ➔ lAmB | No | Alive |
| Bojic et al. [14] | Austria | 2013 | Skin, lung | Proven | AML | Skin biopsy | Histology | CSF ➔ lAmB + VRZ | Yes | Died |
| Corzo-León et al. [15] | USA | 2015 | Lung | Probable | AML | BAL | Culture | VRZ ➔ lAmB | No | Died |
| Heiblig et al. [16] | France | 2015 | Sinus, orbit, CNS | Proven | AML | Sinus biopsy | DE + culture | CSF ➔ PSZ ➔ lAmB + VRZ | Yes | Died |
| Nanno et al. [17] | Japan | 2016 | Lung, CNS, small intestine | Proven | MDS | Lung biopsy | Histology + culture + βDG | ITZ ➔ lAmB + CSF ➔ VRZ + MCF ➔ VRZ + lAmB | No | Died |
| Koncan et al. [18] | Italy | 2016 | Lung | Proven | MPAL | Lung resection |
Culture + PF-PCR + βDG |
PSZ ➔ VRZ | Yes | Alive |
| Correa-Martinez et al. [19] | Germany | 2017 | Skin | Proven | Nephroblastoma | Skin biopsy | Histology + culture | PSZ | Yes | Alive |
| Godet et al. [20] | France | 2017 | Lung | Proven | AML | Lung biopsy | DE + PF-PCR | VRZ ➔ lAmB (IV + nebulized) | Yes | Alive |
| Jain et al. [21] | India | 2019 | Eye | Proven | Intraocular lens implantation | Corneal tissue | DE + culture + PF-PCR | Natamycin + ITZ ➔ VRZ | Yes | Alive (loss of the eye) |
| Chauhan et al. [22] | USA | 2019 | Lung, CNS | Proven | CML | Autopsy | Histology + culture + PF-PCR + βDG | MCF | No | Died |
| Our report HA1 | France | 2019 | Lung | Probable | AML | BAL |
DE + PF-PCR + βDG |
lAmB | No | Died |
Search for previously published cases using the terms “Hormographiella aspergillata” or “Coprinus cinereus infection” in MEDLINE database
*ALL Acute lymphoid leukemia, AML Acute myeloid leukemia, BAL Biphenotypic acute leukemia, X-ALD X-linked adrenoleukodystrophy, CML Chronic myeloid leukemia, MDS Myelodysplasia syndrome, MPAL Mixed phenotype acute leukemia, CNS Central nervous system, AmB Deoxycholate amphotericin B, ITZ Itraconazole, CSF Caspofungin, VRZ Voriconazole, PSZ Posaconazole, lAmB Liposomal amphotericin B, MCF Micafungin, IV Intravenous, RFLP Restriction fragment length polymorphism, DE Direct examination, PF-PCR Pan-fungal-polymerization chain reaction, βDG 1, 3-beta-D glucan
Discussion and conclusions
Hormographiella aspergillata is an environmental filamentous basidiomycete found in numerous substrates including soils, leaves, pressmud compost and in the air [3, 4]. It is the anamorph form of Coprinopsis cinerea (formerly Coprinus cinereus), which commonly grows on horse dung. It can be an opportunistic pathogen and is the second filamentous basidiomycete responsible for human infection after Schizophyllum commune [25]. To date, 22 invasive infections involving H. aspergillata have been reported (Table 2), mostly identified by sequencing of the 28S rDNA or ITS regions [2, 5–22]. Most cases were diagnosed in Europe, but some were documented in the United States, Japan and India, in both rural and urban areas [2, 5–22]. Infection cases occurred mainly in neutropenic patients. Although H. aspergillata is primarily responsible for pulmonary infections it can occasionally cause primary cutaneous lesions [10, 14, 19]. H. aspergillata is able to grow in blood cultures [11] and a few cases of disseminated infections have been reported, affecting the small intestine, the eye and the brain [11, 16, 17, 22]. Interestingly, three cases of IFI have also been reported in immunocompetent patients following cardiac or ophthalmic surgery [2, 9, 21]. The most contributive samples were biopsies, but some cases were diagnosed with BAL. [8, 15] H. aspergillata grows well on different fungal media without cycloheximide at 25 °C or 35 °C. However, diagnosis can be challenging in patients with negative cultures, as for the HA1 patient, whose strain was probably inhibited by the concomitant antifungal treatment. To date, there are insufficient data to draw any conclusions about biomarkers since in all documented reports galactomannan assays were negative and only two observations reported strongly positive β-D-glucan antigens greater than 500 pg/mL [18, 22]. We attempted to evaluate the production of galactomannan, β-D-glucan and glucuronoxylomannan antigens on in vitro cultures. Glucuronoxylomannan is a capsular antigen of Cryptococcus neoformans widely used to diagnose cryptococcosis. Some cross-reactions have already been described with other basidiomycete pathogens such as Trichosporon sp. or even Coprinopsis cinerea [26]. Interestingly, culture supernatants from strains HA2 and HA3 showed that H. aspergillata can produce galactomannan and β-D-glucan but not glucuronoxylomannan (Table 3). Although, as for HA1, results in sera are variable, biomarker assays could provide supplementary evidence in patients with suspected IFI.
Table 3.
Galactomannan (GM), β-D-glucan and glucuronoxylomannan antigen assays on culture supernatant. For each strain, 5 to 10 colonies incubated at 35 °C for 4 days on Sabouraud media were suspended in 1 ml distilled water. After vigorous agitation, the suspensions were centrifuged for 5 min at 10,000 g. 1, 1:10 and 1:100 dilutions of the supernatants were then tested with Platelia® Aspergillus assay (Bio-Rad, France), Fungitell® assay (Associates of Cape Cod Inc., USA) and Biosynex® CryptoPS assay (Biosynex, France) according to the manufacturer’s recommendations
| Isolate | Dilution factor | Galactomannan | β-D-glucan (pg/mL) | Glucuronoxylomannan |
|---|---|---|---|---|
| HA2 | 1 | > 3,5 | > 500 | Negative |
| 10 | > 3,5 | > 500 | n.d. | |
| 100 | 0,2423 | 51,048 | n.d. | |
| HA3 | 1 | > 3,5 | > 500 | Negative |
| 10 | > 3,5 | > 500 | n.d. | |
| 100 | 0,4197 | 98,804 | n.d. |
n.d. Not determined
H. aspergillata can also be a colonizer of the respiratory tract, as illustrated in our three patients, all of whom had an underlying respiratory condition. The weak clinical significance of the isolation of basidiomycetes in healthy subjects, in contrast with their life-threatening potential in immunocompromised patients, has already been described with Schizophyllum commune or Ceriporia lacerata, for example [27, 28]. These fungi are widely present in the environment, and their spores are easily inhaled and can grow in pulmonary alveoli in cases of local or systemic impaired function of alveolar macrophages.
As yet there are no EUCAST nor Clinical and Laboratory Standards Institute (CLSI) breakpoints to interpret the antifungal MICs for H. aspergillata. However, previous articles have reported in vitro resistance to echinocandins, fluconazole along with high MIC for flucytosine (Table 1). We found higher MICs for isavuconazole (4 and 16 mg/L) than what is usually observed for basidiomycetes [28, 29]. In the light of our findings and data from the literature, lAmB and voriconazole have the lowest MICs. However, H. aspergillata infections have a poor prognosis even when surgical debridement is performed.
In conclusion, on isolation of H. aspergillata, its pathogenic potential in clinical samples should be interpreted together with the patient’s history. Formal identification of the fungus can be tricky and usually requires molecular tools in addition to culture. Basidiomycetes can also be contaminants or colonizers and so microscopy examination of samples and/or histology in combination with biomarkers are crucial for diagnosis. Respiratory tract colonization is probably not uncommon given that the fungus is widespread in the environment but seems to be restricted to patients with underlying respiratory diseases. lAmB and voriconazole seem to be the antifungals of choice.
Acknowledgements
Not applicable.
Abbreviations
- AML
Acute myeloid leukemia
- BAL
Bronchoalveolar lavage
- CLSI
Clinical and Laboratory Standards Institute
- CRP
c-reactive protein
- CT
Computed tomography
- EORTC/MSG
European Organization for Research and Treatment of Cancer/ Mycoses Study Group
- EUCAST
European Committee on Antimicrobial Susceptibility Testing
- HSCT
Hematopoietic stem cell transplantation
- IFI
Invasive fungal infection
- ITS
Internal transcribed spacer
- lAmB
Liposomal amphotericin B
- MIC
Minimum inhibitory concentrations
Authors’ contributions
MM did the literature search and drafted the manuscript. MM, RAL, TM did the experimentations. RG, FM and PP provided guidance for drafting the manuscript. CN conceived the case report and oversaw the manuscript. All authors read and approved the final manuscript.
Funding
There was no funding for this study.
Availability of data and materials
New genome sequences obtained in this study have been deposited in GenBank under accession numbers MN841917, MN841918 and MN841919.
Ethics approval and consent to participate
This case report received approval from University Hospital of Clermont-ferrand Hospital Ethics and Research Committee. This document is available upon request.
Consent for publication
Written informed consent was obtained from the next-of-kin of patient HA1 and from patients HA2 and HA3 for publication of this case report and any accompanying images. Copies of the written consents are available for review by the Editor of this journal.
Competing interests
The authors have no conflicts of interest to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brandt ME. Filamentous Basidiomycetes in the clinical laboratory. Curr Fungal Infect Rep. 2013;7(3):219–223. doi: 10.1007/s12281-013-0148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Speller DE, MacIver AG. Endocarditis caused by a Coprinus species: a fungus of the toadstool group. J Med Microbiol. 1971;4(3):370–374. doi: 10.1099/00222615-4-3-370. [DOI] [PubMed] [Google Scholar]
- 3.Gené J, Guillamon JM, Guarro J, Pujol I, Ulfig K. Molecular characterization, relatedness and antifungal susceptibility of the basidiomycetous Hormographiella species and Coprinus cinereus from clinical and environmental sources. Antonie Van Leeuwenhoek. 1996;70(1):49–57. doi: 10.1007/BF00393569. [DOI] [PubMed] [Google Scholar]
- 4.de Oliveira TB, Lopes VCP, Barbosa FN, Ferro M, Meirelles LA, Sette LD, et al. Fungal communities in pressmud composting harbour beneficial and detrimental fungi for human welfare. Microbiology. 2016;162(7):1147–1156. doi: 10.1099/mic.0.000306. [DOI] [PubMed] [Google Scholar]
- 5.Nenoff P, Friedrich T, Schwenke H, Mierzwa M, Horn LC, Haustein UF. Rare fatal simultaneous mould infection of the lung caused by Aspergillus flavus and the basidiomycete Coprinus sp. in a leukemic patient. J Med Vet Mycol Bi-Mon Publ Int Soc Hum Anim Mycol. 1997;35(1):65–69. [PubMed] [Google Scholar]
- 6.Verweij PE, van Kasteren M, van de Nes J, de Hoog GS, de Pauw BE, Meis JF. Fatal pulmonary infection caused by the basidiomycete Hormographiella aspergillata. J Clin Microbiol. 1997;35(10):2675–2678. doi: 10.1128/JCM.35.10.2675-2678.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Surmont I, Van Aelst F, Verbanck J, De Hoog GS. A pulmonary infection caused by Coprinus cinereus (Hormographiella aspergillata) diagnosed after a neutropenic episode. Med Mycol. 2002;40(2):217–219. doi: 10.1080/mmy.40.2.217.219. [DOI] [PubMed] [Google Scholar]
- 8.Lagrou K, Massonet C, Theunissen K, Meersseman W, Lontie M, Verbeken E, et al. Fatal pulmonary infection in a leukaemic patient caused by Hormographiella aspergillata. J Med Microbiol. 2005;54(7):685–688. doi: 10.1099/jmm.0.46016-0. [DOI] [PubMed] [Google Scholar]
- 9.Greer EL, Kowalski TJ, Cole ML, Miller DV, Baddour LM. Truffle’s revenge: a pig-eating fungus. Cardiovasc Pathol. 2008;17(5):342–343. doi: 10.1016/j.carpath.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Abuali MM, Posada R, Del Toro G, Roman E, Ramani R, Chaturvedi S, et al. Rhizomucor variabilis var. regularior and Hormographiella aspergillata infections in a leukemic bone marrow transplant recipient with refractory neutropenia. J Clin Microbiol. 2009;47(12):4176–4179. doi: 10.1128/JCM.00305-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conen A, Weisser M, Hohler D, Frei R, Stern M. Hormographiella aspergillata: an emerging mould in acute leukaemia patients? Clin Microbiol Infect. 2011;17(2):273–277. doi: 10.1111/j.1469-0691.2010.03266.x. [DOI] [PubMed] [Google Scholar]
- 12.Suarez F, Olivier G, Garcia-Hermoso D, Randriamalala E, Ghez D, Bruneau J, et al. Breakthrough Hormographiella aspergillata infections arising in Neutropenic patients treated empirically with Caspofungin. J Clin Microbiol. 2011;49(1):461–465. doi: 10.1128/JCM.01213-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pang K-AP, Godet C, Fekkar A, Scholler J, Nivoix Y, Letscher-Bru V, et al. Breakthrough invasive mould infections in patients treated with caspofungin. J Inf Secur. 2012;64(4):424–429. doi: 10.1016/j.jinf.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Bojic M, Willinger B, Rath T, Tobudic S, Thalhammer F, Böhm A, et al. Fatal skin and pulmonary infection caused by Hormographiella aspergillata in a leukaemic patient: case report and literature overview. Mycoses. 2013;56(6):687–689. doi: 10.1111/myc.12087. [DOI] [PubMed] [Google Scholar]
- 15.Corzo-León DE, Satlin MJ, Soave R, Shore TB, Schuetz AN, Jacobs SE, et al. Epidemiology and outcomes of invasive fungal infections in allogeneic haematopoietic stem cell transplant recipients in the era of antifungal prophylaxis: a single-Centre study with focus on emerging pathogens. Mycoses. 2015;58(6):325–336. doi: 10.1111/myc.12318. [DOI] [PubMed] [Google Scholar]
- 16.Heiblig M, Bozzoli V, Saison J, Thomas X, Croze DD, Traverse-Glehen A, et al. Combined medico-surgical strategy for invasive sino-orbito-cerebral breakthrough fungal infection with Hormographiella aspergillata in an acute leukaemia patient. Mycoses. 2015;58(5):308–312. doi: 10.1111/myc.12305. [DOI] [PubMed] [Google Scholar]
- 17.Nanno S, Nakane T, Okamura H, Nishimoto M, Koh H, Nakamae H, et al. Disseminated Hormographiella aspergillata infection with involvement of the lung, brain, and small intestine following allogeneic hematopoietic stem cell transplantation: case report and literature review. Transpl Infect Dis. 2016;18(4):611–616. doi: 10.1111/tid.12561. [DOI] [PubMed] [Google Scholar]
- 18.Koncan R, Nadali G, Favuzzi V, Ligozzi M, Sorrentino A, Cascio GL. Invasive fungal infection by Hormographyella aspergillata: a tricky diagnosis triggered by (1,3)-Beta-D-Glucan assay. J Microb Biochem Technol. 2016;8(4):1–3. doi: 10.4172/1948-5948.1000309. [DOI] [Google Scholar]
- 19.Correa-Martinez C, Brentrup A, Hess K, Becker K, Groll AH, Schaumburg F. First description of a local Coprinopsis cinerea skin and soft tissue infection. New Microbes New Infect. 2017;21:102–104. doi: 10.1016/j.nmni.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Godet C, Cateau E, Rammaert B, Grosset M, Moal GL, Béraud G, et al. Nebulized liposomal amphotericin B for treatment of pulmonary infection caused by Hormographiella aspergillata: case report and literature review. Mycopathologia. 2017;182(7–8):709–713. doi: 10.1007/s11046-017-0117-9. [DOI] [PubMed] [Google Scholar]
- 21.Jain N, Jinagal J, Kaur H, Ghosh A, Gupta S, Ram J, et al. Ocular infection caused by Hormographiella aspergillata: a case report and review of literature. J Mycol Médicale. 2019;29(1):71–74. doi: 10.1016/j.mycmed.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Chauhan A, Gruenberg J, Arbefeville S, Mettler T, Brent CH, Ferrieri P. Disseminated Hormographiella aspergillata infection with lung and brain involvement after Allogenic hematopoietic stem-cell transplantation in a 54-year-old man. Lab Med. 2019;50:426. doi: 10.1093/labmed/lmz018. [DOI] [PubMed] [Google Scholar]
- 23.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of cancer/invasive fungal infections cooperative group and the National Institute of Allergy and Infectious Diseases mycoses study group (EORTC/MSG) consensus group. Clin Infect Dis. 2008;46(12):1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arendrup MC, Meletiadis J, Mouton JW, Guinea J, Cuenca-Estrella M, Lagrou K, et al. Eucast definitive document E. DEF. 9.3.1. Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia forming Moulds. London: European Committee on Antimicrobial Susceptibility Testing; 2017. [Google Scholar]
- 25.Chowdhary A, Kathuria S, Agarwal K, Meis JF. Recognizing filamentous basidiomycetes as agents of human disease: a review. Med Mycol. 2014;52(8):782–797. doi: 10.1093/mmy/myu047. [DOI] [PubMed] [Google Scholar]
- 26.Tone K, Umeda Y, Makimura K. Cross-reactivity in Cryptococcus antigen latex agglutination test in two commercial kits. Med Mycol. 2016;54(4):439–443. doi: 10.1093/mmy/myv115. [DOI] [PubMed] [Google Scholar]
- 27.Iizasa T, Kamei K, Chiyo M, Suzuki M, Baba M, Toyosaki T, et al. Colonization with Schizophyllum commune of localized honeycomb lung with mucus. Respir Int Rev Thorac Dis. 2001;68(2):201–203. doi: 10.1159/000050493. [DOI] [PubMed] [Google Scholar]
- 28.Chowdhary A, Agarwal K, Kathuria S, Singh PK, Roy P, Gaur SN, et al. Clinical significance of filamentous basidiomycetes illustrated by isolates of the novel opportunist Ceriporia lacerata from the human respiratory tract. J Clin Microbiol févr. 2013;51(2):585–590. doi: 10.1128/JCM.02943-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chowdhary A, Kathuria S, Singh PK, Agarwal K, Gaur SN, Roy P, et al. Molecular characterization and in vitro antifungal susceptibility profile of Schizophyllum commune, an emerging Basidiomycete in Bronchopulmonary mycoses. Antimicrob Agents Chemother. 2013;57(6):2845–2848. doi: 10.1128/AAC.02619-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
New genome sequences obtained in this study have been deposited in GenBank under accession numbers MN841917, MN841918 and MN841919.