Abstract
Aim:
To analyze indoleamine 2,3-dioxygenase (IDO) production in the cell culture supernatant of phytohemagglutinin (PHA)-stimulated peripheral blood mononuclear cells (PBMCs) from type 2 DM (T2DM) patients and investigate IDO’s association to pro- and anti-inflammatory cytokines.
Subjects and methods:
PBMC samples were collected from 21 T2DM patients and 17 normoglycemic participants, then stimulated with PHA for 3 days. Cytokine and IDO concentrations were measured in the PBMC culture supernatants. In vitro production of TNF-α, IL-6, interferon-γ, and IL-10 were measured using multiplex immunoassay. IDO concentration was assessed using ELISA. To assess how PHA stimulation altered IDO production and to minimize the unstimulated baseline effect of T2DM, we subtracted the PHA-stimulated IDO concentration from the unstimulated one. IBM SPSS version 23 was used for statistical analysis.
Results:
The IDO concentrations in the PBMC culture supernatants were significantly higher in T2DM patients regardless of whether they were unstimulated (P < .001) or PHA-stimulated (P = .012). Reduced IDO production was observed in 52.8% of T2DM patients and was associated with older age and lower interferon-γ levels. Conversely, 42.8% of T2DM patients showed increased IDO concentrations, which were correlated with the IL-6/IL-10 ratio (r = 0.683, P = .021) and interferon-γ/IL-10 ratio (r = 0.517, P = .077).
Conclusion:
The interferon-γ level was reduced in the PBMC culture supernatant of T2DM patients with reduced IDO production. Reduced IDO production in T2DM patients following PHA stimulation was associated with older age and, notably, higher baseline IDO concentrations. Since IDO is primarily produced by dendritic cells, reduced IDO production after PHA stimulation may indicate dendritic cell dysfunction.
Keywords: Type 2 diabetes mellitus; cytokines; peripheral blood mononuclear cells; indoleamine 2,3-dioxygenase
Introduction
In 2019, the International Diabetes Foundation estimated that approximately 463 million people globally had been diagnosed with DM, and that this was predicted to rise to 576 million people by 2030.1 Almost 90% of DM were type 2 DM (T2DM), which is characterized by high blood glucose and insulin resistance.2 A chronic low-grade inflammatory condition is often associated with insulin resistance in obesity, pre-diabetes, and diabetes.3-5 This inflammatory condition is driven by chronic hyperglycemia attributed to many intracellular pathways, including oxidative stress, the polyol pathway, advanced glycation end products, and protein kinase C pathway.6 Those pathways increase the production of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-6 from macrophages, hepatocytes, and adipocytes.6,7 In another study, the Th1 and Th17 cells and also their cytokines—interferon-γ and IL-17—increased in response to the activated macrophages.8 Innate and adaptive immune activation over long periods of time disrupts anti-inflammatory response in T2DM, which is characterized by decreasing regulatory T (Treg) cells and IL-10 production.9
Indoleamine 2,3 dioxygenase-1 (IDO, formerly termed IDO-1) is an enzyme produced by dendritic cell after interferon-γ stimulation.10 This enzyme can provide negative feedback following an inflammatory response by enhancing Treg cell proliferation and dampening the effector T cells.10 Interferon-γ upregulates JAK/STAT pathway and interferon regulatory factor-3, thereby activating IDO.11 The IDO enzyme can catalyze tryptophan degradation to kynurenine. Tryptophan deficit leads to effector T cells cycle arrest and apoptosis. Kynurenine, a tryptophan metabolite, also contributes to the differentiation of naïve CD4 T cell to Foxp3+ Treg cells.12
Evaluating IDO’s role as an immunomodulator is of interest due to the relationship between low-grade inflammation and T2DM. Currently, IDO’s role as an immunomodulator in T2DM is uncertain. Several studies suggest that the effect of chronic IDO production and dysregulation of the tryptophan/kynurenine pathway leads to insulin resistance.13-16 Deleting the IDO gene in mice improved insulin sensitivity and alleviated glycemic control.17 Previous studies have also evaluated IDO activity by estimating the serum kynurenine to tryptophan ratio and measuring IDO RNA expression in specific tissues.13-17 These approaches might be less accurate for evaluating IDO as an immunomodulator because they do not measure IDO concentration directly from the immune cells. Therefore, we analyze IDO concentrations in the cell culture supernatant of phytohemagglutinin (PHA)-stimulated peripheral blood mononuclear cells (PBMCs) from T2DM patients and investigate IDO’s association with the pro- and anti-inflammatory cytokines
Subjects and Methods
Subjects
In-vitro experimental study was conducted on PBMCs from 21 T2DM patients and 17 normoglycemic participants. The T2DM patients were 32 to 55-year-old with established T2DM for at least 5 years regardless of the use of anti-diabetic medication. The normoglycemic participants were 32 to 55-year-old with fasting blood glucose (FBG) <100 mg/dL and glycosylated hemoglobin (HbA1c) <5.7%. Exclusion criteria in both groups were being pregnant, steroid use, use of non-steroidal anti-inflammatory drugs, or antibiotics within the 2 weeks preceding the study; and infection at the time of blood collection, including symptoms of fever, sore throat, respiratory tract infection, or urinary tract infection.
Ethic consideration
The study was approved by the ethical committee of the Faculty of Medicine from Universitas Indonesia (reference number 19-03-0221). All participants provided their written, informed consent to take part in this research.
Blood collection and PBMC isolation
All participants were asked to fast overnight for 10 to 12-hours before the examination. Venous blood was taken from all participants and 5 mL was placed into 1 EDTA tube and 1 lithium-heparin tube. Blood collected in EDTA tube was used to measure glucose and lipid profile—FBG, HbA1c, triglyceride, total cholesterol, low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C). Serum FBG and lipids were measured using the spectrophotometric method and HbA1c was measured using a semi-automated analyzer; Quo-Lab HbA1c analyzer (EKF diagnostics, UK) with measuring range of 4% to 15% at the National Institute of Health Research and Development, Ministry of Health, Bogor, Indonesia.
Lithium-heparin whole blood was used for PBMC isolation. Within 1 hour after sampling, 4 mL of lithium-heparin whole blood was added to sterile 15 mL-Eppendorf tubes containing 4 mL (1:1) RPMI 1640 medium. PBMCs were isolated from RPMI diluted blood by centrifugation through 4 mL of Ficoll-Paque Plus (GE Healthcare Life Sciences, USA)
Cell cultures
PBMCs were washed twice in 2 mL RPMI 1640 and resuspended in cell culture medium consisted of RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, and 1% streptomycin at a concentration of 106 cells/mL. A 500 µL cell suspension was placed in a 24-well tissue culture plate. Then, either 500 µL of 20 mM Gibco PHA-M (Thermo Fisher Scientific, California, USA) or 500 µL of cell culture medium (the control medium) was added to the wells. Therefore, the final concentration of PHA-M in the cell culture was 10 mM. Next, culture plates were incubated for 3 days at 37°C, 5% CO2. After incubation, all samples were centrifuged and the supernatants were harvested and stored at −20°C until assay. PHA, a lectin extract from red kidney beans, is used to stimulate T cells in vitro. PHA initiates cell signaling via CD2 and CD3 receptors, which leads to Ca2+ influx through calcium channels.18,19 PHA-activated Th1 cells produce IFN-γ, which eventually induce IDO production.
Cytokines analysis
Cell culture supernatants were measured for in vitro cytokines and IDO production. TNF-α, IL-6, interferon-γ, and IL-10 were analyzed using magnetic bead-based multiplex assay (R&D Systems, Minneapolis, USA).
All samples and reagents were brought to room temperature. Fifty microliters of magnetic microsphere suspension and 50 µL of the samples were added to a black 96 microwell plate. The plate was then incubated on a horizontal orbital microplate shaker at 800 rpm at room temperature for 2 hours. After a washing step, 50 µL of biotin-labeled detection antibody was added to the wells and incubated for 30 minutes on the shaker at room temperature. Following another washing step, the plate was incubated with streptavidin-phycoerythrin for 2 minutes. The samples were measured using Luminex 200 (Luminex, Austin, Texas). Detection limits were 0.6 pg/mL for TNF-α; 1.11 pg/mL for IL-6, 1.27 pg/mL for interferon γ; and 0.3 pg/mL for IL-10.
IDO analysis
The IDO concentration of the cell culture supernatant was measured using sandwich ELISA (Cloud Clone Corp, Texas, USA). All samples and reagents were brought to room temperature. One hundred microliters of the samples and standards were added to the appropriate well and incubated for 1 hour at 37°C. The liquid was removed from each well, added to 100 µL of the detection antibody, then incubated for 1 hour at 37°C. After a washing step, 100 µL of streptavidin-HRP was added and the plate was incubated for 30 minutes at 37°C. Following a second washing step, 90 µL of tetramethylbenzidine solution was added to each well and incubated for 20 minutes. Once the liquid began to turn blue, 50 µL of stop solution was added and the plate was run on the microplate reader at a wavelength of 450 nm. The IDO detection limit was 0.127 ng/mL. To assess the alteration of IDO production after PHA stimulation and to minimize the unstimulated baseline effect of T2DM, we subtracted the PHA-stimulated IDO concentration from the unstimulated one.
Data analysis
Data were analyzed using IBM-Statistical Package for the Social Sciences for Windows, Version 23.0 (IBM SPSS, IBM Corp, Armonk, NY). A Shapiro-Wilk test was used to assess normality between groups. Unpaired Student’s t-tests were used to compare the baseline characteristics between the 2 groups. The IDO concentration between the 2 groups was compared using the Mann-Whitney U test. One-way analysis of variance (ANOVA) with LSD post-hoc tests were used to compare the differences between cytokines and age among the 3 groups—normoglycemic participants, T2DM with increased IDO, and T2DM with reduced IDO—if the data were distributed normally. Otherwise, Kruskal Wallis tests were used. Spearman correlations were used to analyze the correlations between cytokine and IDO concentrations. Covariate analysis was analyzed using linear regression. Statistical significance was accepted at P < .05
Results
Subject characteristics
The baseline characteristics of the 21 T2DM patients and 17 normoglycemic participants enrolled in this study are presented in Table 1. As expected, the T2DM group had higher FBG, HbA1c, and triglyceride levels (P < .001) with lower HDL-C levels (P = .008). There were no significant differences in total cholesterol and LDL-C levels. Moreover, based on FBG and HbA1c, most of our T2DM patients were considered as poorly-controlled T2DM and only 7 out of 21 T2DM patients were well controlled. The normoglycemic group was younger and leaner than the T2DM group, which introduces a high chance of confounding factors. Therefore, further multivariate analyses included age and body mass index (BMI) as covariates.
Table 1.
Baseline characteristics of subject.
| Baseline characteristic of subjects | Normoglycemic group (n = 17) | T2DM group (n = 21) | P |
|---|---|---|---|
| Female sex (n, %) | 11 (64.7) | 11 (52.4) | .521 |
| Male sex (n, %) | 6 (35.2) | 10 (47.6) | |
| Age (years) | 40.2 ± 4.5 | 46,4 ± 6,3 | .002** |
| BMI (kg/m2) | 22.9 ± 2.6 | 27.8 ± 3.8 | <.001*** |
| FBG (mg/dL) | 92.3 ± 13.9 | 214.6 ± 96.7 | <.001*** |
| HbA1c (%) | 4.8 ± 0.5 | 9.1 ± 3.0 | <.001*** |
| Triglyceride (mg/dL) | 87.1 ± 39.5 | 175.3 ± 76.9 | <.001*** |
| Total cholesterol (mg/dL) | 189.7 ± 29.1 | 203.2 ± 33.2 | .197 |
| LDL-C (mg/dL) | 119.1 ± 28.7 | 124.7 ± 32.5 | .577 |
| HDL-C (mg/dL) | 56.1 ± 12.6 | 46.2 ± 7.3 | .008** |
Abbreviations: T2DM, type 2 diabetes mellitus; BMI, body mass index; FBG, fasting blood glucose; HbA1c, glycosylated hemoglobin; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol.
Statistical significances were calculated using unpaired Student’s t-test. Data were represented as mean ± standard deviation.
P < .01. ***P < .001.
IDO production in T2DM-PBMC culture supernatant
Figure 1 shows that supernatant from T2DM patients had significantly increased IDO concentrations in the unstimulated PBMC culture [T2DM median = 0.375 (min 0.168, max 0.730) ng/mL vs normoglycemic median = 0.241 (min 0.143, max 0.322) ng/mL, P < .001] and PHA-stimulated PBMC culture [T2DM median = 0.484 (min 0.155, max 0.817) ng/mL vs normoglycemic median = 0.322 (min 0.074, max 0.385) ng/mL, P = .012]. T2DM patients still had significantly higher IDO concentrations than normoglycemic participants in the unstimulated (P = .041) and PHA-stimulated (P = .033) culture supernatants after adjusting for BMI and age (Supplemental Table 1).
Figure 1.
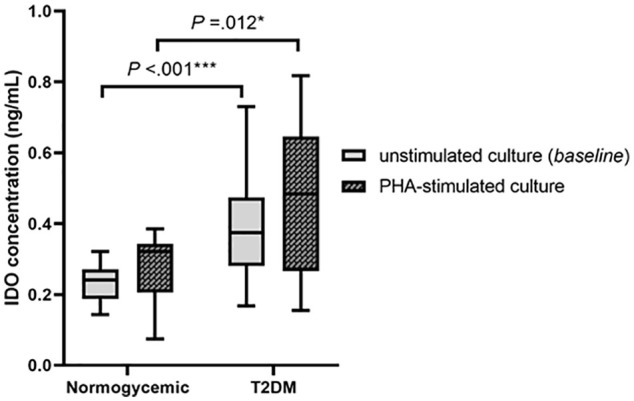
IDO concentration from PHA-stimulated and unstimulated PBMC of control subjects and Type 2 DM patients.
Abbreviations: IDO, indoleamine 2,3 dioxygenase; PHA, phytohemagglutinin, T2DM, type 2 diabetes mellitus.
Statistical significances were calculated using Mann-Whitney test.
*P < .05. ***P < .001.
To assess how PHA stimulation altered IDO production and to minimize the unstimulated baseline effect of T2DM, we subtracted the PHA-stimulated IDO production from the unstimulated one. PHA stimulation altered the IDO concentration in all participants by 0.0389 ± 0.077 ng/mL. As shown in Figure 2, we categorized the IDO productions into 3 groups according to mean ± SD: mean range, increased IDO production, and reduced IDO production. The IDO production of 13 normoglycemic participants (76.5%) was in the mean range, whereas almost all T2DM patients had IDO production outside of the mean range. Eleven T2DM patients (52.8%) had a reduced IDO production, 9 T2DM patients (42.8%) had increased IDO production, and only 1 T2DM patient had an IDO production within the mean range. Thus, we divided T2DM patients into those with increased IDO production and those with reduced IDO production. The IDO production of T2DM patient within mean IDO production range was excluded from further analyses. To reduce bias due to BMI, age, and cytokines concentration in normoglycemic group, the IDO productions of normoglycemic participant outside mean range IDO production range were also excluded.
Figure 2.
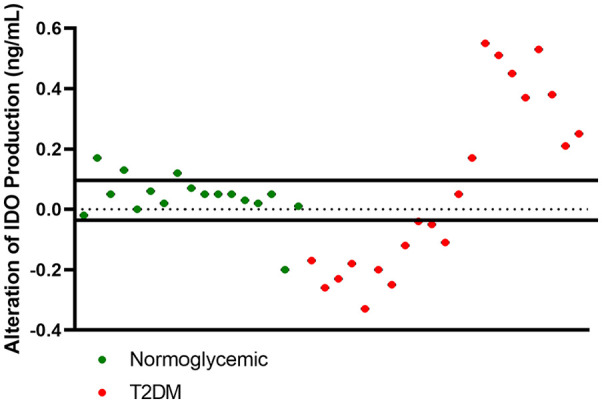
Scatter plot of alteration of IDO in the PBMC cultures supernatant from normoglycemic participants and T2DM patients.
Abbreviations: IDO, indoleamine 2,3 dioxygenase; T2DM, type 2 diabetes mellitus; PBMC, peripheral blood mononuclear cells.
Cytokines and associated IDO production in PBMC culture
T2DM patients had increased obesity rates compared to normoglycemic participants, but there was no BMI difference between the T2DM patients with increased IDO production and those with reduced IDO production. Table 2 also shows a significant effect of age on IDO production in PBMCs after PHA stimulation. Increased IDO production was observed in PBMCs from younger T2DM patients. Meanwhile, PBMCs from older T2DM patients tended to produce lower IDO concentrations after PHA stimulation, even though they had higher baseline IDO concentrations. The T2DM patients with reduced IDO production had significantly lower interferon-γ levels compared to normoglycemic participants (P = .004). Meanwhile, TNF-α, IL-6, and IL-10 production did not significantly differ across groups (Table 2).
Table 2.
Comparison of BMI, age, IDO, and cytokine concentrations of PBMC culture supernatants from mean range-normoglycemic participants, T2DM patients with increased IDO production, and T2DM patients with reduced IDO production.
| Parameters | Normoglycemic participants | T2DM patients with higher IDO production | T2DM patients with reduced IDO production |
|---|---|---|---|
| No of persons (n) | 13 | 9 | 11 |
| BMI (kg/m2) | 23.04 ± 2.98 | 27.4 ± 2.19** | 28.17 ± 5.02** |
| Age (years) | 41.38 ± 4.23 | 43.33 ± 5.85# | 48.27 ± 5.83*** |
| Baseline IDO (ng/mL) | 0.236 ± 0.06 | 0.295 ± 0.09### | 0.498 ± 0.14*** |
| PHA-stimulated IDO (ng/mL) | 0.270 ± 0.07 | 0.674 ± 0.09*** ### | 0.321 ± 0.13 |
| Alteration of IDO production (ng/mL) | 0.034 ± 0.02 | 0.378 ± 0.14*** ### | −0.176 ± 0.09*** |
| TNF-α (pg/mL) | 6,843.25 ± 3,531.24 | 7,555.72 ± 3,450.36 | 5,375.87 ± 3,344.72 |
| IL-6 (pg/mL) | 368.40 [32.73-960.00] | 380.40 [179.27-1,321.50] | 339.32 [9.79-1,189.53] |
| Interferon-γ (pg/mL) | 10,710.83 ± 5,434.34 | 8,614.80 ± 3,039.96 | 5,820.79 ± 1,343.43** |
| IL-10 (pg/mL) | 111.52 [34.56-485.90] | 142 [54.70-982.87] | 89.18 [6.46-385.91] |
Abbreviations: BMI, body mass index; IDO, indoleamine 2,3-dioxygenase; PHA, phytohemagglutinin; TNF-α, tumor necrosis factor-α; IL-6, interleukin 6; IL-10, interleukin 10; T2DM, type 2 diabetes mellitus.
BMI, age, baseline IDO concentration, PHA-stimulated IDO concentration, alteration of IDO production, TNF-α, and interferon-γ data are presented as mean ± standard deviation and analyzed using one-way ANOVAs with LSD for post-hoc analysis. IL-6 and IL-10 data are presented as median [min-max] and statistical significance was calculated using the Kruskal Wallis test.
P < .01 versus normoglycemic participants. ***P < .001 versus normoglycemic participants. #P < .05 versus T2DM with reduced IDO production. ###P<.001 versus T2DM with reduced IDO production.
We compared the IDO concentration from PHA-stimulated culture supernatants and pro/anti-inflammatory cytokines ratio in each group. As shown in Table 3, the IDO concentration in T2DM patients with increased IDO production was moderately and positively correlated with the IL-6/IL-10 ratio (P = .021) and interferon-γ/IL-10 ratio (P = .077). Conversely, the IDO concentration in T2DM patients with reduced IDO production was not correlated with the pro-/anti-inflammatory cytokine ratios.
Table 3.
Correlation between the IDO concentration and ratio of pro-/anti-inflammatory cytokines in PHA-stimulated PBMC supernatant from normoglycemic participants and T2DM patients.
| Parameter | Normoglycemic participants (n = 13) |
T2DM patients with higher IDO production (n = 9) |
T2DM patients with reduced IDO production (n = 11) |
|||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| TNF-α/IL-10 ratio | −0.003 | .496 | 0.383 | .154 | −0.155 | .325 |
| IL-6/IL-10 ratio | 0.340 | .128 | 0.683 | .021* | 0.127 | .355 |
| Interferon-γ/IL-10 ratio | 0.440 | .066 | 0.517 | .077 | 0.009 | .489 |
Abbreviations: IDO, indoleamine 2,3-dioxygenase; TNF-α, tumor necrosis factor-α; IL-6, interleukin 6; IL-10, interleukin 10; T2DM, type 2 diabetes mellitus.
All correlations were calculated using Spearman correlations.
*P < .05.
Discussion
The current study reveals 3 findings of primary significance. First, IDO concentrations were higher in the unstimulated and PHA stimulated-PBMCs of T2DM patients compared to normoglycemic individuals, even after accounting for BMI and age. Second, PHA stimulation in the T2DM patients produced IDO levels that were higher or lower than the mean range. Third, reduced IDO production in PHA-stimulated PBMCs from T2DM patients was associated with higher BMI, older age, and lower interferon-γ concentrations.
To the best of our knowledge, this is the first study that analyzing IDO production following PHA stimulation from PBMCs collected from T2DM patients. The current results provide new insight into IDO’s mechanism underlying regulation of chronic low-grade inflammatory response in type 2 DM. Increased IDO concentrations were observed in PBMCs from T2DM patients compared to normoglycemic participants. This finding is consistent with other studies that showed that IDO enzymatic activity, analyzed by kynurenine/tryptophan ratio increased in T2DM13 and patients with diabetic nephropathy.15 Moreover, the subcutaneous fat of obese patients shows a decreased Th17/Treg ratio that is correlated with an increased kynurenine/tryptophan ratio.20 These results reflect that IDO production acts as the compensatory mechanism to provide negative feedback to reduce low-grade chronic inflammatory condition in T2DM patients. We hypothesize that low-grade chronic inflammatory condition would promote dendritic cells and macrophages to increase IDO enzyme production to suppress the activity of effector T cells, even in the absence of stimulation.
We observed that T2DM patients with reduced IDO production were older and had a higher baseline IDO level with lower PHA-stimulated IDO concentrations compared to T2DM patients with increased IDO production. The IDO concentration in PBMC culture supernatants might be influenced by chronic innate immune activation due to aging, known as inflamm-aging.21 Marttila et al22 found that aging was associated with increased IDO activity in serum collected in non-stimulated and non-acute conditions. The dendritic cells of aged people have increased NF-κB expression, type 1 interferon, and IL-6 levels after stimulation with self-antigen,23,24 without an induction of tolerance.25 The current study revealed a loss of tolerance in aging through reduced IDO production in PHA-stimulated PBMC cultures.
Pro-inflammatory cytokines play an essential role in IDO production.26,27 In T2DM patients with reduced IDO production, the low interferon-γ level is the vital factor that may contribute to decreased IDO production,28 even though there was no correlation with pro-/anti-inflammatory ratio. However, the interferon-γ concentration was not significantly different between the T2DM patients with reduced and increased IDO production, and its concentration in T2DM patients with increased IDO production also appeared lower than interferon-γ level in normoglycemic participants. It appears that altered IDO production in T2DM with increased IDO production is not only influenced by interferon-γ, but also the inflammatory response quantified by pro-/anti-inflammatory ratio of IL-6/IL-10 and interferon-γ/IL-10.
The study limitations include a small sample size after T2DM patients were categorized to T2DM with reduced and increased IDO production group. Further, at the time of the study, we did not measure the number of cytokine-producing cells or analyze the number of functional T cells and dendritic cells. We also did not compare the IDO concentrations with expression of the IDO-1 gene or the kynurenine/tryptophan ratio.
Conclusion
The interferon-γ level was reduced in the PBMC culture supernatant of T2DM patients with reduced IDO production. Low IDO concentration following PHA simulation in T2DM PBMCs was associated with older age and, notably, a higher baseline IDO concentration. Since IDO is primarily produced by dendritic cells, reduced IDO production following PHA stimulation may reflect dendritic cell dysfunction.
Supplemental Material
Supplemental material, sj-pdf-1-try-10.1177_1178646920978236 for Altered Indoleamine 2,3-Dioxygenase Production and Its Association to Inflammatory Cytokines in Peripheral Blood Mononuclear Cells Culture of Type 2 Diabetes Mellitus by Rona Kartika, Heri Wibowo, Dyah Purnamasari, Saraswati Pradipta and Rahma A Larasati in International Journal of Tryptophan Research
Acknowledgments
The authors would like to thank to Dr Ekowati Rahajeng, the participants, nurses, and doctors in a cohort study for non-communicable disease, National Institute of Health Research and Development, Ministry of Health, Indonesia, to all staff in Integrated Laboratory Faculty of Medicine, Universitas Indonesia, and also to all who help this research.
Footnotes
Author Contributions: RK and HW contributed to conception, design, and acquisition; critically drafted the manuscript; revised the manuscript; and gave final approval, agree to be accountable for all aspects of work ensuring integrity and accuracy. DP contributed to acquisition, critically drafted the manuscript, revised the manuscript, and gave final approval, agrees to be accountable for all aspects of work ensuring integrity and accuracy. SP and RAL contributed to interpretation, critically revised the manuscript, and gave final approval, agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by Excellent Higher Education Institutional Research (PDUPT) Grant with reference number NKB-151/UN2.RST/HKP.05.00/2020.
ORCID iDs: Rona Kartika  https://orcid.org/0000-0003-2818-4553
https://orcid.org/0000-0003-2818-4553
Heri Wibowo  https://orcid.org/0000-0001-7708-5135
https://orcid.org/0000-0001-7708-5135
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. [DOI] [PubMed] [Google Scholar]
- 2. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes care. 2013;36(suppl 1):S67-S74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mohiuddin SS. Low grade chronic inflammatory response in pathogenicity of diabetes mellitus. Curr Res Diabetes Obes J. 2018;5: 555666. [Google Scholar]
- 4. Chen L, Chen R, Wang H, Liang F. Mechanisms linking inflammation to insulin resistance. Int J Endocrinol. 2015;2015:508409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes care. 2004;27:813-823. [DOI] [PubMed] [Google Scholar]
- 6. Graves DT, Kayal RA. Diabetic complications and dysregulated innate immunity. Front Biosci. 2008;13:1227-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shanmugam N, Reddy MA, Guha M, Natarajan R. High glucose-induced expression of proinflammatory cytokine and chemokine genes in monocytic cells. Diabetes. 2003;52:1256-1264. [DOI] [PubMed] [Google Scholar]
- 8. Zeng C, Shi X, Zhang B, et al. The imbalance of Th17/Th1/Tregs in patients with type 2 diabetes: relationship with metabolic factors and complications. J Mol Med. 2012;90:175-186. [DOI] [PubMed] [Google Scholar]
- 9. Straczkowski M, Kowalska I, Nikolajuk A, Krukowska A, Gorska M. Plasma interleukin-10 concentration is positively related to insulin sensitivity in young healthy individuals. Diabetes care. 2005;28:2036-2037. [DOI] [PubMed] [Google Scholar]
- 10. Bilir C, Sarisozen C. Indoleamine 2, 3-dioxygenase (IDO): only an enzyme or a checkpoint controller? J Oncol Sci. 2017;3:52-56. [Google Scholar]
- 11. Schmidt SV, Nino-Castro AC, Schultze JL. Regulatory dendritic cells: there is more than just immune activation. Front Immunol. 2012;3:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yan Y, Zhang G-X, Gran B, et al. IDO upregulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis. J Immunol. 2010;185:5953-5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hussain SH. Activity of indoleamine 2, 3 dioxygenase (IDO) in type 2 diabetes mellitus patients in Pakistan. J Rawalpindi Med Coll. 2017;21:136-140. [Google Scholar]
- 14. Nahomi RB, Mailankot M, Kern T, et al. Deletion of IDO prevents diabetes-induced retinal capillary degeneration in mice. Invest Ophthalmol Vis Sci. 2014;55:5830-5830. [Google Scholar]
- 15. Zhang Y, Ruan Y, Zhang P, Wang L. Increased indoleamine 2, 3-dioxygenase activity in type 2 diabetic nephropathy. J Diabetes Complications. 2017;31:223-227. [DOI] [PubMed] [Google Scholar]
- 16. Oxenkrug G. Insulin resistance and dysregulation of tryptophan-kynurenine and kynurenine-nicotinamide adenine dinucleotide metabolic pathways. Mol Neurobiol. 2013;48:294-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Laurans L, Venteclef N, Haddad Y, et al. Genetic deficiency of indoleamine 2,3-dioxygenase promotes gut microbiota-mediated metabolic health. Nat Med. 2018;24:1113-1120. [DOI] [PubMed] [Google Scholar]
- 18. O’flynn K, Russul-Saib M, Ando I, et al. Different pathways of human T-cell activation revealed by PHA-P and PHA-M. Immunol. 1986;57(1):55-60. [PMC free article] [PubMed] [Google Scholar]
- 19. Tiefenthaler G, Hünig T. The role of CD2/LFA-3 interaction in antigen-and mitogen-induced activation of human T cells. Int Immunol. 1989;1:169-175. [DOI] [PubMed] [Google Scholar]
- 20. Wolowczuk I, Hennart B, Leloire A, et al. Tryptophan metabolism activation by indoleamine 2, 3-dioxygenase in adipose tissue of obese women: an attempt to maintain immune homeostasis and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2012;303:R135-R143. [DOI] [PubMed] [Google Scholar]
- 21. Frasca D, Blomberg BB. Inflammaging decreases adaptive and innate immune responses in mice and humans. Biogerontology. 2016;17:7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marttila S, Jylhävä J, Eklund C, Hervonen A, Jylhä M, Hurme M. Aging-associated increase in indoleamine 2,3-dioxygenase (IDO) activity appears to be unrelated to the transcription of the IDO1 or IDO2 genes in peripheral blood mononuclear cells. Immun Ageing. 2011;8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Agrawal A, Tay J, Ton S, Agrawal S, Gupta S. Increased reactivity of dendritic cells from aged subjects to self-antigen, the human DNA. J Immunol. 2009;182:1138-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Agrawal A, Agrawal S, Gupta S. Role of dendritic cells in inflammation and loss of tolerance in the elderly. Front Immunol. 2017;8:896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Agrawal S, Ganguly S, Tran A, Sundaram P, Agrawal A. Retinoic acid treated human dendritic cells induce T regulatory cells via the expression of CD141 and GARP which is impaired with age. Aging (Albany NY). 2016;8:1223-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rožman P, Švajger U. The tolerogenic role of IFN-γ. Cytokine Growth Factor Rev. 2018;41:40-53. [DOI] [PubMed] [Google Scholar]
- 27. Grant RS. Indoleamine 2, 3-dioxygenase activity increases NAD+ production in IFN-γ-stimulated human primary mononuclear cells. Int J Tryptophan Res. 2018;11:1178646917751636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Robinson CM, Hale PT, Carlin JM. The role of IFN-γ and TNF-α-responsive regulatory elements in the synergistic induction of indoleamine dioxygenase. J Interferon Cytokines Res. 2005;25:20-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-try-10.1177_1178646920978236 for Altered Indoleamine 2,3-Dioxygenase Production and Its Association to Inflammatory Cytokines in Peripheral Blood Mononuclear Cells Culture of Type 2 Diabetes Mellitus by Rona Kartika, Heri Wibowo, Dyah Purnamasari, Saraswati Pradipta and Rahma A Larasati in International Journal of Tryptophan Research


