Abstract
This study aimed to assess the impact of a culturally sensitive, comprehensive women-centered intervention on psychoactive substance use among women in the Republic of Georgia. Study participants included 128 women who had injected psychoactive substance(s) in the past 30 days and had enrolled in a randomized controlled trial that compared Reinforcement Based Treatment and the Women’s CoOp (RBT+WC) to usual care (UC). RBT+WC provided a structured 12-session intervention designed to reduce HIV/HCV risk and psychoactive substance and alcohol use, and to improve mental and physical health; whereas UC provided information booklets on the same topics and case management for 12 sessions. Urine drug screening was conducted at baseline, treatment completion, and 3-month follow-up. The findings showed that RBT+WC was not more effective than UC, although both treatments positively impacted opioid, benzodiazepine, and amphetamine/methamphetamine use. The findings suggest that RBT+WC represents a promising comprehensive women-centered intervention for reducing substance use and HIV risks for Georgian women who use substances.
Keywords: Women-centered intervention, Injection drug use, Republic of Georgia, HIV prevention, Risky injection behavior
Despite the low prevalence of HIV in the general population of the Republic of Georgia (<0.1%), the epidemic is concentrated among key risk groups (National Center for Disease Control and Public Health, 2015). Injection drug use represents the major route for transmission of HIV infection, with 44.7% of cumulative registered cases attributed to injection drug use (Georgia Infectious Diseases AIDS and Clinical Immunology Research Center, 2016). Women represent 25.7% of the total cumulative 6,038 HIV cases registered in Georgia by November 2016. Of particular concern is the steadily increasing rate of new HIV diagnoses, as the number of annually registered cases doubled between 2008 and 2015 and women represent a significant proportion of these cases (Georgia Infectious Diseases AIDS and Clinical Immunology Research Center, 2016).
Women who use psychoactive substances in Georgia represent 2% of clients in substance use services (Javakhishvili, Otiashvili, & Tabatadze, 2016). A wide range of factors contributes to this low rate of service utilization (Kirtadze et al., 2015; Kirtadze et al., 2013; Otiashvili et al., 2013). For example, cultural norms coupled with societal stigma, unwillingness to admit substance use, lack of motivation to seek treatment, and absence of gender-specific treatment programs have been identified as impediments to substance use service access for Georgian women (Kirtadze et al., 2015; Kirtadze et al., 2013; Otiashvili et al., 2013).
The Georgian word for “hope” is imedi. The IMEDI (Investigating Methods for Enhancing Development in Individuals) project was designed to address the unmet needs of women who inject psychoactive substances, to provide women-specific and women-centered treatment services, and to create feelings of hope and promise for the future of the study participants.
The IMEDI project had two overarching goals. The first goal was to ascertain information from women who inject substances and treatment providers about the current status of psychoactive substance use, HIV risk behaviors, and substance abuse treatment in Georgia. Based on this formative research, the second goal was to adapt, integrate, and implement a comprehensive treatment program to slow HIV and hepatitis C virus (HCV) transmission in Georgia.
The project involved four separate studies. The first three studies involved formative and pilot research to inform the development of the intervention program. The fourth study conducted a small-scale randomized controlled trial to assess the feasibility and efficacy of this culturally sensitive, women-centered intervention relative to usual care. The goal of the intervention was to reduce HIV and HCV sex-risk behaviors and reduce psychoactive substance use. Complete details regarding the IMEDI project itself, including a description of this fourth study, can be found in Jones et al. (2014).
The results of this intervention on sex-risk behaviors have been reported previously (Jones et al., 2015). The present study reports the analysis of secondary outcomes that compared the frequency of psychoactive substance use (as measured by urine drug screening at baseline, treatment completion, and 3-month follow-up) between the intervention condition and the usual care condition. We hypothesized that compared to usual care participants, participants in the intervention condition would, on average, show significant decreases in non-prescribed use of (1) buprenorphine, (2) methadone, (3) opioids other than buprenorphine and methadone, (4) cannabis, (5) benzodiazepines, (6) amphetamines/methamphetamines, and (7) any use of alcohol.
METHODS
The IMEDI study received approvals from the Office of Human Research Ethics Institutional Review Board (IRB) of the University of North Carolina at Chapel Hill (USA) and the Maternal and Child Care Union (Georgia). All participants were consented in written form at the study entry phase.
Treatment Conditions
Reinforcement-based Treatment + the Women’s CoOp (RBT+WC)
The RBT+WC condition provided a structured 12-session intervention designed to reduce HIV/HCV risk and substance use and to improve mental and physical health. RBT is a social-learning-theory-driven, evidence-based substance abuse treatment based on social learning theory. It uses life skills training, recreational therapy, and employment as components of a comprehensive treatment model (Tuten, Jones, Schaffer, & Stitzer, 2011). Additionally, RBT addresses the multiple needs of clients, a required treatment component for addressing the complex life issues of Georgian women who inject substances.
RBT has been found to reduce injection and noninjection substance use and related risk behaviors. However, it does not focus on sex-risk behaviors. Consequently, to develop a comprehensive women-centered intervention that effectively addressed sex-risk behaviors, selected treatment modules from the Russian Women’s CoOp intervention (WC) (Wechsberg et al., 2012) were integrated into RBT.
The WC is a best-evidence intervention based in feminist theory and empowerment theory (Wechsberg, 1998). Like RBT, it uses principles of social cognitive theory (Wechsberg & Luseno, 2010). The Women’s CoOp goals include education regarding substance use, sexual risk, and gender-based violence, and it focuses on reducing risk by helping women to develop assertive skills and a harm reduction plan within a supportive environment (Wechsberg, Luseno, Kline, Browne, & Zule, 2010).
RBT and the WC have complementary strengths that held the promise of creating a comprehensive, women-centered intervention for substance-using women. However, it was necessary to revise the integrated RBT+WC intervention in order to yield an intervention sensitive to the values and beliefs of the Georgian culture. The first three IMEDI studies, described in detail in Jones et al. (2014), provided the basis for the revision and adaptation of RBT+WC.
Usual Care (UC)
The UC condition provided information booklets on the same topics as in the RBT+WC condition paired with case management for 12 sessions involving referrals to all available services in the city for injection-drug-using women.
Participants
Participants were recruited via several sources. One source was the network of organizations delivering specialized substance abuse treatment and harm reduction services in Tbilisi, Georgia, and in several surrounding cities. A second source was the pool of women that used psychoactive substances who participated in interviews at the formative and pilot stages of this study. An additional source was through a Community Advisory Board (CAB) and a Beneficiary Advisory Board (BAB) established as part of the project. The CAB was chaired by the study’s principal investigator in Georgia, and comprised 11 professionals in women’s health and services that are provided to women who inject substances. It also served to identify and liaise with local organizations that might serve as places where potential study participants could be recruited. The BAB was established to solicit input from Georgian women who inject substances. In addition to providing input on all aspects of the study design and helping interpret the findings, the four women on the BAB identified local sites where potential participants could be recruited.
Study participants were offered an incentive in the amount of $12 USD as compensation for their time and effort to visit the research site and an additional $8 USD for providing a urine sample. Outreach workers were trained in the recruitment procedures and protocols, and the recruitment procedure was scripted in a manual. A field screening instrument was used to make the initial determination of eligibility and refer potential participants to the study field office for the final determination. The research site rented for this trial was located in the central residential district of the capital city Tbilisi and was easily accessible by public transportation. The research site offered a comfortable atmosphere to women by fostering a nonjudgmental and nonstigmatizing approach. This environment was created by a research team of young women—project director, research assistant, three consultants, and two recreational teachers—that were trained in advance by the US research team. At the field office, a research assistant screened potential participants to determine final eligibility. The research assistant then opened a sealed envelope that indicated the participant’s randomized assignment to a treatment condition. We used a block randomization procedure to assign each successive pair of participants to each of the treatment conditions.
Eligibility criteria to participate in the study included the following: conversant in Georgian; minimum 18 years of age; able to provide informed consent; injection of illicit substances in the past 30 days, as verified by venipuncture stigmata; and self-report of heterosexual activity at least once in the past 30 days.
Outcome Measures
Survey assessments were conducted at baseline, at the end of treatment (end of the scheduled treatment, regardless of whether or not the participant completed treatment), and 3-months following treatment entry (whether or not the participant completed treatment). Additionally, urine drug screening was conducted at each session. The ACON Laboratories test strip detected the presence of opiates (200 ng/mL cut-off level), buprenorphine (10 ng/mL cut-off level), methadone (200 ng/mL cut-off level), cannabis (50 ng/mL cut-off level), amphetamines and methamphetamines (300 ng/mL cut-off level), and benzodiazepines (100 ng/mL cut-off level). A breathalyzer reading for alcohol also was collected at the same time as collection of a urine sample.
Statistical Analysis
The study design was a 2 (Treatment Condition: RBT+WC vs. Usual Care) × 3 (assessment Time point: baseline vs. post-treatment vs. 3-month follow-up). Effects of interest were the main effects of Treatment Condition and Time, and their interaction. The 7 outcome measures were each binary variables assumed to follow a binomial distribution. Because all outcomes were measured repeatedly, all effects were tested using a Generalized Estimating Equations (GEE) approach. Statistical analyses were conducted with SAS 9.3 (SAS, 2011).
RESULTS
Out of 173 women screened, 128 women met the eligibility criteria and were assigned to one of the two treatment conditions, and 113 women completed treatment. Data collection took place between January 2013 and July 2014. A CONSORT diagram providing a study flow chart is presented in Figure 1.
Figure 1.
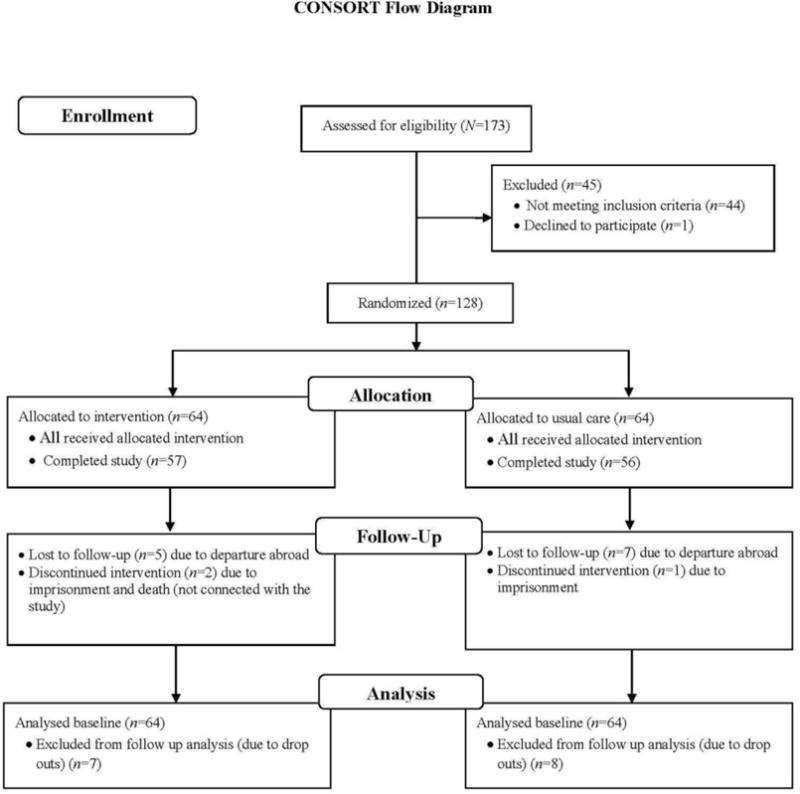
Participants’ Background Characteristics
Participants’ detailed baseline characteristics have appeared elsewhere (Jones et al., 2015) and are only summarized briefly here. A majority (89%) of women were Georgian. Age ranged from 20 to 64 years, with a mean of 41.7 (SD=9.8) years. Approximately half of participants (52.4%) had 16 or more years of education (i.e., completed university or higher education). About one-third of participants were married. A majority of participants were unemployed (82%), and 52 women (40.6%) responded that they live with someone who was using psychoactive substances, including marijuana or alcohol. Table 1 summarizes participants’ baseline substance use history and risk behavior history.
Table 1.
Baseline Substance Use History and Risk Behavior History for the Study Entry Sample, the Samples that Completed and Failed to Complete Treatment, and Reinforcement-based Treatment + Women’s Coop (RBT+WC) and Usual Care (UC) Samples at Baseline (N=128)
| Study entry Sample (N=128) | Completed Treatment Sample (n=113) | Failed to Complete Treatment Sample (n=15) | Test of Treatment Completion Status | RBT+WC Sample (n=64) | UC Sample (n=64) | Test of Treatment Condition | |
|---|---|---|---|---|---|---|---|
| Variable | M (SD) or n (%) | p | M (SD) or n (%) | P | |||
| Age of first use: | |||||||
| Tobacco (n=125) | 18.3 (5.2) | 18.4 (5.3) | 17.5 (4.1) | 0.515 | 18.0 (5.1) | 18.6 (5.3) | 0.549 |
| Alcohol (n=123) | 16.9 (3.2) | 16.9 (3.2) | 16.3 (3.3) | 0.509 | 16.8 (3.6) | 16.9 (2.9) | 0.928 |
| First substance use excluding tobacco and alcohol | 21.4 (6.2) | 21.5 (6.4) | 20.6 (5.1) | 0.589 | 20.6 (5.8) | 22.2 (6.6) | 0.141 |
| First injection (n=127) | 26.3 (8.1) | 26.6 (8.3) | 24.3 (6.6) | 0.295 | 26.6 (8.5) | 26.0 (7.7) | 0.697 |
| Assistance with first injection: | |||||||
| Alone | 11 (8.6%) | 10 (8.9%) | 1 (6.7%) | 6 (9.4%) | 5 (7.8%) | ||
| Main sex partner | 26 (20.3%) | 24 (21.2%) | 2 (13.3%) | 12 (18.8%) | 14 (21.9%) | ||
| Secret sex partner | 3 (2.3%) | 3 (2.7%) | 0 | 3 (4.7%) | 0 | ||
| Family member | 1 (0.8%) | 1 (0.9%) | 0 | 1 (1.6%) | 0 | ||
| Friend | 75 (58.6%) | 63 (55.8%) | 12 (80.0%) | 35 (54.7%) | 40 (62.5%) | ||
| Past-30-day amount of money spent on drugs | 417.7 (892.7) | 328.0 (534.5) | 1081.0 (2082.2) | 0.002 | 554.1 (1202.7) | 276.9 (315.4) | 0.081 |
| Lifetime needle/syringe sharing: yes | 43 (33.6%) | 36 (31.9) | 7 (46.7%) | † | 20 (31.3%) | 23 (35.9%) | 0.708 |
| Past-30-day number of times sharing needles/syringes | 1.0 (8.9) | 1.1 (9.4) | 0.4 (1.5) | 0.777 | .3 (1.3) | 1.7 (12.5) | 0.362 |
| Past-30-day number of times sharing other equipment | 1.0 (4.1) | 1.0 (4.3) | 1.1 (2.8) | 0.948 | 1.6 (5.6) | 0.4 (1.7) | 0.098 |
| Source of needles/syringes: | † | † | |||||
| Harm reduction program | 2 (1.6%) | 1 (0.9%) | 1 (6.7%) | 0 | 2 (3.1%) | ||
| Pharmacy | 118 (92.2%) | 105 (92.9%) | 13 (86.7%) | 61 (95.3%) | 57 (89.1%) | ||
| Main sexual partner | 5 (3.9%) | 4 (3.5%) | 1 (6.7%) | 2 (3.1%) | 3 (4.7%) | ||
| Friends | 1 (0.8%) | 1 (0.9%) | 0 | 0 | 1 (1.6%) | ||
| Other | 2 (1.6%) | 2 (1.8%) | 0 | 1 (1.6%) | 1 (1.6%) | ||
| Ever injected from preloaded (by someone else) syringe: yes | 94 (73.4%) | 82 (72.6%) | 12 (80.0%) | † | 47 (73.4%) | 47 (73.4%) | 1 |
| Lifetime number of overdose episodes | 1.4 (2.6) | 1.4 (2.7) | 1.2 (1.6) | 0.724 | 1.2 (1.8) | 1.6 (3.2) | 0.339 |
| Perceived treatment need: yes | 21 (16.4%) | 19 (16.8%) | 2 (13.3%) | † | 8 (12.5%) | 13 (20.3%) | 0.34 |
Note: Test of Treatment Completion Status compares the 113 participants who completed treatment to the 15 participants who failed to complete treatment at baseline. Test of Treatment Conditions compares the 64 participants in the RBT+WC condition to the 64 participants in the UC condition at baseline. Amount of money spent on drugs has been converted into $USD.
indicates that the expected cell count was less than 5 in one or more cells.
There were no statistically significant differences in demographic characteristics between study completers and dropouts and between the RBT+UC and UC conditions. Mean age of first psychoactive substance use (excluding tobacco and alcohol) was 21.4 years (SD=6.2) and age of first injection was 26.3 years (SD=8.1). In a majority of cases (n=75; 58.6%) the first injection occurred in the presence of (and with assistance from) a friend. Of the sample of 128 women, 112 (87%) had most commonly injected opioids during the past 30 days (mainly desomorphine, nicknamed “crocodile”), followed by homemade stimulants (n=59; 46%). Lifetime rates of attempt to quit tobacco smoking and heroin injection are quite similar, 40 (31%) and 39 (33.3%), respectively. For the vast majority of participants (92.2%), the main source of needles and syringes were pharmacies. Past-30-day sharing of needles and injection paraphernalia rarely occurred in the sample (M=1; SD=8.9). However, 73.4% reported ever injecting from a preloaded syringe. Only 8 (6%) women had ever been in substance use treatment yet 16.4% of the sample indicated they were in need of substance-use-related treatment.
Substance Use Outcomes
Table 2 presents the results of the analyses of the seven main psychoactive substance use outcomes.
Table 2.
Test Statistics, p Values, and Means (Standard Errors) from Intent-to-treat Inferential Analyses for Substance Use Outcome Measures (N=128)
| Treatment Condition |
Time | Treatment Condition × Time | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RBT | Usual Care | ||||||||||||||||
| R B T |
Us ua l Ca re |
Bas elin e |
Post- Treat ment |
3- mo nth Foll ow- up |
Bas elin e |
Post- Trea tmen t |
3- mo nth Foll ow- up |
Bas elin e |
Post- Trea tmen t |
3- mo nth Foll ow- up |
|||||||
| χ 2 |
p | Mean (SE) |
χ2 | p | Mean (SE) | χ 2 |
p | Mean (SE) | |||||||||
| Urine drug screening test result for buprenorphine: yes | |||||||||||||||||
| Urine drug screening test result for methadone: yes | |||||||||||||||||
| Urine drug screening test result for opioids: yes | 3.6 | 0.6 | 20 (.04) | .33 (.05) | 107 | <.001 | .67 (.04) | .18 (.04) | .09 (.03) | .9 | .65 | .63 (06) | .15 (.05) | .05 (.03) | .72 (.06) | .21 (05) | .14 (.05) |
| Urine drug screening test result for benzodiazepines : yes | .0 | .93 | .29 (.05) | .29 (.04) | 22.4 | <.001 | .46 (.04) | .22 (.04) | .22 (.04) | 1.3 | .52 | .47 (.06) | .20 (.05) | .25 (.06) | .45 (.06) | .25 (.06) | .20 (.05) |
| Urine drug screening test result for amphetamines: yes | .8 | .39 | .10 (.03) | .13 (.03) | 29.6 | <.001 | .28 (.04) | .06 (.02) | .08 (.03) | 1.7 | .44 | .30 (.06) | .04 (.03) | .07 (.04) | .27 (.06) | .09 (.04) | .09 (.04) |
| Urine drug screening test result for cannabis : yes | 2.3 | .13 | .06 (.02) | .12 (.03) | 4.4 | .11 | .12 (.03) | .04 (.02) | .11 (.03) | 2.7 | .26 | .11 (.04) | .02 (.02) | .11 (.04) | .14 (.04) | .11 (.04) | .13 (.04) |
| Breathalyzer test result for alcohol : yes | .1 | .74 | .04 (.02) | .05 (.02) | 2.7 | .26 | .04 (.02) | .03 (.02) | .08 (.03) | 2.5 | 2.9 | .06 (.03) | .02 (.02) | .05 (.03) | .03 (.02) | .04 (.02) | .11 (.04) |
Notes. For the Treatment Condition main effect, df = 1; for the Time main effect df = 2; for the Treatment Condition × Time, df = 2. Analyses for buprenorphine and methadone failed to converge due to cells with 0 values.
The analyses of buprenorphine use and methadone use failed to converge, because the nonprescribed use of buprenorphine and methadone declined precipitously over the course of treatment in both conditions, resulting in some conditions with 0 values. Buprenorphine use decreased from 18% use (with 20.3% use in the RBT+WC condition and 15.6% use in the UC condition) at baseline to 3.6% use (with 0% use in the RBT+WC condition) at treatment completion to .9% (with the only use by 1 participant in the UC condition) at 3-month follow up. This pattern was even more pronounced for methadone, with methadone use at baseline of 32.8% (with 31.3% use in the RBT+WC and 34.4% use in the UC condition), decreasing to 3.6% use (with 0% use in the RBT+WC condition) at treatment completion and 3.5% use at 3-month follow-up.
For the remaining 5 outcomes, neither the Treatment Condition main effect nor the Treatment Condition × Time interaction effect was significant. The Time main effect was significant for opioids, benzodiazepines, and amphetamines/methamphetamines. Post hoc simple mean comparisons of the means, shown in Table 2, indicated that the decrease from baseline to treatment completion was significant for these 3 outcomes; however, the change from treatment completion to 3-month follow-up was nonsignificant.
DISCUSSION
This study was the first randomized controlled trial examining a culturally sensitive women-centered comprehensive intervention designed for Georgian women who use substances. However, conducting this study presented certain challenges. As previously reported (Jones et al., 2014), in contrast to research in the United States, this type of research in Georgia is a relatively new phenomenon. As a result, after being study participants, Georgian women that used substances whom we recruited into our studies have often been highly motivated to support our research and be part of data collection. This level of interest and support may have been a factor in our ability to initially recruit participants into the present study and to retain them in treatment at a relatively high level, and may have helped in collection of follow-up data. Moreover, this motivation to support the conduct of our research may have had the unintended, nonspecific effect of motivating participants in both conditions toward improvement, obviating the impact of RBT+WC relative to UC.
A majority of participants reported a history of opioid use. On average, the first injection of a psychoactive substance among women occurs at age 25 or later, whereas men start injection use in their late teens (Curatio International Foundation & Bemoni Public Union, 2015). The mean age of first injection in the present sample is consistent with this previous research. Moreover, the sample reported almost no sharing of needles and syringes, and likewise no sharing of other injection paraphernalia. However, the lifetime incidence of the practice of injection with a preloaded syringe is high. Notably, the use of benzodiazepines occurred at a similar frequency to that of opioids. However, the use of both amphetamines/methamphetamines, marijuana, and, markedly, alcohol trailed the use of opioids and benzodiazepines.
Regarding the impact of treatment, there were reductions in the use of buprenorphine, methadone, opioids, benzodiazepines, and amphetamines/methamphetamines in the total sample from baseline to treatment completion 12 weeks following treatment entry. However, the changes from treatment completion to 3-month follow-up were negligible. Given the generally positive impact of treatment, the fact that there was no change in substance use from treatment completion to 3-month follow-up suggests that the impact of treatment, both RBT+WC and UC, might be enduring. Conversely, there were no significant differential changes between the RBT+WC and UC conditions over the course of the study, as none of the 5 Treatment Condition × Time interactions were significant. Nor were there statistically significant differences between RBT+WC and UC on any of the 5 outcomes. The findings for the other two outcomes, buprenorphine use and methadone use, suggest a pattern of change similar to that of these 5 outcomes.
There are several limitations to the present study. First, it is possible that the sample of women recruited to participate in the study may not have been representative of Georgian women who inject substances, given that some form of random or systematic sampling was not used to select the sample. However, the number of women who inject substances who are either in substance use treatment or seeking substance use treatment in Georgia is so small (Kirtadze et al., 2015) that recruiting a sample of such women would not be possible. Consequently, our ability to generalize the findings to Georgian women who inject substances is limited by our recruitment strategy and the resulting sample. Second, given that the goal of the IMEDI project was to develop a culturally sensitive, comprehensive, women-centered intervention for Georgian women who inject substances, the sample size in this study was relatively small, as a future goal is to use the initial feasibility and efficacy information derived from the four studies conducted as part of the IMEDI project to further revise the intervention and then conduct a larger scale trial. The small sample size limited our ability to detect small effect sizes associated with the intervention. Third, the UC condition, considered to be a control condition, may have contained active treatment components such that the differences between the RBT+WC condition and UC condition were not as large as anticipated, thereby limiting our ability to detect an effect. However, ethical standards required us to offer some form of treatment to participants in the control condition. Fourth, although the development of the RBT+WC intervention was based on the results of three previous IMEDI studies, and was specifically designed to meet the needs of Georgian women who inject substances, the RBT+WC intervention may not have been suitably customized for this population.
In summary, study findings suggest that RBT+WC is a promising treatment for reducing substance use in Georgian women who inject substances. The fact that the RBT+WC did not differentially impact outcomes relative to UC requires further investigation. This line of research would need two foci. The first focus would be on determining whether it would be necessary to refine the RBT+WC to better address the needs of Georgian women who inject substances. The second focus would be on comparing the RBT+WC to other active treatment conditions in large samples to establish the extent to which RBT+WC addresses the unique treatment needs of Georgian women who inject substances.
Acknowledgments
The authors thank IMEDI project staff for making the study possible, and study participants for their valuable time and effort.
Financial Support: NIDA R01 DA029880 (Hendrée E. Jones, PI).
Financial Disclosures:
Supported by National Institute on Drug Abuse (NIDA) grant R01 DA________ (_________, PI). NIDA played no role in the study design; the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit or where to submit the article for publication.
Footnotes
Conflicts of Interest:
All authors declare no competing interests related to this article.
References
- Curatio International Foundation & Bemoni Public Union. Bio-Behavioral Surveillance Surveys Among People Who Injecting Drug Users in 7 Cities of Georgia, (December), 2008–2009. 2015 Retrieved from http://curatiofoundation.org/wp-content/uploads/2009/09/229.pdf.
- Georgia Infectious Diseases AIDS and Clinical Immunology Research Center. HIV/AIDS epidemiology in Georgia. 2016 Retrieved 24 November, 2016, from http://aidscenter.ge/epidsituation_eng.html.
- Javakhishvili D, Otiashvili D, Tabatadze M. Drug Situation in Georgia 2014. Tbilisi: 2016. [Google Scholar]
- Jones HE, Kirtadze I, Otiashvili D, Murphy K, O’Grady KE, Zule W, Wechsberg WM. Feasibility and initial efficacy of a culturally sensitive women-centered substance use intervention in Georgia: Sex risk outcomes. Subst Abuse Treat Prev Policy. 2015;10:47. doi: 10.1186/s13011-015-0043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE, Kirtadze I, Otiashvili D, Murphy K, O’Grady KE, Zule W, Wechsberg WM. Feasibility and initial efficacy of a culturally sensitive women-centered substance use intervention in Georgia: Sex risk outcomes. Substance Abuse Treatment, Prevention, and Policy. 2015;10(1):1–15. doi: 10.1186/s13011-015-0043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE, Kirtadze I, Otiashvili D, O’Grady KE, Murphy K, Zule W, Wechsberg WM. Process and product in cross-cultural treatment research: development of a culturally sensitive women-centered substance use intervention in georgia. J Addict. 2014;2014:163603. doi: 10.1155/2014/163603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE, Kirtadze I, Otiashvili D, O’Grady KE, Murphy K, Zule W, Wechsberg WM. Process and product in cross-cultural treatment research: development of a culturally sensitive women-centered substance use intervention in Georgia. J Addict, Article ID 163603. 2014 doi: 10.1155/2014/163603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirtadze I, Otiashvili D, O’Grady K, Zule W, Krupitsky E, Wechsberg W, Jones H. Women Who Inject Drugs in the Republic of Georgia: In Their Own Words. Journal of Psychoactive Drugs. 2015;47(1):71–79. doi: 10.1080/02791072.2014.990174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirtadze I, Otiashvili D, O’Grady K, Zule W, Krupitsky E, Wechsberg W, Jones HE. Women who inject drugs in the republic of Georgia: in their own words. J Psychoactive Drugs. 2015;47(1):71–79. doi: 10.1080/02791072.2014.990174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirtadze I, Otiashvili D, O’Grady KE, Zule WA, Krupitskii EM, Wechsberg WM, Jones HE. Twice stigmatized: Health service provider’s perspectives on drug-using women in the Republic of Georgia. Journal of Psychoactive Drugs. 2013;45(1):1–9. doi: 10.1080/02791072.2013.763554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Disease Control and Public Health. Georgia UNGASS Progress Report, Reporting Period January – December, 2014. Tbilisi, Georgia: 2015. [Google Scholar]
- Otiashvili D, Kirtadze I, O’Grady KE, Zule W, Krupitsky E, Wechsberg WM, Jones HE. Access to treatment for substance-using women in the Republic of Georgia: socio-cultural and structural barriers. Int J Drug Policy. 2013;24(6):566–572. doi: 10.1016/j.drugpo.2013.05.004. doi: S0955-3959(13)00078-9 [pii] 10.1016/j.drugpo.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS. User’s Guide. SAS Institute Inc; Cary, NC: 2011. SAS/STAT 9.3 User’s Guide. [Google Scholar]
- SAS. User’s Guide. SAS Institute Inc; Cary, NC: 2011. SAS/STAT 9.3 User’s Guide. [Google Scholar]
- Tuten LM, Jones HE, Schaffer CM, Stitzer M. Reinforcement-Based Treatment for Substance Use Disorders: A Comprehensive Behavioral Approach. Washington, DC: American Psychological Association; 2011. [Google Scholar]
- Wechsberg WM. Facilitating empowerment for women substance abusers at risk for HIV. Pharmacology Biochemistry and Behavior. 1998;61(1):158. [Google Scholar]
- Wechsberg WM, Krupitsky E, Romanova T, Zvartau E, Kline TL, Browne FA, Jones HE. Double jeopardy - drug and sex risks among Russian women who inject drugs: initial feasibility and efficacy results of a small randomized controlled trial. Subst Abuse Treat Prev Policy. 2012;7:1. doi: 10.1186/1747-597X-7-1. doi: 1747-597X-7-1 [pii] 10.1186/1747-597X-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsberg WM, Luseno WK. The need for culturally appropriate, gender-specific global HIV prevention efforts with vulnerable women. J Prev Interv Community. 2010;38(2):85–88. doi: 10.1080/10852351003640559. doi: 921001334 [pii] 10.1080/10852351003640559. [DOI] [PubMed] [Google Scholar]
- Wechsberg WM, Luseno WK, Kline TL, Browne FA, Zule WA. Preliminary findings of an adapted evidence-based woman-focused HIV intervention on condom use and negotiation among at-risk women in Pretoria, South Africa. J Prev Interv Community. 2010;38(2):132–146. doi: 10.1080/10852351003640799. doi: 921001349 [pii] 10.1080/10852351003640799. [DOI] [PMC free article] [PubMed] [Google Scholar]


