Abstract
Recent clinical successes using therapeutic nucleic acids (TNAs) have accelerated the transition of nucleic acid nanotechnology toward therapeutic applications. Significant progress in the development, production, and characterization of nucleic acid nanomaterials and nucleic acid nanoparticles (NANPs), as well as abundant proof-of-concept data, are paving the way toward biomedical applications of these materials. This recent progress has catalyzed the development of new strategies for biosensing, imaging, drug delivery, and immunotherapies with previously unrecognized opportunities and identified some barriers that may impede the broader clinical translation of NANP technologies. A recent workshop sponsored by the Kavli Foundation and the Materials Research Society discussed the future directions and current challenges for the development of therapeutic nucleic acid nanotechnology. Herein, we communicate discussions on the opportunities, barriers, and strategies for realizing the clinical grand challenge of TNA nanotechnology, with a focus on ways to overcome barriers to advance NANPs to the clinic.
Graphical Abstract
In the past two decades, the broad biomedical applications of nucleic acids, given their innate functions and high degree of programmability, gave rise to two independently established fields known as DNA and RNA nanotechnology. In each of these disciplines, rationally designed oligonucleotides and long, single-stranded DNA or RNA molecules are programmed to organize into supramolecular nucleic acid nanoparticles (NANPs), with controlled physicochemical properties and biological activities. Nucleic acid nanoparticles can be designed for biosensing, nanoscale material organization, biocomputing, and biocompatible nanoscaffolding with embedded functionalities for controlled and targeted delivery to diseased cells and tissues. To categorize the main strategic directions covering all potential applications of these innovative technologies, two scientific societies were established. The International Society for Nanoscale Science, Computation and Engineering (ISNSCE), was established in 2004 to cover the major research endeavors in DNA nanotechnology. More recently, the International Society of RNA Nanotechnology and Nanomedicine (ISRNN) was organized in 2016 to exploit the current advances in RNA nanotechnology and therapeutics. Despite some conceptual overlap in the overall missions of these societies, ISNSCE mainly supports the research areas of material sciences, molecular computation, and nanoengineering from DNA oligomers used as building blocks, whereas ISRNN mostly focuses on biomedicine, pharmaceutical sciences, imaging, and diagnostic applications of RNA nanoassemblies that widely employ architectural principles found in natural RNA structures.
Many interdisciplinary research groups worldwide from both fields have made breakthroughs in the computer-assisted design1–3 and large-scale fabrication3–7 of NANPs, advanced their characterization both in vitro and in vivo, and generated significant amounts of proof-of-concept data for a variety of therapeutic applications of these novel nanomaterials.
Despite the scientific breadth and rigor of these interconnected fields, the translation of these innovative, promising technologies to clinical settings has not yet progressed, with no candidates currently approaching clinical trials. However, the recent release of Onpattro,8 the first U.S. Food and Drug Administration (FDA)-approved lipid-nanoparticle-formulated small interfering RNA (siRNA), marked a milestone for TNAs, and furthered their development with a second RNA interference (RNAi) therapy (GalNac-conjugated siRNA, Givlaari)9 entering the market just one year later. Currently, there are six RNAi therapies in phase III10–15 and eight others in phase I and II clinical trials.16 In addition to specific RNAi inducers, there are several other classes of TNAs, such as antisense oligonucleotides, mRNAs, aptamers, ribozymes, and gene-editing therapeutics, which are either already approved (12 TNAs) or in clinical trials (33 TNAs).16
In this Nano Focus, we relate discussions pertaining to the clinical translation of DNA and RNA nanotechnology and propose a strategy for overcoming key challenges in this area.
All of these ongoing advances open a plethora of new opportunities for NANP technologies and have paved the way for their more focused development and clinical translation. However, poor therapeutic index and species variability both in the manufacturing of TNAs and in their in vivo performance create additional major hurdles for their clinical translation.17–20 Among other commonly accepted challenges,17–20 potential barriers substantially impeding the translation of NANP technologies include the lack of unified protocols and standards for their synthesis and characterization and gaps both in mechanistic understanding of their immunological properties and in published proof-of-concept in vivo studies demonstrating novel efficacy or mode of action.
To discuss these barriers and to chart a path toward overcoming them by identifying the most promising further directions in therapeutic RNA and DNA nanotechnology, we organized a workshop with support from the Materials Research Society and the Kavli Foundation in Boston, Massachusetts on December 7, 2019. Leading researchers from the fields of DNA and RNA nanotechnology convened for constructive discussion. In this Nano Focus, we relate discussions pertaining to the clinical translation of DNA and RNA nanotechnology and propose a strategy for overcoming key challenges in this area.
Identified Opportunities To Be Explored.
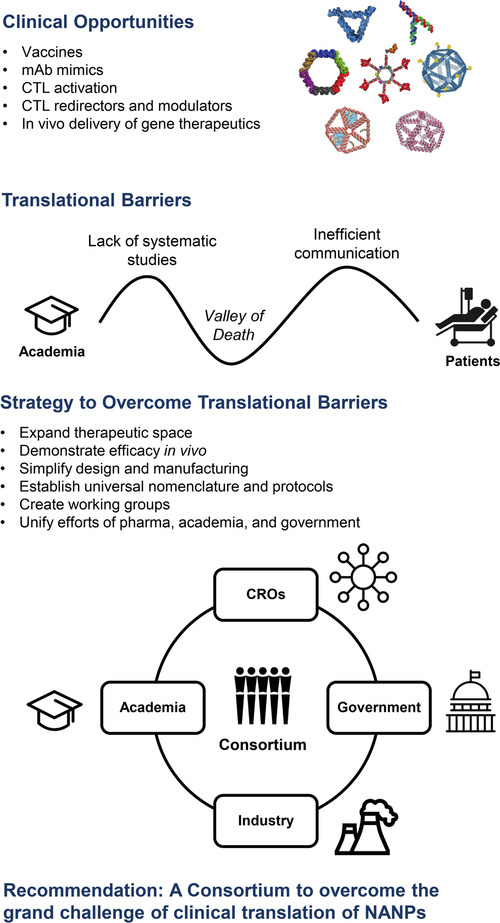
Specific opportunities for NANPs include but are not limited to the following product categories: (i) subunit and peptide vaccines; (ii) artificial antigen-presenting cells (APCs) that promote cytotoxic T lymphocyte (CTL) activation; (iii) caged and multispecific affinity platforms as CTL redirectors and modulators; (iv) monoclonal antibody (mAb) mimics; and (v) virus-like nanoparticles for targeted delivery of mRNAs, antisense oligonucleotides (ASOs), and other therapeutic nucleic acid payloads including clustered regularly interspaced short palindromic repeats (CRISPR) ribonucleoproteins (Figure 1).
Figure 1.
Overview of (1) clinical opportunities for nucleic acid nanoparticles (NANP) therapeutics include small interfering RNAs, antisense oligonucleotides, mRNAs, CRISPR ribonucleoproteins, and vaccine vectors; (2) translational barriers that are limiting progress from academia to the clinic to benefit patients; (3) proposed strategy to overcome these translational barriers; and (4) our recommendation of a consortium to help translate NANPs to the clinic.
The overall goals of prophylactic vaccines and immunotherapies are to stimulate innate and adaptive immunity and to restore immune homeostasis without affecting the patient. Although various types of immune cells are involved in mounting the response against a pathogen, APCs, and particularly dendritic cells (DCs), represent the most common targets for vaccine delivery. Substances that help APCs to recognize, to process, and to present antigenic fragments to T cells, also known as adjuvants, are commonly used to optimize immune response and to promote the generation of memory B cells. Typical cellular targets for immunotherapies are DCs, macrophages, and cytotoxic T lymphocytes. Various nanoparticles are commonly considered for vaccines and immunotherapy delivery, and the benefits of their use in this field have been extensively discussed elsewhere.21,22 Recent advances in vaccines and immunotherapies,23,24 along with beneficial properties of NANPs for these indications,25 have also been reviewed in detail. Herein, we briefly review several of them to highlight this opportunity.
Antigen-presenting cells exposed to NANPs typically secrete type I and type III interferons (IFNs),26 which are known for their ability to induce DC maturation.27–29 The magnitude of IFN induction in response to NANPs depends in part on their physicochemical properties (e.g., molecular weight, CpG composition, size, and geometry);26 therefore, the ability of NANPs to induce an IFN response may help to personalize vaccines so that IFN levels that are optimal for a given individual may be induced. This property may be combined with subunit or peptide delivery and is particularly important because inflammation triggered by adjuvants varies between individuals.30 Cytokine levels in response to the same adjuvant may be insufficient for effective vaccination for one individual and too toxic for another. Customizing vaccines to the individual’s immune system is, therefore, an attractive avenue for personalized vaccination that is both efficacious and free from adverse immune-mediated effects. Both types I and III IFNs in the form of recombinant protein therapeutics are used to combat viral infections and cancer.31–35 Despite proven efficacy, systemic administration of recombinant proteins may cause side effects (e.g., fever or fever-like reactions and chills)36 and induce antidrug antibodies (ADAs).37 Such ADAs may affect drug efficacy and cause toxicity and, in some instances, neutralize recombinant protein therapeutics.38 A variety of approaches are used to minimize the immunogenicity of recombinant IFNs, but none is completely efficient at eliminating the ADA response.38 For example, one of the most commonly used approaches—conjugation of protein to polyethylene glycol (PEG)—often fails due to the immunogenicity of the PEG itself and the presence of pre-existing antiPEG antibodies in healthy donors’ blood.39–41 Due to their ability to induce IFN responses, NANPs could potentially provide a solution to these problems by directing a patient’s immune system to produce its own IFNs that are not immunogenic. However, significant research is still needed to understand the induction of ADAs by NANPs themselves, which may lead to neutralization or, in worse cases, lupus-like pathology and disease.
Substances that activate Toll-like receptors (TLRs) are popular adjuvants in the vaccine and immunotherapy fields.42 However, some of these substances, particularly TLR7/8 agonists, are too toxic to be injected into the blood, so an alternative method of delivery must be utilized.42 Hong et al. demonstrated that NANPs made of RNA activate an IFN response via TLR7.43 When exposed to human blood cells, NANPs do not cause inflammation or an IFN response unless their uptake by the blood cells is directed by a delivery carrier.26 These findings emphasize an important property of RNA-based NANPs, namely, a potent TLR7 agonist ability that manifests only after NANPs are internalized by APCs, thus creating an opportunity for NANPs to be used as delivery-controlled adjuvants. This agonistic activity makes NANPs an alternative to resiquimod, a well-known TLR 7/8 agonist that is only safe for use in topical applications due to the overt immunostimulation that occurs immediately when this adjuvant enters the systemic circulation.44
The ability of NANPs to act as scaffolds for in vivo delivery of TNAs45–52 can be leveraged to alter the expression of genes that are responsible for altered immune responses against tumors, the so-called cancer immunity cycle.53 Moreover, NANPs may be designed to possess split functionalities whereby they carry no function when used independently but acquire functionality only after co-delivery into the same cell.47,54,55 This modality enables researchers to use NANPs to turn on and off biological responses when such controls are needed. The advantage of such dual-functionality NANPs is the elimination of off-target toxicity when an individual NANP is delivered into the off-target cell. One design of the dual-functional NANPs includes so-called split functionality in which a first NANP aimed at performing one function (e.g., activation of the immune response) is administered as a single treatment and then a second NANP with alternative functionality (e.g., inhibition of the immune response) is administered to neutralize the effect of the first NANP.54,55 This modality enables additional control over the immune cells activated by immunotherapy (e.g., CTLs) and, therefore, is instrumental in immunotherapy to avoid adverse effects arising due to activation of the immune system, which is often overstimulated and may lead to autoimmunity. Another example of controlled immune activation is via NANPs releasing NF-kB decoy oligonucleotides.55 Because altered NF-kB function is often observed in various types of cancer,56–59 the inhibition of NF-kB by decoy-releasing NANPs has the potential to create a new therapeutic modality for cancer therapy. The same property would also benefit vaccines that contain TLR agonists inducing a robust tumor necrosis factor (TNF) response. Although TNF is needed to activate APCs, high levels of this cytokine may lead to tissue necrosis at the site of vaccine injection. Having NANPs release NF-kB decoy oligonucleotide and activating type I IFN in such vaccines would help to control undesirable cytokine levels and provide optimal levels of desirable cytokines. Proof of efficacy of NF-kB-controlling NANPs has been recently reported55 and warrants further research in this area.
Nucleic acid nanoparticles may be designed to possess split functionalities whereby they carry no function when used independently but acquire functionality only after co-delivery into the same cell.
Specific areas of research to explore this opportunity include NANPs optimized for organ biodistribution60 and active, specific cellular targeting and uptake61 followed by intracellular action in the form of (i) programmable scaffolds for therapeutic nucleic acids; (ii) targeted delivery of nucleic acid gene therapeutics (CRISPR, siRNAs, miRNAs, etc);62,63 (iii) organization, control (activation or inhibition), and delivery vehicles for endosomal escape and unique tissue and intracellular distribution; and (iv) controlled induction of beneficial immune responses for cancer therapeutics64 and prophylactic vaccines.65 Lessons learned from clinical translation of traditional nucleic acid therapeutics suggest that, in situations when more proven approaches like GalNAc are not an option, delivery using nanocarriers might be beneficial.
Barriers and Strategies for Overcoming Them.
Several barriers were prioritized as critical to overcome in order to accelerate the translation of therapeutic nucleic acid nanotechnology to the clinical settings, including overcoming (1) the translational Valley of Death; (2) the lack of systematic studies required for regulatory submissions; and (3) inefficient communication between stakeholders (Figure 1).
Valley of Death.
Translation from the bench to the clinic requires both resources and a specialized knowledge set encompassing good laboratory practices (GLPs), good manufacturing practices (GMPs), the FDA guidance for the industry relevant to the given drug product category and indication, and a solid GLP-validated bioanalytical suite of assays for nanoparticle characterization, as well as a workforce qualified to perform studies compliant with the GLP/GMP regulatory requirements. Such a knowledge set and workforce are typically not available in academia because most academic investigators have neither resources nor sufficient budgets to support the requisite translational infrastructure. Likewise, industrial and regulatory organizations equipped for translational studies compliant with FDA requirements often do not have the skillset and understanding of sophisticated technologies such as NANPs. These disparities in part contribute to the gap known as the “Valley of Death” faced by many new technologies, which must be overcome to benefit patients in need. Additional hurdles include the high costs and limited access to non-human primate models that are commonly used to evaluate safety of traditional TNAs. Crossing the Valley of Death, therefore, is critical to accelerate the translation of DNA and RNA NANPs to clinical settings and requires a concerted, coordinated effort across multiple stakeholders. In particular, the following resources and activities may lower this barrier: (i) leveraging existing resources such as the Nanotechnology Characterization Laboratory (NCL) to provide knowledgebase and characterization support for cancer nanomedicines; (ii) introducing GMPs and GLPs into laboratories that are aiming to translate their products to preclinical studies, or offering clear commercial research organization (CRO) partners and paths to assist with translation; (iii) scaling up production of pyrogen-free NANPs with defined physicochemical and immunological properties and high batch-to-batch consistency in partnership with CROs and other interested parties and laboratories; (iv) obtaining support from government-funded programs such as PAR-20–116 and the Biomedical Advanced Research and Development Authority are needed to enable translational studies in larger animal models including dogs and non-human primates.66 Mechanisms for translational funding are required to enable these translational efforts, and they must specifically appreciate the need to support the major financial and personnel commitments needed to cross the Valley of Death between basic, fundamental research and translational clinical material production and validation.
Crossing the Valley of Death is critical to accelerate the translation of DNA and RNA nucleic acid nanoparticles to clinical settings and requires a concerted, coordinated effort across multiple stakeholders.
Lack of Systematic Studies Required for Regulatory Submissions.
The FDA reviews efficacy and safety data for various drug products (small molecules, biologics, nucleic acids), medical devices, and combination products.67 As of January 20, 2020, the FDA released five guidance documents for industry to express the Agency’s view of cosmetic, veterinary, and human pharmaceutical products containing nanotechnology.68 Currently, products containing nanomaterials are regulated according to the safety and efficacy regulatory suite established for other drug products but with some nuances. For example, if a nanotechnology product contains both small molecular drug and biologics, then the studies required for drugs and for biologics would both have to be undertaken to characterize that nanomaterial.67 The FDA has a series of indications- and product-specific guidance documents for gene therapies;69 however, specific guidance recommendations for NANPs are not yet among these documents. Bioavailability, barrier penetration, in vivo delivery, and unwanted toxicity create safety concerns that are among the major obstacles preventing the field from entering clinical stages. Studies investigating NANP absorption, distribution, metabolism, excretion, and toxicity (ADME/Tox), as well as understanding of clearance rates and safety in rodent and non-rodent species are needed prior to clinical studies.
These barriers can be eliminated by (i) developing NANP-based formulations targeted to organs and tissues other than the liver (i.e., extrahepatic targeting of NANPs); (ii) sensing and actuation for improving therapeutic index; (iii) performing in vivo studies in rats and dogs or non-human primates and comparing the findings to those with traditional nucleic acid therapeutics; (iv) organizing seminars and workshops between academic and industrial researchers working on RNA and DNA NANPs and regulatory scientists; and (v) promoting FDA reviewers to interact with and to provide guidance to academic investigators regarding study design for ADME/Tox and the immunological safety of drug products and vaccines.
Inefficient Communication between Stakeholders.
The gap in communication between clinicians and nanotechnologists further delays the understanding and timely identification of important therapeutic challenges. This barrier may be reduced or eliminated by the following activities: (i) creating non-monetary incentives for clinicians to present achievable webinars on unmet needs in particular therapeutic areas; (ii) creating forums for clinicians and scientists to brainstorm ideas and to discuss potential collaborations; (iii) initiating new funding opportunities for driving these translational collaborations.
In each of these cases, an overarching need for academic researchers and basic scientists involved in NANP studies is to demonstrate both clear efficacy and translational capability of their materials for the Valley of Death to be crossed. Whereas traditional funding mechanisms and academic publication rewards are well-suited to the former, they are not typically oriented toward supporting the latter. Moreover, academic researchers are not typically trained, equipped, nor supported financially for translation, which will require a new, collaborative model to emerge for NANPs to translate successfully to the clinic in the near future (Figure 1).
CONCLUSIONS AND PROSPECTS
The overarching sentiment of this meeting highlighted the need to improve our mechanistic understanding of the interaction of NANPs with non-human primate and other model organisms, as well as eventually with humans, including but not limited to mechanisms of metabolism, clearance, immunotoxicity, and distribution to organs and target tissues. Another important conclusion was the urgent need for the development of standardized methods and reference materials to assist the global nucleic acid nanotechnology community in translating this technology to clinical stages. In all cases, benchmarking NANPs’ efficacy, toxicity, and immunogenicity and, therefore, their translational potential and impact for well-established delivery and vaccine technologies including lipid nanoparticles, viral vectors, and polymeric formulations will also be essential for the field to demonstrate its potential value and benefits to patients and clinicians.
Paths Forward.
To promote the potential of highly promising and innovative NANP therapies further, additional efforts should be placed on (i) expanding the functional, therapeutic chemical space of nucleic acid nanotechnology; (ii) simplifying the design and improving the yields of functional NANP assemblies; (iii) establishing universal nomenclature and protocols for production and characterization of NANPs; (iv) creating working groups of MDs, PhDs, and biomedical engineers and unifying the efforts of pharma, academia, and the government (FDA, NIH) to identify timely and important disease targets, therapeutic challenges, and regulatory path-ways; (v) introducing feedback loops for patients, clinicians, academics, and industry partners; and, perhaps most importantly, (vi) using comparative studies to demonstrate the relative clinical benefit and value of NANPs over competing, pre-existing gene therapeutic and vaccine formulations (Figure 1). These goals might be achieved by creating an interdisciplinary and international consortium of academics, contract research organizations, industrial partners, and government agency counterparts, specifically focusing on overcoming the grand challenge of clinical translation of NANPs (Figure 1).
ACKNOWLEDGMENTS
We gratefully acknowledge fruitful discussions with Paul Burke, Hanadi Sleiman, Jorgen Kjems, William Shih, Bjoern Hogberg, Hendrik Dietz, Thomas Hermann, Nils Walter, Chenxiang Lin, and Sarah Woodson, among other participants at the MRS-Kavli Future of Materials Workshop on Nucleic Acid Nanotechnology, which motivated the preceding piece. This study was supported in part by federal funds from the National Cancer Institute and the National Institutes of Health, under Contract Nos. HHSN261200800001E and 75N91019D00024 to M.A.D. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. Research reported in this publication was also supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R01GM120487 (to K.A.A.). M.B. is grateful for funding from the National Institutes of Health (R21-EB026008 and R01-MH112694), the Office of Naval Research (N00014-16-1-2953), the Army Research Office (W911NF1810436), and the National Science Foundation (CCF-1564025 and CBET-1729397).
Footnotes
Notes
The authors declare no competing financial interest.
Contributor Information
Kirill A. Afonin, Nanoscale Science Program, Department of Chemistry, The University of North Carolina at Charlotte, Charlotte, North Carolina 28223, United States.
Marina A. Dobrovolskaia, Nanotechnology Characterization Lab, Cancer Research Technology Program, Frederick National Laboratory for Cancer Research sponsored by the National Cancer Institute, Frederick, Maryland 21702, United States.
George Church, Department of Genetics, Harvard Medical School, Boston, Massachusetts 02115, United States; Wyss Institute for Biologically Inspired Engineering, Boston, Massachusetts 02115, United States; Harvard Graduate Program in Biological and Biomedical Sciences, Boston, Massachusetts 02115, United States.
Mark Bathe, Department of Biological Engineering, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, United States.
REFERENCES
- (1).Jun H; Shepherd TR; Zhang KM; Bricker WP; Li SS; Chiu W; Bathe M Automated Sequence Design of 3D Polyhedral Wireframe DNA Origami with Honeycomb Edges. ACS Nano 2019, 13, 2083–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Veneziano R; Ratanalert S; Zhang K; Zhang F; Yan H; Chiu W; Bathe M Designer Nanoscale DNA Assemblies Programmed from the Top Down. Science 2016, 352, 1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Wamhoff EC; Banal JL; Bricker WP; Shepherd TR; Parsons MF; Veneziano R; Stone MB; Jun HM; Wang X; Bathe M Programming Structured DNA Assemblies To Probe Biophysical Processes. Annu. Rev. Biophys 2019, 48, 395–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Shepherd TR; Du RR; Huang H; Wamhoff EC; Bathe M Bioproduction of Pure, Kilobase-Scale Single-Stranded DNA. Sci. Rep 2019, 9, 6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Veneziano R; Shepherd TR; Ratanalert S; Bellou L; Tao CQ; Bathe M In Vitro Synthesis of Gene-Length Single-Stranded DNA. Sci. Rep 2018, 8, 6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Praetorius F; Kick B; Behler KL; Honemann MN; Weuster-Botz D; Dietz H Biotechnological Mass Production of DNA Origami. Nature 2017, 552, 84–87. [DOI] [PubMed] [Google Scholar]
- (7).Minev D; Guerra R; Kishi JY; Smith C; Krieg E; Said K; Hornick A; Sasaki HM; Filsinger G; Beliveau BJ; Yin P; Church GM; Shih WM Rapid in Vitro Production of Single-Stranded DNA. Nucleic Acids Res 2019, 47, 11956–11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Adams D; Gonzalez-Duarte A; O’Riordan WD; Yang CC; Ueda M; Kristen AV; Tournev I; Schmidt HH; Coelho T; Berk JL; Lin KP; Vita G; Attarian S; Plante-Bordeneuve V; Mezei MM; Campistol JM; Buades J; Brannagan TH 3rd; Kim BJ; Oh J; et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N. Engl. J. Med 2018, 379, 11–21. [DOI] [PubMed] [Google Scholar]
- (9).Chan A; Liebow A; Yasuda M; Gan L; Racie T; Maier M; Kuchimanchi S; Foster D; Milstein S; Charisse K; Sehgal A; Manoharan M; Meyers R; Fitzgerald K; Simon A; Desnick RJ; Querbes W Preclinical Development of a Subcutaneous ALAS1 RNAi Therapeutic for Treatment of Hepatic Porphyrias Using Circulating RNA Quantification. Mol. Ther.–Nucleic Acids 2015, 4, No. e263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Liebow A; Li X; Racie T; Hettinger J; Bettencourt BR; Najafian N; Haslett P; Fitzgerald K; Holmes RP; Erbe D; Querbes W; Knight J An Investigational RNAi Therapeutic Targeting Glycolate Oxidase Reduces Oxalate Production in Models of Primary Hyperoxaluria. J. Am. Soc. Nephrol 2017, 28, 494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Sardh E; Harper P; Balwani M; Stein P; Rees D; Bissell DM; Desnick R; Parker C; Phillips J; Bonkovsky HL; Vassiliou D; Penz C; Chan-Daniels A; He Q; Querbes W; Fitzgerald K; Kim JB; Garg P; Vaishnaw A; Simon AR; Anderson KE Phase 1 Trial of an RNA Interference Therapy for Acute Intermittent Porphyria. N. Engl. J. Med 2019, 380, 549–558. [DOI] [PubMed] [Google Scholar]
- (12).Janas MM; Zlatev I; Liu J; Jiang Y; Barros SA; Sutherland JE; Davis WP; Liu J; Brown CR; Liu X; Schlegel MK; Blair L; Zhang X; Das B; Tran C; Aluri K; Li J; Agarwal S; Indrakanti R; Charisse K; et al. Safety Evaluation of 2′-Deoxy-2′-Fluoro Nucleotides in GalNAc-siRNA Conjugates. Nucleic Acids Res 2019, 47, 3306–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Stoekenbroek RM; Kallend D; Wijngaard PL; Kastelein JJ Inclisiran for the Treatment of Cardiovascular Disease: The ORION Clinical Development Program. Future Cardiol 2018, 14, 433–442. [DOI] [PubMed] [Google Scholar]
- (14).Solano EC; Kornbrust DJ; Beaudry A; Foy JW; Schneider DJ; Thompson JD Toxicological and Pharmacokinetic Properties of QPI-1007, a Chemically Modified Synthetic siRNA Targeting Caspase 2 mRNA, Following Intravitreal Injection. Nucleic Acid Ther 2014, 24, 258–266. [DOI] [PubMed] [Google Scholar]
- (15).Moreno-Montanes J; Bleau AM; Jimenez AI Tivanisiran, a Novel siRNA for the Treatment of Dry Eye Disease. Expert Opin. Invest. Drugs 2018, 27, 421–426. [DOI] [PubMed] [Google Scholar]
- (16).Weng Y; Huang Q; Li C; Yang Y; Wang X; Yu J; Huang Y; Liang XJ Improved Nucleic Acid Therapy with Advanced Nanoscale Biotechnology. Mol. Ther.–Nucleic Acids 2020, 19, 581–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Herrera VL; Colby AH; Ruiz-Opazo N; Coleman DG; Grinstaff MW Nucleic Acid Nanomedicines in Phase II/III Clinical Trials: Translation of Nucleic Acid Therapies for Reprogramming Cells. Nanomedicine (London, U. K.) 2018, 13, 2083–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Jackson AL; Levin AA Developing MicroRNA Therapeutics: Approaching the Unique Complexities. Nucleic Acid Ther 2012, 22, 213–225. [DOI] [PubMed] [Google Scholar]
- (19).Kornbrust D; Cavagnaro J; Levin A; Foy J; Pavco P; Gamba-Vitalo C; Guimond A Oligo Safety Working Group Exaggerated Pharmacology Subcommittee Consensus Document. Nucleic Acid Ther 2013, 23, 21–28. [DOI] [PubMed] [Google Scholar]
- (20).Schubert D; Levin AA; Kornbrust D; Berman CL; Cavagnaro J; Henry S; Seguin R; Ferrari N; Shrewsbury SB The Oligonucleotide Safety Working Group (OSWG). Nucleic Acid Ther 2012, 22, 211–212. [DOI] [PubMed] [Google Scholar]
- (21).Grimaldi AM; Incoronato M; Salvatore M; Soricelli A Nanoparticle-Based Strategies for Cancer Immunotherapy and Immunodiagnostics. Nanomedicine (London, U. K.) 2017, 12, 2349–2365. [DOI] [PubMed] [Google Scholar]
- (22).Shao K; Singha S; Clemente-Casares X; Tsai S; Yang Y; Santamaria P Nanoparticle-Based Immunotherapy for Cancer. ACS Nano 2015, 9, 16–30. [DOI] [PubMed] [Google Scholar]
- (23).Hong E; Dobrovolskaia MA Addressing Barriers to Effective Cancer Immunotherapy with Nanotechnology: Achievements, Challenges, and Roadmap to the Next Generation of Nanoimmunotherapeutics. Adv. Drug Delivery Rev 2019, 141, 3–22. [DOI] [PubMed] [Google Scholar]
- (24).Milling L; Zhang Y; Irvine DJ Delivering Safer Immunotherapies for Cancer. Adv. Drug Delivery Rev 2017, 114, 79–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Dobrovolskaia MA Nucleic Acid Nanoparticles at a Crossroads of Vaccines and Immunotherapies. Molecules 2019, 24, 4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Hong E; Halman JR; Shah AB; Khisamutdinov EF; Dobrovolskaia MA; Afonin KA Structure and Composition Define Immunorecognition of Nucleic Acid Nanoparticles. Nano Lett 2018, 18, 4309–4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Radvanyi LG; Banerjee A; Weir M; Messner H Low Levels of Interferon-Alpha Induce CD86 (B7.2) Expression and Accelerates Dendritic Cell Maturation from Human Peripheral Blood Mononuclear Cells. Scand. J. Immunol 1999, 50, 499–509. [DOI] [PubMed] [Google Scholar]
- (28).Tam MA; Wick MJ MyD88 and Interferon-Alpha/Beta are Differentially Required for Dendritic Cell Maturation but Dispensable for Development of Protective Memory against Listeria. Immunology 2009, 128, 429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Trepiakas R; Pedersen AE; Met O; Svane IM Addition of Interferon-Alpha to a Standard Maturation Cocktail Induces CD38 Up-Regulation and Increases Dendritic Cell Function. Vaccine 2009, 27, 2213–2219. [DOI] [PubMed] [Google Scholar]
- (30).Duffy D Milieu Interieur: Defining the Boundaries of a Healthy Immune Response for Improved Vaccination Strategies. Hum. Vaccines Immunother 2018, 14, 2217–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Floros T; Tarhini AA Anticancer Cytokines: Biology and Clinical Effects of Interferon-Alpha2, Interleukin (IL)-2, IL-15, IL-21, and IL-12. Semin. Oncol 2015, 42, 539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Cheknev SB; Kobyakina NA; Mezentseva MV; Skvortsova VI Long-Term Study of Interferon System State in Patients with Multiple Sclerosis Received the Individual Immune Therapy with Human Recombinant IFN-alpha. Russ J. Immunol 2001, 6, 39–46. [PubMed] [Google Scholar]
- (33).Bongioanni MR; Durelli L; Ferrero B; Imperiale D; Oggero A; Verdun E; Aimo G; Pagni R; Geuna M; Bergamasco B Systemic High-Dose Recombinant-Alpha-2a-Interferon Therapy Modulates Lymphokine Production in Multiple Sclerosis. J. Neurol. Sci 1996, 143, 91–99. [DOI] [PubMed] [Google Scholar]
- (34).Kujawski LA; Talpaz M The Role of Interferon-Alpha in the Treatment of Chronic Myeloid Leukemia. Cytokine Growth Factor Rev 2007, 18, 459–471. [DOI] [PubMed] [Google Scholar]
- (35).Rong L; Perelson AS Treatment of Hepatitis C Virus Infection with Interferon and Small Molecule Direct Antivirals: Viral Kinetics and Modeling. Crit. Rev. Immunol 2010, 30, 131–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Filipi ML; Beavin J; Brillante RT; Costello K; Hartley GC; Hartley K; Namey M; O’Leary S; Remington G Nurses’ Perspective on Approaches to Limit Flu-Like Symptoms During Interferon Therapy for Multiple Sclerosis. Int. J. MS Care 2014, 16, 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Rosenberg AS Immunogenicity of Biological Therapeutics: A Hierarchy of Concerns. Dev. Biol. (Basel) 2003, 112, 15–21. [PubMed] [Google Scholar]
- (38).Baker MP; Reynolds HM; Lumicisi B; Bryson CJ Immunogenicity of Protein Therapeutics: The Key Causes, Consequences and Challenges. Self Nonself 2010, 1, 314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Chang CJ; Chen CH; Chen BM; Su YC; Chen YT; Hershfield MS; Lee MM; Cheng TL; Chen YT; Roffler SR; Wu JY A Genome-Wide Association Study Identifies a Novel Susceptibility Locus for the Immunogenicity of Polyethylene Glycol. Nat. Commun 2017, 8, 522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Chen BM; Su YC; Chang CJ; Burnouf PA; Chuang KH; Chen CH; Cheng TL; Chen YT; Wu JY; Roffler SR Measurement of Pre-Existing IgG and IgM Antibodies against Polyethylene Glycol in Healthy Individuals. Anal. Chem 2016, 88, 10661–10666. [DOI] [PubMed] [Google Scholar]
- (41).Hsieh YC; Wang HE; Lin WW; Roffler SR; Cheng TC; Su YC; Li JJ; Chen CC; Huang CH; Chen BM; Wang JY; Cheng TL; Chen FM Pre-Existing Anti-Polyethylene Glycol Antibody Reduces the Therapeutic Efficacy and Pharmacokinetics of PEGylated Liposomes. Theranostics 2018, 8, 3164–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Dowling DJ Recent Advances in the Discovery and Delivery of TLR7/8 Agonists as Vaccine Adjuvants. Immunohorizons 2018, 2, 185–197. [DOI] [PubMed] [Google Scholar]
- (43).Hong E; Halman JR; Shah A; Cedrone E; Truong N; Afonin KA; Dobrovolskaia MA Toll-Like Receptor-Mediated Recognition of Nucleic Acid Nanoparticles (NANPs) in Human Primary Blood Cells. Molecules 2019, 24, 1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Tomai MA; Miller RL; Lipson KE; Kieper WC; Zarraga IE; Vasilakos JP Resiquimod and Other Immune Response Modifiers as Vaccine Adjuvants. Expert Rev. Vaccines 2007, 6, 835–847. [DOI] [PubMed] [Google Scholar]
- (45).Halman JR; Kim KT; Gwak SJ; Pace R; Johnson MB; Chandler MR; Rackley L; Viard M; Marriott I; Lee JS; Afonin KA A Cationic Amphiphilic Co-Polymer as a Carrier of Nucleic Acid Nanoparticles (Nanps) for Controlled Gene Silencing, Immunostimulation, and Biodistribution. Nanomedicine 2020, 23, 102094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Afonin KA; Viard M; Koyfman AY; Martins AN; Kasprzak WK; Panigaj M; Desai R; Santhanam A; Grabow WW; Jaeger L; Heldman E; Reiser J; Chiu W; Freed EO; Shapiro BA Multifunctional RNA Nanoparticles. Nano Lett 2014, 14, 5662–5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Afonin KA; Viard M; Martins AN; Lockett SJ; Maciag AE; Freed EO; Heldman E; Jaeger L; Blumenthal R; Shapiro BA Activation of Different Split Functionalities Upon Re-Association of RNA-DNA Hybrids. Nat. Nanotechnol 2013, 8, 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Lee H; Lytton-Jean AK; Chen Y; Love KT; Park AI; Karagiannis ED; Sehgal A; Querbes W; Zurenko CS; Jayaraman M; Peng CG; Charisse K; Borodovsky A; Manoharan M; Donahoe JS; Truelove J; Nahrendorf M; Langer R; Anderson DG Molecularly Self-Assembled Nucleic Acid Nanoparticles for Targeted in Vivo siRNA Delivery. Nat. Nanotechnol 2012, 7, 389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Binzel DW; Shu Y; Li H; Sun M; Zhang Q; Shu D; Guo B; Guo P Specific Delivery of MiRNA for High Efficient Inhibition of Prostate Cancer by RNA Nanotechnology. Mol. Ther 2016, 24, 1267–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Shu D; Li H; Shu Y; Xiong G; Carson WE; Haque F; Xu R; Guo P Systemic Delivery of Anti-miRNA for Suppression of Triple Negative Breast Cancer Utilizing RNA Nanotechnology. ACS Nano 2015, 9, 9731–9740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Feng L; Li SK; Liu H; Liu CY; LaSance K; Haque F; Shu D; Guo P Ocular Delivery of pRNA Nanoparticles: Distribution and Clearance After Subconjunctival Injection. Pharm. Res 2014, 31, 1046–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Shukla GC; Haque F; Tor Y; Wilhelmsson LM; Toulmé J-J; Isambert H; Guo P; Rossi JJ; Tenenbaum SA; Shapiro BA A Boost for the Emerging Field of RNA Nanotechnology. ACS Nano 2011, 5, 3405–3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Chen DS; Mellman I Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [DOI] [PubMed] [Google Scholar]
- (54).Halman JR; Satterwhite E; Roark B; Chandler M; Viard M; Ivanina A; Bindewald E; Kasprzak WK; Panigaj M; Bui MN; Lu JS; Miller J; Khisamutdinov EF; Shapiro BA; Dobrovolskaia MA; Afonin KA Functionally-Interdependent Shape-Switching Nanoparticles with Controllable Properties. Nucleic Acids Res 2017, 45, 2210–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Ke W; Hong E; Saito RF; Rangel MC; Wang J; Viard M; Richardson M; Khisamutdinov EF; Panigaj M; Dokholyan NV; Chammas R; Dobrovolskaia MA; Afonin KA RNA-DNA Fibers and Polygons with Controlled Immunorecognition Activate RNAi, FRET and Transcriptional Regulation of NF-KappaB in Human Cells. Nucleic Acids Res 2019, 47, 1350–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Dolcet X; Llobet D; Pallares J; Matias-Guiu X NF-kB in Development and Progression of Human Cancer. Virchows Arch 2005, 446, 475–482. [DOI] [PubMed] [Google Scholar]
- (57).Li F; Zhang J; Arfuso F; Chinnathambi A; Zayed ME; Alharbi SA; Kumar AP; Ahn KS; Sethi G NF-KappaB in Cancer Therapy. Arch. Toxicol 2015, 89, 711–731. [DOI] [PubMed] [Google Scholar]
- (58).Pramanik KC; Makena MR; Bhowmick K; Pandey MK Advancement of NF-KappaB Signaling Pathway: A Novel Target in Pancreatic Cancer. Int. J. Mol. Sci 2018, 19, 3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Tilborghs S; Corthouts J; Verhoeven Y; Arias D; Rolfo C; Trinh XB; van Dam PA The Role of Nuclear Factor-Kappa B Signaling in Human Cervical Cancer. Crit. Rev. Oncol. Hematol 2017, 120, 141–150. [DOI] [PubMed] [Google Scholar]
- (60).Jasinski DL; Li H; Guo PX The Effect of Size and Shape of RNA Nanoparticles on Biodistribution. Mol. Ther 2018, 26, 784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Douglas SM; Bachelet I; Church GM A Logic-Gated Nanorobot for Targeted Transport of Molecular Payloads. Science 2012, 335, 831–834. [DOI] [PubMed] [Google Scholar]
- (62).Hoiberg HC; Sparvath SM; Andersen VL; Kjems J; Andersen ES An RNA Origami Octahedron with Intrinsic siRNAs for Potent Gene Knockdown. Biotechnol. J 2019, 14, No. 1700634. [DOI] [PubMed] [Google Scholar]
- (63).Liu Q; Wang D; Xu Z; Huang CJ; Zhang C; He BF; Mao CD; Wang GS; Qian H Targeted Delivery of Rab26 siRNA with Precisely Tailored DNA Prism for Lung Cancer Therapy. ChemBioChem 2019, 20, 1139–1144. [DOI] [PubMed] [Google Scholar]
- (64).Qi X; Liu X; Matiski L; Rodriguez Del Villar R; Yip T; Zhang F; Sokalingam S; Jiang S; Liu L; Yan H; Chang Y RNA Origami Nanostructures for Potent and Safe Anticancer Immunotherapy. ACS Nano 2020, 14, 4727–4740. [DOI] [PubMed] [Google Scholar]
- (65).Veneziano R; Moyer TJ; Stone MB; Wamhoff E-C; Read BJ; Mukherjee S; Shepherd TR; Das J; Schief WR; Irvine DJ; Bathe M Role of Nanoscale Antigen Organization on B-Cell Activation Probed Using DNA Origami. Nat. Nanotechnol 2020, DOI: 10.1038/s41565-020-0719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).https://grants.nih.gov/grants/guide/pa-files/PAR-20-116.html (accessed 2020-06-16).
- (67).Tyner K; Sadrieh N Considerations When Submitting Nanotherapeutics to FDA/CDER for Regulatory Review. Methods Mol. Biol 2011, 697, 17–31. [DOI] [PubMed] [Google Scholar]
- (68).https://www.fda.gov/science-research/nanotechnology-programs-fda/nanotechnology-guidance-documents (accessed 2020-06-16).
- (69).https://www.fda.gov/vaccines-blood-biologics/biologics-guidances/cellular-gene-therapy-guidances (accessed 2020-06-16).