Abstract
Returning veterans often face multiple concurrent psychiatric and behavioral conditions that negatively impact reintegration into civilian life and are associated with functional disability. Understanding how conditions interact to negatively impact functioning is an important step toward developing holistic treatment approaches that are optimized for this population. This study utilized a cross-sectional and prospective longitudinal cohort design applying regression algorithms to understand the relative contribution of common clinical issues to functional disability in U.S. veterans who served after the September 11, 2001 (9/11) terror attacks. Community-dwelling post-9/11 veterans (N = 397) completed detailed assessments, including common clinical condition diagnoses, combat experience, and demographics, which were used to predict functional disability (World Health Organization Disability Assessment Schedule); 205 participants were reassessed approximately 1–2 years after enrollment. Regression analyses showed a strong association between the predictor variables and functional disability, f 2 = 1.488. Validation analyses showed high prediction ability of functional disability both to independent samples, r = .719, and across time in the same individuals, r = .780. The strongest predictors included current posttraumatic stress disorder, depressive disorder, sleep disturbance, and pain diagnoses. These results demonstrate the importance of considering multiple common co-occurring conditions when assessing functional disability in post-9/11 veterans, and suggest that certain syndromes contribute the most unique information for predicting functional disability with high confidence. As most U.S. veterans utilize private healthcare systems, these results have clinical utility for both Veterans Affairs and civilian healthcare practitioners in assessing and monitoring functional disability in post-9/11 veterans over time.
A substantial percentage of U.S. veterans who served in the recent conflicts after the September 11, 2001 [9/11] terror attacks (e.g., Afghanistan and Iraq) were exposed to physical and psychological trauma while deployed and present to primary care clinics with multiple psychiatric, behavioral, and/or neurological conditions that interfere with successful reintegration into civilian life. In addition to the two signature injuries of these wars—traumatic brain injury (TBI) and posttraumatic stress disorder (PTSD; Atkinson, Guetz, & Wein, 2009; Hoge, et al., 2004; Terrio, et al., 2009), numerous studies have identified common comorbidities that often accompany PTSD and TBI, such as chronic pain and sleep disturbances, as well as additional psychiatric conditions, such as depressive, anxiety, and substance use disorders (Amick, et al., 2018; Beckham, et al., 1997; Bleich, Koslowsky, Dolev, & Lerer, 1997; McGlinchey, Milberg, Fonda, & Fortier, 2017; Thomas, et al., 2010; Walker, Clark, & Sanders, 2010). These comorbid diagnoses likely interact with concurrent diagnoses of PTSD or TBI to further impair cognitive, social, and functional outcomes both during reintegration from military to civilian cultures and well into the future (Amick, et al., 2018; Fonda, et al., 2017; Lew, et al., 2008; Schult, 2011). Previous work has shown that the totality of these complex clinical presentations may be larger than the sum of symptoms or features associated with each individual diagnosis (Amick, et al., 2018; Lew, et al., 2008; Lippa, et al., 2015; Walker, et al., 2010).
Often, clinical assessment and treatment focuses on individual conditions (e.g., PTSD). However, this approach may not be appropriate in complex clinical populations in which there are multiple concurrent clinical factors in play. Rather than attending to a single diagnosis, it has been proposed that attention to commonly occurring comorbid conditions among this veteran cohort provides a more holistic understanding of the underlying causes of reintegration difficulty and overall functional disability over time (Amick, et al., 2013; Amick, et al., 2018; Fortier, et al., 2018; Lippa, et al., 2015; McGlinchey, et al., 2017). Further, it has been shown that in addition to their relatively high co-occurrence, at least six comorbid conditions are associated with increases in self-reported functional disability (Lippa, et al., 2015). These include mild TBI (mTBI), PTSD, depressive disorder, anxiety disorder, current pain, and sleep disturbances. Although findings reported to date have noted significant associations among these conditions, there remains a need to determine the extent to which associative relations with these clinical issues are replicable and predictive of outcomes. Additionally, there is a need for longitudinal assessments to determine the temporal stability of these associations to strengthen confidence in findings that influence the development of future therapeutic interventions.
The present study sought to extend and validate recent findings regarding the relative influence of co-occurring clinical disorders on overall functional disability by leveraging a large cohort of post-9/11veterans that includes longitudinal assessments over a 1–2-year time period. First, we assessed the ability of the multivariable regression model proposed by Lippa et al., (2015), which utilizes diagnostic statuses readily available in most clinical settings, to predict functional disability in novel participant samples and within the same participants over time. Second, using a novel hierarchical cross-validation algorithm, we tested the hypothesis that combining information across multiple diagnoses provides better predictive ability of functional disability than individual diagnoses alone and aimed to determine which subsets of diagnoses would optimize prediction ability within the cohort tested.
Method
Participants and Procedure
Participants were community-dwelling post-9/11 veterans enrolled in the longitudinal cohort study conducted by the Translational Research Center for TBI and Stress Disorders (TRACTS). This ongoing longitudinal cohort study employs a convenience sample, with participants self-selected based on interest in the study. Participants were not selected a priori for any specific clinical diagnoses. Thus, this sample also includes a subset of healthy veterans with no clinical issues and is demographically representative of the post-9/11 veteran population who utilize the Veterans Affairs (VA) Health Care system (Lippa, et al., 2015). However, the sample does show higher rates of TBI and mental health issues than the larger post-9/11 veteran population. At the time of this study, the full TRACTS cohort included 475 consecutively run veterans who met the major inclusion and exclusion criteria. All participants recruited to TRACTS complete comprehensive psychiatric and neuropsychological assessments (McGlinchey, et al., 2017). Diagnostic interviews were completed by doctoral-level psychologists, with cases reviewed by at least three doctoral-level psychologists or psychiatrists to achieve consensus. Inclusion criteria for the TRACTS cohort was that participants were deployed or to-be-deployed post-9/11 service members between 18 and 65 years of age. General exclusion criteria included prior seizure disorders, cognitive disorder due to general medical conditions, and/or neurological illness unrelated to TBI; active suicidal and/or homicidal ideation requiring intervention; or a current diagnosis of bipolar disorder or psychotic disorder unrelated to PTSD. Following the methodology outlined by Lippa et al. (2015), participants were additionally excluded if they had not been deployed to a combat zone (n = 22), in order to focus on deployment-related trauma, and/or had a history of moderate or severe TBI (n = 16). Four additional participants were excluded due to non–combat-related factors that could significantly impact cognitive (n = 1), psychiatric (n = 1), or neurological (n = 2) function, such as low premorbid IQ, possible psychotic or personality disorder diagnoses, and congenital neurological irregularities, respectively. Participants were then excluded for missing data on the functional disability questionnaire used as our dependent variable (n = 26) or any clinical assessments used as independent variables outlined herein (n = 10). We note that rerunning all analyses including the 10 participants with missing clinical assessments, using 10-fold multiple imputation with pooled estimates, did not alter any of the results outlined later and are thus not reported. Table 1 shows demographic information for the remaining 397 participants. From this sample, 205 participants returned for follow-up reassessment 1–2 years following their enrollment (i.e., baseline) assessment. This study was approved by the VA Boston Healthcare System internal review board, and all participants completed written informed consent before testing.
Table 1.
Demographic, Current Psychiatric, and Behavioral Status for Participants Who Completed Initial Enrollment and Follow-Up Assessments
| Initial Assessment (n = 397) | Follow-Up Assessment (n = 205) | Statistical Test | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | M | SD | n | % | M | SD | ||
| Variable | |||||||||
| Age (years) | 32.34 | 8.58 | 34.21 | 8.58 | t(600) = −2.546* | ||||
| Male gender | 364 | 91.7 | 182 | 88.8 | χ2(1, n = 205) =1.347 | ||||
| Education (years) | 14.0 | 1.96 | 14.38 | 2.22 | t(600) = −2.140* | ||||
| Race/ethnicity | |||||||||
| White | 297 | 74.8 | 150 | 73.2 | χ2(1, n = 205) = 0.181 | ||||
| Black | 29 | 7.3 | 15 | 7.3 | χ2(1, n = 205) = 0.000 | ||||
| Hispanic | 58 | 14.6 | 33 | 16.1 | χ2(1, n = 205) = 0.237 | ||||
| Other | 7 | 1.8 | 3 | 1.5 | χ2(1, n = 205) = 0.073 | ||||
| Unknown/missing | 6 | 1.5 | 4 | 2.0 | χ2(1, n = 205) = 0.205 | ||||
| Service branch | |||||||||
| Army | 113 | 28.5 | 50 | 24.4 | χ2(1, n = 205) = 1.148 | ||||
| Navy | 14 | 3.5 | 7 | 3.4 | χ2(1, n = 205) = 0.004 | ||||
| Air Force | 16 | 4.0 | 7 | 3.4 | χ2(1, n = 205) = 0.133 | ||||
| Marines | 76 | 19.1 | 35 | 17.1 | χ2(1, n = 205) = 0.359 | ||||
| National Guard | 130 | 32.7 | 89 | 43.4 | χ2(1, n = 205) = 6.679** | ||||
| Reserves | 48 | 12.1 | 17 | 8.3 | χ2(1, n = 205) = 2.022 | ||||
| Deployments | |||||||||
| Number | 1.49 | 0.81 | 1.42 | 0.77 | t(600) = 1.012 | ||||
| Months | 14.36 | 8.55 | 14.26 | 9.01 | t(600) = 0.122 | ||||
| Months since last deployment | 42.27 | 33.71 | 61.18 | 38.59 | t(600) = −6.200*** | ||||
| Combat exposurea | 17.43 | 11.82 | 16.04 | 11.44 | t(600) = 1.380 | ||||
| N of military mTBIs | 0.84 | 1.59 | 0.76 | 1.51 | t(600) = 0.654 | ||||
| PTSD | 241 | 60.7 | 104 | 50.7 | χ2(1, n = 205) = 5.516* | ||||
| PTSD severity | 51.10 | 29.64 | 45.18 | 28.74 | t(600) = 2.347* | ||||
| ΔPTSD severityb | −3.51 | 20.77 | t(600) = −2.359* | ||||||
| Depressive disorders | 110 | 27.7 | 44 | 21.5 | χ2(1, n = 205) = 2.725 | ||||
| Major | 98 | 24.7 | 39 | 19.0 | χ2(1, n = 205) = 2.464 | ||||
| Other | 12 | 3.0 | 4 | 2.0 | χ2(1, n = 205) = 0.599 | ||||
| Anxiety disorders | 84 | 21.2 | 40 | 19.5 | χ2(1, n = 205) = 0.238 | ||||
| Panic | 36 | 9.1 | 15 | 7.3 | χ2(1, n = 205) = 0.535 | ||||
| Social phobia | 23 | 5.8 | 8 | 3.9 | χ2(1, n = 205) = 0.990 | ||||
| GAD | 34 | 8.6 | 16 | 7.8 | χ2(1, n = 205) = 0.102 | ||||
| Other | 23 | 5.8 | 17 | 8.3 | χ2(1, n = 205) = 1.361 | ||||
| Substance use disorder | 61 | 15.4 | 30 | 14.6 | χ2(1, n = 205) = 0.067 | ||||
| Alcohol | 53 | 13.4 | 21 | 10.3 | χ2(1, n = 205) = 1.210 | ||||
| Other | 13 | 3.3 | 12 | 5.9 | χ2(1, n = 205) = 2.259 | ||||
| Current pain | 283 | 71.3 | 149 | 72.7 | χ2(1, n = 205) = 0.131 | ||||
| 30-day averagec | 31.08 | 26.11 | 30.48 | 26.68 | t(600) = 0.263 | ||||
| Sleep disturbance | 315 | 79.3 | 161 | 78.5 | χ2(1, n = 205) = 0.052 | ||||
| Sleep qualityd | 10.09 | 4.75 | 9.56 | 4.35 | t(600) = 1.346 | ||||
| Number of psychiatric/behavioral conditions | 3.21 | 1.69 | 2.98 | 1.74 | t(600) = 1.589 | ||||
| ≥ 3 comorbidities | 255 | 64.2 | 121 | 59.0 | χ2(1, n = 205) = 1.556 | ||||
| NSI validitye | 15 | 3.8 | 5 | 2.4 | χ2(1, n = 205) = 0.755 | ||||
| Measured disabilityf | 18.84 | 17.23 | 17.38 | 17.23 | 0.31 | ||||
Note. GAD = generalized anxiety disorder; mTBI = mild traumatic brain injury; PTSD = posttraumatic stress disorder; NSI = Neurobehavioral Symptom Inventory.
Combat exposure was total score from the Deployment Risk and Resilience Inventory (DRRI) combat module.
Difference in PTSD severity scores, calculated by subtracting initial score from follow-up score; significance measured using one-sample t test.
30-day average pain severity from the Short Form McGill Pain Questionnaire.
Sleep quality represents the Pittsburgh Sleep Quality Index (PSQI) global score.
Validity-10 symptom exaggeration score derived from NSI (score ≥ 23).
Square root of World Health Organization Disability Assessment Schedule 2.0 overall score.
p < .05.
p < .01.
p < .001.
Measures
Demographic characteristics.
Demographic information on age, race/ethnicity, gender, and other variables was collected via a questionnaire administered at baseline and follow-up assessent.
Combat exposure.
Combat exposure was assessed using the Deployment Risk and Resilience Inventory (DRRI) Combat Experience subscale (King, King, Vogt, Knight, & Samper, 2006). The DRRI combat module is a 16-item self-report questionnaire that uses a Likert scale ranging from 0 (never) to 4 (daily or almost daily), with higher scores indicating a larger number of combat experiences. In the present sample, the Combat Experience total score ranged from 0 to 64, Cronbach’s α = .91. Evidence for the internal consistency reliability, criterion-related validity, discriminative validity, and test–retest reliability of the DRRI scales has been demonstrated (King, et al., 2006; Vogt, Proctor, King, King, & Vasterling, 2008).
Symptom validity.
The Neurobehavioral Symptom Inventory (NSI: Cicerone & Kalmar, 1995) is a 22-item self-report questionnaire that uses a Likert scale ranging from 0 (never) to 4 (very severe). In the current sample, the NSI total score ranged from 0 to 88, Cronbach’s α = .95. The Validity-10 Scale (Vanderploeg, et al., 2014), which is embedded in the NSI, was used to examine possible symptom overreporting or exaggeration on self-report measures. Validity-10 scores can range from 0 to 40, and a cutoff score of 23 or higher was used to classify groups (Lange, Brickell, Lippa, & French, 2015).
Traumatic brain injury.
The Boston Assessment of TBI–Lifetime (BAT-L: Fortier, et al., 2014) was used to assess participants’ history of TBI that occurred pre-deployment, during deployment, and post-deployment. A history of military mTBI was defined as a period of self-reported loss of consciousness that lasted less than 30 minutes, and/or posttraumatic amnesia that lasted less than 24 hours, and/or altered mental status that lasted less than 24 hours following a credible injury mechanism that occurred during military service (U.S. Department of Veterans Affairs and U.S. Department of Defence, 2009).
PTSD.
To evaluate the presence, history, and severity of PTSD, participants were assessed using the Clinician-Administered PTSD Scale (CAPS: Blake, et al., 1990) based on criteria from the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; Weathers, Ruscio, & Keane, 1999). Interrater reliability for the CAPS-IV was examined in 5% of the TRACTS cohort. Interrater reliabilities were strong (range κ = 0.687 to 0.691), for diagnoses for each time epoch (current and post-deployment) as compared to established CAPS-IV interrater reliability (i.e., κ = 0.58; Blake, et al., 1995). In our sample, CAPS-IV total scores ranged from 0 to 125, Cronbach’s α = .96.
Psychiatric disorders.
The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID: First, Spitzer, Gibbon, & Williams, 1996; Lobbestael, Leurgans, & Arntz, 2011; Williams, et al., 1992), nonpatient edition, was used to assess current depressive, anxiety, and substance abuse disorders. Note that we refer to the assessment of Axis 1 mood disorders as depressive disorders in this sample as it includes major depressive disorder, dysthymic disorder, substance-induced depressive disorder, or depressive disorder not otherwise specified, but not bipolar disorder, which is an exclusionary criterion for the TRACTS sample.
Sleep quality.
The Pittsburgh Sleep Quality Index (PSQI: Carpenter & Andrykowski, 1998) was administered, and, following the format outlined by Lippa et al., (2015), a global cutoff score of 6 or higher was used to define the presence of sleep disturbance. In the present sample, PSQI global scores ranged from 0 to 20 with higher values indicating poorer sleep, Cronbach’s α = .79.
Pain.
Using the Short Form McGill Pain Questionnaire (SFMPQ: Grafton, Foster, & Wright, 2005; Melzack, 1987), participants reported current overall pain levels (ordinal, with response options ranging from none to excruciating) and 30-day average pain severity (range: 0–100, with higher scores indicating a higher level of pain). Participants who self-reported current overall pain levels as mild or higher were classified as having current pain.
Functional disability.
The World Health Organization Disability Assessment Schedule 2.0 (WHODAS: World Health Organization, 2010) was used to measure self-reported functional disability. The WHODAS-2 is a 36-item self-report questionnaire that uses a Likert scale ranging from 0 (never) to 4 (extreme/cannot do). It has been shown to have high internal consistency (Cronbach’s α = 0.86), stable factor structure, and high test–retest reliability (intraclass correlation coefficient = 0.98; (Üstün, et al., 2010). Within this 36-item questionnaire, there are six subscales: Understanding and Communicating, Getting Around, Self-Care, Getting Along with People, Life Activities, and Participation in Society. Overall and subdomain scores range from 0 to 100, with higher scores indicating worse functioning. Each item contributes to the respective subdomain score equally, and each subdomain score is divided by the total possible points. Each subdomain score contributes equally to the overall score. In our sample, the WHODAS overall total ranged from 0 to 75, Cronbach’s α = .97. For all analyses, the square root of the overall WHODAS and subscale scores was used to normalize the positively skewed distribution (normalized range: 0–10). The normalized overall WHODAS score is subsequently referred to as the measured disability score.
Data Analysis
Descriptive statistics for demographics and clinical characteristics during initial enrollment and the follow-up testing sessions were calculated. Independent sample t tests and chi-square tests were calculated using SPSS (Version 24) to test for sample differences across the two testing periods. The main analyses used the Statistics Toolbox and custom scripts written in Matlab (MathWorks Inc., Natick, MA) to examine the degree to which clinical diagnoses were related to disability. TRACTS employs periodic dataset releases, with approximately 50 newly enrolled participants included in each release. Utilizing this, we separated the initial enrollment data (N = 397) into our model development group (N = 355), including all participants from the December 2015 data release, and a novel enrollment validation dataset (N = 42) that included all newly enrolled participants in the February 2017 data release. For the initial model development, a multiple linear regression analysis was performed on the 2015 data release (N = 355). Following the method described by Lippa et al. (2015), 12 independent predictors were used to model the participant-reported disability. These predictors included general demographic information (gender, age, years of education), the DRRI combat exposure score, and diagnoses (yes or no) for potential symptom exaggeration (NSI), military mTBI, current PTSD, current sleep disturbance and pain, and current depressive, anxiety, and substance disorders.
Model validation in novel groups.
Even with low parameter numbers relative to observations, regression models can overfit data for a number of reasons (Arlot & Celisse, 2010; Babyak, 2004; Hawkins, 2004). Ultimately, for a model to be of clinical relevance, it should be able to predict performance in independent datasets. To assess the quality and prediction ability of the initial regression model, the beta weights from the regression model on the 2015 dataset were used to predict reported disability in two novel samples of participants. First, the beta weights were applied to the measured data in the 42 novel participants from the 2017 dataset to predict their measured disability scores. The same process was then used to predict the measured disability scores for the 205 participants in the longitudinal follow-up dataset. This process allowed us to assess the validity of the model in predicting functional disability in unique participants and to track functional disability within individuals over time.
Full cross-validation modeling approach.
As the TRACTS cohort may have varied systematically over time, a full 10-fold cross-validation approach was also used to model the data. Here, all 397 initial enrollment participants were randomly assigned to one of 10 groups. In each iteration, one dataset was left out and a regression model was fit to the 9 remaining training datasets. The resulting beta weights were then applied to the participants in the left-out test dataset. After all 10 iterations, the quality of the regression fit was assessed by correlating the measured versus predicted disability scores across participants, using Pearson r. The average beta weights across the 10 iterations were then used to predict reported disability in the follow-up testing dataset. For this approach, we first used the overall measured disability score as the dependent variable and then repeated the analysis using each of the six WHODAS subscale scores.
Relative contributions of individual conditions to predicted disability.
Since assessing all predictors may not be feasible in clinical settings, our final hierarchical cross-validation analysis assessed the extent to which individual diagnoses contribute to overall predictive ability to determine whether reduced models can provide sufficient prediction ability. First, the hierarchical order in which variables would be added was determined from the percent of variance in disability explained by each of the 12 independent variables separately in the initial enrollment dataset. Thus, the order was not defined in a fully independent manner for the enrollment dataset but is independent when the model was applied to the follow-up dataset. Second, in each of 12 iterative steps, the full 10-fold cross-validation approach outlined earlier was used to calculate the correlation between observed and predicted disability scores for both the initial enrollment and follow-up datasets. The percent of variance explained (R2) was then calculated from the Pearson’s r. In each iterative step, a new predictor variable was added to the set of predictors, and the procedure was repeated. The order in which parameters were added to the model began with the independent variable that explained the largest proportion of variance independently (PTSD) and proceeded to the variable that explained the least proportion of variance independently (gender).
Random permutation testing approaches, such as Monte-Carlo permutations, are appropriate when the number of possible permutations is large. Given the limited number of potential permutations in our dataset, which contained only 12 potential features, we instead used exact permutation testing, where the expected R2 value is calculated from all possible permutations at each level in the hierarchy (Table 4), to assess the quality of our model statistically (Berry, Mielke Jr, & Mielke, 2002). The null distribution here assumes that the selected model performs no better than the other possible permutations of n randomly selected variables from the 12 potential parameters. For example, for the level of the regression model that includes three parameters (current PTSD, depressive disorders, and pain), the variance explained by the a priori model was assessed relative to all possible three-parameter models. This addresses whether the selected three-parameter model provides more information than all other possible combinations of three diagnoses (e.g., current PTSD, military mTBI, and current substance use disorder).
Table 4.
Possible Combinations and Results of Exact Permutation Testing
| Parameters in Model | Unique Combinations | Minimum p value | Initial Enrollment Observed p value | No. Equal or Higher | Follow-Up Observed p value | No. Equal or Higher |
|---|---|---|---|---|---|---|
| 1 | 12 | .083 | .083 | 1 | < .083 | 0 |
| 2 | 66 | .015 | .015 | 1 | < .015 | 0 |
| 220 | .005 | .005 | 1 | < .005 | 0 | |
| 4 | 495 | .002 | .004 | 2 | < .002 | 0 |
| 5 | 792 | .001 | .004 | 3 | < .001 | 0 |
| 6 | 926 | .001 | .001 | 1 | < .001 | 0 |
| 7 | 792 | .001 | .009 | 7 | < .001 | 0 |
| 8 | 495 | .002 | .004 | 2 | < .002 | 0 |
| 9 | 220 | .005 | .032 | 7 | < .005 | 0 |
| 10 | 66 | .015 | .015 | 1 | < .015 | 0 |
| 11 | 12 | .083 | .167 | 2 | < .083 | 0 |
| 12 | 1 | 1 |
As with our a priori model, for each null distribution comparative model, the variance explained (R2) was calculated from a 10-fold cross-validation procedure comparing the observed and predicted disability scores for all 397 participants in the enrollment dataset. The average beta weights from the 10-fold procedures were then applied to the data of the 205 participants in the follow-up dataset to calculate the variance explained (R2) between observed and predicted disability scores in this sample.
For each dataset, p values, representing the probability that an R2 value as high as that seen in the a priori model would have been observed with a randomly selected model of the same number of parameters, were calculated as the proportion of permutations with an R2 value equal to or higher than the R2 value of the a priori model. For all n levels of the hierarchy, the permutation approach was applied except for the final 12-parameter model, for which there was only one potential combination (i.e., all available parameters). As seen in Table 4, for the single parameter and 11-parameter models, only 12 potential permutations were possible, meaning the minimum p value of p = .083 falls above criterion (α = .05) for significance.
Results
Aside from increases in age and months since last deployments, group-level differences across the two datasets showed an increase in the average level of educational attainment and decreases in PTSD severity scores and proportion of the sample diagnosed with current PTSD (Table 1). The level of PTSD severity at initial enrollment did not differ across participants who did and did not return for follow-up prior to the 2017 release. This suggests individuals who had yet to return for follow up (due to timing or declining to return) did not have more severe PTSD symptoms, t(319.96) = 1.30, p = .196, corrected for unequal variance. Additionally, PTSD severity scores were positively correlated across the initial and follow-up testing sessions in those participants who did return for additional testing, r = 0.730, p < .001. Similar to previous findings (Lippa, et al., 2015), functional disability as scored on the WHODAS was higher than would be expected in 75% of the general international population (World Health Organization, 2010) and showed stability across the two testing periods, r = 0.734, p < .001.
The initial regression model fit to the December 2015 dataset (Figure 1 and Table 2) showed positive associations with reported functional disability, with all clinical diagnoses except for current substance use contributing significantly to model (Table 2). Overall, the model showed a strong correlation between the observed and predicted functional disability scores, F(12, 342) = 42.445, p < .001, R2 = .598, 90% CI [.525 – .638], f2 = 1.488. Although similar patterns were seen for the beta weights on the six WHODAS subdomain scores, the number of diagnoses that reached significance varied from two to six (Table 3).
Figure 1.
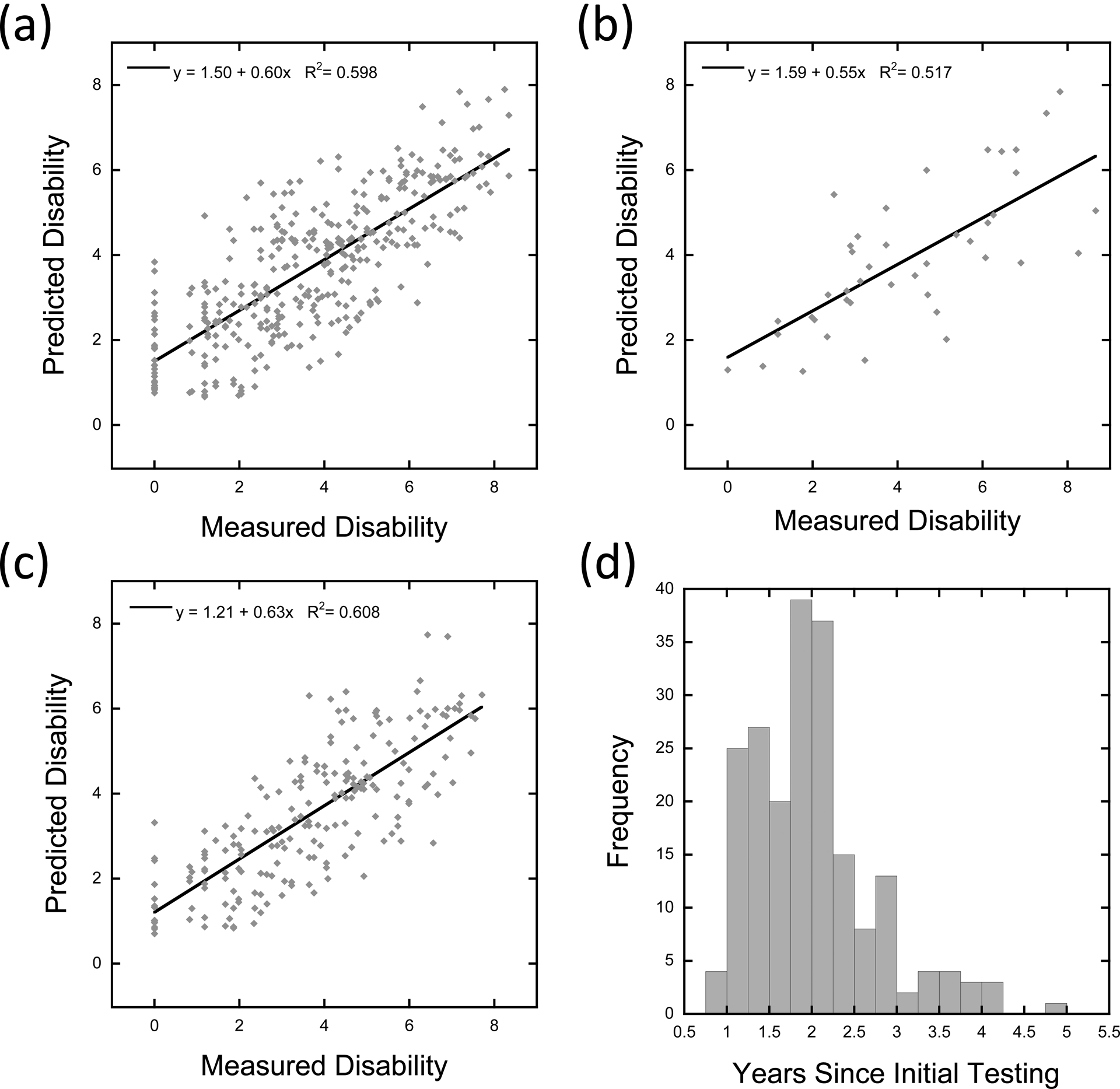
Regression model results and predictions of functional disability in novel datasets. Panel A depicts a scatterplot showing the association between measured and predicted disability scores on the World Health Organization Disability Assessment Schedule 2.0 (WHODAS) from the regression model fit to 355 participants’ data during their initial enrollment testing period. Each dot shows the data of a single participant. The solid line shows the best-fitting linear function. Panel B depicts a scatterplot showing the association between measured and predicted functional disability scores in 42 novel participants, applying the beta weights derived from the initial enrollment testing dataset. Panel C depicts a scatterplot showing the association between measured and predicted functional disability scores in 205 participants at their first follow-up session, applying the beta weights derived from the initial enrollment testing dataset. Panel D depicts a histogram showing the range of time between the initial enrollment testing session and the first follow-up testing session, in years, for the 205 repeat participants (as shown in Panel C).
Table 2.
Multiple Linear Regression of Transformed World Health Organization Disability Assessment Schedule 2.0 (WHODAS) Total Score and Demographic, Psychiatric, and Behavioral Conditionsa
| Variable | B | SE | β | t(342) |
|---|---|---|---|---|
| Age | 0.018 | 0.009 | .073 | 1.983 |
| Gender | 0.223 | 0.265 | .030 | 0.841 |
| Education (years) | −0.045 | 0.041 | −.041 | −1.092 |
| DRRIb | 0.009 | 0.007 | .050 | 1.238 |
| NSIc | 1.208 | 0.425 | .103 | 2.839** |
| mTBI | 0.398 | 0.163 | .094 | 2.436* |
| PTSD | 1.252 | 0.186 | .288 | 6.722*** |
| Depressive disorder | 1.396 | 0.182 | .293 | 7.651*** |
| Anxiety disorder | 0.504 | 0.185 | .099 | 2.726** |
| Substance use disorder | −0.045 | 0.203 | −.008 | −0.223 |
| Current Pain | 0.985 | 0.175 | .213 | 5.634*** |
| Sleep disturbance | 0.684 | 0.200 | .133 | 3.414*** |
Note. N = 355. R2 =.598, Adjusted R2 =.584. DRRI = Deployment Risk and Resilience Inventory; NSI = Neurobehavioral Symptom Inventory; mTBI = mild traumatic brain injury; PTSD = posttraumatic stress disorder.
WHODAS total score transformed by taking the square root to normalize the distribution across participants.
Combat Exposure score.
Validity-10 symptom exaggeration score derived from NSI (score ≥ 23).
p < .05.
p < .01.
p < .001
Table 3.
Standardized Beta Values from Multiple Linear Regression of the Six Transformed World Health Organization Disability Assessment Schedule 2.0 (WHODAS) Subscores and Demographic, Psychiatric, and Behavioral Conditions
| Variable | UC | GA | SC | GAWP | LA | PS |
|---|---|---|---|---|---|---|
| Age | 0.074 | 0.115* | 0.028 | 0.084* | 0.022 | 0.071 |
| Gender | −0.003 | −0.023 | 0.050 | 0.057 | 0.040 | 0.013 |
| Education (years) | −0.023 | −0.094* | −0.011 | −0.038 | 0.000 | −0.019 |
| DRRI | 0.039 | 0.094 | 0.053 | 0.052 | −0.045 | 0.045 |
| NSI | 0.128** | 0.113* | −0.025 | 0.048 | 0.123** | 0.064 |
| mTBI | 0.09* | 0.052 | 0.133* | 0.078 | 0.033 | 0.099* |
| PTSD | 0.257*** | 0.106 | 0.104 | 0.34*** | 0.229*** | 0.301*** |
| Depressive disorder | 0.199*** | 0.172*** | 0.315*** | 0.311*** | 0.221*** | 0.315*** |
| Anxiety disorder | 0.121** | 0.041 | 0.051 | 0.076 | 0.096* | 0.066 |
| Substance use disorder | 0.038 | −0.031 | −0.003 | −0.057 | 0.021 | −0.030 |
| Pain | 0.21*** | 0.263*** | 0.098 | 0.054 | 0.22*** | 0.191*** |
| Sleep disturbance | 0.134** | 0.085 | 0.070 | 0.107* | 0.095 | 0.108** |
| Model Adjusted R2 | 0.474 | 0.308 | 0.239 | 0.473 | 0.344 | 0.534 |
Note. For all six regression models, overall model ps < .001. UC = Understanding and Communicating (Cognition domain); GA = Moving and Getting Around (Mobility domain); SC = Self-Care domain; GAWP = Getting Along with People domain; LA = Life Activities domain; PS = Participating in Society (Participation domain).
p < .05.
p < .01.
p < .001.
The coefficients from the initial model were then applied to the 42 novel participants in the February 2017 dataset. Results showed a strong correlation between the observed and predicted functional disability scores in this new sample of participants, r = .719, p < .001 (Figure 1, Panel B). Next, the coefficients were applied to the data from the participants in the follow-up dataset. As seen in Panel C of Figure 1, the results again showed a strong correlation between the observed and predicted functional disability scores in these longitudinal participants, r = .780, p < .001. This suggests temporal stability in prediction ability across time (Figure 1, Panel D).
A 10-fold cross-validation approach was then applied to the enrollment dataset for overall disability as well as the six subscale scores from the WHODAS (Figure 2, Panel A). For the overall score in the initial enrollment dataset, a strong correlation was still observed between the measured and predicted disability scores, r = .752, p < .001. When the average beta weights across the 10-fold procedures were used to predict functional disability in the follow-up dataset, a strong positive correlation was observed, again demonstrating temporal stability in prediction ability, r = .763, p < .001. Though lower than the overall score, significant positive associations were also observed for all six subscales for both the initial enrollment and follow-up datasets, p’s < .001.
Figure 2.
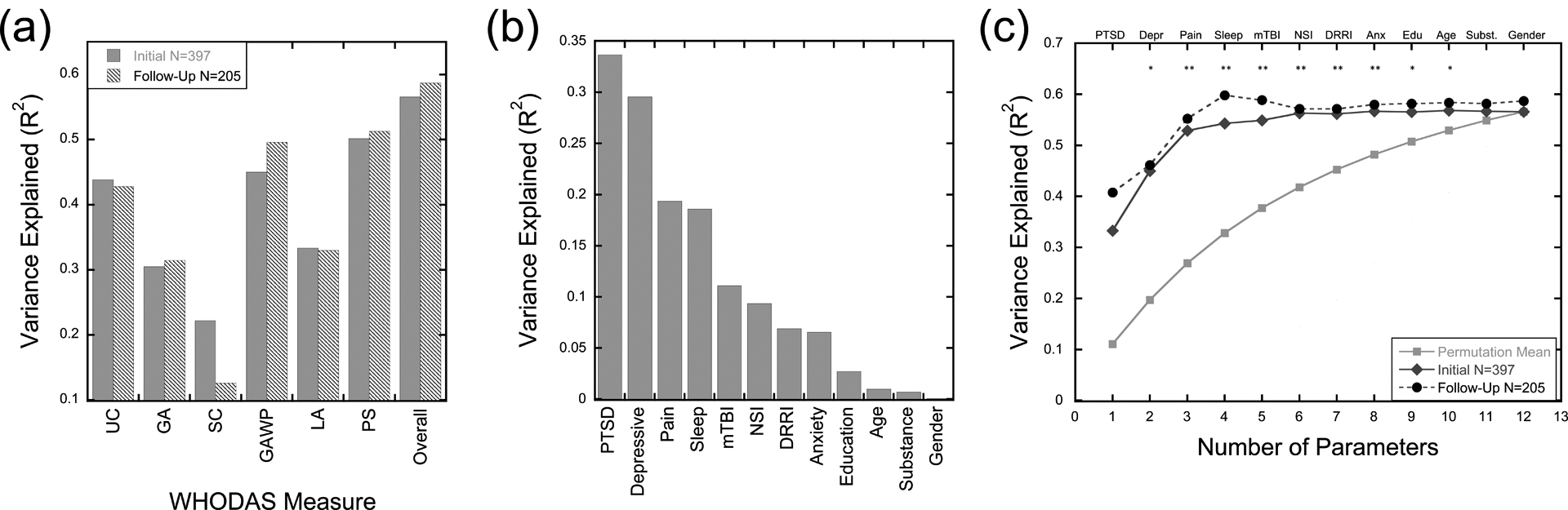
10-Fold cross-validation and contribution of individual predictor variables. Panel A depicts a bar graph showing the proportion of variance explained in the 10-fold cross-validation analyses predicting the six subscales and the overall disability score on the World Health Organization Disability Assessment Schedule 2.0 (WHODAS) in the initial enrollment and follow-up datasets. Panel B depicts a bar graph showing the proportion of variance in overall disability scores explained in the initial enrollment dataset by the 12 predictor variables independently using bivariate Pearson r correlations. Predictors are shown in the descending order used in the following iterative analysis. Panel C depicts the proportion of variance in overall disability scores explained when independent predictors are iteratively added to the 10-fold cross-validation regression analyses starting with current PTSD diagnosis and progressively adding predictors. Cross-validation was run on initial enrollment data (grey diamonds), and the average beta weights. were used to predict functional disability scores in the follow-up dataset (black circles). The light grey squares show the average proportion of variance explained from all permutations of N predictors at each level.
*p < .05 and ** p < .01 for the initial enrollment dataset. UC = Understanding and Communicating (Cognition domain); GA = Moving and Getting Around (Mobility domain); SC = Self-Care domain; GAWP = Getting Along with People domain; LA = Life Activities domain; PS = Participating in Society (Participation domain); Depr = Depressive disorders; Anx = anxiety disorders; Edu = education; Subst = substance use disorder
Finally, a hierarchical cross-validation approach was used to assess the prediction ability of reduced models with subsets of diagnoses included (Figure 2, Panel B). Starting with just current PTSD diagnosis, on each iteration the full 10-fold cross-validation approach was repeated, moving from one to all 12 independent predictors included in the model. Permutation testing showed that the selected model order out-performed randomly selected features in the two-parameter model up to the 10-parameter model. Prediction ability in the follow-up dataset out-performed random model selections at all iterations. As seen in Figure 2 (Panels B and C) and Table 4, prediction ability increased, adding current PTSD, depressive disorder, pain, sleep disturbance diagnoses, mTBI, and our symptom exaggeration flag. However, after including these variables, prediction ability began to asymptote, with relatively small or no changes in prediction ability in both the initial enrollment and longitudinal datasets as more variables are added to the model.
Discussion
The present results demonstrate strong associations between reported functional disability and a set of common clinical and behavioral disorders in a large sample of post-9/11 U.S. veterans. Importantly, we also demonstrate that these clinical variables can be used to predict functional disability in independent samples and across time. These validation analyses support that previous assessments of these associations (Lippa et al., 2015) are robust and have clinical implications for assessing and tracking disability in both public and private clinical settings, as over 50% of veterans receive healthcare outside of the VA healthcare system (Epidemiology Program Post-Deployment Health Group, 2012; National Center for Veterans Analysis and Statistics, 2017).
A great deal of resources from both the VA and the U.S. Department of Defense have been expended to understand and treat PTSD and mTBI in post-9/11veterans. However, there is accumulating evidence that these disorders alone do not wholly account for the substantial reintegration issues and functional disability experienced among veterans over the long term (Carlson, et al., 2010; Sayer, et al., 2009; Sayer, Noorbaloochi, Frazier, Carlson, & Gravely, 2010) and are not easily isolated in such a highly polymorbid population (Carlson, et al., 2010; Hoge, et al., 2008). Determining which combination of conditions are associated most strongly with functional disability may aid in the development of interventions for the individuals most at risk for poor functional outcomes, by targeting symptoms that occur across diagnoses. The present results support findings across the literature that relates PTSD diagnosis to poor functional outcomes (Amick, et al., 2018; Hoge, et al., 2004; Schult, 2011; Zatzick, et al., 1997). Additionally, consistent with an emerging body of literature (Amick, et al., 2018; Fonda, et al., 2017; Hoge, Goldberg, & Castro, 2009; Lippa, et al., 2015; Pugh, et al., 2018), we found a history of mTBI as a single, isolated diagnosis to be rare and only mildly predictive of increased disability. In contrast, other behavioral conditions, such as sleep disturbance and pain, were found to be more predictive. We note, however, that within our sample, a positive history of mTBI was highly associated with current sleep disorders and pain, with only 1.5% of veterans with a history of mTBI not reporting concurrent issues with sleep or pain. Although this represents a higher comorbidity rate than expected at the population level (Lew, et al., 2008), this result suggests that within our sample, it may not be a question of whether an individual experiences an mTBI per se but rather whether a history of mTBI is accompanied by sleep and pain conditions due to physical trauma that determines future functional disabilities.
Although collecting as much information as possible about issues that patients may be facing has advantages, such broad assessments are simply not feasible in the current milieu of fast-paced, time-limited clinical settings. Our final analysis suggests that with regards to prioritizing clinical assessments in this veteran population, certain diagnoses provide more information regarding functional disability than others and, thus, may be sufficient regarding identification of individuals at high risk for poor outcome. Specifically, assessments of current PTSD status, depressive disorder, pain, and sleep disturbances were the most informative (Figure 2, Panel C). Of interest, however, PTSD showed significant associations with subscales measuring communication, social, and work functioning but not with measures of mobility (e.g., getting around and self-care), whereas depressive disorders were associated with all aspects of functioning (Table 3). Although they were significant contributors in the regression model, a positive history of mTBI during military service and a current diagnosis of anxiety disorder provided far less information regarding disability. However, mTBI showed an association with difficulties in self-care that was not seen with PTSD and thus may be tapping into unique aspects of disability not typically associated with PTSD. Current substance abuse disorder appears to provide the least unique information in this regard. Of note, however, this does not suggest that these latter diagnoses are not related to functional impairments (Fonda, et al., 2017). Rather, within such a highly comorbid sample, it may be that “yes or no” diagnostic categories in these areas fail to provide unique information regarding functional disability that is not already captured by diagnoses of PTSD, depression, pain, and sleep. Although there are evidence-based treatments for the individual diagnoses included in this sample, even well-validated treatments, like those for PTSD, face issues such as higher treatment dropout rates in post-9/11 veterans than other veteran populations (Goetter, et al., 2015). The present results support the hypothesis that post-9/11 veterans are a highly comorbid population with co-occurring multiple diagnoses that interact to negatively impact daily functioning. Thus, treatments designed to address multiple co-occurring conditions, such as the Polytrauma Integrative Medicine Initiative (Cornis-Pop & Reddy, 2019), or those that are transdiagnostic in nature (Fortier, et al., 2018) may best address the underlying symptoms that are driving dysfunction in this population.
There are several limitations to the current study. First, the current approach was data-driven and identified the pattern of conditions most predictive of functional disability within the current cohort. Although the current study focused on community-dwelling veterans with a range of issues and symptom severity, deviations of our sample from the larger post-9/11 veteran population may limit the degree to which the current model generalizes to the larger population. Future studies that assess the validity of the model in larger samples would help to further validate the present results and allow for more detailed analysis of specific subgroups within diagnostic categories on prediction ability (e.g., generalized anxiety disorder vs. all anxiety disorders) or medical conditions (e.g., amputation or shrapnel) that could mediate the clinical diagnoses. As only 33 women were enrolled in the current study, testing larger groups of female veterans is needed to determine if the patterns of comorbidities and functional disability observed hold for female veterans. Although the gender distribution is representative of the larger population, understanding any gender-specific disability patterns is important for addressing unique challenges female veterans may face after leaving the military.
Longer follow-up assessments would also be of value. Five-year follow-up assessments and recruitment at different sites is currently underway. The current longitudinal assessments also lack detailed information regarding treatments that participants may have sought for specific conditions (e.g., PTSD, substance use) between the initial and follow-up assessments. Although the models were robust despite this shortcoming, adding this information will allow for further assessments of potential treatment impacts on the overall model performance and whether their status at the initial assessment is predictive of future additional issues. For example, considering just anxiety disorders, whereas 18 participants presented with anxiety disorders across both assessments, 19 participants presented with an anxiety disorder only at the initial assessment, and 22 participants presented with an anxiety disorder only at the follow-up assessment. Though beyond the scope of the current paper, additional information about treatments and other potential mediating life events may allow additional analyses regarding whether and how these events impact changes seen in specific disorders across time within individuals and their potential concurrent impact on the other disorders assessed in the present study.
Finally, although the WHODAS is well-validated (Gold, 2014; Üstün, 2010; World Health Organization, 2010), additional research is needed to determine if the current assessments can predict other objective measures of functional outcome (e.g., employment status). Of note is the significant beta weight for the NSI Validity-10 scale, which is thought to assess overreporting or exaggeration of neurobehavioral symptoms and could also indicate overreporting of functional disability in our sample. There is currently disagreement in the literature on the optimal cutoff for this validity measure (Lippa, et al., 2016) and whether these symptoms, including those comprising the Validity-10, are due to mTBI or concurrent psychiatric and/or behavioral conditions (Andrews, Fonda, Levin, McGlinchey, & Milberg, 2018). Additionally, only 2%–4% of the current sample showed signs of overreporting. However, the significance of this predictor in the current model highlights the need for validity measurements when the outcome measure is itself based on self-report, especially in clinical samples in which the goal is to help screen for potential exaggeration but not exclude representative patients from a sample or future care.
We report robust evidence that taking a holistic approach to characterizing multiple clinical and behavioral conditions provides the best indicator of functional disability, both now and in the future, in post-9/11veterans. Similar to geriatric populations, post-9/11 veterans often face several concurrent clinical and behavioral issues. Although understanding each condition independently can aid in the characterization and delineation of clinical conditions, assessing multiple clinical domains may provide the most comprehensive and accurate picture of a patient’s current functional status and better inform future treatment approaches.
Acknowledgments
This research was supported by the U.S. Department of Veterans Affairs (VA) through the Translational Research Center for TBI and Stress Disorders (TRACTS), a VA Rehabilitation Research & Development Traumatic Brain Injury National Research Center (B9254-C), a Career Development award from the Department of Veterans Affairs Rehabilitation Research & Development (1IK2RX002268-01A2) to F.C.F., a VA Clinical Science Research & Development Merit Review (CX001327) to R.E.M., and a National Institute of Health NCCIH grant (R21 AT009430-01) to W.P.M. The contents within do not represent the views of the Department of Veterans Affairs or the United States government.
References
- Amick MM, Clark A, Fortier CB, Esterman M, Rasmusson AM, Kenna A, et al. (2013). PTSD modifies performance on a task of affective executive control among deployed OEF/OIF veterans with mild traumatic brain injury. Journal of the International Neuropsychological Society, 19(7), 792–801. 10.1017/s1355617713000544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amick MM, Meterko M, Fortier CB, Fonda JR, Milberg WP, & McGlinchey RE (2018). The deployment trauma phenotype and employment status in veterans of the wars in Iraq and Afghanistan. The Journal of Head Trauma Rehabilitation, 33(2), E30–E40. 10.1097/htr.0000000000000308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews RJ, Fonda JR, Levin LK, McGlinchey RE, & Milberg WP (2018). Comprehensive analysis of the predictors of neurobehavioral symptom reporting in veterans. Neurology, 91, e732–e745. 10.1212/wnl.0000000000006034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlot S, & Celisse A (2010). A survey of cross-validation procedures for model selection. Statistics Surveys, 4, 40–79. 10.1214/09-ss054 [DOI] [Google Scholar]
- Atkinson MP, Guetz A, & Wein LM (2009). A dynamic model for posttraumatic stress disorder among US troops in Operation Iraqi Freedom. Management Science, 55(9), 1454–1468. 10.1287/mnsc.1090.1042 [DOI] [Google Scholar]
- Babyak MA (2004). What you see may not be what you get: a brief, nontechnical introduction to overfitting in regression-type models. Psychosomatic Medicine, 66(3), 411–421. 10.1097/00006842-200405000-00021 [DOI] [PubMed] [Google Scholar]
- Beckham JC, Crawford AL, Feldman ME, Kirby AC, Hertzberg MA, Davidson JRT, et al. (1997). Chronic posttraumatic stress disorder and chronic pain in Vietnam combat veterans. Journal of Psychosomatic Research, 43(4), 379–389. 10.1016/S0022-3999(97)00129-3 [DOI] [PubMed] [Google Scholar]
- Berry KJ, Mielke PW Jr, & Mielke HW (2002). The Fisher-Pitman permutation test: an attractive alternative to the F test. Psychological Reports, 90(2), 495–502. https://doi.org/10.2466%2Fpr0.2002.90.2.495 [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers F, Nagy L, Kaloupek D, Klauminzer G, Charney D, et al. (1990). A clinician rating scale for assessing current and lifetime PTSD: The CAPS-1. Behavior Therapist, 13, 187–188. [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. (1995). The development of a clinician-administered PTSD scale. Journal of Traumatic Stress, 8, 75–90. 10.1007/BF02105408 [DOI] [PubMed] [Google Scholar]
- Bleich A, Koslowsky M, Dolev A, & Lerer B (1997). Post-traumatic stress disorder and depression. An analysis of comorbidity. The British Journal of Psychiatry, 170(5), 479–482. 10.1192/bjp.170.5.479 [DOI] [PubMed] [Google Scholar]
- Carlson KF, Nelson D, Orazem RJ, Nugent S, Cifu DX, & Sayer NA (2010). Psychiatric diagnoses among Iraq and Afghanistan war veterans screened for deployment-related traumatic brain injury. Journal of Traumatic Stress, 23(1), 17–24. 10.1002/jts.20483 [DOI] [PubMed] [Google Scholar]
- Carpenter JS, & Andrykowski MA (1998). Psychometric evaluation of the Pittsburgh sleep quality index. Journal of Psychosomatic Research, 45(1), 5–13. 10.1016/s0022-3999(97)00298-5 [DOI] [PubMed] [Google Scholar]
- Cicerone KD, & Kalmar K (1995). Persistent postconcussion syndrome: the structure of subjective complaints after mild traumatic brain injury. The Journal of Head Trauma Rehabilitation, 10(3), 1–17. 10.1016/S0022-3999(97)00298-5 [DOI] [Google Scholar]
- Cornis-Pop M, & Reddy KP (2019). Integrative medicine and health coaching in polytrauma rehabilitation. Physical Medicine & Rehabilitation Clinics of North America, 30, 261–274. 10.1016/j.pmr.2018.08.007 [DOI] [PubMed] [Google Scholar]
- Epidemiology Program Post-Deployment Health Group. (2012). Analysis of VA Health Care Utilization among Operation Enduring Freedom (OEF), Operation Iraqi Freedom (OIF), and Operation New Dawn (OND) Veterans: Cumulative from 1st Qtr FY 2002 through 1st Quarter FY 2012 (October 1, 2001 - December 31, 2011).
- First MB, Spitzer RL, Gibbon M, & Williams JB (1996). Structured Clinical Interview for DSM-IV Axis I Disorders. Washington, DC: American Psychiatric Press. [Google Scholar]
- Fonda JR, Fredman L, Brogly SB, McGlinchey RE, Milberg WP, & Gradus JL (2017). Traumatic brain injury and attempted suicide among veterans of the wars in Iraq and Afghanistan. American Journal of Epidemiology, 186(2), 220–226. 10.1093/aje/kwx044 [DOI] [PubMed] [Google Scholar]
- Fortier CB, Amick MM, Grande L, McGlynn S, Kenna A, Morra L, et al. (2014). The Boston Assessment of Traumatic Brain Injury–Lifetime (BAT-L) semistructured interview: evidence of research utility and validity. The Journal of Head Trauma Rehabilitation, 29(1), 89–98. https://doi.org/10.1097%2FHTR.0b013e3182865859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier CB, Kenna A, Dams-O’Connor K, Fonda J, Levin LK, Hursh C, et al. (2018). Feasibility of a Skills-Based Group Reintegration Workshop for OEF/OIF Veterans: STEP-Home. The Journal of Head Trauma Rehabilitation, 33(4), E17–E23. 10.1097/HTR.0000000000000362 [DOI] [PubMed] [Google Scholar]
- Goetter EM, Bui E, Ojserkis RA, Zakarian RJ, Brendel RW, & Simon NM (2015). A systematic review of dropout from psychotherapy for posttraumatic stress disorder among Iraq and Afghanistan combat veterans. Journal of Traumatic Stress, 28(5), 401–409. 10.1002/jts.22038 [DOI] [PubMed] [Google Scholar]
- Gold LH (2014). DSM-5 and the Assessment of Functioning: The World Health Organization Disability Assessment Schedule 2.0 (WHODAS 2.0). Journal of the American Academy of Psychiatry & the Law Online, 42(2), 173–181. [PubMed] [Google Scholar]
- Grafton KV, Foster NE, & Wright CC (2005). Test-retest reliability of the Short-Form McGill Pain Questionnaire: Assessment of intraclass correlation coefficients and limits of agreement in patients with osteoarthritis. The Clinical Journal of Pain, 21(1), 73–82. 10.1097/00002508-200501000-00009 [DOI] [PubMed] [Google Scholar]
- Hawkins DM (2004). The problem of overfitting. Journal of Chemical Information & Computer Sciences, 44(1), 1–12. 10.1002/chin.200419274 [DOI] [PubMed] [Google Scholar]
- Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, & Koffman RL (2004). Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. New England Journal of Medicine, 351(1), 13–22. 10.1056/nejmoa040603 [DOI] [PubMed] [Google Scholar]
- Hoge CW, Goldberg HM, & Castro CA (2009). Care of war veterans with mild traumatic brain injury - flawed perspectives. The New England Journal of Medicine, 360(16), 1588–1591. 10.1056/nejmp0810606 [DOI] [PubMed] [Google Scholar]
- Hoge CW, McGurk D, Thomas JL, Cox A, Engel CC, & Castro CA (2008). Mild traumatic brain injury in U.S. soldiers returning from Iraq. New England Journal of Medicine, 358, 453–463. 10.1056/nejmc086083 [DOI] [PubMed] [Google Scholar]
- King LA, King DW, Vogt DS, Knight J, & Samper RE (2006). Deployment Risk and Resilience Inventory: A collection of measures for studying deployment-related experiences of military personnel and veterans. Military Psychology, 18(2), 89–120. 10.1207/s15327876mp1802_1 [DOI] [Google Scholar]
- Lange RT, Brickell TA, Lippa SM, & French LM (2015). Clinical utility of the Neurobehavioral Symptom Inventory validity scales to screen for symptom exaggeration following traumatic brain injury. Journal of Clinical & Experimental Neuropsychology, 37(8), 853–862. 10.1080/13803395.2015.1064864 [DOI] [PubMed] [Google Scholar]
- Lew HL, Vanderploeg RD, Moore DF, Schwab K, Friedman L, Yesavage J, et al. (2008). Overlap of mild TBI and mental health conditions in returning OIF/OEF service members and veterans. Journal of Rehabilitation Research & Development, 45(3), xi–xvi. [PubMed] [Google Scholar]
- Lippa SM, Fonda JR, Fortier CB, M.A. A, A. K, Milberg WP, et al. (2015). Deployment-related psychiatric and behavioral conditions and their association with functional disability in OEF/OIF/OND Veterans. Journal of Traumatic Stress, 28, 155–184. 10.1002/jts.21979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippa SM, Lange RT, Bailie JM, Kennedy JE, Brickell TA, & French LM (2016). Utility of the Validity-10 scale across the recovery trajectory following traumatic brain injury. Journal of Rehabilitation Research & Development, 53(3), 379–390. 10.1682/JRRD.2015.01.0009 [DOI] [PubMed] [Google Scholar]
- Lobbestael J, Leurgans M, & Arntz A (2011). Inter‐rater reliability of the Structured Clinical Interview for DSM‐IV Axis I disorders (SCID I) and Axis II disorders (SCID II). Clinical Psychology & Psychotherapy, 18(1), 75–79. 10.1002/cpp.693 [DOI] [PubMed] [Google Scholar]
- McGlinchey RE, Milberg WP, Fonda JR, & Fortier CB (2017). A methodology for assessing deployment trauma and its consequences in OEF/OIF/OND veterans: The TRACTS longitudinal prospective cohort study. International Journal of Methods in Psychiatric Research, e1556, 1–15. 10.1002/mpr.1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R (1987). The short-form McGill pain questionnaire. Pain, 30(2), 191–197. 10.1016/0304-3959(87)91074-8 [DOI] [PubMed] [Google Scholar]
- National Center for Veterans Analysis and Statistics. (2017). Profile of Veterans 2015: Data from the American Community Survey.
- Pugh MJ, Swan AA, Carlson KF, Jaramillo CA, Eapen BC, Dillahunt-Aspillaga C, et al. (2018). Traumatic brain injury severity, comorbidity, social support, family functioning, and community reintegration among veterans of the Afghanistan and Iraq wars. Archives of Physical Medicine & Rehabilitation, 99(2), S40–S49. 10.1016/j.apmr.2017.05.021 [DOI] [PubMed] [Google Scholar]
- Sayer NA, Cifu DX, McNamee S, Chiros CE, Sigford BJ, Scott S, et al. (2009). Rehabilitation needs of combat-injured service members admitted to the VA Polytrauma Rehabilitation Centers: the role of PM&R in the care of wounded warriors. Physical Medicine & Rehabilitation, 1(1), 23–28. 10.1016/j.pmrj.2008.10.003 [DOI] [PubMed] [Google Scholar]
- Sayer NA, Noorbaloochi S, Frazier P, Carlson K, & Gravely A (2010). Reintegration problems and treatment interests among Iraq and Afghanistan combat veterans receiving VA medical care. Psychiatric Services, 61(6), 589–597. 10.1176/appi.ps.61.6.589 [DOI] [PubMed] [Google Scholar]
- Schult T (2011). Mental health diagnosis and occupational functioning in National Guard/Reserve veterans returning from Iraq. Journal of Rehabilitation Research & Development, 48(10), 1159–1170. 10.1682/jrrd.2010.11.0212 [DOI] [PubMed] [Google Scholar]
- Terrio H, Brenner LA, Ivins BJ, Cho JM, Helmick K, Schwab K, et al. (2009). Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. The Journal of Head Trauma Rehabilitation, 24(1), 14–23. 10.1097/HTR.0b013e31819581d8 [DOI] [PubMed] [Google Scholar]
- Thomas JL, Wilk JE, Riviere LA, McGurk D, Castro CA, & Hoge CW (2010). Prevalence of mental health problems and functional impairment among active component and national guard soldiers 3 and 12 months following combat in Iraq. Archives of General Psychiatry, 67(6), 614–623. 10.1001/archgenpsychiatry.2010.54 [DOI] [PubMed] [Google Scholar]
- U.S. Department of Veterans Affairs and U.S. Department of Defence. (2009). VA/DOD clinical practice guidelines for the management of concussion/mild traumatic brain injury. from http://www.healthquality.va.gov/mtbi/concussion_mtbi_full_1_0.pdf
- Üstün TB (2010). Measuring health and disability: Manual for WHO Disability Assessment Schedule WHODAS 2.0: World Health Organization. [Google Scholar]
- Üstün TB, Chatterji S, Kostanjsek N, Rehm J, Kennedy C, Epping-Jordan J, et al. (2010). Developing the World Health Organization disability assessment schedule 2.0. Bulletin of the World Health Organization, 88, 815–823. 10.2471/blt.09.067231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderploeg RD, Cooper DB, Belanger HG, Donnell AJ, Kennedy JE, Hopewell CA, et al. (2014). Screening for postdeployment conditions: Development and cross-validation of an embedded validity scale in the neurobehavioral symptom inventory. The Journal of Head Trauma Rehabilitation, 29(1), 1–10. 10.1097/htr.0b013e318281966e [DOI] [PubMed] [Google Scholar]
- Vogt DS, Proctor SP, King DW, King LA, & Vasterling JJ (2008). Validation of scales from the Deployment Risk and Resilience Inventory in a sample of Operation Iraqi Freedom veterans. Assessment, 15(4), 391–403. 10.1177/1073191108316030 [DOI] [PubMed] [Google Scholar]
- Walker RL, Clark ME, & Sanders SH (2010). The “postdeployment multi-symptom disorder”: An emerging syndrome in need of a new treatment paradigm. Psychological Services, 7(3), 136–147. 10.1037/a0019684 [DOI] [Google Scholar]
- Weathers FW, Ruscio AM, & Keane TM (1999). Psychometric properties of nine scoring rules for the Clinician-Administered Posttraumatic Stress Disorder Scale. Psychological Assessment, 11(2), 124–133. 10.1037/1040-3590.11.2.124 [DOI] [Google Scholar]
- Williams JB, Gibbon M, First MB, Spitzer RL, Davies M, Borus J, et al. (1992). The structured clinical interview for DSM-III-R (SCID): II. Multisite test-retest reliability. Archives of General Psychiatry, 49(8), 630–636. 10.1001/archpsyc.1992.01820080038006 [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2010). Measuring health and disability: Manual for WHO Disability Assessment Schedule. Geneva, Switzerland. [Google Scholar]
- Zatzick DF, Marmar CR, Weiss DS, Browner WS, Metzler TJ, Golding JM, et al. (1997). Posttraumatic stress disorder and functioning and quality of life outcomes in a nationally representative sample of male Vietnam veterans. American Journal of Psychiatry, 154(12), 1690–1695. 10.1176/ajp.154.12.1690 [DOI] [PubMed] [Google Scholar]


