Abstract
Background
Bacteremia in adult patients has traditionally been treated with extended courses of intravenous antibiotics. Data on the use of (or rapid transition to) oral therapy are limited.
Methods
Adult infectious disease physicians participating in the Infectious Diseases Society of America Emerging Infections Network (EIN) were surveyed regarding their use of oral antibiotics in patients with bacteremia. Respondents were asked to assume that patients were hemodynamically stable, recovered bacteria were susceptible to potential antibiotics, adequate source control had been achieved, and patients had adequate gastrointestinal absorption. Variables of specific bacteria, oral agent, and associated infection were included.
Results
A total of 655 (50%) of 1321 EIN participants responded. Under certain conditions, 88% would transition patients with Gram-negative bacteremia to complete a course of therapy with oral antibiotics; 71% would transition patients with Gram-positive bacteremia to oral agents. Only 78 (12%) respondents would not treat any bacteremic patient with oral agents. Most respondents (≥75%) were comfortable treating infections secondary to Enterobacteriaceae, Salmonella, Pseudomonas, Stenotrophomonas, Streptococcus pneumoniae, and β-hemolytic streptococci with oral agents. Fewer than 20% endorsed use of oral antibiotics for Staphylococcus aureus or in cases of endocarditis. Fluoroquinolones and trimethoprim-sulfamethoxazole were the preferred agents in Gram-negative bacteremia; linezolid and β-lactams were the preferred agents in Gram-positive bacteremia.
Conclusions
In select circumstances, the majority of respondents would transition patients to oral antibiotics, in both Gram-negative and Gram-positive bacteremia. Most agreed with the use of oral agents in Gram-negative bacteremia caused by Enterobacteriaceae, but they would not use oral agents for Gram-positive bacteremia caused by S aureus or in endocarditis.
Keywords: bacteremia, oral antibiotics, oral antimicrobial agents
Infections complicated by bacteremia have traditionally been treated with intravenous (IV) antimicrobial agents. Data supporting the use of (or rapid transition to) oral antimicrobial agents in these infections are quite limited. Intravenously infused antibiotics carry multiple advantages, including high blood levels, delivered to the site of infection, with assurance that patients are receiving adequate therapy, through avoidance of potential issues with drug absorption, distribution, and adherence. Once the patient’s infection is controlled and the cause (pathogen, antimicrobial susceptibility of the pathogen, source, etc) is known, continued treatment with IV antibiotics may not be the most beneficial choice. In addition to the cost of these agents and the expense of placement and maintenance of IV access, catheter-related infections and thrombosis are untoward effects of continued IV therapy. Oral treatment, when possible, obviates these negative impacts. Multiple factors influence the efficacy of transitioning to oral antimicrobial agents in these serious infections, including bioavailability of the agent and whether therapeutic levels of drug are achievable at the site of infection. Additional concerns include patient adherence to treatment plans.
The Infectious Disease Society of America (IDSA) Emerging Infections Network (EIN) is a provider-based emerging infections sentinel network that includes infectious disease (ID) specialist physicians from across the United States [1]. We conducted a survey to assess the practice patterns of these ID specialists in transitioning patients to oral antibiotics in the treatment of bacteremia. Our survey examined which bacterial pathogens our respondents felt comfortable treating with oral agents and with which antibiotics. We also included the source of infection as a variable in these questions.
METHODS
A 10-question multiple choice/open comment survey was developed by the authors, with input and pilot testing by ID physicians with additional content expertise. On September 18, 2018, we distributed the survey by e-mailed link or by facsimile to all 1441 IDSA EIN IDs physician members in active adult-based practice. Two reminders were sent to nonrespondents and the survey remained open until October 14, 2018.
The survey included 2 clinical vignettes. The first case was a patient with Gram-negative bacteremia secondary to acute pyelonephritis. The second was the case of a patient with Gram-positive, central-line associated bloodstream infection (see Supplementary Appendix A). Questions associated with each vignette asked respondents to select oral antibiotics/antibiotic classes, specific organisms/organism group, and infectious sources of bacteremia for which they would be comfortable transitioning patients to oral therapy. For both clinical vignettes, the survey included a note stating, “For all questions assume a hemodynamically stable patient with known susceptible bacteria, adequate source control, and presumed adequate gastrointestinal absorption.” An open-text field was provided following each answer to allow survey respondents to comment on the choices.
Practice characteristics for participants, including employment, geographic location, and years of practice were imported from the EIN database. Similar to previous EIN surveys, the response rate was calculated from EIN members who had ever responded to a survey [1]. Descriptive statistics were calculated as percentages for each response category. Statistical analyses were performed using SAS version 9.4 software (SAS Institute, Cary, NC).
RESULTS
Participants
Of 1321 adult ID physician IDSA EIN participants who had ever responded to an EIN survey, 655 (50%) responded to this survey (Table 1). This included physicians from all regions of the United States, ranging in experience from fellows-in-training to those with at least 25 years of IDs experience. Hospital types represented by respondents included community, nonuniversity teaching, university, Veterans’ Affairs or other federal (eg, military), and city/county.
Table 1.
Practice Characteristics for Infectious Diseases (ID) Physician Respondents (N = 655) Categorized by Whether There Were Scenarios in Which They Would Transition Patients With Gram-Negative and Gram-Positive Bacteremias to Oral Antibiotics to Complete a Course of Therapy
| Variable | Would Use Oral Antibiotics in Gram-Negative Bacteremia | Would Use Oral Antibiotics in Gram-Positive Bacteremia | Total |
|---|---|---|---|
| Total number (%) | 575 (87.8) | 466 (71.2) | 655 (100) |
| US Census Bureau Region | |||
| South | 163 (84.9) | 128 (66.7) | 192 |
| Midwest | 153 (90.0) | 121 (71.2) | 170 |
| Northeast | 122 (87.8) | 104 (74.8) | 139 |
| West | 133 (88.7) | 109 (72.7) | 150 |
| Canada and Puerto Rico | 4 (100) | 4 (100) | 4 |
| Years of ID Experience | |||
| <5 years | 118 (89.4) | 93 (70.5) | 132 |
| 5–14 years | 202 (91.0) | 164 (73.9) | 222 |
| 15–24 years | 91 (87.5) | 69 (66.4) | 104 |
| ≥25 years | 164 (83.3) | 140 (71.1) | 197 |
| Primary Hospital Type | |||
| Community | 160 (89.9) | 120 (67.4) | 178 |
| Nonuniversity teaching | 162 (90.5) | 134 (74.9) | 179 |
| University | 181 (83.8) | 155 (71.8) | 216 |
| Veterans’ Affairs or other Federal | 44 (88.0) | 35 (70.0) | 50 |
| City/county | 28 (87.5) | 22 (68.8) | 32 |
| Primary Hospital Bed Size | |||
| <200 | 58 (80.6) | 43 (59.7) | 72 |
| 200–350 | 135 (90.6) | 113 (75.8) | 149 |
| 351–450 | 90 (84.9) | 71 (67.0) | 106 |
| 451–600 | 124 (90.5) | 100 (73.0) | 137 |
| >600 | 168 (88.0) | 139 (72.8) | 191 |
Gram-Negative Bacteremia
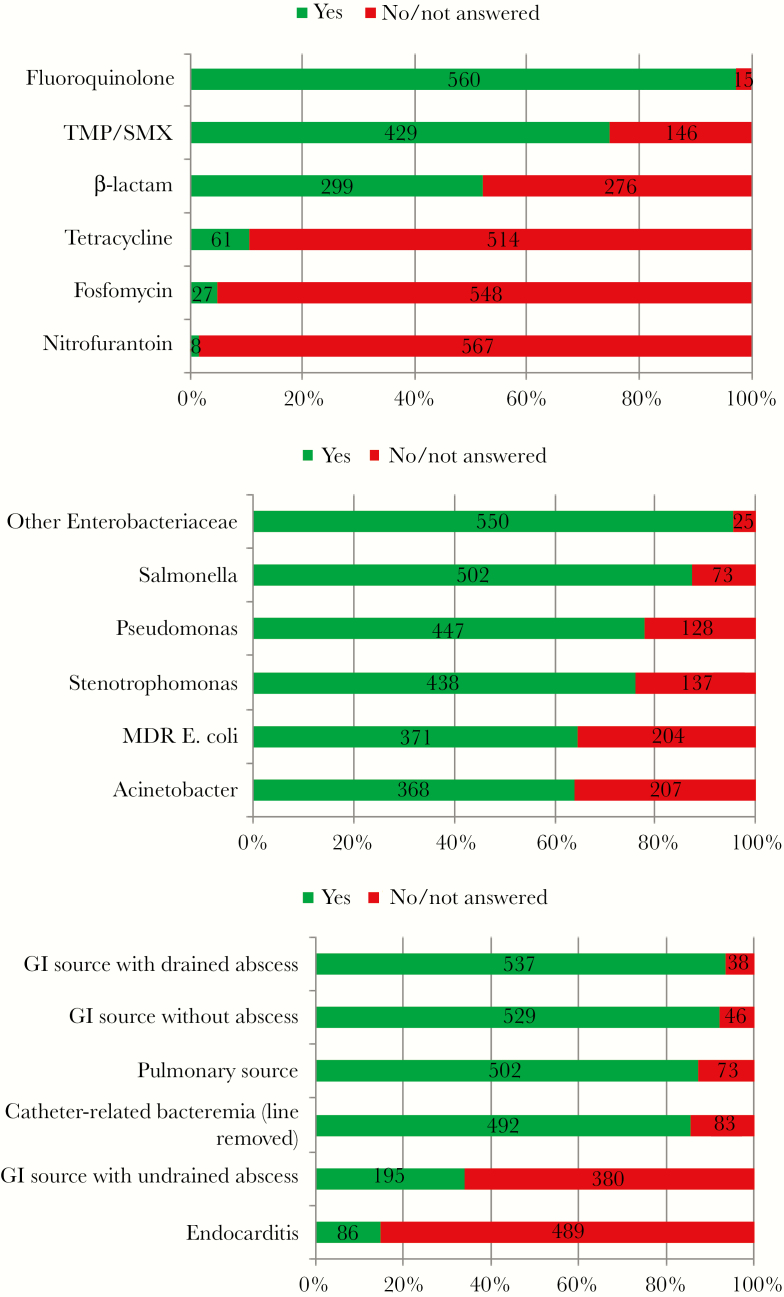
A total of 575 of 655 (88%) of participants responded yes to the question, “In your clinical practice, are there scenarios in which you transition patients with gram-negative bacteremia to oral antibiotics to complete a course of therapy?” In a clinical vignette describing a 36-year-old woman with acute pyelonephritis and Escherichia coli bacteremia, more than 50% of the 575 respondents felt comfortable transitioning this patient to an oral fluoroquinolone, trimethoprim/sulfamethoxazole, or beta-lactam antibiotics (Figure 1A). When queried about duration of total therapy for Gram-negative bacteremia, 64 of 557 reported treating for ≤7 days (11%), 234 reported treating from 8 to 13 days (42%), 254 reported treating for 14 days (46%), and 5 reported treating for more than 14 days (0.9%).
Figure 1.
Patient vignette of a 36-year-old woman who presented with symptoms of acute pyelonephritis, who responded to initial intravenous antibiotics, and had Escherichia coli recovered in both blood and urine cultures, susceptible to all listed agents; N = 575. (A) Which of the listed oral agents would respondents feel comfortable transitioning to. (B) Would respondents be willing to use an oral antibiotic if the organism was not an E coli, but rather _____? (C) Would respondents feel comfortable using an oral agent given the following sources of the Gram-negative bacteremia? MDR, multidrug-resistant; TMP/SMX, trimethoprim/sulfamethoxazole.
Respondents were asked if their willingness to use an oral agent would change if the bacterial pathogen were altered. More than 60% of participants felt comfortable with oral antibiotic therapy in patients bacteremic with other Enterobacteriaceae, Salmonella, Pseudomonas, Stenotrophomonas, multidrug-resistant E coli, and Acinetobacter (Figure 1B). When queried about other sources of Gram-negative bacteremia, more than 80% of participants felt comfortable using oral agents in bacteremias from gastrointestinal sources, without abscess or with drained abscess, pulmonary sources, and catheter-related infections (line removed) (Figure 1C). Only 34% felt comfortable using oral agents in gastrointestinal sources with undrained abscesses; 15% reported they would feel comfortable using oral agents in Gram-negative endocarditis.
Gram-Positive Bacteremia
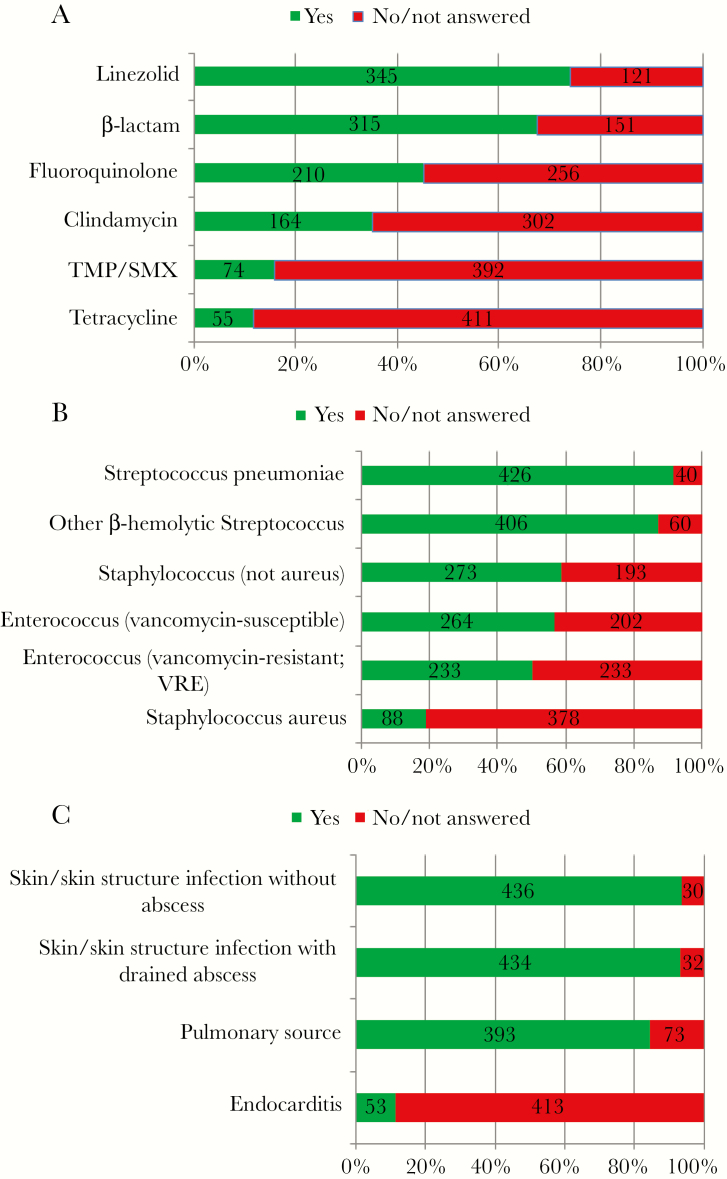
In response to the general question, “In your clinical practice, are there scenarios in which you transition patients with gram-positive bacteremia to oral antibiotics to complete a course of therapy?”, 71% (466 of 655) of participants responded in the affirmative. When provided with a scenario in which a 50-year-old man presented with group B Streptococcus bacteremia that was associated with a central venous catheter infection, more than 60% of participants felt comfortable transitioning this patient to oral linezolid or a beta-lactam antibiotic (Figure 2A). When queried how their practice would change in response to other Gram-positive bacteria, more than 80% indicated that they would feel comfortable treating with oral agents if the Gram-positive bacteria were Streptococcus pneumoniae or other beta-hemolytic streptococci. This declined to 50% or more feeling comfortable if non-aureus Staphylococcus or Enterococcus (including vancomycin-resistant enterococci) were recovered. Less than 20% felt comfortable using oral antibiotics to treat Staphylococcus aureus bacteremia (Figure 2B). Most participants (more than 90%) would use oral antibiotics in the treatment of Gram-positive bacteremia when the source was skin and skin structure infection without abscess or with abscess drained (Figure 2C). Greater than 80% would use oral antibiotics in bacteremias with a pulmonary source. Only 12% reported they would use oral antibiotics in the setting of endocarditis.
Figure 2.
Patient vignette of a 50-year-old man admitted with fevers, chills, and leukocytosis correlated with central venous catheter infusions. He is started in intravenous vancomycin and the catheter is removed. Symptoms and leukocytosis resolve; blood cultures recovered group B Streptococcus, susceptible to all listed antibiotics. Follow-up blood cultures are negative; N = 466. (A) Which of the listed oral agents respondents would feeling comfortable transitioning to. (B) Would respondents be willing to use an oral antibiotic if the organism was not a group B Streptococcus, but rather ____? (C) Would respondents feel comfortable using an oral agent given the following sources of the Gram-positive bacteremia? TMP/SMX, trimethoprim/sulfamethoxazole.
DISCUSSION
Based on our results, the majority of ID physicians in the United States seem to be comfortable transitioning patients with both Gram-negative and Gram-positive bacteremia from IV to oral antibiotics under many clinical scenarios. Whether and when to use oral antibiotics in the treatment of patients with infections complicated by bacteremia has not been clearly defined by randomized clinical trials. Thus, clinicians who choose to transition to oral therapy in treatment of bacteremia are forced to select the best oral antimicrobial agent and to decide when to transition patients away from IV therapy, all without the benefit of robust data from randomized controlled trials. What data exist are chiefly from retrospective studies, small randomized trials, and data extracted (ie, subanalysis) for bacteremic patients identified in larger randomized studies. Most data are from urinary tract infections secondary to Enterobacteriaceae, community-acquired pneumonias secondary to S pneumoniae, and endocarditis secondary to Gram-positive bacteria. Timing of transition to oral agents and total length of therapy varies greatly amongst these reports.
Gram-Negative Bacteremia
The greatest quantity of data currently exists for oral stepdown therapy in patients with Enterobacteriaceae bacteremia. The majority of these data are from pyelonephritis/complicated urinary tract infections, but the literature does include data from gastrointestinal infections and, less commonly, from central line-associated, pulmonary and skin and skin structure infections [2–10]. Only the study by Mombelli et al [10] directly compares oral and IV therapy in a randomized fashion—comparing oral and IV ciprofloxacin as initial empirical therapy in severe pyelonephritis or complicated urinary tract infection. In this study of 163 patients, 83 received oral therapy. Bacteremia was noted in 53 participating patients, and outcomes were noted to be similar in bacteremic patients with oral or IV ciprofloxacin. Another randomized clinical trial reported the use of early oral antibiotics (after 6 days of IV therapy) in the treatment of acute cholangitis with bacteremia [7]. This small study of 59 patients found noninferiority in the orally transitioned patient group when compared with those who received all IV therapy. Comparison of the available retrospective data is also made difficult by varying times of transition to oral antimicrobials (ranging from 3 to 6 days). This interpretation is further confounded by emerging data on the use (ie, efficacy) of shorter course antimicrobial therapy in Enterobacteriaceae bacteremia (6–10 days versus 11–16 days) [11]. In our survey, courses of therapy from 8 to 14 days were preferred by most ID physicians. Courses of 7 days or less were acceptable to only 11% of respondents. Therefore, if shortened courses of antibiotic therapy are ultimately proven to be effective, a transition to oral antibiotics after 6 or more days may become irrelevant.
Gram-Positive Bacteremia
Current data available to inform use of oral antimicrobials in the treatment of Gram-positive infections with bacteremia are also limited. Virtually no data on using initial oral therapy in Gram-positive bacteremia exists. Agreement on transition to oral therapy in Gram-positive bacteremia appears most consistent in pneumonia (with bacteremia) caused by S pneumoniae. Based on small retrospective studies and a single randomized controlled trial, clinical practice guidelines for community-acquired pneumonia currently suggest that a “patient should be switched from intravenous to oral therapy when they are hemodynamically stable and improving clinically” [12–14]. In a recent study, a randomized clinical trial supporting transition to oral antibiotics in endocarditis was published. This study of 400 subjects included follow-up on oral therapy in patients with Gram-positive endocarditis after receiving at least 10 days of IV therapy [15]. The authors found that transition to oral was noninferior to continued IV therapy. In another large study, 214 patients were switched to oral therapies after a median 21 days of IV therapy [16]. Similar results were reported in another retrospective cohort study, which required only 3 days of IV therapy before change [17], and a recent single-center retrospective review of outpatient therapy of methicillin-resistant S aureus bacteremia [18]. Older data also support the use of combination oral antibiotic regimens in the treatment of right-sided endocarditis secondary to Staphylococcus [19–21]. Use of IV linezolid in the treatment of S aureus and enterococcal bacteremia is the subject of several studies [22–25]. Although the use of oral linezolid has not been included in these, the approximately 100% bioavailability of the oral formulation of this drug has been interpreted by many as supportive of oral linezolid use in these infections. In a recent subanalysis of a nonrandomized, noncontrolled S aureus bacteremia study, Willekens et al [26] identified 45 patients transitioned to oral linezolid between 3 and 9 days of therapy. Compared with a propensity score-matched group of 90 patients who received standard parenteral therapy (both groups treated for a median of 15 days), there was no difference in 30-day all-cause mortality or 90-day relapse, and median length of hospital stay was 11 days less.
The overall burden of bloodstream infections in the United States has been estimated to be 536–628 000 per year, with 70–85 000 deaths per year in the United States [27]. Management of these infections in the safest, most effective and cost-efficient manner is imperative. In addition to preventing unnecessary central line-associated infections and thromboses, antimicrobial stewardship programs also promote early transition to oral antimicrobials as a cost-saving measure [28]. Unfortunately, no data from randomized clinical trials inform clinicians’ decisions to select the best oral antimicrobial agent and decide when to transition patients away from IV therapy. Selection of when to transition, and which agents to transition to, is based on many factors including bioavailability of drugs, source of patient’s bacteremia, patient’s ability to absorb antimicrobial agents, perceived adherence, and other underlying comorbidities. Previous reviews and surveys (including of European ID specialists) have examined this issue in recent years [29–31].
Our study has 4 major limitations. First, we recruited a convenience sample of ID physicians, so the opinions of respondents may not be generalizable to other IDs physicians. Second, we relied on self-report, and individual responses to questions may be subject to recall bias. For example, we did not do chart reviews to validate expressed treatment approaches. Third, we limited the number of questions for this query to minimize response fatigue; however, this approach also limited the number of different antimicrobial-prescribing scenarios considered, limiting the overall scope of inquiry. Finally, although our 50% physician response rate was relatively high, a response bias may still exist. Respondents may have been more likely to transition to oral therapy than the general IDs physician population.
CONCLUSIONS
Despite the limitations listed above, our findings indicate that the vast majority of respondents would transition patients with Gram-negative bacteremia from IV to oral antibiotics, and a majority of respondents would transition patients with some Gram-positive bacteremias, provided that source control is achieved. In contrast, most respondents would not use oral agents for Gram-positive bacteremia caused by S aureus or for patients with endocarditis. Our results clearly indicate the need for future randomized controlled trials to inform optimal treatment choices for patients with bacteremia. Without guidance from randomized clinical trials, expert assistance by an ID physician and IDs pharmacists in managing many or most of these cases is warranted.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author
Acknowledgments
Disclaimer. The contents in this paper are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services.
Financial support. This publication was supported by the Cooperative Agreement Number 1 U50 CK000477, funded by the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Pillai SK, Beekmann SE, Santibanez S, Polgreen PM. The Infectious Diseases Society of America Emerging Infections Network: bridging the gap between clinical infectious diseases and public health. Clin Infect Dis 2014; 58:991–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tamma PD, Conley AT, Cosgrove SE, et al. Association of 30-day mortality with oral step-down vs continued intravenous therapy in patients hospitalized with Enterobacteriaceae bacteremia. JAMA Intern Med 2019; doi: 10.1001/jamainternmed.2018.6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mercuro NJ, Stogsdill P, Wungwattana M. Retrospective analysis comparing oral stepdown therapy for enterobacteriaceae bloodstream infections: fluoroquinolones versus β-lactams. Int J Antimicrob Agents 2018; 51:687–92. [DOI] [PubMed] [Google Scholar]
- 4. Rieger KL, Bosso JA, MacVane SH, et al. Intravenous-only or intravenous transitioned to oral antimicrobials for Enterobacteriaceae-associated bacteremic urinary tract infection. Pharmacotherapy 2017; 37:1479–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kutob LF, Justo JA, Bookstaver PB, et al. Effectiveness of oral antibiotics for definitive therapy of Gram-negative bloodstream infections. Int J Antimicrob Agents 2016; 48:498–503. [DOI] [PubMed] [Google Scholar]
- 6. Yan LZ, Herrington JD. Outcomes of hospitalized neutropenic oncology patients with Pseudomonas aeruginosa bloodstream infections: focus on oral fluoroquinolone conversion. J Oncol Pharm Pract 2016; 22:584–90. [DOI] [PubMed] [Google Scholar]
- 7. Park TY, Choi JS, Song TJ, et al. Early oral antibiotic switch compared with conventional intravenous antibiotic therapy for acute cholangitis with bacteremia. Dig Dis Sci 2014; 59:2790–6. [DOI] [PubMed] [Google Scholar]
- 8. Eliakim-Raz N, Yahav D, Paul M, Leibovici L. Duration of antibiotic treatment for acute pyelonephritis and septic urinary tract infection– 7 days or less versus longer treatment: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother 2013; 68:2183–91. [DOI] [PubMed] [Google Scholar]
- 9. Peterson J, Kaul S, Khashab M, et al. A double-blind, randomized comparison of levofloxacin 750 mg once-daily for five days with ciprofloxacin 400/500 mg twice-daily for 10 days for the treatment of complicated urinary tract infections and acute pyelonephritis. Urology 2008; 71:17–22. [DOI] [PubMed] [Google Scholar]
- 10. Mombelli G, Pezzoli R, Pinoja-Lutz G, et al. Oral vs intravenous ciprofloxacin in the initial empirical management of severe pyelonephritis or complicated urinary tract infections: a prospective randomized clinical trial. Arch Intern Med 1999; 159:53–8. [DOI] [PubMed] [Google Scholar]
- 11. Chotiprasitsakul D, Han JH, Cosgrove SE, et al. Comparing the outcomes of adults with enterobacteriaceae bacteremia receiving short-course versus prolonged-course antibiotic therapy in a multicenter, propensity score-matched cohort. Clin Infect Dis 2018; 66:172–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44(Suppl 2):S27–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oosterheert JJ, Bonten MJ, Schneider MM, et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomised trial. BMJ 2006; 333:1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ramirez JA, Bordon J. Early switch from intravenous to oral antibiotics in hospitalized patients with bacteremic community-acquired Streptococcus pneumoniae pneumonia. Arch Intern Med 2001; 161:848–50. [DOI] [PubMed] [Google Scholar]
- 15. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med 2019; 380:415–24. [DOI] [PubMed] [Google Scholar]
- 16. Mzabi A, Kernéis S, Richaud C, et al. Switch to oral antibiotics in the treatment of infective endocarditis is not associated with increased risk of mortality in non-severely ill patients. Clin Microbiol Infect 2016; 22:607–12. [DOI] [PubMed] [Google Scholar]
- 17. Rodriguez-Pardo D, Pigrau C, Campany D, et al. Effectiveness of sequential intravenous-to-oral antibiotic switch therapy in hospitalized patients with Gram-positive infection: the SEQUENCE cohort study. Eur J Clin Microbiol Infect Dis 2016; 35:1269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jorgensen SC, Lagnf AM, Bhatia S, et al. Sequential intravenous-to-oral outpatient antibiotic therapy for MRSA bacteraemia: one step closer. J Antimicrob Chemother 2019; 74:489–98. [DOI] [PubMed] [Google Scholar]
- 19. Al-Omari A, Cameron DW, Lee C, Corrales-Medina VF. Oral antibiotic therapy for the treatment of infective endocarditis: a systematic review. BMC Infect Dis 2014; 14:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heldman AW, Hartert TV, Ray SC, et al. Oral antibiotic treatment of right-sided staphylococcal endocarditis in injection drug users: prospective randomized comparison with parenteral therapy. Am J Med 1996; 101:68–76. [DOI] [PubMed] [Google Scholar]
- 21. Dworkin RJ, Lee BL, Sande MA, Chambers HF. Treatment of right-sided Staphylococcus aureus endocarditis in intravenous drug users with ciprofloxacin and rifampicin. Lancet 1989; 2:1071–3. [DOI] [PubMed] [Google Scholar]
- 22. Zhao M, Liang L, Ji L, et al. Similar efficacy and safety of daptomycin versus linezolid for treatment of vancomycin-resistant enterococcal bloodstream infections: a meta-analysis. Int J Antimicrob Agents 2016; 48:231–8. [DOI] [PubMed] [Google Scholar]
- 23. Britt NS, Potter EM, Patel N, Steed ME. Comparison of the effectiveness and safety of linezolid and daptomycin in vancomycin-resistant enterococcal bloodstream infection: a National Cohort Study of Veterans Affairs Patients. Clin Infect Dis 2015; 61:871–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shorr AF, Kunkel MJ, Kollef M. Linezolid versus vancomycin for Staphylococcus aureus bacteraemia: pooled analysis of randomized studies. J Antimicrob Chemother 2005; 56:923–9. [DOI] [PubMed] [Google Scholar]
- 25. Stevens DL, Herr D, Lampiris H, et al. Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis 2002; 34:1481–90. [DOI] [PubMed] [Google Scholar]
- 26. Willekens R, Puig-Asensio M, Ruiz-Camps I, et al. Early oral switch to linezolid for low-risk patients with Staphylococcus aureus bloodstream infections: a propensity-matched cohort study. Clin Infect Dis 2019; doi: 10.1093/cid/ciy916. [DOI] [PubMed] [Google Scholar]
- 27. Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect 2013; 19:501–9. [DOI] [PubMed] [Google Scholar]
- 28. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection 2017; 45:263–8. [DOI] [PubMed] [Google Scholar]
- 29. Diallo K, Thilly N, Luc A, et al. Management of bloodstream infections by infection specialists: an international ESCMID cross-sectional survey. Int J Antimicrob Agents 2018; 51:794–8. [DOI] [PubMed] [Google Scholar]
- 30. Diallo K, Kern WV, de With K, et al. Management of bloodstream infections by infection specialists in France and Germany: a cross-sectional survey. Infection 2018; 46:333–9. [DOI] [PubMed] [Google Scholar]
- 31. Hale AJ, Snyder GM, Ahern JW, et al. When are oral antibiotics a safe and effective choice for bacterial bloodstream infections? An evidence-based narrative review. J Hosp Med 2018; 13:328–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.