Abstract
Despite the success of preventive vaccination, the Human Papilloma Virus still accounts for 266,000 deaths annually, as the main causative factor of cervical, vaginal, anal, penile and oropharyngeal cancers. Human Papilloma Virus infects epithelial cells, driving tumourigenesis primarily from incorporation of DNA into the host cellular genome. Translation of two particular Human Papilloma Virus–specific oncoproteins, E6 and E7, are the key drivers of malignancy. If diagnosed early cervical, vaginal and vulval cancers have good prognosis and are treated with curative intent. However, metastatic disease carries a poor prognosis, with first-line systemic treatment providing only modest increase in outcome. Having shown promise in other solid malignancies, immune checkpoint inhibition and therapeutic cancer vaccines have been directed towards Human Papilloma Virus–associated gynaecological cancers, mindful that persistent Human Papilloma Virus infection drives malignancy and is associated with immunosuppression and lack of T-cell immunity. In this review, we discuss novel therapeutic approaches for targeting Human Papilloma Virus–driven gynaecological malignancies including vaccination strategies, use of immunomodulation, immune checkpoint inhibitors and agents targeting Human Papilloma Virus–specific oncoproteins. We also highlight the evolving focus on exciting new treatments including adoptive T-cell therapies.
Keywords: cervical cancer, Human Papilloma Virus, Human Papilloma Virus therapeutic vaccine, HPVE6/E7, T-cell therapy
Introduction
Up to 5% of all human cancers can be attributed to Human Papilloma Virus (HPV) infections.1,2 It is estimated that HPV causes 528,000 incident cancer cases and 266,000 deaths annually with the majority of the global burden in less developed regions.3 HPV infection is associated with pre-malignant and malignant lesions of the cervix, anogenital area (vulvar, vaginal, penile, anal), head and neck and oesophagus.4–8 HPV infection is one of the main causative factors in female genital tract cancer, and epidemiological studies show the presence of HPV in up to 99% of cervical cancers and between 40%–85% of all vaginal and vulvar carcinomas.9 This review will discuss the mechanisms of HPV-induced tumourigenesis and how this can be exploited therapeutically in the context of gynaecological cancers, including vaccine, T cell, immunotherapy and combination therapy.
HPV infection
HPV is the most common sexually transmitted disease worldwide, with 14 million individuals infected annually and a lifetime risk of infection greater than 80%.10 The majority of individuals will clear the virus spontaneously, however infections persist in up to 10% and leads to cancer initiation in 1% of infected people.11 Over 170 subtypes of the HPV virus have been described, of which over 40 are sexually transmitted and infect the anal, genital and oropharyngeal tracts.12 Thirteen HPV subtypes are classified as high-risk HPV (hrHPV).13 HPV-16 and 18 are the most prevalent hrHPV types and respectively account for approximately 63% and 16% of invasive cervical cancers14 and collectively for 80%–86% of vulvar and vaginal cancers, 93% of anal cancers, 63%–80% of penile cancers and 89%–95% oropharyngeal cancers.15
HPV and tumourigenesis
HPV is a circular, non-enveloped double-stranded deoxyribonucleic acid (dsDNA) virus. The HPV genome consists of approximately 8000 base pairs which encode for eight genes. These are divided according to their expression patterns into six early genes (E1, E2, E4, E5, E6, E7) and two late genes (L1 and L2).16 Early genes are associated with viral replication and transcription, whereas late genes code for the viral capsid proteins (Figure 1). Genes E6 and E7 are responsible for oncogenic transformation of host cells.
Figure 1.
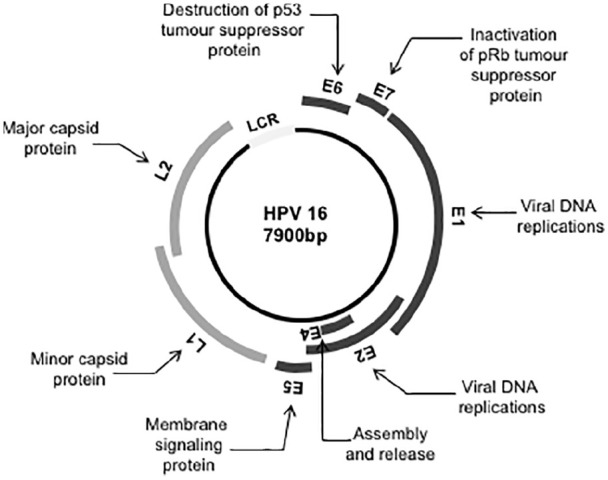
HPV16 genomic organization. The early region of the HPV genome contains six open reading frames (E1, E2, E4, E5 and E7 genes). These encode proteins required for viral replication and contribute to cell transformation. The late region codes for two proteins of the viral capsid (L1 and L2). LCR, locus control region. pRb, retinoblastoma protein.
The virus infects skin or mucosal cells by entering microlesions in the epithelium and spreading to the basal cell layer. After entering the cell, viral replication requires activation of the viral gene expression with the ultimate aim to increase the viral genome copy number. HPV is known to exist genetically either autonomously within the cytoplasm, in an episomal state, or integrated with its DNA incorporated into that of the infected host cell. Many low-grade lesions, for example CIN1 and CIN2, often only show evidence of episomal viral genetic information. Genomic integration is more likely to occur with increasing stages of carcinogenesis. Though not thought to be essential, the integration of the HPV viral genome into host DNA and the associated chromosomal instability have indeed been recognized as key events in cervical cancer pathogenesis and the transition from pre-cancerous to malignant phenotypes. This is especially apparent with HPV-16 and 18.17–19 For both types of infection, neoplastic transformation results as the HPV oncoproteins alter intrinsic DNA damage repair (DDR) systems in a bid to drive host cellular proliferation (and thus viral replication).
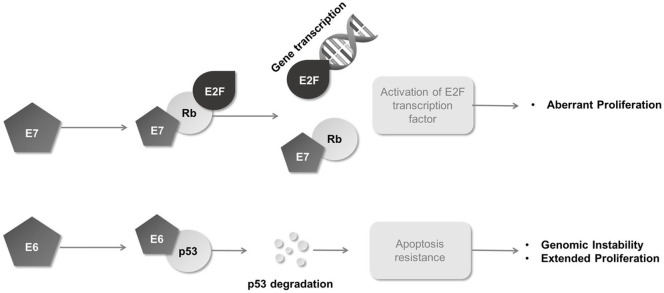
The HPV genes code for proteins involved in viral replication; HPV E7 binds to the Rb family proteins and by doing so, the cellular transcription factor E2F is released to activate expression of cell cycle promoting proteins. This allows viral replication to co-exist with cellular replication (Figure 2). In addition, HPV E6 assists in p53 degradation, thus inhibiting p53-mediated apoptosis, allowing an ongoing cell cycle for viral replication (Figure 2).20 HPV E2 protein negatively regulates E6 and E7 expression (Figure 1) and is commonly lost during integration,21 leading to a further increase in expression of E6 and E7 oncoproteins. There are now collective data to show that expression of E6 and E7 proteins in HPV infection contributes to malignant transformation by affecting almost all of the essential hallmarks of cancer, including apoptosis, proliferation, growth and invasion/motility.16 These result from HPV-driven exploitation of cell cycle checkpoints, suppressing apoptosis or promoting DNA synthesis leading to impaired DDR in host cells, making malignant transformation more likely. In cervical cancer specifically, the cellular transcription factor Activator Protein-1 (AP-1) is understood to play a vital role in carcinogenesis. Expression of E6 and E7 oncogenes is dependent on the availability of AP-1 that binds to the upstream regulatory region (URR) of HPV and in the cervical cancer cell line HeLa, an antioxidant-induced change to the AP-1 complex correlates with a tumourigenic phenotype, implying a key role for this transcription factor in malignant transformation of HPV infected cervical cells.22
Figure 2.
E6 and E7 oncogenes. E6 binds to p53 and induces its degradation. E7 binds the Rb gene product and causes the transcription factor E2F to become unbound and free to induce the cell cycle activation/proliferation. Rb, retinoblastoma protein.
Current management of recurrent or metastatic HPV-related gynaecological cancers
Early-stage cervical, vaginal and vulvar cancer are treated with radical intent with either surgery or combination chemo-radiotherapy. However, recurrent or metastatic disease is almost universally fatal and treatment is aimed at controlling symptoms and prolonging survival. Historically cisplatin chemotherapy was the standard of care for cervical cancer, with an overall response rate (ORR) between 20% and 30%, progression-free survival (PFS) of approximately 3 months and overall survival (OS) between 6.5 and 9.0 months.23 Various studies have examined the benefit of adding a second chemotherapeutic agent with some modest improvement in clinical outcomes observed. The addition of paclitaxel or topotecan to cisplatin improves ORR and PFS compared to cisplatin monotherapy, but OS remains poor at less than 10 months.24,25
The GOG 240 trial investigated the addition of bevacizumab, a monoclonal antibody against vascular endothelial growth factor, to platinum-based chemotherapy in patients with metastatic, persistent or recurrent cervical carcinoma. This increased median OS by 3.5 months compared with chemotherapy alone (16.8 vs 13.3 months, HR: 0.77 (95% CI: 0.62–0.95)),26 and thus, the combination of paclitaxel–cisplatin with bevacizumab is now considered the standard first-line therapy for metastatic or recurrent cervical cancer.
There is no standard second-line therapy for advanced cervical cancer following failure of first-line treatment. Numerous agents have been trialled including vinorelbine, topotecan, gemcitabine, nanoparticle albumin-bound paclitaxel, with a median PFS between 2 and 4 months and response rates as low as 0%–19%.27 Recent approval of pembrolizumab, an immune checkpoint inhibitor (ICI), may add improvement to these figures (discussed below), but such is the lack of conclusive and comparative efficacy data to support second-line therapy that international guidelines such as those from the European Society of Medical Oncology (ESMO) state that ‘no recommendation can be given about the most effective second-line treatment’.27
In the case of recurrent or metastatic vaginal or vulvar cancer, chemotherapy is considered when no other effective treatments (surgery or radiotherapy) are available. Several phase II studies have reported on the effects of chemotherapy for recurrent diseases but no treatment is regarded as standard of care.28–31
As there is a lack of effective treatment strategies and poor survival in these groups of patients, urgent novel approaches are required to improve outcome. Due to the almost ubiquitous presence of HPV within these malignancies, one approach is to target HPV as a therapeutic strategy.
The immune response and HPV
Bivalent and quadrivalent HPV vaccines became available in 2006 with the aim of primary prevention of HPV infection via the generation of neutralizing antibody. Each has shown more than 90% efficacy in preventing HPV type 16 and 18.32 In 2018, a nine-valent vaccine was introduced, having demonstrated efficacy at 6 years in women aged 16–26 years.33 However, the uptake of these vaccines is limited in some countries, particularly developing countries which suffer the greatest burden of this disease. Prophylactic vaccination programmes can be costly to deploy in these regions and have limited cross-protection (i.e. do not cover other hrHPV types). Though likely only effective in HPV naïve individuals, there is some debate over whether the vaccine may also prevent reinfection in those who have spontaneously cleared the virus, and in those vaccinated post-treatment for intraepithelial neoplasia.34–38 Preventive vaccines have no therapeutic action for those already infected with HPV, as they operate by mounting a humoral immune response directed against viral capsid proteins which are lost as HPV is integrated into the host genome.39 In the case of established infections, a cell-mediated immune response is required. Persistent hrHPV infections and preinvasive lesions are significantly more frequent in immunosuppressed individuals, such as untreated HIV patients 40 or recipients of anti-rejections therapies post-transplant.41 This is supported by two large studies which show that viral persistence corresponds with a lack of demonstrable HPV-specific T-cell immunity and that (transient) clearance or regression of lesions is indeed associated with the presence of HPV-specific immunity.42,43 In patients with high-grade cervical lesions, HPV 18-specific Th1 immunity is associated with higher production of IFN-gamma and thus a more dense T cell infiltrate and better clinical outcomes.44 Therefore, these data support the concept that cellular (i.e. T cell) immunity in HPV infection is important, particularly in malignant transformation.
For HPV-driven cancers, regulating the immune system is viewed as critical for controlling tumour growth. Strategies to manipulate the immune system in HPV-driven cancers can be either by direct manipulation of the immunogenicity associated with HPV oncoproteins (e.g. vaccines) or indirectly by targeting a component of the HPV-induced phenotype (e.g. ICIs).
Direct targeting of HPV in cervical cancer
The E6 and E7 oncoproteins pose attractive targets for cancer therapy. They are constitutively expressed in HPV-positive tumours, specific to the tumour, functionally important to the tumour cells and recognized by the adaptive immune system as tumour antigens. In clinical studies, T-cells specific to E6 and E7 are detectable in patients and are associated with more favourable outcomes.42,45 There are two ways in which one can induce a T-cell response against tumour antigens: either by vaccinating with the proposed antigen in a bid to create a natural T cell response in vivo or by artificially generating tumour antigen specific T-cells ex vivo and infusing them back to the patient. Both methods have been developed in treatment for HPV-induced malignancies as a form of direct targeting.
HPV Therapeutic Vaccines
The ideal vaccine activates effector killer-cells while silencing regulatory factors, generating memory cells that can mount a strong immune response and prevents reinfection by HPV and cancer relapse. Several different classes of vaccine have been developed including live vectors, protein and nucleic acid vaccines (Table 1). The majority of these vaccines are currently in the pre-clinical setting, but below we discuss those that have been tested in early phase clinical trials.
Table 1.
Therapeutic vaccine targeting HPV E6 ± E7.
| Type | Vaccine | Target |
|---|---|---|
| Live (bacterial and viral) vector-based vaccine | ADXS11-011 (bacterial) TA-HPV (viral) |
HPV-16 E7 fusion protein HPV-16 E6 and E7 peptide |
| Peptide | ISA 101 | HPV-16 E7 peptide |
| Protein | SGN-00101 | HPV-16 E7 fusion protein |
| Nucleic acid | ZYC101A VGV-3100a |
HPV-16 E7 HLA-A2 peptide Plasmid targeting HPV16/18 E6/E7 |
ADXS11-011 is a live, highly attenuated Listeria monocytogenes (Lm) bioengineered to secrete an HPV-16 E7 fusion protein. Listeria monocytogenes has been utilized for decades as an immunotherapeutic bacterial vector for the delivery of foreign antigen. In the case of ADXS11-011, Lm has been bioengineered to secrete an HPV-16 E7 fusion protein which targets HPV transformed cells, but itself acts as a natural adjuvant. The Lm vectors enter the blood stream and are internalized by antigen presenting cells (APCs) and also stimulates an innate immune response. Once inside the APCs, they secrete the pore-forming toxin listeriolysin O (LLO), a virulence factor that destroys the phagosomal membrane and therefore allows escape of the live organism from the phagolysosome. LLO is released into the APC cytosol as multiple copies of the fusion protein Lm-LLO-E7, which is then processed into peptides for presentation to and activation of tumour-associated antigen (TAA)-specific T-cells. Lm-LLO-E7 is highly immunogenic and drives the immune system by promoting the differentiation of TAA-specific T-cells against the E7 proto-oncogene, as well as innate immunity.46–48
The safety of ADXS11-011 was first assessed in a Phase 1 trial of 15 patients with previously treated recurrent or metastatic cervical cancer. The vaccine was administered at dose levels of 1 × 109, 3.3 × 109, or 1 × 1010 colony-forming units (CFU) as an intravenous infusion followed by a second dose at 3 weeks.49 Grade 3 toxicity was observed in 6 (40%) of patients, with no Grade 4 events. The highest dose was associated with fever and dose limiting hypotension; 13 patients were evaluable for response, one partial response (PR) was observed and 7 patients had stable disease (SD).
Two Phase 2 studies of ADXS11-011 in women with persistent, recurrent and/or refractory cervical cancer have been undertaken. In the GOG/NRG-0265 trial, ADXS11-011 was administered as single agent, given for three doses at 1 × 109 CFU every 28 days.50 Fifty patients were included, half of them had received more than two lines of prior therapy. One response was observed (ORR: 2%), with a disease control rate (DCR) of 32% and 12-month survival of 38%.33 In the second study, 110 women with recurrent or metastatic cervical cancer were randomized to receive either three doses of ADXS11-001 at 1 × 109 CFU or four doses of ADXS11-001 at 1 × 109 CFU with concurrent cisplatin chemotherapy.51 There was an ORR of 11% with a DCR of 44% and 12-month survival of 34%. The addition of cisplatin had no effect. Activity was observed against all hrHPV strains detected.51 Whether early introduction of ADXS11-011 can delay or prevent recurrences is currently under evaluation in the Phase 3 trial, AIM2CERV (NCT02853604). Here, 450 patients with high-risk locally advanced cervical cancer will be randomized to receive ADXS11-011 vaccine or placebo following standard chemoradiation.
While the majority of work involving HPV targeting in gynaecological cancers has been performed in cervical cancer, the same principles of treatment can be applied to HPV-associated vaginal and vulvar cancer. An example of this is the ISA101 peptide vaccine consisting of 12 synthetic long peptides derived from E6 and E7 of HPV 16.52 ISA101 was initially tested in HPV16-positive high-grade vulvar intraepithelial neoplasia (VIN).53 In a Phase 2 trial, vaccines were administered three or four times at three weekly intervals. The most common adverse events were local swelling in 100% of patients and fever in 64%, but none of these toxicities exceeding Grade 2 in severity. At 12 months of follow-up, 79% of patients had a clinical response, with 47% showing a complete response (CR; maintained for 24 months follow-up). All patients had vaccine-induced T cell responses; in an ad hoc analysis the patients with CR at 3 months had a significantly stronger T cell immune response compared to those without a CR.
Following this early data, this vaccine has been applied to advanced cervical cancer, and a Phase 1/2 of ISA101 given for three doses 2 weeks after a second, third and fourth cycles cycle of carboplatin and paclitaxel demonstrated a promising ORR of 69% (CR 6%, PR 63%) in chemotherapy naïve patients and an ORR of 26% (CR 4%, PR 21%) following two or more lines of previous chemotherapy.54 Clinical correlation of improved response was seen in patients with robust HPV 16 specific T-cell response (as measured by enzyme-linked immunospot assay), thus proving treatment intent. Further studies in cervical cancer are required to confirm these results.
Combination therapy of vaccines with other immune modulatory agents are likely to be investigated in future. Recent reports from Phase 2 testing of combination nivolumab (an ICI targeting programmed cell death 1 (PD-1)) with ISA101 has shown promising results in a HPV 16 positive patients.55 The study population consisted of 24 cancer patients who had presence of HPV 16 on archival tumour tissue analysis; 22 with oropharyngeal cancer, 1 anal cancer and 1 cervical cancer. Combination treatment resulted in an ORR of 33% (90% CI: 19%–50%). The one patient with cervical cancer achieved SD by RECIST criteria. This clinical study therefore supports the concept of combining cancer vaccination with immune checkpoint blockade. Further ISA101 combination studies are recruiting, and specifically in cervix cancer, the vaccine is being trialled with Phase 1/2 with carboplatin and paclitaxel with or without bevacizumab (NCT02128126).
Other novel methods to directly target the E6/7 include MEDI0457, a combination of synthetic plasmids targeting HPV-16 and HPV-18 E6/E7 antigens and a recombinant interleukin-12 (IL-12) encoding molecular adjuvant. This DNA vaccine is aimed to enhance immunogenicity with the addition of the plasmid cytokine adjuvant and was safe in the Phase 1 setting when given to patients with HPV-associated head and neck SCC.56 A Phase 2 in combination with the anti-PD-L1 durvalumab is currently recruiting and includes cervical cancer patients (NCT03439085).
Adoptive T-cell therapy
Adoptive T-cell therapy is a highly personalized cancer therapy whereby tumour targeted T-cells (tumour infiltrating lymphocytes or TILs) are infused directly into the patient. Durable, CRs have been observed in haematological malignancies and ongoing studies are underway in patients with solid malignancies (e.g. NCT03374839, NCT03725605, NCT03175705). It is hypothesized that the use of HPV targeted TILs will be effective in HPV-associated cancers, increasing the response. HPV-TIL therapy has many advantages over vaccine therapy; T-cells can be manipulated and selected in vitro, levels of antigen specific T-cells produced are more than 10 times that seen with traditional vaccine therapy and HPV specific T-cells persist for several months following infusion.
Stevanovic and colleagues performed a small proof of concept study and explored the use of adoptive T cell therapy in a cohort of patients with recurrent HPV-positive cervical cancer.57,58 Here, patients were treated with a single infusion of TILs cells with selected HPV E6 and E7 reactivity (HPV-TILs). Cell infusion was preceded by lymphocyte-depleting chemotherapy and was followed by administration of aldesleukin. From the initial cohort of nine patients, three objective tumour responses (two CRs and one PR) were observed. The two CRs were ongoing 67 and 53 months after treatment.57,58 The one PR was 3 months in duration. On the basis of the initial results, the cohort was expanded to include an additional nine patients with a further two PRs of 3 month duration observed.57 The HPV reactivity of T-cells in the infusion product (as measured by interferon gamma production, enzyme-linked immunospot, and CD137 upregulation assays) correlated positively with clinical response and the frequency of HPV-reactive T-cells in peripheral blood 1 month after treatment was positively associated with clinical response.58 While the HPV reactivity of TILs were predictive of response, subsequent analysis demonstrated that HPV-reactive TILs were not the immunodominant T-cells in the two patients with prolonged CR.59 Instead, T-cell reactivities directed against mutated neoantigens or a cancer germline antigen, rather than canonical viral antigens predominated.59 Thus, while this approach began as an HPV directed therapy, subsequent analysis revealed the potential of a novel treatment paradigm of targeting non-viral tumour antigens in HPV-associated cancers.59 Nevertheless, the remarkable, prolonged CR observed in two patients in this study following a single infusion of TILs suggests that cellular therapy for HPV directed cancers merits further investigation.
An alternative approach to TILs is administration of peripheral blood T-cells genetically engineered to express a tumour-targeting chimeric antigen receptor (CAR) or T-cell receptor (TCR). While encouraging results have been observed in patients with haematological malignancies, strategies utilized in epithelial cancer have generally targeted antigens shared by the tumour and normal tissue resulting in off tumour toxicity.60 The HPV oncoprotein represents an attractive target for TCR gene engineered T-cells, due to the lack of expression within normal tissue. T-cells genetically engineered to express a restricted HPV epitope have demonstrated specific recognition and killing of HPV-positive cancer cell lines in vitro.61
Indirect targeting of HPV in cervical cancer
Following HPV infection, the development of cancer is a multistep process resulting in deregulation of critical molecular processes and immune surveillance. As such, the presence of HPV infection induces a number of phenotypic changes which can be exploited therapeutically and will be explored in this section.
ICIs
In addition to the vaccine-based therapy described above, there has been an increased interest in the use of ICIs in an effort to reverse the immune privileges state seen in the tumour microenvironment. A number of ICI monoclonal antibodies are licenced for use in solid malignancies with significant improvement in clinical outcome.62,63 Essentially, ICIs work by blocking the inhibitory signals that prevent T cell activation, thus switching on the cellular immune response against tumour antigens. To date, the primary therapeutic targets of immune modulation in clinical practice include CTL antigen-4 (CTLA-4) and PD-1 which play a nonredundant role as negative regulators of immune function.64 CTLA-4, through engagement with its ligands CD80 and CD86, plays a pivotal role in attenuating early activation of naive and memory T-cells. Conversely, PD-1 is involved in modulating T-cell activity in peripheral tissues. HPV evades host immune surveillance through increased expression of programmed death ligand 1 (PD-L1), enabling viral persistence and the development of malignant lesions.65
There have been a number of studies examining the role of ICIs in advanced cervical cancer with response rates to single agent therapy ranging from 8.8% to 26.3%, with the greatest responses observed with earlier lines of treatment and PD-L1 positive tumours.66–69 On the basis of an observed ORR of 14.6% (95% CI: 7.8–24.2) in PD-L1 positive tumours within the KEYNOTE-158 trial (NCT02628067), pembrolizumab received FDA regulatory approval for use in advanced cervical cancer. Of note, the trial observed no responses in PD-L1 negative tumours. The dose of 200mg, at three-weekly intervals, is specifically licenced for patients with metastatic cervical cancer who have tumours expressing PD-L1 with evidence of disease progression post-chemotherapy.69,70 Following on from this, the Phase 3 KEYNOTE-826 trial (NCT03635567) is exploring pembrolizumab plus chemotherapy compared to chemotherapy alone in the first-line metastatic setting.
The Checkmate 358 early phase trial (NCT02488759) examined the role of nivolumab (anti-PD-1) either alone or in combination with ipililumab (anti-CTLA-4), relatlimab (anti-LAG3) or daratumumab (binds to CD38) in HPV-driven cancers (including cervical and vaginal/vulvar cancer). Results with single agent nivolumab in cervical (n = 19) and vaginal/vulvar (n = 5) patients demonstrate an ORR of 26.3% (95% CI: 9.1–51.2) and 20.0% (95% CI: 0.5–71.6) respectively. The duration of response (DOR) has yet to be reached in the five responding cervical patients (range: 23.3–29.5+ months), and was 5 months in the single vaginal/vulvar patient.68 Preliminary results from cervical patients treated with combination nivolumab 3mg/kg 2 weekly plus ipililumab 1mg/kg 6 weekly (regimen A) or nivolumab 1mg/kg plus ipililumab 3mg/kg 3 weekly ×4, followed by nivolumab 240 mg two weekly maintenance (regimen B) shows an ORR of 23.1% (n = 26; 95% CI: 9.0–43.6) and 36.4% (n = 22, 95% CI: 17.2–59.3), respectively, in previously treated patients. In patients who had received no prior therapy for their recurrent or metastatic disease, the ORR were 31.6% (n = 19, 95% CI: 12.6–56.6) for regimen A and 45.8% (n = 24, 95% CI: 25.6–67.2) for regimen B. Responses appear to be durable, and at a median follow-up of >10 months, the median DOR has yet to be reached in patients who have had no prior treatment for both regimens and was 14.6 months (regimen A) and 9.5 months (regimen B) for previously treated patients. Of note, unlike pembrolizumab, responses were noted regardless of tumour cell PD-L1 expression.71 Results from the other combination arms are awaited.
A number of prospective, randomized trials involving ICI either alone or in combination with chemotherapy or other ICIs are ongoing in both advanced and early stage disease. This includes GOG 3016 EMPOWER-Cervical 1 (NCT03257267) comparing the PD-1 inhibitor cemiplimab with investigator choice chemotherapy in patients with recurrent, persistent, or metastatic cervical cancer and SKYSCRAPER-04 (NCT04300647) comparing the PD-L1 inhibitor atezolizumab alone versus in combination with the anti-TIGIT antibody tiragolumab in patients with recurrent or metastatic PD-L1 cervical cancer.
The observation that the FDA approved pembrolizumab on the basis of a non-randomized Phase 2 trial demonstrates the significant unmet clinical need for this group of patients. Combination strategies are clearly promising, and further data are needed to increase the number of patients who benefit.
Defective signalling pathways
There are several distinct signalling pathways implicated in the deregulation of critical molecular processes that affect cell proliferation and differentiation in HPV-induced malignancies and targeted therapy against these pathways could serve as a therapeutic option for treating these cancers.
The DDR pathway represents one such approach for HPV-induced malignancies. During the oncogenic process the E7 oncoprotein inactivates Rb, while E6 promotes degradation of p53 (Figure 2), an integral component of DDR. As a consequence, HPV-positive cervical cancer cells display significantly impaired ability to activate cell cycle checkpoints and to induce apoptosis upon DNA damage.72 Furthermore, the expression of HPV E6 and E7 oncoproteins induces numerous structural chromosomal aberrations, anaphase bridges as well as elevated levels of γ-H2AX, all pointing at defective DNA repair.73–75 These observations render HPV-induced malignancies potentially vulnerable to DDR targeted therapy (reviewed extensively by Wieringa et al.).76 Many agents targeting DDR including inhibitors of ATR, ATM and DNAPK are in early phase clinical development.76 As such, in advanced cervical cancer there is interest in several early phase trials trialling modulators of DNA repair (such as PARP, ATM and ATR inhibitors) in combination with standard chemotherapy or radiotherapy (NCT01281852, NCT02223923, NCT02595879, NCT02466971).
Future directions and conclusions
Despite vaccination programmes, HPV-driven cancers are likely to remain an ongoing challenge worldwide. The prognosis for advanced, metastatic HPV-related gynaecological cancers is poor with limited treatment options. Hence, there is a major requirement to develop effective treatments to improve outcomes. An improved understanding of the critical molecular events induced by HPV-related oncoproteins has offered the opportunity to develop HPV-specific therapies. This review has summarized these novel approaches, including therapeutic vaccines, TILs, CAR-T cells, ICIs and DDR agents.
Results of ongoing clinical trials are required to conclusively determine the impact of these novel agents. To date, there are no data from large randomized Phase 3 trials to confirm the promising results observed in non-randomized studies, and thus, firm comparisons to the current standard of care are difficult. However, based on existing early-phase trial data, and extrapolating from other cancers in which immune-based therapies have proven successful, there is the promise of long-term responses in patients with advanced HPV-driven gynaecological cancers.
As with any systemic anticancer therapy, resistance, tolerance and relapse are likely. Therefore, it is important that this is explored in ongoing, prospective studies with a focus on predictive biomarkers, for example, immune cell infiltrate or immunosuppressive factors with immune therapy.77 One way to overcome resistance is a multimodal approach, and as such these novel therapies should not only be compared to the current standard of care but also tested in combination with them. This is already the case for chemotherapy and HPV vaccines.51,54 Pre-clinical data with combination radiotherapy and cancer vaccines show radiotherapy induces immunogenic tumour cell stress and death that results in enhanced sensitivity to T-cells,78,79 thus combining the two treatments could more effective than just vaccination alone for locally advanced disease. After combination with current treatment agents, the next likely pathway will be combining successful novel agents. This approach has already shown an almost doubling of median OS when vaccine and ICI are combined (compared to ICI alone),55 and as such further combination trials are likely to be proposed.
It is recognized that the greatest burden of HPV-driven malignancy is in low income countries, where many of the treatment strategies discussed above will be prohibitable for widespread use due to expense. Therefore, it is imperative that efforts remain focused on preventive strategies alongside the development of novel treatments.
In conclusion, by furthering our understanding of HPV tumourigenesis, we are now on the cusp of meaningful translational clinical progress. As such, there should be ongoing optimism for the future treatment field of HPV-driven gynaecological cancers.
Footnotes
Declaration of conflicting interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: RM reports personal fees and non-financial support from AstraZeneca, personal fees from Tesaro-GlaxoSmithKline and Roche, grants and personal fees from MSD, and personal fees from Clovis Oncology outside the submitted work. SC reports personal fees from BMS.
References
- 1. National Cancer Institute at the National Institutes of Health. HPV and cancer – National Cancer Institute. National Cancer Institute, 2015, https://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-and-cancer%0Ahttps://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-fact-sheet%0Ahttps://www.cancer.gov/about-cancer/causes-prevention/risk/infectio (accessed 27 April 2020). [Google Scholar]
- 2. de Martel C, Plummer M, Vignat J, et al. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer 2017; 141(4): 664–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO. Human papillomavirus (HPV), 2018, https://www.who.int/immunization/diseases/hpv/en/ (accessed 17 June 2019).
- 4. Gillison ML, Alemany L, Snijders PJF, et al. Human papillomavirus and diseases of the upper airway: head and neck cancer and respiratory papillomatosis. Vaccine 2012; 30: F34–F54. [DOI] [PubMed] [Google Scholar]
- 5. Serrano B, de Sanjose S, Tous S, et al. Human papillomavirus genotype attribution for HPVs 6, 11, 16, 18, 31, 33, 45, 52 and 58 in female anogenital lesions. Eur J Cancer 2015; 51(13): 1732–1741. [DOI] [PubMed] [Google Scholar]
- 6. Hansen BT, Campbell S, Nygård M. Long-term incidence trends of HPV-related cancers, and cases preventable by HPV vaccination: a registry-based study in Norway. BMJ Open 2018; 8(2): e019005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arbyn M, Weiderpass E, Bruni L, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health 2020; 8(2): e191–e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bosch FX, Manos MM, Muñoz N, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International Biological Study on Cervical Cancer (IBSCC) Study Group. J Natl Cancer Inst 1995; 87(11): 796–802. [DOI] [PubMed] [Google Scholar]
- 9. Araldi RP, Sant’Ana TA, Módolo DG, et al. The human papillomavirus (HPV)-related cancer biology: an overview. Biomed Pharmacother 2018; 106: 1537–1556. [DOI] [PubMed] [Google Scholar]
- 10. Milner D, Pecora N, Solomon I, et al. Diagnostic pathology. Infectious diseases. Amsterdam: Elsevier, 2015. [Google Scholar]
- 11. Moscicki A-B, Schiffman M, Burchell A, et al. Updating the natural history of human papillomavirus and anogenital cancers. Vaccine 2012; 30: F24–F33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bzhalava D, Guan P, Franceschi S, et al. A systematic review of the prevalence of mucosal and cutaneous human papillomavirus types. Virology 2013; 445(1–2): 224–231. [DOI] [PubMed] [Google Scholar]
- 13. Bouvard V, Baan R, Straif K, et al. A review of human carcinogens – part B: biological agents. Lancet Oncol 2009; 10(4): 321–322. [DOI] [PubMed] [Google Scholar]
- 14. Guan P, Howell-Jones R, Li N, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer 2012; 131(10): 2349–2359. [DOI] [PubMed] [Google Scholar]
- 15. Chaturvedi AK. Beyond cervical cancer: burden of other HPV-related cancers among men and women. J Adolesc Health 2010; 46(4Suppl): S20–S26. [DOI] [PubMed] [Google Scholar]
- 16. McLaughlin-Drubin ME, Münger K. Oncogenic activities of human papillomaviruses. Virus Res 2009; 143(2): 195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vinokurova S, Wentzensen N, Kraus I, et al. Type-dependent integration frequency of human papillomavirus genomes in cervical lesions. Cancer Res 2008; 68(1): 307–313. [DOI] [PubMed] [Google Scholar]
- 18. Wentzensen N, Vinokurova S, Doeberitz M, et al. Systematic review of genomic integration sites of human papillomavirus genomes in epithelial dysplasia and invasive cancer of the female lower genital tract. Cancer Res 2004; 64(11): 3878–3884. [DOI] [PubMed] [Google Scholar]
- 19. McBride AA, Warburton A. The role of integration in oncogenic progression of HPV-associated cancers. PLoS Pathog 2017; 13(4): e1006211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boulet G, Horvath C, Broeck D, et al. Human papillomavirus: E6 and E7 oncogenes. Int J Biochem Cell Biol 2007; 39(11): 2006–2011. [DOI] [PubMed] [Google Scholar]
- 21. Choo KB, Pan CC, Han SH. Integration of human papillomavirus type 16 into cellular DNA of cervical carcinoma: preferential deletion of the E2 gene and invariable retention of the long control region and the E6/E7 open reading frames. Virology 1987; 161(1): 259–261. [DOI] [PubMed] [Google Scholar]
- 22. Rösl F, Das BC, Lengert M, et al. Antioxidant-induced changes of the AP-1 transcription complex are paralleled by a selective suppression of human papillomavirus transcription. J Virol 1997; 71(1): 362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kumar L, Harish P, Malik PS, et al. Chemotherapy and targeted therapy in the management of cervical cancer. Curr Probl Cancer 2018; 42(2): 120–128. [DOI] [PubMed] [Google Scholar]
- 24. Moore DH, Blessing JA, McQuellon RP, et al. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol 2004; 22(15): 3113–3119. [DOI] [PubMed] [Google Scholar]
- 25. Long HJ, Bundy BN, Grendys EC, et al. Randomized phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix: a gynecologic oncology group study. J Clin Oncol 2005; 23(21): 4626–4633. [DOI] [PubMed] [Google Scholar]
- 26. Tewari KS, Sill MW, Long HJ, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med 2014; 370(8): 734–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marth C, Landoni F, Mahner S, et al. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018; 29(Suppl. 4): iv262. [DOI] [PubMed] [Google Scholar]
- 28. Saito T, Tabata T, Ikushima H, et al. Japan Society of Gynecologic Oncology guidelines 2015 for the treatment of vulvar cancer and vaginal cancer. Int J Clin Oncol 2018; 23(2): 201–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tropé C, Johnsson JE, Larsson G, et al. Bleomycin alone or combined with mitomycin C in treatment of advanced or recurrent squamous cell carcinoma of the vulva. Cancer Treat Rep 64(4–5): 639–642. http://www.ncbi.nlm.nih.gov/pubmed/6159078 (accessed 17 June 2019). [PubMed] [Google Scholar]
- 30. Wagenaar HC, Colombo N, Vergote I, et al. Bleomycin, methotrexate, and CCNU in locally advanced or recurrent, inoperable, squamous-cell carcinoma of the vulva: an EORTC Gynaecological Cancer Cooperative Group Study. Gynecol Oncol 2001; 81(3): 348–354. [DOI] [PubMed] [Google Scholar]
- 31. Witteveen PO, van der Velden J, Vergote I, et al. Phase II study on paclitaxel in patients with recurrent, metastatic or locally advanced vulvar cancer not amenable to surgery or radiotherapy: a study of the EORTC-GCG (European Organisation for Research and Treatment of Cancer – Gynaecological Cancer Group). Ann Oncol 2009; 20(9): 1511–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Crosbie EJ, Einstein MH, Franceschi S, et al. Human papillomavirus and cervical cancer. Lancet (London, England) 2013; 382(9895): 889–899. [DOI] [PubMed] [Google Scholar]
- 33. Huh WK, Joura EA, Giuliano AR, et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16–26 years: a randomised, double-blind trial. Lancet 2017; 390(10108): 2143–2159. [DOI] [PubMed] [Google Scholar]
- 34. Beachler DC, Kreimer AR, Schiffman M, et al. Multisite HPV16/18 Vaccine Efficacy Against Cervical, Anal, and Oral HPV Infection. J Natl Cancer Inst 2016; 108(1): djv302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kang WD, Choi HS, Kim SM. Is vaccination with quadrivalent HPV vaccine after loop electrosurgical excision procedure effective in preventing recurrence in patients with high-grade cervical intraepithelial neoplasia (CIN2-3). Gynecol Oncol 2013; 130(2): 264–268. [DOI] [PubMed] [Google Scholar]
- 36. Joura EA, Garland SM, Paavonen J, et al. Effect of the human papillomavirus (HPV) quadrivalent vaccine in a subgroup of women with cervical and vulvar disease: retrospective pooled analysis of trial data. BMJ 2012; 344(7851): e1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Miltz A, Price H, Shahmanesh M, et al. Systematic review and meta-analysis of L1-VLP-based human papillomavirus vaccine efficacy against anogenital pre-cancer in women with evidence of prior HPV exposure. PLoS ONE 2014; 9(3): e0090348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hildesheim A, Gonzalez P, Kreimer AR, et al. Impact of human papillomavirus (HPV) 16 and 18 vaccination on prevalent infections and rates of cervical lesions after excisional treatment. Am J Obstet Gynecol 2016; 215(2): 212.e1–212.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hildesheim A, Herrero R, Wacholder S, et al. Effect of Human Papillomavirus 16/18 L1 Viruslike Particle Vaccine Among Young Women With Preexisting Infection. JAMA 2007; 298(7): 743. [DOI] [PubMed] [Google Scholar]
- 40. De Vuyst H, Lillo F, Broutet N, et al. HIV, human papillomavirus, and cervical neoplasia and cancer in the era of highly active antiretroviral therapy. Eur J Cancer Prev 2008; 17(6): 545–554. [DOI] [PubMed] [Google Scholar]
- 41. Hinten F, Meeuwis KAP, van Rossum MM, et al. HPV-related (pre)malignancies of the female anogenital tract in renal transplant recipients. Crit Rev Oncol Hematol 2012; 84(2): 161–180. [DOI] [PubMed] [Google Scholar]
- 42. Woo YL, van den Hende M, Sterling JC, et al. A prospective study on the natural course of low-grade squamous intraepithelial lesions and the presence of HPV16 E2-, E6- and E7-specific T-cell responses. Int J Cancer 2010; 126(1): 133–141. [DOI] [PubMed] [Google Scholar]
- 43. Farhat S, Nakagawa M, Moscicki A-B. Cell-mediated immune responses to human papillomavirus 16 E6 and E7 antigens as measured by interferon gamma enzyme-linked immunospot in women with cleared or persistent human papillomavirus infection. Int J Gynecol Cancer 2009; 19(4): 508–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Seresini S, Origoni M, Lillo F, et al. IFN-gamma produced by human papilloma virus-18 E6-specific CD4+ T cells predicts the clinical outcome after surgery in patients with high-grade cervical lesions. J Immunol 2007; 179(10): 7176–7183. [DOI] [PubMed] [Google Scholar]
- 45. Kim KH, Greenfield WW, Cannon MJ, et al. CD4+ T-cell response against human papillomavirus type 16 E6 protein is associated with a favorable clinical trend. Cancer Immunol Immunother 2012; 61(1): 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lin C-W, Lee J-Y, Tsao Y-P, et al. Oral vaccination with recombinantListeria monocytogenes expressing human papillomavirus type 16 E7 can cause tumor growth in mice to regress. Int J Cancer 2002; 102(6): 629–637. [DOI] [PubMed] [Google Scholar]
- 47. Sewell DA, Douven D, Pan Z-K, et al. Regression of HPV-positive tumors treated with a new listeria monocytogenes vaccine. Arch Otolaryngol Head Neck Surg 2004; 130(1): 92–97. [DOI] [PubMed] [Google Scholar]
- 48. Sewell DA, Pan ZK, Paterson Y. Listeria-based HPV-16 E7 vaccines limit autochthonous tumor growth in a transgenic mouse model for HPV-16 transformed tumors. Vaccine 2008; 26(41): 5315–5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Maciag PC, Radulovic S, Rothman J. The first clinical use of a live-attenuated Listeria monocytogenes vaccine: a phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine 2009; 27(30): 3975–3983. [DOI] [PubMed] [Google Scholar]
- 50. Huh W, Brady WE, Dizon DS, et al. A prospective phase II trial of the listeria-based human papillomavirus immunotherpay axalimogene filolisbac in second- and third-line metastatic cervical cancer: a NRG oncology group trial. Gynecol Oncol 2017; 145: 220. [Google Scholar]
- 51. Petit R, Basu P. ADXS11-001 immunotherapy targeting HPV-E7: preliminary survival data from a P2 study in Indian women with recurrent/refractory cervical cancer, 2017. https://www.semanticscholar.org/paper/ADXS11-001-immunotherapy-targeting-HPV-E7%3A-survival-Petit-Basu/bfea567e71ddaa4695b171dcc4e4918ac05fb3b8 (accessed 17 June 2019).
- 52. Kenter GG, Welters MJP, Valentijn ARPM, et al. Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin Cancer Res 2008; 14(1): 169–177. [DOI] [PubMed] [Google Scholar]
- 53. Kenter GG, Welters MJP, Valentijn ARPM, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med 2009; 361(19): 1838–1847. [DOI] [PubMed] [Google Scholar]
- 54. Melief CJ, Gerritsen WR, Welters M, et al. Correlation between strength of T-cell response against HPV16 and survival after vaccination with HPV16 long peptides in combination with chemotherapy for late-stage cervical cancer. ASCO-SITC Clin Immuno-Oncology Symp 2017; 35: 140. [Google Scholar]
- 55. Massarelli E, William W, Johnson F, et al. Combining immune checkpoint blockade and tumor-specific vaccine for patients with incurable human papillomavirus 16–related cancer. JAMA Oncol 2019; 5: 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Aggarwal C, Cohen RB, Morrow MP, et al. Immunotherapy targeting HPV16/18 generates potent immune responses in HPV-associated head and neck cancer. Clin Cancer Res 2019; 25(1): 110–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stevanović S, Draper LM, Langhan MM, et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus–targeted tumor-infiltrating T cells. J Clin Oncol 2015; 33(14): 1543–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stevanovic S, Helman SR, Wunderlich JR, et al. Treatment of metastatic human papillomavirus-associated epithelial cancers with adoptive transfer of tumor-infiltrating T cells. J Clin Oncol 2018; 36(15_suppl): 3004–3004. [Google Scholar]
- 59. Stevanović S, Pasetto A, Helman SR, et al. Landscape of immunogenic tumor antigens in successful immunotherapy of virally induced epithelial cancer. Science 2017; 356(6334): 200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hinrichs CS, Rosenberg SA. Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunol Rev 2014; 257(1): 56–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Draper LM, Kwong MLM, Gros A, et al. Targeting of HPV-16+ epithelial cancer cells by TCR gene engineered T cells directed against E6. Clin Cancer Res 2015; 21(19): 4431–4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017; 377(14): 1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med 2015; 373(2): 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer 2019; 19(3): 133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yang W, Song Y, Lu Y-L, et al. Increased expression of programmed death (PD)-1 and its ligand PD-L1 correlates with impaired cell-mediated immunity in high-risk human papillomavirus-related cervical intraepithelial neoplasia. Immunology 2013; 139(4): 513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lheureux S, Butler MO, Clarke B, et al. A phase I/II study of ipilimumab in women with metastatic or recurrent cervical carcinoma: a study of the Princess Margaret and Chicago N01 Consortia. J Clin Oncol 2015; 33(15_Suppl.): 3061–3061. [Google Scholar]
- 67. Frenel J-S, Le Tourneau C, O’Neil B, et al. Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1-positive cervical cancer: results from the phase Ib KEYNOTE-028 trial. J Clin Oncol 2017; 35(36): 4035–4041. [DOI] [PubMed] [Google Scholar]
- 68. Wendel Naumann R, Hollebecque A, Meyer T, et al. Safety and efficacy of nivolumab monotherapy in recurrent or metastatic cervical, vaginal, or vulvar carcinoma: results from the phase I/II CheckMate 358 trial. J Clin Oncol 2019; 37(31): 2825–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chung HC, Ros W, Delord J-P, et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol 2019; 37(17): 1470–1478. [DOI] [PubMed] [Google Scholar]
- 70. FDA. FDA approves pembrolizumab for advanced cervical cancer with disease progression during or after chemotherapy, 2018, https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-advanced-cervical-cancer-disease-progression-during-or-after-chemotherapy (accessed 17 June 2019).
- 71. Naumann RW, Oaknin A, Meyer T, et al. Efficacy and safety of nivolumab (Nivo) + ipilimumab (Ipi) in patients (pts) with recurrent/metastatic (R/M) cervical cancer: results from CheckMate 358. Ann Oncol 2019; 30(5630): v898–v899. [Google Scholar]
- 72. Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer 2010; 10(8): 550–560. [DOI] [PubMed] [Google Scholar]
- 73. Duensing S, Münger K. The human papillomavirus type 16 E6 and E7 oncoproteins independently induce numerical and structural chromosome instability. Cancer Res 2002; 62(23): 7075–7082. [PubMed] [Google Scholar]
- 74. Havre PA, Yuan J, Hedrick L, et al. p53 inactivation by HPV16 E6 results in increased mutagenesis in human cells. Cancer Res 1995; 55(19): 4420–4424. [PubMed] [Google Scholar]
- 75. Deng W, Tsao SW, Guan X-Y, et al. Pericentromeric regions are refractory to prompt repair after replication stress-induced breakage in HPV16 E6E7-expressing epithelial cells. PLoS ONE 2012; 7(10): e48576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wieringa HW, van der Zee AG, de Vries EG, et al. Breaking the DNA damage response to improve cervical cancer treatment. Cancer Treat Rev 2016; 42: 30–40. [DOI] [PubMed] [Google Scholar]
- 77. Nowicki TS, Hu-Lieskovan S, Ribas A. Mechanisms of Resistance to PD-1 and PD-L1 Blockade. Cancer J (United States) 2018; 24(1): 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ferrara TA, Hodge JW, Gulley JL. Combining radiation and immunotherapy for synergistic antitumor therapy. Curr Opin Mol Ther 2009; 11(1): 37–42. [PMC free article] [PubMed] [Google Scholar]
- 79. Gameiro SR, Jammeh ML, Wattenberg MM, et al. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killign. Oncotarget 2014; 5(2): 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]