Abstract
Thyroid cancer is a common malignant tumour of the endocrine system and ranks ninth in cancer incidence worldwide. An extensive body of evidence has demonstrated that lncRNAs play a critical role in the progression of thyroid cancer. The lncRNA MAPKAPK5-AS1 has been reported to be abnormally expressed and to play a role in the development of various human cancers. However, MAPKAPK5-AS1’s potential role in thyroid cancer progression remains unknown. The objective of our study was to explore the role and mechanism of MAPKAPK5-AS1 in thyroid cancer cells and provide a potential target for its biological diagnosis and treatment. We transfected sh-MAPKAPK5-AS1 and sh-NC into BCPAP and TPC-1 cells for loss-of-function assays. Results of RT-qPCR analysis demonstrated that MAPKAPK5-AS1 was more highly expressed in thyroid cancer cells compared to normal cells. Functional assays demonstrated that interfering with the expression of MAPKAPK5-AS1 notably repressed proliferation and invasion and accelerated apoptosis of BCPAP and TPC-1 cells. Mechanistically, we found that miR-519e-5p was negatively regulated by MAPKAPK5-AS1 and that tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein eta (YWHAH) was a target of miR-519e-5p. Additionally, rescue assays demonstrated that downregulation of MAPKAPK5-AS1 expression inhibited cell proliferation, migration, and invasion and promoted apoptosis by sponging miR-519e-5p, thereby increasing YWHAH expression. Ultimately, our study revealed that MAPKAPK5-AS1 promotes proliferation and migration of thyroid cancer cells by targeting the miR-519e-5p/YWHAH axis, which provides novel insight into the development and progression of thyroid cancer.
Key words: IncRNA MAPKAPK5-AS1, MiR-519e-5p, YWHAH, thyroid cancer cell
Introduction
Thyroid cancer is a common malignant tumour of the endocrine system, and the incidence and mortality of thyroid cancer have increased in women worldwide, especially in developed countries.1,2 Global cancer statistics estimate that approximately 567,000 thyroid cancers were diagnosed in 2018 and that approximately 15.9% of these cases were in China.3 Although therapeutic strategies, including radioiodine therapy, thyroidectomy and thyroid- stimulating hormone inhibition therapy, have been achieved for the clinical treatment of thyroid cancer, their efficacy is dissatisfactory. Therefore, it is of great importance to investigate the underlying mechanisms of thyroid cancer, which may reveal more effective strategies for diagnosis and therapy.
Long noncoding RNAs (lncRNAs) are RNA molecules greater than 200 nt in length. Growing evidence indicates that lncRNAs function as crucial mediators in the regulation of gene expression levels.4-7 Mounting investigations demonstrate that aberrantly expressed lncRNAs play important roles in the development of malignancies, including thyroid cancer.8-12 Many studies have revealed that lncRNAs are involved in regulating cancer biological activities, such as cell proliferation, differentiation, metastasis and apoptosis.6,13-16 For example, lncRNA HOTTIP accelerates papillary thyroid carcinoma progression by regulating miR-637.10 Moreover, lncRNA SNHG20 facilitates cervical cancer cell proliferation and invasion via the miR-140-5p-ADAM10 axis.17 IncRNA ZEB1-AS1 accelerates cell migration and metastasis by posttranscriptional activation of ZEB1 in bladder cancer.18 Notably, lncRNA MAPKAPK5-AS1 (MAPKAPK5-AS1) has been proven to exert its carcinogenic activity in tumour progression. For example, MAPKAPK5-AS1 has been found to promote colorectal cancer proliferation partly by silencing p21 expression.19 Moreover, Yang et al. indicated that MAPKAPK5-AS1 promotes colorectal cancer progression by cis-regulating the nearby gene MK5 and acting as a let-7f-1-3p sponge.20 However, whether MAPKAPK5-AS1 participates in thyroid cancer tumorigenesis and progression is still unknown.
Various microRNAs (miRNAs; miRs) have been found to be involved in the onset and progression of tumours, including thyroid cancer.21-24 The miR-519 family is located on human chromosome 9 and includes miR-519a-3p, miR-519b-3p, miR-519c-3p, miR-519a-5p and miR-519b-5p. miR-519a-3p, miR-519b-3p and miR-519c-3p have similar sequences and share the same seed sequence.19 It has been reported that the miR-519 family is associated with cancer development.25,26 For example, miR-519a functions as a tumour suppressor in glioma by targeting the oncogenic STAT3 pathway. In addition, miR-519 facilitates cervical cancer progression and metastasis by directly targeting Smad7.27 However, the function of miR-519 in thyroid cancer remains unclear.
In the present study, we aimed to explore the biological function and molecular mechanism of MAPKAPK5-AS1 in the progression of thyroid cancer through function experiments, including cell proliferation, migration, invasion and apoptosis. Our study illustrated that MAPKAPK5-AS1 promotes proliferation and migration of thyroid cancer cell lines by targeting miR-519e- 5p/YWHAH, which provided evidence that MAPKAPK5-AS1 function as a potential target for the treatment of thyroid cancer.
Materials and methods
Cell culture
Human thyroid cancer cell lines (BCPAP, BHP5-16, TPC-1 and CGTH-W3) and human normal cell lines (Nthy-ori3-1) were obtained from the Chinese Academy of Medical Science (Shanghai, China). Cells were grown in DMEM with 10% foetal bovine serum (FBS, Gibco, NY, USA) at 37°C in a humidified incubator under 5% CO2.
Cell transfection
Specific siRNAs against MAPKAPK5-AS1 or YWHAH and their corresponding siNCs were constructed by Genechem (Shanghai, China). Moreover, miR-519e-5p mimic, miR-519e-5p inhibitor and their respective NCs were synthesized by GenePharma (Shanghai, China). Cell transfection was performed using Lipofectamine 3000 (Thermo Fisher Scientific, Waltham, MA, USA) at a final concentration of approximately 10 μM according to the manufacturer’s instructions. The sequences were as follows: si- MAPKAPK5-AS1: 5’-CGAGAAGUGGUCAAGCUAGAA-3’; si- NC: 5’-TTCTCCGAACGTGTCACGT-3’.
Cell counting kit-8 assay
We detected cell viability using the cell counting kit-8 (CCK- 8) assay. In brief, cells (1×104) were inoculated into each well in 96-well plates. Then, cells were cultured for 0, 24, 48 and 72 h, processed with 10 μL CCK-8 reagent and incubated for an additional 4 h at 37°C. Finally, optical density was determined at a wavelength of 450 nm by a microplate reader.
EdU analysis
Cell proliferation was determined using a 5-ethynyl-20- deoxyuridine (EdU) assay kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. Cells (1×105) were maintained in 6-well plates. After 48 h, 100 μL EdU was added for 2 h. Afterwards, cells were treated with 4% paraformaldehyde, and then 0.5% Triton X-100 was added. Finally, cells were stained with anti-EdU solution. For analysis, EdU-positive cells were analysed using fluorescence microscopy (Olympus, Tokyo, Japan), using the 20× objective.
RT-qPCR analysis
Total RNA from cells was extracted using TRIzol reagent (Invitrogen; Thermo Fisher Scientific). RNA (1 mg) was reverse transcribed to cDNA with TaqMan one-step reverse transcription (Applied Biosystems, USA). qRT-PCR experiments were performed with SYBR Green qPCR Master Mix (Thermo Fisher Scientific).
GAPDH and U6 were used as internal standards. RNA expression was assessed using the 2-ΔΔCt method. The specific primers were as follows: MAPKAPK5-AS1: 5’-AAGCCCGAGTCTGATGCTAA- 3’ (forward) and 5’-CTGCACACCTCTTCTGGTCA-3’ (reverse); GAPDH: 5’-TATGATGATATCAAGAGGGTAGT-3’ (forward) and 5’-TGTATCCAAACTCATTGTCATAC-3’ (reverse); U6: 5’-CTCGCTTCGGCAGCACA-3’ (forward) and 5’-AACGCTTCACGAATTTGCGT-3’ (reverse).
Western blot assay
Total protein from cells was isolated with RIPA buffer (Invitrogen), and protein concentration was determined using a BCA kit (Beyotime, Haimen, China). Samples (1 mg) were separated using 10% SDS-PAGE and transferred to PVDF membranes, which were blocked in 5% non-fat milk for 1 h at 37°C. Subsequently, membranes were incubated with primary antibodies at 4°C overnight. After washing in TBS with Tween-20, cells were probed with HRP-conjugated secondary antibody (cat. no. ab97040) for another 2 h at 37°C. Protein bands were visualized by an ECL kit (Millipore, Bedford, MA, USA) and quantified using ImageJ software. GAPDH was used as an internal reference. The primary antibodies were as follows: cyclin D1 (cat. no. ab238625, 1:1000), p21 (cat. no. ab188224, 1:1000), Bax (cat. no. ab182733, 1:1000), Bcl-2 (cat. no. ab692, 1:1000), cleaved caspase-3 (cat. no. ab2302, 1:1000), cleaved caspase-9 (cat. no. ab2324, 1:1000), MMP-2 (cat. no. ab86607, 1:1000), MMP-9 (cat. no. ab58803, 1:1000), YWHAH (cat. no. ab138463, 1:1000) and GAPDH (cat. no. ab181602, 1:1000). All antibodies were purchased from Abcam (Cambridge, UK).
Wound healing assay
Cells were inoculated in 6-well plates. Scratches were created using a 200 μL pipette tip, with the scratch width remaining the same when cell confluence was up to 90%. Then, cells were continuously cultured for 48 h after washing twice. Cells were imaged using a light microscope, and scratch width was measured to calculate the wound healing rate: relative scratch width = (number of cells at T48 − number of cells at T0)/number of cells at T0 × 100%, where T0 was 0 h and T48 was 48 h.
Transwell chamber assay
A Transwell chamber assay was performed to assess cell migration and invasion abilities. Cells were plated into 24-well upper uncoated chambers (BD Biosciences, CA, USA) with serum-free medium for migration analysis, while the upper chamber was loaded with Matrigel for invasion analysis. Culture medium was added to the lower chamber to culture for 48 h. Then, cells that did not migrate and invaded to the lower chamber were removed. Remaining cells were fixed in 4% paraformaldehyde and stained with 1% crystal violet. The numbers of migrated or invasive cells were counted under a light microscope at 40× magnification (Olympus Corporation) in 5 random fields.
Flow cytometry assays
The effect of PRMT5 on the cell cycle was detected with a cell cycle kit and the effect of MAPKAPK5-AS1 on apoptosis of BCPAP and TPC-1 cells was evaluated by flow cytometry using the Annexin V Apoptosis Detection kit I (BD Biosciences, San Jose, CA, USA). In brief, transfected BCPAP and TPC-1 cells were placed into 6-well plates and harvested 48 h after by mild trypsinization. Then, the cells were centrifuged at 150 g for 5 min and washed twice with pre-chilled 1×PBS. Finally, cells were incubated with 5 μL of Annexin V and 5 μL of propidium iodide (PI) for 15 min at room temperature in the dark. The percentage of apoptotic cells and the cell cycle were evaluated by flow cytometry (FACScanR; BD Biosciences, Franklin Lakes, NJ, USA).
Luciferase reporter assay
We predicted miR-519e-5p as a target of MAPKAPK5-AS1 and YWHAH as a target of miR-519e-5p using StarBase tools. WT/Mut MAPKAPK5-AS1 or WT/Mut YWHAH 3’UTR were subcloned into the pmirGLO dual-luciferase vector and then cotransfected into cells with miR-519e-5p mimic or their respective NC mimics. Relative luciferase activities were assessed utilizing a dual-luciferase reporter assay system (Promega) after cotransfection for 48 h. Renilla luciferase activity was normalized.
Immunofluorescence assay
BCPAP and TPC-1 cells were cultured in 6-well plates at a density of 3×105 cells/well, fixed in 4% paraformaldehyde and permeabilized with 0.2% Triton X-100. Then, cells were probed with primary antibody against YWHAH (cat. no. ab138463, 1:1000, Abcam) at 4°C overnight. Subsequently, cells were treated with secondary antibody (cat. no. ab150113, 1: 1000, Abcam). Protein expression was observed by fluorescence microscopy (Zeiss, Germany) at 40× magnification.
Statistical analysis
All experiments were performed in three independent experiments, and results are presented as the mean ±SD. Statistical analyses were analysed using SPSS 19.0 (IBM Corp.). Differences among multiple groups were analysed using one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test for analysis. Student’s t-test was performed to evaluate significant differences between two independent groups of samples. p<0.05 was considered statistically significant.
Results
Knockdown of MAPKAPK5-AS1 inhibits proliferation and induces apoptosis of thyroid cancer cells
To identify the expression levels of MAPKAPK5-AS1 in thyroid cancer cells, we performed RT-qPCR analysis to examine MAPKAPK5-AS1 expression in thyroid cancer cells (BCPAP, BHP5-16, TPC-1 and CGTH-W3) and normal human cells (Nthyori3-1). Our findings revealed that MAPKAPK5-AS1 expression was markedly higher in cancer cells than in Nthy-ori3-1 cells (Figure 1A). In addition, BCPAP and TPC-1 cells showed the highest expression of ARAP1-AS1 compared to BHP5-16 and CGTHW3 cells; thus, BCPAP and TPC-1 cells were selected for loss-offunction assays to explore the biological role of MAPKAPK5-AS1 in thyroid cancer cells. We next knocked down expression of MAPKAPK5-AS1 in BCPAP and TPC-1 cells. Transfection efficiency was verified by RT-qPCR analysis (Figure 1B). More importantly, CCK-8 assay revealed that downregulation of MAPKAPK5- AS1 expression inhibited the viability of BCPAP and TPC-1 cells (Figure 1C). These results were further supported by EdU assays (Figure 1D). Subsequently, we performed flow cytometry and western blot assays to explore the effects of MAPKAPK5- AS1 on the cell cycle. As shown in Figure 1E, a significantly greater proportion of G0/G1 phase cells were observed in BCPAP and TPC-1 cells. Western blot analysis indicated that knockdown of MAPKAPK5-AS1 repressed the expression levels of cyclin D1, while enhancing levels of p21 compared to sh-NC (Figure 1F).
In addition, MAPKAPK5-AS1 knockdown promoted apoptosis, as evidenced by flow cytometry analysis (Figure 1G). At the molecular level, Western blot assay indicated that Bax, cleaved caspase-3 and cleaved caspase-9 expression levels were enhanced, while expression levels of Bcl-2 were diminished when expression of MAPKAPK5-AS1 was decreased (Figure 1H). Collectively, these data demonstrated that knockdown of MAPKAPK5-AS1 inhibits the oncogenicity of thyroid cancer by affecting cell proliferation and apoptosis.
Knockdown of MAPKAPK5-AS1 inhibits cell migration and invasion of thyroid cancer cells
Given that cell migration and invasion are two processes involved in cancer metastasis, we performed wound healing and transwell chamber assays to investigate the effects of MAPKAPK5- AS1 on these two processes. Results revealed that knockdown of MAPKAPK5-AS1 remarkably repressed cell migration and invasion in BCPAP and TPC-1 cells (Figure 2 A,B). Moreover, Western blot analysis indicated that si-MAPKAPK5-AS1 repressed migration and invasion abilities by inhibiting expression levels of MMP-2 and MMP-9 in thyroid cancer cells (Figure 2C). Hence, MAPKAPK5-AS1 knockdown exerts suppressive impacts on cell migration and invasion in thyroid cancer.
MiR-519e-5p is a direct target of MAPKAPK5-AS1 in thyroid cancer cells
As shown in Figure 3A, we predicted that miR-519e-5p could be a target of MAPKAPK5-AS1 using the StarBase database, since miR-519e-5p has been reported to participate in the regulation of various types of tumour progression. To further determine the causal relationship between miR-519e-5p and MAPKAPK5-AS1, we performed dual luciferase activity analysis using miR-519e-5p mimic and MAPKAPK5-AS1-Luc. Transfection efficiency of the miR-519e-5p mimic is presented in Figure 3B. Results of the luciferase reporter assay showed that miR-519e-5p mimic markedly decreased the luciferase activity of MAPKAPK5-AS1-WT, while MAPKAPK5-AS1-Mut-Luc exhibited modest alterations in BCPAP and TPC-1 cells (Figure 3C). Consistently, downregulation of MAPKAPK5-AS1 increased miR-519e-5p expression (Figure 3D). In addition, we verified levels of miR-519e-5p in thyroid cancer cell lines and found that miR-519e-5p expression was dramatically decreased in thyroid cancer cells (BCPAP, BHP5-16, TPC-1 and CGTH-W3) compared to Nthy-ori3-1 cells (Figure 3E). These data revealed that MAPKAPK5-AS1 negatively regulates miR- 519e-5p expression.
YWHAH is a downstream target of miR-519e-5p
To validate the possible targets of miR-519e-5p, bioinformatics tool miRanda, TargetScan, and PicTar were jointly employed to predict the target genes, and finally YWHAH was selected as the target gene by cross-selection. Meanwhile, we identified putative binding sites for YWHAH with miR-519e-5p (Figure 4A). Moreover, YWHAH gene is 14-3-3 eta protein, one member of the 14-3-3 protein family. The previous studies have found that YWHAH contribute to cell proliferation, tumorigenesis and arthritis disease.24-26 In addition, the luciferase activity of YWHAH-WT was reduced by the miR-519e-5p mimic, while no obvious alteration was observed in response to the mutant form of the 3’-UTR of YWHAH (Figure 4B). RT-qPCR analysis was used to measure mRNA expression of YWHAH. We demonstrated that ectopic expression of miR-519e-5p decreased expression levels of YWHAH in BCPAP and TPC-1 cells. These results were further confirmed by Western blot and immunofluorescence analyses (Figure 4 C-E). In addition, we detected expression of YWHAH in BCPAP, BHP5-16, TPC-1 and CGTH-W3 cells and in normal Nthy-ori3-1 cells. Results revealed that YWHAH expression was higher in thyroid cancer cell lines compared to normal cells (Figure 4F).
Figure 1.
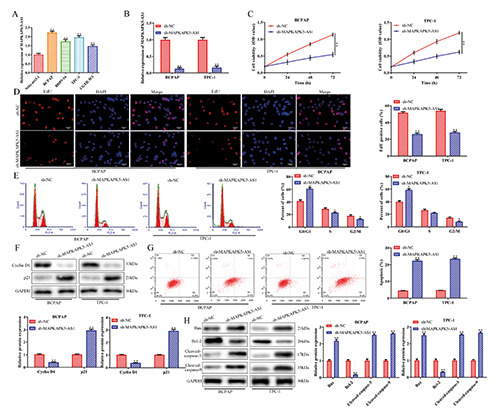
Knockdown of MAPKAPK5-AS1 inhibits proliferation and induces apoptosis of thyroid cancer cells. A) RT-qPCR analysis of expression levels of lncRNA MAPKAPK5-AS1 in thyroid cancer cells (BCPAP, BHP5-16, TPC-1 and CGTH-W3) and Nthy-ori3-1 cells. **p<0.01 vs Nthy-ori3-1 cells. B) Transfection efficiency was verified by RT-qPCR analysis. CCK-8 (C) and EdU (D) assays were adopted to identify the effects of MAPKAPK5-AS1 on cell proliferation. E-H) Flow cytometry and Western blot assays were performed to determine the effects of MAPKAPK5-AS1 on the cell cycle and apoptosis. *p<0.05, **p<0.01 vs sh-NC.
lncRNA MAPKAPK5-AS1 promotes thyroid cancer progression via the miR-519e-5p/YWHAH axis
Based on the above findings, rescue assays were conducted to verify whether MAPKAPK5-AS1 elicited its performance in thyroid cancer through the miR-519e-5p/YWHAH axis. RT-qPCR analysis revealed that expression levels of miR-519e-5p were decreased after transfection with the miR-519e-5p inhibitor, and MAPKAPK5-AS1 expression was repressed in the sh-MAPKAPK5- AS1 group compared to the sh-NC group in BCPAP cells (Figure 5A).
CCK-8 and EdU assays showed that the MAPKAPK5-AS1 knockdown-induced reduction in cell viability and proliferation abilities of BCPAP cells was promoted by miR-519e-5p inhibition, while the effect of the miR-519e-5p inhibitor was subsequently recovered by YWHAH depletion (Figure 5 B,C). Flow cytometry assays demonstrated that MAPKAPK5-AS1 knockdown-induced apoptosis was abolished by the miR-519e-5p inhibitor, while knockdown of YWHAH antagonized the impact of the miR-519e- 5p inhibitor in MAPKAPK5-AS1 knockdown cells (Figure 5D). Similar results were observed for cell migration and invasion (Figure 5 E,F). These results provide strong evidence that MAPKAPK5- AS1 promotes thyroid cancer progression by targeting the miR-519e-5p/YWHAH axis.
Figure 2.
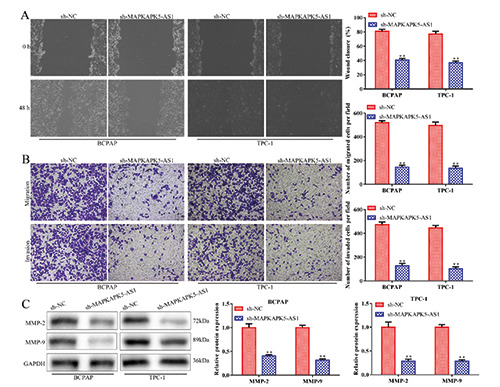
Knockdown of MAPKAPK5-AS1 impedes cell migration and invasion of thyroid cancer cells. Wound healing assay (A) and transwell invasion assay (B) were adopted to identify the functional role of MAPKAPK5-AS1 on cell migration and invasion in thyroid cancer cells. C) Western blot assay was used to assess expression of MMP-2 and MMP-9. **p<0.01 vs sh-NC.
Discussion
In the past few decades, abnormal expression of lncRNAs has been widely observed and plays a vital role in multiple tumours. Mu et al. demonstrated that lncRNA AFAP1-AS1 promotes cell proliferation and metastasis in clear cell renal cell carcinoma.24 lncRNA TDRG1 accelerates cervical cancer progression by targeting miR-326.28 However, little is known about the expression of MAPKAPK5-AS1 and its underlying molecular mechanism in thyroid cancer. In the present study, we investigated the malignant properties of MAPKAPK5-AS1 in thyroid cancer, which might provide a novel therapeutic target in thyroid cancer. In the present study, we demonstrated that expression levels of MAPKAPK5- AS1 in thyroid cancer are, increased and that MAPKAPK5-AS functions as a critical oncogene in thyroid cancer.
Recent studies have demonstrated that the interaction between lncRNAs and miRNAs can affect each other’s expression and form a complex regulatory network, which plays a vital role in the biological process of various malignant tumour cells. lncRNA H19 affects laryngeal squamous cell cancer progression via the miR- 148a-3p/DNMT1 axis.29 Moreover, lncRNA SPRY4-IT1 enhances cell proliferation and metastasis through the miR-101-3p/EZH2 axis in bladder cancer.30 In addition, lncRNA PTCSC3/miR-574-5p modulates papillary thyroid carcinoma cell proliferation and migration via Wnt/β-catenin signalling.31
Previous studies have described the involvement of miRNAs in thyroid cancer pathogenesis. For example, miR-299-3p functions as a tumour suppressor in hepatocellular carcinoma by regulating Sirtuin 5.32 Zhang et al. demonstrated that miR-574-5p mediates the cell cycle and apoptosis in thyroid cancer cells vioea Wnt/β-catenin signalling by repressing the expression of Quaking proteins.33 Moreover, miR-524 inhibits cell proliferation and induces apoptosis in thyroid cancer cells by targeting SPAG9.34 The objective of this study was to explore the molecular mechanism of MAPKAPK5-AS1 in thyroid cancer cells. Bioinformatics analysis showed that miR-519e-5p possesses binding sites with MAPKAPK5-AS1. MiR-519e-5p has been found to be decreased in multiple cancers, which might be a potential target of MAPKAPK5-AS1.35,36 However, to the best of our knowledge, the role of miR-363-3p in thyroid cancer has not yet been studied. In the current study, results of the luciferase reporter assay and RT-qPCR assay demonstrated that miR-519e- 5p is a direct target of MAPKAPK5-AS. Furthermore, we identified YWHAH as a direct target of the MAPKAPK5-AS1/miR- 519e-5p axis in thyroid cancer cells. The direct binding relationship between miR-519e-5p and the 3’UTR of YWHAH was assessed through a luciferase assay. Moreover, expression levels of miR-519e-5p and YWHAH were negatively correlated in thyroid cancer cells. Mechanistically, rescue experiments revealed that the inhibitory effect of MAPKAPK5-AS1 knockdown on thyroid cancer tumorigenicity was mediated by the miR-519e- 5p/YWHAH axis. In general, our findings demonstrated that downregulation of MAPKAPK5-AS1 impeded cell proliferation, migration, and invasion and accelerated apoptosis by targeting the miR-519e-5p/YWHAH axis in thyroid cancer cells. In conclusion, our findings unveiled that MAPKAPK5-AS1 promotes thyroid cancer progression by targeting the miR-519e- 5p/YWHAH axis, which shed light on new insight into the progression and clinical treatment of thyroid cancer. In the future, the important molecular mechanism of how MAPKAPK5-AS1 works in thyroid cancer in vivo needs to be further verified.
Figure 3.
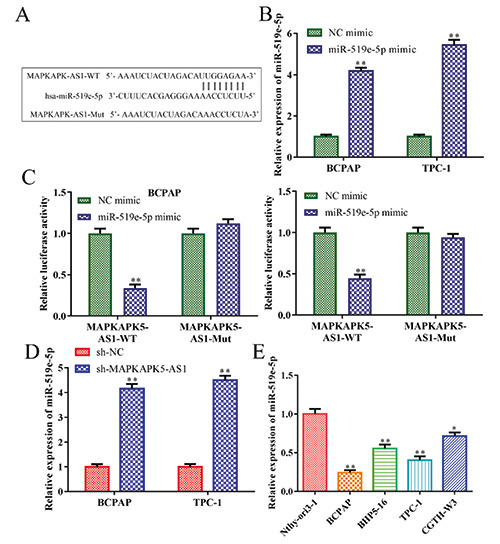
MiR-519e-5p is a direct target of MAPKAPK5-AS1 in thyroid cancer cells. A) StarBase was used to predict miR-519e-5p as a target of MAPKAPK5-AS1. B) Levels of miR-519e-5p were measured by RT-qPCR in BCPAP and TPC-1 cells. C) To estimate the interaction between MAPKAPK5-AS1 and miR-519e-5p, a luciferase reporter assay was implemented; **p<0.01 vs NC mimic. D,E) MiR-519e-5p expression was measured by RT-qPCR; *p<0.05, **p<0.01 vs sh-NC.
Figure 4.
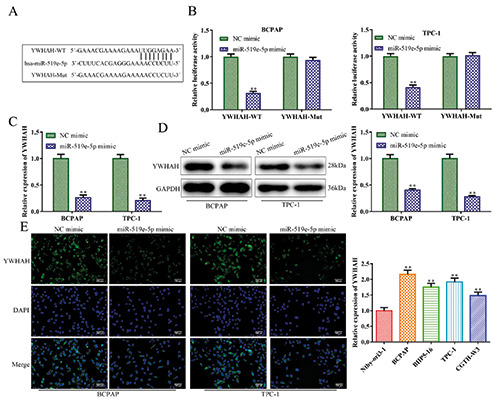
YWHAH is a downstream target of miR-519e-5p. A) Putative miR-519e-5p binding sites in the 3’UTR of YWHAH. () A luciferase reporter assay was implemented to certify the association between miR-519e-5p and YWHAH. C-E) RT-qPCR, Western blot and immunofluorescence analyses were used to determine the effects of miR-519e-5p mimic on YWHAH mRNA and protein levels. F) Expression of YWHAH was detected in thyroid cancer cell lines; **p<0.01 vs NC mimic.
Figure 5.
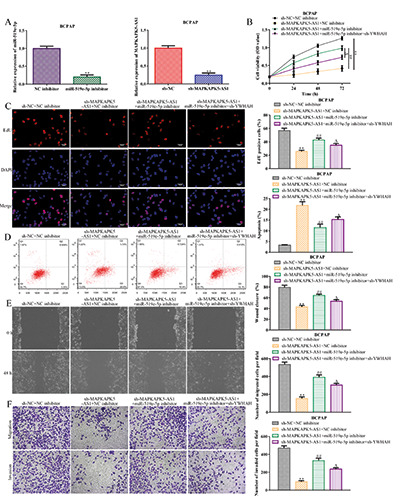
lncRNA MAPKAPK5-AS1 promotes thyroid cancer progression by targeting miR-519e-5p/YWHAH. A) RT-qPCR assay was implemented to certify transfection efficiency for miR-519e-5p inhibitor and MAPKAPK5-AS1 siRNAs. B-F) CCK-8, EdU, flow cytometry, wound healing, and transwell assays were employed to estimate the role of the MAPKAPK5-AS1/miR-519e-5p/YWHAH axis in BCPAP cell proliferation, migration, invasion and apoptosis; **p<0.01 vs sh-NC + NC inhibitor, ##p<0.01 vs sh-MAPKAPK5- AS1 + NC inhibitor, △p<0.01 vs sh-MAPKAPK5-AS1 + miR-519e-5p inhibitor.
Funding Statement
Funding: This study received no funding.
References
- 1.Zaballos MA, Santisteban P. Key signaling pathways in thyroid cancer. J Endocrinol 2017;235:R43-61. [DOI] [PubMed] [Google Scholar]
- 2.Cramer JD, Fu P, Harth KC, Margevicius S, Wilhelm SM. Analysis of the rising incidence of thyroid cancer using the Surveillance, Epidemiology and End Results National Cancer Data Registry. Surgery 2010;148:1147-53. [DOI] [PubMed] [Google Scholar]
- 3.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [DOI] [PubMed] [Google Scholar]
- 4.Zhang M, Wu WB, Wang ZW, Wang XH, Zhang M. lncRNA NEAT1 is closely related with progression of breast cancer via promoting proliferation and EMT. Eur Rev Med Pharmacol Sci 2017;21:1020-6. [PubMed] [Google Scholar]
- 5.Liu Q, Guo X, Que S, Yang X, Fan H, Liu M, et al. LncRNA RSU1P2 contributes to tumorigenesis by acting as a ceRNA against let-7a in cervical cancer cells. Oncotarget 2017;8: 43768-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang K, Geng J, Wang J. Long non-coding RNA RP11-552M11.4 promotes cells proliferation, migration and invasion by targeting BRCA2 in ovarian cancer. Cancer Sci 2018;109: 1428-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Li XY, Hu P, Ding YS. lncRNA NORAD contributes to colorectal cancer progression by inhibition of miR-202-5p. Oncol Res 2018;26:1411-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun MY, Zhu JY, Zhang CY, Zhang M, Zhang H. Autophagy regulated by lncRNA HOTAIR contributes to the cisplatin-induced resistance in endometrial cancer cells. Biotechnol Lett 2017;39:1477-84. [DOI] [PubMed] [Google Scholar]
- 9.Han P, Li JW, Zhang BM, Lv JC, Li YM, Gu XY, et al. The lncRNA CRNDE promotes colorectal cancer cell proliferation and chemoresistance via miR-181a-5p-mediated regulation of Wnt/β-catenin signaling. Mol Cancer 2017;16:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan Q, Liu Y, Fan Y, Liu Z, Wang X, Jia M, et al. LncRNA HOTTIP promotes papillary thyroid carcinoma cell proliferation, invasion and migration by regulating miR-637. Int J Biochem Cell Biol 2018;98:1-9. [DOI] [PubMed] [Google Scholar]
- 11.Zhanga HJ, Weia QF, Wanga SJ, Zhangb HJ, Zhanga XY. LncRNA HOTAIR alleviates rheumatoid arthritis by targeting miR-138 and inactivating NF-κB pathway. Int Immunopharmacol 2017;50:283-90. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Wang S, Li ZZ, Long XY, Wen LZ. The lncRNA NEAT1 facilitates cell growth and invasion via the miR-211/HMGA2 axis in breast cancer. Int J Biol Macromol 2017;105:346. [DOI] [PubMed] [Google Scholar]
- 13.Jin Y, Feng SJ, Qiu S, Shao N, Zheng JH. LncRNA MALAT1 promotes proliferation and metastasis in epithelial ovarian cancer via the PI3K-AKT pathway. Eur Rev Med Pharmacol 2017;21:3176-84. [PubMed] [Google Scholar]
- 14.Chen W, Zhu HY, Yin L, Wang TT, He X. lncRNA-PVT1 facilitates invasion through upregulation of MMP9 in nonsmall cell lung cancer cell. DNA Cell Biol 2017;36:787-93. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Li XY, Hu P, Ding YS. lncRNA NORAD contributes to colorectal cancer progression by inhibition of miR-202-5p. Oncol Res 2018;26:1411-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu ZH, Hou ZH, Zheng LB, Wang XQ, Wu LB, Zhang C. LncRNA DICER1-AS1 promotes the proliferation, invasion and autophagy of osteosarcoma cells via miR-30b/ATG5. Biomed Pharmacother 2018;104:110-8. [DOI] [PubMed] [Google Scholar]
- 17.Guo H, Yang S, Li S, Yan MT, Li L, Zhang HX. LncRNA SNHG20 promotes cell proliferation and invasion via miR-140-5p-ADAM10 axis in cervical cancer. Biomed Pharmacother 2018;102:749-57. [DOI] [PubMed] [Google Scholar]
- 18.Zhao X, Wang D, Ding Y, Zhou JM, Liu GH, Ji ZG. LncRNA ZEB1-AS1 promotes migration and metastasis of bladder cancer cells by post-transcriptional activation of ZEB1. Int J Mol Med 2019;44:196-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ji H, Hui B, Wang J, Zhu Y, Tang L, Peng P, et al. Long noncoding RNA MAPKAPK5-AS1 promotes colorectal cancer proliferation by partly silencing p21 expression. Cancer Sci 2019;110:72-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang T, Chen WC, Shi PC, Liu MR, Jiang T, Song H, et al. Long noncoding RNA MAPKAPK5-AS1 promotes colorectal cancer progression by cis-regulating the nearby gene MK5 and acting as a let-7f-1-3p sponge. Clin Cancer Res 2020;39:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aprelikova O, Yu X, Palla J, Wei BR, Niederhuber J. The role of miR-31 and its target gene SATB2 in cancer-associated fibroblasts. Cell Cycle 2010;9:4387-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng X, Wu B, Xiao K, Kang J, Xie J, Zhang XP, et al. MiR-146b-5p promotes metastasis and induces epithelial-mesenchymal transition in thyroid cancer by targeting ZNRF3. Cell Physiol Biochem 2015;35:71-82. [DOI] [PubMed] [Google Scholar]
- 23.Carroll M. Relationship of YWHAH single nucleotide polymorphisms to markers of rheumatoid arthritis disease severity. J Med Biol Stud 2018;1:101. [Google Scholar]
- 24.Lu YC, Cheng AJ, Lee LY, You GR, Li YL, Chen HY, et al. MiR-520b as a novel molecular target for suppressing stemness phenotype of head-neck cancer by inhibiting CD44. Sci Rep 2017;7:2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haonon O, Rucksaken R, Pinlaor P, et al. Upregulation of 14-3-3 eta in chronic liver fluke infection is a potential diagnostic marker of cholangiocarcinoma. Proteomics Clin Appl 2016; 10:248-56. [DOI] [PubMed] [Google Scholar]
- 26.Ren L, Li Y, Zhao Q, Fan L, Tan B, Zang A, et al. miR-519 regulates the proliferation of breast cancer cells via targeting human antigen R. Oncol Lett 2020;19:1567-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou JY, Zheng SR, Liu J, Shi R, Yu HL, Wei M, et al. MiR-519d facilitates the progression and metastasis of cervical cancer through direct targeting Smad7. Cancer Cell Int 2016;16:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang H, Liang M, Jiang Y, Zhang T, Zhou RJ. The lncRNA TDRG1 promotes cell proliferation, migration and invasion by targeting miR-326 to regulate MAPK1 expression in cervical cancer. Cancer Cell Int 2019;19:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu TY, Qu LM, He GQ, Tian LL, Li L, Zhou H, et al. Regulation of laryngeal squamous cell cancer progression by the lncRNA H19/miR-148a- 3p/DNMT1 axis. Oncotarget 2016;7:11553-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu D, Li Y, Luo G, Xiao XY, Tao D, Wu XC, et al. LncRNA SPRY4-IT1 sponges miR-101-3p to promote proliferation and metastasis of bladder cancer cells through up-regulating EZH2. Cancer Lett 2017;388:281-91. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Lu X, Geng Z, Yang GY, Shi Y. LncRNA PTCSC3/miR-574-5p governs cell proliferation and migration of papillary thyroid carcinoma via Wnt/β-catenin signaling. J Cell Biochem 2017;118:4745-52. [DOI] [PubMed] [Google Scholar]
- 32.Dang S, Zhou J, Wang ZY, Dai SJ, He SX. MiR-299-3p functions as a tumor suppressor via targeting Sirtuin 5 in hepatocellular carcinoma. Biomed Pharmacother 2018;106:966-75. [DOI] [PubMed] [Google Scholar]
- 33.Zhang ZJ, Li XY, Xiao Q, Wang ZM. MiR-574-5p mediates the cell cycle and apoptosis in thyroid cancer cells via Wnt/β-catenin signaling by repressing the expression of Quaking proteins. Oncology Lett 2018;15:5841-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhen Z, Dong F, Shen H, Wang QG, Hu J. MiR-524 inhibits Cell proliferation and induces cell apoptosis in thyroid cancer via targeting SPAG9. Eur Rev Med Pharmacol Sci 2018;22:3812-8. [DOI] [PubMed] [Google Scholar]
- 35.Yu G, Zhang T, Jing Y, Bao QM, Tang Q, Zhang Y. miR-519 suppresses nasopharyngeal carcinoma cell proliferation by targeting oncogene URG4/URGCP. Life Sci 2017;15:47-51. [DOI] [PubMed] [Google Scholar]
- 36.Li YY, Shao JP, Zhang SP, Xing GQ, Liu HJ. miR-519d-3p inhibits cell proliferation and invasion of gastric cancer by downregulating B-cell lymphoma 6. Cytogenet Genome Res 2018;154:12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


