Abstract
The field of language research has seen tremendous progress in the last two decades. Advances in neuro-imaging and stimulation mapping have changed the way we conceive the neural basis of speech and language processing. In the past, the Wernicke-Lichtheim model was the most influential model explaining the neuro-anatomical basis of language. More recently, the concept of dual stream language processing has emerged, wherein separate dorsal and ventral networks are synergistically involved in phonological (sound to articulation) and semantic (sound to meaning) processing respectively. In this review article, we highlight new insights and approaches to the neurobiology of language, across different aspects of language processing like perception, comprehension, production, hemisphere lateralization, role of subcortical structures and effect of damage to language networks.
Keywords: Aphasia, dual-stream, language, networks, neuro-biology
Guest editor's notes: The models of language organisation in brain have undergone major revisions during the last two decades, with the advent of newer techniques of neuroimaging, electrophysiology, and refined testable theories in neurolinguistics, psycho-linguistics and cognitive neurology. Clinicians must keep themselves abreast with new developments, not only for the sack of science but also for practical implications.
BACKGROUND
The neurobiology of language has remained an enigmatic area for researchers since decades. From the first language models, particularly the 'Wernicke-Lichtheim-Geschwind model' to the current 'dual stream models', the field of language neurobiology has made great strides and is now ready to adopt a modern integrative approach. In this review, we will discuss the classic models of language function, their fallacies, and evolving paradigms of language neurobiology. We will also review the role of subcortical structures, the non-dominant hemisphere and the clinical application of the current models of language.
Classic model of language function
The classic model of language neurobiology focussed primarily on localization. Broca, Wernicke, Lictheim and others provided this fundamental knowledge in 19th century based on observational and autopsy studies. Thus aphasiology in the 20th century was based on the Broca-Wernicke-Lichtheim-Geschwind model. Certain cortical centres in the brain were upheld as sacrosanct for a particular function, repudiating the role of subcortical connections and networks. This model consisted of Broca's area (motor speech centre), Wernicke's area (sensory speech centre) and the arcuate fasciculus connecting the two, in the dominant cerebral hemisphere. Brodmann areas 44 (pars opercularis) and 45 (pars triangularis), in the posterior part of the inferior frontal gyrus in left hemisphere (for majority of human beings) were designated as the Broca's area. Brodmann area 22, corresponding to the posterior part of superior temporal gyrus and a part of the supramarginal gyrus, was named as Wernicke's area. As per this approach, sounds of words travel through the auditory pathways to the primary auditory cortex. Meaning is then extracted in the Wernicke's area and sent to the Broca's area through the arcuate fasciculus. Morphemes are formed here and passed to the motor cortex. Visual information was hypothesized to pass from the visual cortex to the angular gyrus and then to the Wernicke's area.
Fallacies of old concepts
Functional imaging of the brain and clinico-radiological correlations over last few decades have raised many questions about the classical aphasia models and rekindled a new interest in language neurobiology. Dronkers et al. restudied Paul Broca's original patients with magnetic resonance imaging of the brain and found astounding results.[1] The lesions extended far deeper than the cortical Broca's area and involved the superior longitudinal fasciculus (a major association tract connecting frontal and parietal lobe). Broca's aphasia is now thought to involve injury to wider cortical areas (middle and inferior precentral gyrus) and their white matter connections.
Ambiguity is even higher for Wernicke's area. Speech comprehension is no longer considered to be a single process localized to a single 'comprehension centre', as previously thought. Wernicke's area lesions are now thought to result in conduction aphasia, where comprehension remains intact. Electrical cortical stimulation studies of this region have shown to elicit phonemic paraphasias without disrupting speech comprehension.[2,3] Functional neuroimaging studies have linked neural activation of this region to activation of speech sound forms (phonemic retrieval) and online holding of retrieved phonemes in the auditory short term memory.[4] In a lesion-deficit correlation study, patients with damage to this area were not able to silently match written rhyming words (example, snow rhymes with blow, not with plow), but were able to match written words which had similar meaning, indicating defects in retrieving the correct phonological representation but intact comprehension.[5] Also, cortical degeneration of this area, as seen in logopenic variant primary progressive aphasia, is characterized by phonemic paraphasias, anomia (both explained by impaired phonemic retrieval) and impaired verbal short term memory explaining impaired sentence comprehension (inability to maintain a string of words in short term memory). Single word comprehension remains intact.[6] Mesulam et al. studied patterns of brain atrophy in patients of primary progressive aphasia with comprehension deficits and reported heterogeneous results.[7] Impaired comprehension of single words was associated with left temporal pole and adjacent anterior temporal cortex atrophy, sparing the Wernicke's area. Impaired comprehension of sentences was associated with Wernicke's (sentence comprehension affected possibly due to impaired verbal short-term memory as explained above), Broca's and dorsal premotor cortex atrophy. Thus, data from patients of primary progressive aphasia, stroke and functional neuroimaging studies draws a conclusion that a widespread network is involved in comprehension, while the classical Wernicke's area plays a little role.
Although the classic model gives a simplified understanding of classical aphasia syndromes (frontal lesions – motor aphasia, temporal/temporo-parietal lesions – sensory aphasia, arcuate fasciculus – conduction aphasia, deeper cortical lesions – disconnection syndromes), it cannot support the full range of aphasic syndromes. Also, patients with similar symptoms may not have identical lesions in the brain. The focus on cortical centres and lack of information on relevant networks is another major limitation of the classical model.
Evolving paradigms of language biology
The current model of language function (spoken language) consists of two pathways – the ventral and the dorsal stream. This model is supported by studies based on intraoperative direct electrical stimulation, diffusion tensor imaging, and functional MRI.[4,8,9]
The dual stream model, composed of parallel and interconnected streams connecting both cortical and subcortical areas, was first proposed by Hickock et al.[10]
The main cortical hubs of the dorsal stream include Wernicke's area [posterior superior temporal gyrus (pSTG) and adjacent supramarginal gyrus (SMG)] and the Broca's area (Pars triangularis and opercularis of the inferior frontal gyrus and middle-inferior part of precentral gyrus). The important white matter tracts connecting these areas are subcomponents of superior longitudinal fasciculus (SLF) and the arcuate fasciculus (AF). The SLF has 4 major subcomponents – SLF I, II, III join the frontal and parietal cortices, SLF temporoparietal (SLF-tp) joins the parietal and temporal lobes. Out of all, SLF-III and SLF-tp are important in language processing. The arcuate fasciculus lies deeper and connects the frontal opercular cortex to the posterior temporal cortex.
The main cortical hubs of the ventral stream (semantic hubs) include anterior temporal lobe or temporal pole [anterior middle temporal gyrus (MTG)], posterior MTG (pMTG), inferior temporal gyrus (ITG) and the angular gyrus (AG). The inferior frontal gyrus plays a role in initiating and controlling activation of semantics,[11,12,13] while the temporal and inferior parietal components are the actual repositories of concepts. The important white matter tracts connecting these areas are the middle longitudinal fasciculus (MLF - connects anterior and posterior temporal regions), inferior longitudinal fasciculus (ILF - connects temporal pole to occipital lobe) and the inferior frontal occipital fasciculus (IFOF).[14] Of these, the IFOF, a white matter bundle connecting inferior frontal cortex to the middle and inferior temporal gyri and the occipital cortex, is of prime importance in mediating inter-lobe complex semantic processing. It passes through the floor of the external capsule. The role of uncinate fasciculus (UF - connects temporal pole to inferior frontal region) in semantics is not certain due to conflicting reports in literature.
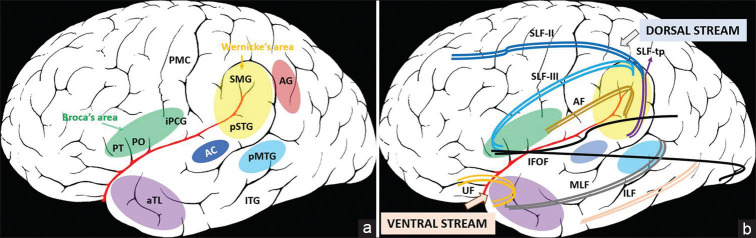
Ventral pathways connecting the inferior frontal and temporal cortices have been suggested to play a role in syntactic (grammatical) processing also. Which is the predominant pathway – the IFOF or UF needs clarification from further studies. These major cortical hubs and the connecting white matter tracts are shown in Figure 1.
Figure 1.
Illustration of dual stream model of spoken language, left hemisphere, lateral view 1A:. Important cortical hubs involved in the two streams 1B: Connecting white matter tracts. The auditory cortex (AC) represents the initial sound perception area. The dorsal stream consists of the Wernicke's area connected to Broca's area by the superior longitudinal fasciculus III (SLF-III) and the arcuate fasciculus (AF). The Wernicke's area includes the posterior superior temporal gyrus (pSTG) and adjacent supramarginal gyrus (SMG) and the Broca's area includes the Pars triangularis (PT), pars opercularis (PO) and the middle and inferior precentral gyrus (iPCG). The superior longitudinal fasciculus temporo-parietal (SLF-tp), which joins the posterior temporal to inferior parietal cortex, is also a part of the dorsal stream. The superior longitudinal fasciculus II (SLF-II) connects the inferior parietal cortex to the pre motor cortex and prefrontal cortices. The ventral stream consists of important semantic hubs – anterior temporal lobe (aTL) or temporal pole (formed by the anterior middle temporal gyrus), posterior middle temporal gyrus (pMTG), inferior temporal gyrus (ITG) and the angular gyrus (AG), connected by middle longitudinal fasciculus (MLF), inferior longitudinal fasciculus (ILF) and inferior frontal occipital fasciculus (IFOF). The uncinate fasciculus (UF) mediates a frontotemporal network and may have a role in syntactic (grammar) processing. PMC – Primary motor cortex. The diagram is a schematic illustration of the cortical hubs and subcortical tracts involved in language processing, and the areas marked may not correspond to exact anatomical boundaries. The background brain image has been taken from en.wikipedia.org, which is in public domain
As per this model, initial sound perception takes place in the auditory cortex, situated in the transverse temporal gyrus of Heschl and part of superior temporal gyrus. The information enters Wernicke's area through U fibres, which serves as the auditory short term memory and constitutes the phonemic retrieval system. Phonemes (speech sound forms) are retrieved, sequenced and held online here. For the process of repetition alone, retrieved phonemes are transferred to the supramarginal gyrus and Broca's area via the superficial superior longitudinal fasciculus and the deep arcuate fasciculus. This dorsal stream (posterior superior temporal to inferior frontal cortices, left hemisphere) is involved in phonological processing, chiefly repetition and articulation (maps sound to articulation). The supplementary motor area connected via the frontal aslant tract to the Broca's area is important for initiation and spontaneity of speech.
The ventral stream (temporal pole to the basal occipito-temporal cortex) is involved in semantic processing (maps sound to meaning). The process takes place mainly in the temporal lobe, but complex semantic processing (like sentences) involves multiple cortical areas (frontal and parietal also).[15] This information processing is supported by the U fibres, middle longitudinal fasciculus and inferior longitudinal fasciculus (intra-temporal processing) and the inferior frontal occipital fasciculus (inter-lobe processing).
For speech comprehension, meaning of single words is retrieved from the middle temporal gyrus (anterior part or temporal pole) through the middle longitudinal fasciculus and inferior longitudinal fasciculus. For understanding sentences and context, the posterior middle temporal gyrus, inferior parietal lobule (angular gyrus), inferior frontal cortex (part of Broca's area) and interlobar connections mediated via inferior frontal occipital fasciculus are involved. Turken and Dronkers studied sentence comprehension in 64 chronic aphasia patients post left hemisphere stroke, using fibre tractography and resting state functional connectivity.[16] They demonstrated that the posterior middle temporal gyrus has extensive connections with rest of the temporal lobe, angular gyrus, inferior and superior frontal gyri, along with homologus nodes in the right hemisphere, due to its strategic location at the crossroad of several large white matter tracts like middle longitudinal fasciculus, inferior longitudinal fasciculus, inferior frontal occipital fasciculus and arcuate fasciculus. Hence, it is the hub which facilitates comprehension of sentences.
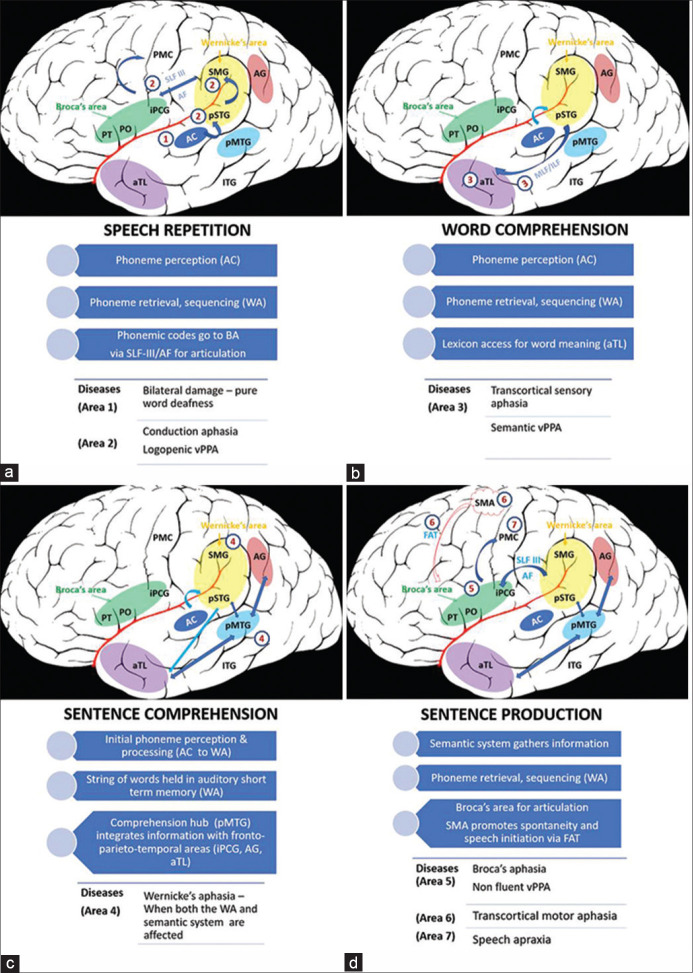
For spontaneous speech, the internal semantic system sends information to the phonemic retrieval system. For visually presented information, after visual recognition (by separate word and object recognition areas in basal occipito-temporal region), the information passes to the ventral and dorsal streams through inferior longitudinal fasciculus. The ventral stream maps visual information to meaning, while the dorsal stream maps visual information to articulation through visuo-phonological conversion. Duffao et al. proposed that the processing of information in the semantic, and phonological streams is parallel and dynamic. They based this concept on results of intraoperative direct electrical stimulation during awake surgery within the left dominant hemisphere.[8] They also postulated a separate fronto-temporal cortico-subcortical sub network involved in syntactic (grammar) processing with interconnections to parallel semantic and phonological circuits. Figure 2 illustrates various individual steps of language processing and effects of damage to the underlying networks.
Figure 2.
Schematic illustration of individual steps of language processing and effects of damage to the involved networks, left hemisphere, lateral view. (a) Speech Repetition: Auditory information or sound is first perceived by the primary auditory cortex (AC, area 1) in the transverse temporal gyrus of Heschl and part of superior temporal gyrus. Bilateral injury to this area causes pure word deafness wherein verbal discrimination of words is affected specifically, reading, writing and ability to appreciate elementary sounds is preserved. Through U fibres the information enters the auditory short-term memory [Wernicke's area (WA) – posterior superior temporal gyrus (pSTG) and adjacent supramarginal gyrus (SMG)] which constitutes the phonemic retrieval system. Phonemes (speech sound forms) are retrieved and sequenced here. For the process of repetition alone, retrieved phonemes are transferred to the Broca's area (BA) via the superficial superior longitudinal fasciculus (SLF) and the deep arcuate fasciculus (AF). Damage to the superior temporal gyrus leads to deficits in phoneme retrieval leading to repetition and naming difficulty, and inability to maintain a string of words in short term memory during sentence comprehension, as seen in logopenic variant primary progressive aphasia (vPPA). Stroke in this region (pSTG/SMG/SLF/AF: areas 2) produces conduction aphasia characterized by disturbance of auditory short-term memory, poor repetition and phonemic paraphasias, with intact comprehension. (b) Word comprehension: meaning of single words is retrieved from the middle temporal gyrus [anterior part/temporal pole (aTL) – a semantic hub] through the middle longitudinal fasciculus (MLF) and inferior longitudinal fasciculus (ILF). Damage to this area (area 3) causes semantic variant of PPA, while stroke in this region produces transcortical sensory aphasia. Repetition is preserved due to sparing of phonemic retrieval system in the superior temporal gyrus. (c) Sentence comprehension: For understanding sentences and context, the semantic system is activated. The posterior middle temporal gyrus P (MTG) is the central hub. It integrates information with the anterior temporal lobe (aTL), angular gyrus (AG), inferior precentral gyrus (iPCG, part of Broca's area). The inter-lobe connections are mediated via inferior frontal occipital fasciculus (not shown). Wernicke's aphasia results from a more widespread lesion involving both the phonemic retrieval (WA, area 4) and the semantic system (area 4). (d) Sentence production: Semantic system sends information to the phonemic system followed by the Broca's area for articulation. The ventral fronto-temporal network does the syntactic (grammar) processing. Stroke involving the Broca's area (area 5) and its white matter connections leads to Broca's aphasia, while degeneration in this region causes non-fluent vPPA. Injury to the supplementary motor area (SMA, area 6) or the frontal aslant tract (FAT, area 6) which connects SMA to Broca's area results in transcortical motor aphasia. SMA and FAT are important for spontaneity and initiation of speech. Since the STG/SMG/SLF/AF network is preserved, repetition is spared in transcortical motor aphasia. Damage to the precentral gyrus (middle inferior part) and its subcortical area (area 7) results in speech apraxia (distortion and mal-connection of phonemes)
Clinical evidence for the dual language model
Lesion deficit correlation studies from stroke patients with aphasia also support the dual stream pathway, moving from a nodular to network perspective.[4,17,18,19] Mirman et al. assessed language in 99 patients with persistent acquired deficits along with high quality structural neuroimaging analysis.[19] They found that suprasylvian regions were associated with speech production (in agreement with dorsal stream), infrasylvian regions were associated with speech recognition (in agreement with ventral stream) and semantic production/recognition deficits were associated with damage in extra-sylvian regions. For semantic production the middle and inferior temporal gyri (anterior temporal lobe) were involved, while for multimodality semantic recognition – 'bottleneck' frontal white matter region where information from other brain regions converges, was found to be the most important.
Fridriksson et al. examined the effect of cortical damage and disconnection involving the dorsal and ventral streams on aphasic impairment in 159 chronic stroke survivors (at 36 months post stroke) using speech and language testing, lesion symptom mapping (3T MRI) and connectome symptom mapping (DTI).[18] Results revealed that damage to the dorsal stream correlated to measures of motor speech impairment, while ventral stream damage was associated with impaired speech comprehension. Naming, repetition and grammar relied on interactions between the two streams, hence impairment could occur due to damage in broader networks. Similar findings were seen for reading and writing subtests.
Role of subcortical structures
Aphasia secondary to predominant or exclusive basal ganglia and thalamic lesions, wherein the language cortices and their white matter connections are largely spared, is categorized as subcortical aphasia. The caudate nucleus by virtue of its frontal connections with the supplementary motor area and the cingulum through the subcallosal fasciculus plays an important role in initiation of motor actions. Intraoperative stimulation of the subcallosal fasciculus produces deficits in speech initiation with intact repetition, similar to what is seen after resection of tumours in the dominant SMA.[14] The putamen is also involved in motor functions. Despite lot of heterogeneity, it is widely accepted that subcortical aphasias are milder and have a better long-term prognosis.
Radanovic and Mansur et al. reviewed 57 studies on language disturbances after vascular lesions in the basal ganglia (involving 303 patients) and reported weak clinic-anatomical correlations.[20] In this review, patients with left basal ganglia lesions demonstrated language disturbance in acute phase (information available from 180 patients) in 60.6% cases (46.6% - anomia, 30% - comprehension deficits, 26% - repetition deficit, 20.5% - paraphasias). Between 1-6 months of ictus (information from 69 cases), 85.5% had language symptoms (47.8% - anomia, 39.1% - comprehension deficits and 28.9% - repetition deficits) and 6 months after the ictus (information from 39 cases) 74.4% had language dysfunction (56.4% - anomia, 53.8% - comprehension deficits, and 43.5% - repetition deficits). At least 50% cases had evidence that persistent language dysfunction was due to associated hypoperfusion of language cortices, remaining lacked a complete information.
Multiple mechanisms – direct and indirect, have been proposed over decades to explain the effects of basal ganglia on language functions. The indirect mechanisms include the disconnection hypothesis [interruption of connecting pathways between the classic language areas, initially advocated by Wernicke and Lichtheim, later revisited by Alexander et al.,[21]] diaschisis [cortical hypoperfusion disclosed by SPECT studies[22]] and, impaired release of cortical language segments.[23,24] As per the latter hypothesis, basal ganglia regulate the flow of excitatory impulses from the anterior ventral nucleus of thalamus to the language cortex. Lesions of the caudate nucleus impair the normal inhibition of the caudate on the globus pallidus, leading to thalamic over-inhibition by the globus pallidus, resultant reduced cortical activation and non-fluent aphasia. Lesions of the globus pallidus impair the normal inhibition of the globus pallidus on the thalamus, leading to thalamic disinhibition, increased cortical activation and resultant fluent aphasia.
On the other hand, direct effect of lesions of subcortical structures on language functions are difficult to quantify, due to small size of the structures involved and, vascularization patterns which render the involvement of subcortical structures alone impossible except for lacunar infarctions. Nevertheless, some authors have tried to investigate the role of basal ganglia in aphasia genesis through the study of circumscribed lesions. In a study of 9 patients with infarcts in the caudate head, putamen and anterior limb of internal capsule in the dominant hemisphere, authors found dysarthria, dysprosody, non-fluent aphasia and rapid resolution of symptoms.[25] The authors postulated that striatal lesions affect movement programming and perception organization, and capsular lesions interrupt several critical pathways involved in language genesis (thalamus to motor and prefrontal cortex, frontal cortex to pons and auditory cortex to head of caudate nucleus). This work was reinforced by the findings of Mega and Alexander who highlighted deficits in language production (with preserved comprehension and repetition) in striato-capsular lesions.[26]
Three aphasic syndromes have been proposed after putamino-capsular lesions by Naeser et al.[27]
Putamino-capsular lesion with antero-superior extension lead to impaired articulation and naming with preserved comprehension.
Putamino-capsular lesion with posterior extension produce fluent aphasia with paraphasias and severely affected comprehension.
Putamino-capsular lesions with anterior and posterior extension produce global aphasia.
Other authors have similarly tried to establish a correlation between anterior lesions and non-fluent aphasia, and posterior lesions with fluent aphasia.[28]
Recently role of haemodynamics is being put forth to explain subcortical aphasias.[29,30] As per this theory, the occlusion of proximal MCA or ICA leads to hemodynamic compromise of the entire vascular territory which includes the language cortex (insula and surrounding areas) and their connecting pathways in the white matter. Collateral circulation precludes the development of infarct in the cortical areas. Subcortical structures are devoid of collateral benefits and hence get infarcted. The transient hemodynamic compromise of the language cortex and the connecting white matter is enough to produce language dysfunction in the absence of a visible cortical infarct on imaging. Similarly, occlusion of the anterior choroidal artery affects the posterior limb of internal capsule, disconnecting the thalamus from the cortex, leading to thalamic aphasia. Hillis et al. prospectively evaluated a consecutive series of 115 patients who presented within 24 h of onset or progression of stroke symptoms, with MRI sequences including diffusion weighted imaging (DWI) and perfusion weighted imaging (PWI), and detailed testing for aphasia or hemispatial neglect. Of 44 patients with only subcortical lesions on DWI, all with aphasia or neglect had cortical hypoperfusion on PWI. In 6 intervention successfully restored perfusion, with complete resolution of aphasia.[31]
Haemorrhagic lesions produce mass effect and ischemic injury in the surrounding structures. So in haemorrhagic lesions, mass effect precludes the establishment of meaningful anatomic-clinical correlations. Studies based on tractography might yield novel information regarding the role of subcortical structures in language function.
Role of thalamus
The role of thalamus in language function has been known since long. Hillemand (1925) and Lhermitte (1936) first described atypical aphasic symptoms after a pathologically confirmed left thalamic haemorrhage. Later Fisher (1959) confirmed that aphasia is just next to gnostic and sensory deficits, amongst the main clinical features of left thalamic haemorrhages.[32] In the sixties data from stereotactic surgery, thalamotomies and electric stimulation of the thalamus brought in the role of certain thalamic nuclei in cognition. This was followed by insights from structural and functional neuroimaging studies, including diffusion tensor imaging in the recent times.
In 2011, De Witte et al., critically reviewed 465 patients with vascular thalamic lesions published in the literature since 1980.[33] 75% had a thalamic infarction, 25% haemorrhage, and 90% patients had a left thalamic lesion. Those who met at least four out of the following six operational criteria: 1) fluent output, 2) normal or mildly impaired comprehension skills, 3) normal or mildly impaired repetition, 4) moderate to severe anomia characterised by semantic paraphasias, neologisms and perseverations, 5) hypophonia and/or mild articulation deficits and 6) reduction of spontaneous speech or verbal aspontaneity, were given the diagnosis of thalamic aphasia. A detailed neurocognitive analysis in 42 out of 465 (9%) cases with isolated thalamic lesions revealed that 64% with left thalamic lesion met 4 out of 6 designated features of the above semiological prototype. The study also found a left hemisphere specialisation for language skills for right-handed adults.
In a study by Osawa et al. on 71 patients with new onset acute left thalamic haemorrhage (excluding those with a prior neurodegenerative disease or stroke, traumatic brain injury, those who received surgical treatment or tracheostomy) 59 patients had language dysfunction (evaluated at a median of 4 days, range 1-21 days).[34] Wernicke's aphasia was the most common (26 patients) followed by anomic (15 patients) and transcortical sensory types (7 patients). Non-fluent aphasia was seen in 6 patients and global in 4, both correlated to larger hematoma volumes.
Also, lesions in the anterior thalamic nuclei (ventral and medial thalamic nuclei) are associated with non-fluent aphasia, while lesions in the pulvinar cause fluent aphasia.[35] This can be understood from the fact that the anterior thalamic nuclei receive input from the globus pallidus and constitute the motor loop that projects to the cortical motor, supplementary motor and the premotor area including Broca's area. The pulvinar bidirectionally communicates with the temporal (including Wernicke's area), parietal and occipital lobe.[36]
Mechanistically, it is proposed that the dominant thalamus activates the cortical language areas and integrates the linguistic function. In the 'dichotomous state' described by Mohr et al., patients with thalamic lesions became paraphasic when sleepy, but spoke clearly when awake.[37] Luria had also described thalamic aphasia as a 'quasiaphasic disturbance of vigilance'.[38] Nadeau et al. have also tried to explain thalamic aphasia via disruption of attentional gating or working memory.[30] In a study by Sebastian et al., twenty patients with isolated acute thalamic infarcts (10 right and 10 left) underwent MRI scanning and detailed cognitive testing.[39] Results revealed that 5 out of 10 patients with left thalamic infarcts had aphasia and only one had cortical hypoperfusion. These findings indicated that, unlike other subcortical regions, aphasia in isolated left thalamic infarcts may not be associated with cortical hypoperfusion.
Role of right (non-dominant) hemisphere
Electrophysiological studies of the Broca's area have revealed a sequential processing of language information within a time period of 450 ms.[40] However, in reality, the processes may occur simultaneously, with dynamic interactions amongst various cortical areas and even bilateral hemispheres. Recent studies reveal that the processes of voice perception, phoneme processing and sensorimotor transformations of speech involve both hemispheres.[41,42,43] Cogan et al. used direct neural recordings in human subjects during a word repetition task. They found that electrodes over bilateral inferior frontal, inferior parietal, superior temporal, premotor, and somatosensory cortices exhibited robust sensory-motor neural responses during both perception and production of words.[41] Obleser et al. used independent manipulations of the temporal and the spectral detail of spoken words in a listening task, and demonstrated differential sensitivity of the left and right auditory cortex in discriminating the temporal and spectral dimensions of speech, respectively.[42] The role of bilateral superior temporal gyri in prelexical speech perception is also supported by a review of 100 fMRI studies of speech comprehension and production published in 2009.[43] The higher order language functions like semantics and grammar are thought to be lateralized to the dominant hemisphere.
As far as the subcortical structures are concerned, there is little evidence in literature supporting role of non-dominant hemisphere. In the review by Radanovic and Mansur, right hemispheric lesions did not exhibit language disturbances.[44] The evidence is anecdotal. Repetition deficits were reported in 4 right-handed Chinese patients with right putaminal lesions.[45] Repetition deficits due to lesion in right lentiform nucleus and internal capsule were also reported in another case, but the patient also had cortical hypoperfusion on SPECT.[46] Transcortical sensory aphasia was reported in the acute phase in a right handed patient with right hemorrhagic lentiform lesion.[47]
To summarize, voice perception and some functions of the ventral (phoneme processing) and dorsal stream (motor transformation) are bilaterally organized. Rest of the language functions are more left hemisphere dominant. It is possible that when the core language areas in left hemisphere are intact, the homologue areas in right hemisphere remain silent, but if the left hemisphere is already lesioned, the role of right hemisphere becomes important.[48]
Role of cerebellum
Although the role of cerebellum does not figure in either the classical or the modern models, it has been reported to result in aphasia and play a role in language functions.[49,50] The cognitive affective role of cerebellum has been attributed to its connections with the cortical and limbic association areas. This role is supported by clinical studies identifying language impairment after cerebellar lesions in children and adults, and functional MRI studies identifying the impact of cerebellar lesions on remote structurally intact cortical areas. Transcranial direct current stimulation of right posterolateral cerebellum has shown to enhance effects on verbal fluency.[51]
Clinical implications
Aphasias are a common consequence of stroke and some neurodegenerative brain disorders like primary progressive aphasias. The anatomical and functional localization of important aphasic syndromes, in the context of new models is summarized in Table 1.
Table 1.
Summary of localization of important aphasic syndromes
| Aphasia type | Localization | Characteristics | |
|---|---|---|---|
| Functional | Anatomical | ||
| Wernicke’s aphasia | Phonemic retrieval Plus Semantic system* |
pSTG, SMG (G) SLF, AF (WM) MTG, ITG, AG (G) MLF, IFOF (WM) |
Phonemic paraphasias Impaired repetition and naming Semantic paraphasias Impaired word and sentence comprehension Intact fluency due to preserved dorsal stream |
| Transcortical sensory aphasia | Semantic system** | MTG, ITG, AG (G) MLF, IFOF (WM) |
Impaired comprehension Semantic paraphasias Intact repetition due to preserved phonemic retrieval syste Intact fluency due to preserved dorsal stream |
| Conduction aphasia | Phonemic retrieval | pSTG, SMG (G) SLF, AF (WM) |
Phonemic paraphasias Impaired repetition and naming Intact comprehension and fluency |
| Transcortical motor aphasia | Dorsal stream | SMA (G) FAT (WM) |
Impaired fluency Intact comprehension and repetition due to preserved ventral stream and phonemic retrieval respectively |
| Broca’s aphasia | Dorsal stream Fronto-temporal subnetwork |
IFG, Middle and inferior precentral gyrus (G) SLF, AF (WM) UF/IFOF mediated |
Impaired fluency Impaired repetition and naming Impaired syntax (grammar) Intact comprehension due to preserved ventral stream, except for comprehension of sentences with complex syntax |
| Non fluent PPA | Dorsal stream | Broca’s area STG, anterior insula |
Effortful speech Agrammatism Impaired comprehension of sentences with complex syntax Intact single word comprehension and object knowledge |
| Semantic PPA | Semantic system | Anterior MTG, ITG (temporal pole) | Impaired single word comprehension, object knowledge and confrontation naming Intact repetition, grammar and motor speech |
| Logopenic variant PPA | Phonemic retrieval | Posterior STG, adjacent SMG | Impaired repetition, single word retrieval in spontaneous speech and naming, phonemic paraphasias Intact single word comprehension, object knowledge and motor speech |
*Left inferior frontal cortex plays a role in initiating and controlling activation of semantics,[11,12,13] while the temporal and inferior parietal components are the actual repositories of concepts ** left prefrontal lesions have also been reported to produce transcortical sensory aphasia.[52,53,54] pSTG = posterior superior temporal gyrus, SMG = supramarginal gyrus, SLF = superior longitudinal fasciculus, AF = arcuate fasciculus, MTG = middle temporal gyrus, ITG = inferior temporal gyrus, AG = angular gyrus, IFOF = inferior frontal occipital fasciculus, SMA = supplementary motor area, FAT = frontal aslant tract, UF = uncinate fasciculus, PPA = primary progressive aphasia, G = grey, WM = white matter
Many patients with post stroke aphasia show some spontaneous recovery, the rate of which slows over time. In the study by Fridriksson et al. mentioned previously,[18] damage to specific cortical hubs like the Broca's area, supramarginal gyrus, angular gyrus and posterior superior temporal gyrus was associated with persistent language deficits after 6 months of stroke. In the dual stream language model, damage to one pathway can induce plasticity related changes in the intact pathway to take up the function of the damaged pathway. For example, transcranial magnetic stimulation mediated inhibition of the angular gyrus (a key semantic region) in healthy individuals has been shown to increase semantic task-related activity in the supramarginal gyrus (a phonological region). Collecting this form of data in post stroke aphasia patients may contribute to aphasia rehabilitation in the future.
Understanding the dual language model can also provide better insights to neurosurgeons in planning resection boundaries when operating within the peri-sylvian region or near other subcortical systems involved in processing of language. Earlier, the focus was largely on preserving the eloquent cortex through cortical mapping studies without real time evaluation of white matter. But considering the network approach, evaluation of the white matter with electrical stimulation should be considered equally important. Selection of the language task for cortical and subcortical mapping should be based on the functions of the area planned for surgery and patient's background. Picture naming is the most commonly used task during awake surgeries as it involves multiple steps in language processing.[55] Counting tasks are used for articulation. For temporal and parietal lobe lesions semantic tasks like pyramid and palm tree test can be used, for lesions around the supramarginal gyrus and SLF, repetition task is used. The IFOF which is an important association fibre bundle in semantic processing needs to be kept in mind during deeper surgical fields while operating insular or opercular gliomas. The frontal aslant tract may be encountered underneath the middle frontal gyrus and superior frontal sulcus, during frontal lobe tumour resection. Electrical stimulation of this area produces slowness or speech arrest.
CONCLUSIONS
To conclude, the classic models of language organization upheld a motor focus, a sensory focus and a connecting fasciculus for auditory motor interaction. Advances in neuroimaging and stimulation mapping have brought new insights to the neurobiology of language. Recent models emphasize two parallel, interconnected dynamic pathways – the dorsal stream involved in phonological processing, the ventral stream involved in semantic processing and also underscore the importance of connecting white matter pathways. The patho-physiology behind basal ganglia lesions and language dysfunction is largely linked to cortical hypoperfusion, but the same does not hold true for thalamic aphasias. Although language functions are predominantly sub-served by the dominant hemisphere, both hemispheres play a role in sound perception, phoneme processing and sensorimotor transformations. A thorough understanding of the functional anatomy of language will not only enhance the understanding of various language syndromes, but help in good neurosurgical planning and in designing rehabilitation studies for patients of aphasia.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Dronkers NF, Plaisant O, Iba-Zizen MT, Cabanis EA. Paul Broca's historic cases: High resolution MR imaging of the brains of Leborgne and Lelong. Brain. 2007;130:1432–41. doi: 10.1093/brain/awm042. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JM, Gilmore R, Roper S, Crosson B, Bauer RM, Nadeau S, et al. Conduction aphasia and the arcuate fasciculus: A reexamination of the Wernicke–Geschwind model. Brain Lang. 1999;70:1–12. doi: 10.1006/brln.1999.2135. [DOI] [PubMed] [Google Scholar]
- 3.Quigg M, Fountain NB. Conduction aphasia elicited by stimulation of the left posterior superior temporal gyrus. J NeurolNeurosurg Psychiatry. 1999;66:393–6. doi: 10.1136/jnnp.66.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binder JR. Current controversies on Wernicke's area and its role in language. Curr Neurol Neurosci Rep. 2017;17:58. doi: 10.1007/s11910-017-0764-8. [DOI] [PubMed] [Google Scholar]
- 5.Pillay SB, Stengel BC, Humphries C, Book DS, Binder JR. Cerebral localization of impaired phonological retrieval during rhyme judgment. Ann Neurol. 2014;76:738–46. doi: 10.1002/ana.24266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leyton CE, Ballard KJ, Piguet O, Hodges JR. Phonologic errors as a clinical marker of the logopenic variant of PPA. Neurology. 2014;82:1620–7. doi: 10.1212/WNL.0000000000000387. [DOI] [PubMed] [Google Scholar]
- 7.Mesulam M-M, Thompson CK, Weintraub S, Rogalski EJ. The Wernicke conundrum and the anatomy of language comprehension in primary progressive aphasia. Brain. 2015;138:2423–37. doi: 10.1093/brain/awv154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duffau H, Moritz-Gasser S, Mandonnet E. A re-examination of neural basis of language processing: Proposal of a dynamic hodotopical model from data provided by brain stimulation mapping during picture naming. Brain Lang. 2014;131:1–10. doi: 10.1016/j.bandl.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 9.DeWitt I, Rauschecker JP. Phoneme and word recognition in the auditory ventral stream. PNAS. 2012;109:E505–14. doi: 10.1073/pnas.1113427109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hickok G, Poeppel D. Dorsal and ventral streams: A framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Thompson-Schill SL, D'Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proc Natl Acad Sci USA. 1997;94:14792–7. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner AD, Paré-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: Left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–38. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- 13.Novick JM, Trueswell JC, Thompson-Schill SL. Cognitive control and parsing: Reexamining the role of Broca's area in sentence comprehension. Cogn Affect Behav Neurosci. 2005;5:263–81. doi: 10.3758/cabn.5.3.263. [DOI] [PubMed] [Google Scholar]
- 14.Chang EF, Raygor KP, Berger MS. Contemporary model of language organization: An overview for neurosurgeons. J Neurosurg. 2015;122:250–61. doi: 10.3171/2014.10.JNS132647. [DOI] [PubMed] [Google Scholar]
- 15.Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19:2767–96. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turken AU, Dronkers NF. The neural architecture of the language comprehension network: Converging evidence from lesion and connectivity analyses. Front Syst Neurosci. 2011;5:1. doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kümmerer D, Hartwigsen G, Kellmeyer P, Glauche V, Mader I, Klöppel S, et al. Damage to ventral and dorsal language pathways in acute aphasia. Brain. 2013;136:619–29. doi: 10.1093/brain/aws354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fridriksson J, den Ouden D-B, Hillis AE, Hickok G, Rorden C, Basilakos A, et al. Anatomy of aphasia revisited. Brain. 2018;141:848–62. doi: 10.1093/brain/awx363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mirman D, Chen Q, Zhang Y, Wang Z, Faseyitan OK, Coslett HB, et al. Neural organization of spoken language revealed by lesion-symptom mapping. Nat Commun. 2015;6:6762. doi: 10.1038/ncomms7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radanovic M, Mansur LL. Aphasia in vascular lesions of the basal ganglia: A comprehensive review. Brain Lang. 2017;173:20–32. doi: 10.1016/j.bandl.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Alexander MP, Naeser MA, Palumbo CL. Correlations of subcortical CT lesion sites and aphasia profiles. Brain. 1987;110:961–91. doi: 10.1093/brain/110.4.961. [DOI] [PubMed] [Google Scholar]
- 22.Perani D, Vallar G, Cappa S, Messa C, Fazio F. Aphasia and neglect after subcortical stroke. A clinical/cerebral perfusion correlation study. Brain. 1987;110:1211–29. doi: 10.1093/brain/110.5.1211. [DOI] [PubMed] [Google Scholar]
- 23.Crosson B. Subcortical functions in language: A working model. Brain Lang. 1985;25:257–92. doi: 10.1016/0093-934x(85)90085-9. [DOI] [PubMed] [Google Scholar]
- 24.Wallesch CW, Papagno C. Subcortical aphasia. In: Rose FC, Whurr R, Wyke MA, editors. Aphasia. London: Whurr; 1988. pp. 256–287. [Google Scholar]
- 25.Damasio AR, Damasio H, Rizzo M, Varney N, Gersh F. Aphasia with nonhemorrhagic lesions in the basal ganglia and internal capsule. Arch Neurol. 1982;39:15–24. doi: 10.1001/archneur.1982.00510130017003. [DOI] [PubMed] [Google Scholar]
- 26.Mega MS, Alexander MP. Subcortical aphasia: The core profile of capsulostriatal infarction. Neurology. 1994;44:1824–9. doi: 10.1212/wnl.44.10.1824. [DOI] [PubMed] [Google Scholar]
- 27.Naeser MA, Alexander MP, Helm-Estabrooks N, Levine HL, Laughlin SA, Geschwind N. Aphasia with predominantly subcortical lesion sites: Description of three capsular/putaminal aphasia syndromes. Arch Neurol. 1982;39:2–14. doi: 10.1001/archneur.1982.00510130004002. [DOI] [PubMed] [Google Scholar]
- 28.Cappa SF, Cavallotti G, Guidotti M, Papagno C, Vignolo LA. Subcortical aphasia: Two clinical-CT scan correlation studies. Cortex. 1983;19:227–41. doi: 10.1016/s0010-9452(83)80016-1. [DOI] [PubMed] [Google Scholar]
- 29.Weiller C, Willmes K, Reiche W, Thron A, Isensee C, Buell U, et al. The case of aphasia or neglect after striatocapsular infarction. Brain. 1993;116:1509–25. doi: 10.1093/brain/116.6.1509. [DOI] [PubMed] [Google Scholar]
- 30.Nadeau SE, Crosson B. Subcortical aphasia. Brain Lang. 1997;58:355. doi: 10.1006/brln.1997.1707. [DOI] [PubMed] [Google Scholar]
- 31.Hillis AE, Wityk RJ, Barker PB, Beauchamp NJ, Gailloud P, Murphy K, et al. Subcortical aphasia and neglect in acute stroke: The role of cortical hypoperfusion. Brain. 2002;125:1094–104. doi: 10.1093/brain/awf113. [DOI] [PubMed] [Google Scholar]
- 32.Fisher CM. The pathologic and clinical aspects of thalamic hemorrhage. Trans Am Neurol Assoc. 1959;84:56–9. [PubMed] [Google Scholar]
- 33.De Witte L, Brouns R, Kavadias D, Engelborghs S, De Deyn PP, Mariën P. Cognitive, affective and behavioural disturbances following vascular thalamic lesions: A review. Cortex. 2011;47:273–319. doi: 10.1016/j.cortex.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Osawa A, Maeshima S. Aphasia and unilateral spatial neglect due to acute thalamic hemorrhage: Clinical correlations and outcomes. Neurol Sci. 2016;37:565–72. doi: 10.1007/s10072-016-2476-2. [DOI] [PubMed] [Google Scholar]
- 35.Vertebrobasilar Occlusive Disease: Review of Selected Aspects-Abstract-Cerebrovascular Diseases 1993, Vol. 3, No. 4-Karger Publishers [Internet]. [cited 2020 Apr 06] Available from: https://www.karger.com/Article/Abstract/108701 .
- 36.Maeshima S, Osawa A. Thalamic lesions and aphasia or neglect. Curr Neurol Neurosci Rep. 2018;18:39. doi: 10.1007/s11910-018-0844-4. [DOI] [PubMed] [Google Scholar]
- 37.Mohr JP, Watters WC, Duncan GW. Thalamic hemorrhage and aphasia. Brain Lang. 1975;2:3–17. doi: 10.1016/s0093-934x(75)80050-2. [DOI] [PubMed] [Google Scholar]
- 38.Luria AR. On quasi-aphasic speech disturbances in lesions of the deep structures of the brain. Brain Lang. 1977;4:432–59. doi: 10.1016/0093-934x(77)90036-0. [DOI] [PubMed] [Google Scholar]
- 39.Sebastian R, Schein MG, Davis C, Gomez Y, Newhart M, Oishi K, et al. Aphasia or neglect after thalamic stroke: The various ways they may be related to cortical hypoperfusion. Front Neurol. 2014;5:231. doi: 10.3389/fneur.2014.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sahin NT, Pinker S, Cash SS, Schomer D, Halgren E. Sequential processing of lexical, grammatical, and phonological information within Broca's area. Science. 2009;326:445–9. doi: 10.1126/science.1174481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cogan GB, Thesen T, Carlson C, Doyle W, Devinsky O, Pesaran B. Sensory-motor transformations for speech occur bilaterally. Nature. 2014;507:94–8. doi: 10.1038/nature12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Obleser J, Eisner F, Kotz SA. Bilateral speech comprehension reflects differential sensitivity to spectral and temporal features. J Neurosci. 2008;28:8116–23. doi: 10.1523/JNEUROSCI.1290-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Price CJ. The anatomy of language: A review of 100 fMRI studies published in 2009. Ann N Y Acad Sci. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- 44.Radanovic M, Mansur LL. Aphasia in Vascular Lesions of the Basal Ganglia: A Comprehensive Review. Brain Lang. 2017;173:20–32. doi: 10.1016/j.bandl.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Hua MS, Chen ST, Chu YC. Chinese writing function in patients with left versus right putaminalhemorrhage. J Clin Exp Neuropsychol. 2001;23:372–85. doi: 10.1076/jcen.23.3.372.1182. [DOI] [PubMed] [Google Scholar]
- 46.Radanovic M, Mansur LL, Azambuja MJ, Porto CS, Scaff M. Contribution to the evaluation of language disturbances in subcortical lesions: A piloty study. Arq Neuropsiquiatr. 2004;62:51–7. doi: 10.1590/s0004-282x2004000100009. [DOI] [PubMed] [Google Scholar]
- 47.Fromm D, Holland AL, Swindell CS, Reinmuth OM. Various consequences of subcortical stroke. Prospective study of 16 consecutive cases. Arch Neurol. 1985;42:943–50. doi: 10.1001/archneur.1985.04060090025009. [DOI] [PubMed] [Google Scholar]
- 48.Nasios G, Dardiotis E, Messinis L. From Broca and Wernicke to the Neuromodulation Era: Insights of Brain language networks for neurorehabilitation? Behav Neurol. 2019;2019:9894571. doi: 10.1155/2019/9894571. doi: 10.1155/2019/9894571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Smet HJ, Paquier P, Verhoeven J, Mariën P. The cerebellum: Its role in language and related cognitive and affective functions. Brain Lang. 2013;127:334–42. doi: 10.1016/j.bandl.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 50.Mariën P, Borgatti R. Language and the cerebellum. Handb Clin Neurol. 2018;154:181–202. doi: 10.1016/B978-0-444-63956-1.00011-4. [DOI] [PubMed] [Google Scholar]
- 51.Turkeltaub PE, Swears MK, D'Mello AM, Stoodley CJ. Cerebellar tDCS as a novel treatment for aphasia? Evidence from behavioral and resting-state functional connectivity data in healthy adults. Restor Neurol Neurosci. 2016;34:491–505. doi: 10.3233/RNN-150633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kwon M, Shim WH, Kim S-J, Kim JS. Transcortical sensory aphasia after left frontal lobe infarction: Loss of functional connectivity. Eur Neurol. 2017;78:15–21. doi: 10.1159/000477167. [DOI] [PubMed] [Google Scholar]
- 53.Sethi NK, Burke L, Torgovnick J, Arsura E. Transcortical sensory aphasia as a result of left frontal cortical-subcortical infarction. A case report. Eur Neurol. 2007;57:52–3. doi: 10.1159/000097012. [DOI] [PubMed] [Google Scholar]
- 54.Maeshima S, Osawa A, Nakayama Y, Miki J-I. Transcortical sensory aphasia following infarction in the left frontal lobe. Eur Neurol. 2004;52:125–8. doi: 10.1159/000080273. [DOI] [PubMed] [Google Scholar]
- 55.Fernández Coello A, Moritz-Gasser S, Martino J, Martinoni M, Matsuda R, Duffau H. Selection of intraoperative tasks for awake mapping based on relationships between tumor location and functional networks. J Neurosurg. 2013;119:1380–94. doi: 10.3171/2013.6.JNS122470. [DOI] [PubMed] [Google Scholar]