Abstract
Background and purpose
Do children have an increased risk for brain AVM recurrence compared to adults and does this risk vary depending on initial presentation with AVM rupture?
Methods
We retrospectively studied 115 patients initially presenting with brain AVM under age 25 years who underwent complete surgical resection of the AVM as documented by digital subtraction angiography (DSA) and had delayed follow-up DSA to evaluate for AVM recurrence after apparent initial cure.
Results
The mean time from baseline DSA to follow-up DSA was 2.3 years, ranging from 0 to 15 years. Twelve patients (10.4% of the 115 patient cohort and 16.7% of 72 patients with hemorrhage at initial presentation) demonstrated AVM recurrence on follow up DSA. All patients with recurrence initially presented with intracranial hemorrhage, and intracranial hemorrhage was a significant predictor of recurrence (log rank p=0.037). Among patients with initial hemorrhage, the five-year recurrence rate was 17.8% (95% CI: 8.3% – 35.7%). All recurrences occurred in patients who were children at the time of their initial presentation; the oldest was 15 years of age at the time of initial AVM surgery. The five-year recurrence rate for children (0 to 18 years of age) with an initial presentation of hemorrhage was 21.4% (95% CI: 10.1% – 41.9%). Using Cox regression, we found the risk of AVM recurrence decreased by 14% per each year increase in age at the time of initial surgical resection (HR=0.86, 95% CI: 0.75–0.99, p=0.031).
Conclusions
There is a high rate of recurrence of apparently cured brain AVMs in children who initially present with AVM rupture. Imaging follow-up is warranted to prevent re-rupture.
Keywords: AVM, arteriovenous malformation, surgery, angiography, pediatrics
INTRODUCTION
Brain arteriovenous malformations (AVMs) are traditionally considered congenital lesions arising from abnormal development of the brain vasculature prior to birth. Accordingly, an AVM was considered “cured” upon a negative imaging study, typically a digital subtraction catheter angiogram (DSA), after definitive treatment. Emerging evidence, however, suggests that AVMs are dynamic lesions with the potential for de novo formation and growth postnatally [1]. AVM recurrence following complete surgical resection as documented by angiographic cure is thought to be rare but is increasingly recognized, particularly in children [2, 3]. Such a recurrence presumably confers an unknown risk of significant morbidity and mortality from an intracranial hemorrhage.
Clinicians lack data to guide clinical practice regarding surveillance imaging after angiographic cure of AVMs. An analysis of predictors of AVM recurrence after angiographic cure could help select patients for surveillance imaging and inform timing. We retrospectively analyzed a consecutive cohort of patients with brain AVMs seen at our large referral center over two decades (1996–2018) to identify rates and risk factors for recurrence and develop follow up recommendations. Based on our experience, we hypothesized that children have an increased risk for AVM recurrence, compared to adults, and that this risk may vary depending on initial presentation with AVM rupture.
MATERIALS AND METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Patient Selection
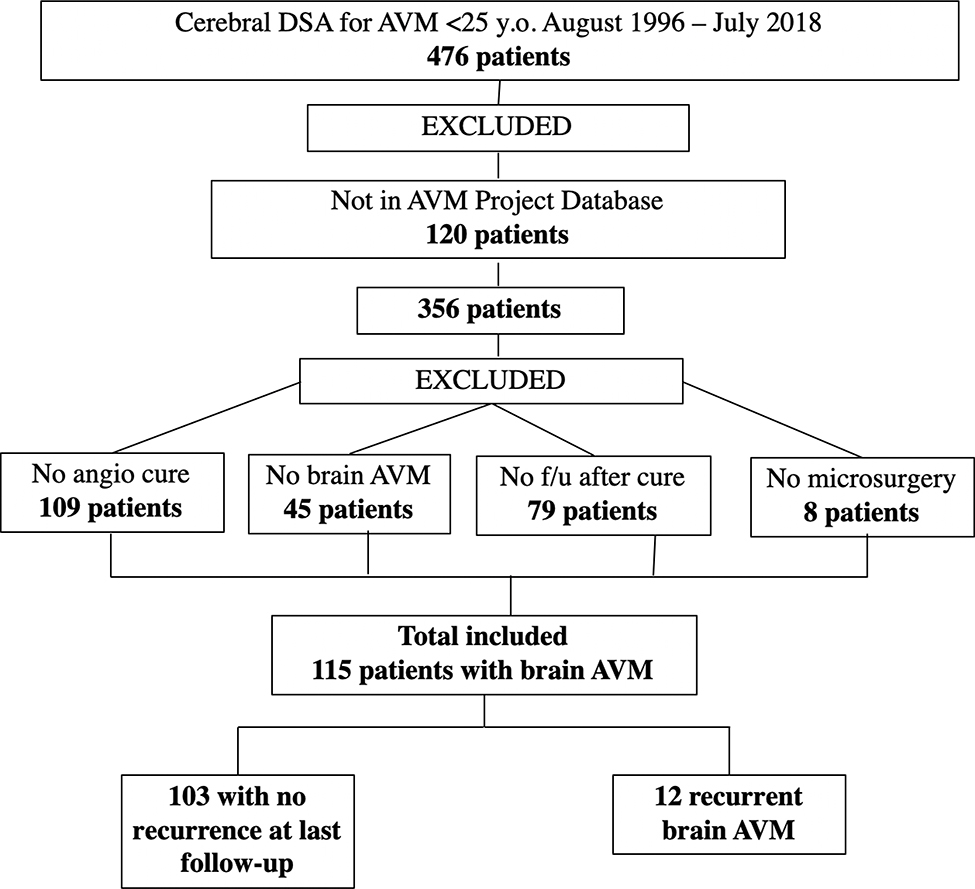
Under an IRB-approved protocol, a retrospective analysis of medical records and imaging studies of all patients under 25 years of age at the time of clinical presentation with a diagnosis of cerebral AVM who underwent DSA at our institution from August 1996 through July 2018 was performed. We have had a consistent team of cerebrovascular neurosurgeons (MTL and AAA), pediatric neurosurgeons (NG), and interventional neuroradiologists (DLC, MRA, CFD, VVH, RTH, and SWH) over the study period. This cohort of patients was cross referenced with The University of California, San Francisco Brain AVM Project database, an IRB-approved, prospectively maintained resource that collects demographic, clinical, imaging, and outcome data for patients diagnosed with brain AVMs who were evaluated and/or treated at our institution. We excluded from the analysis patients who, i) did not achieve complete angiographic cure following initial treatment as evidenced by the presence of a residual nidus or persistent arteriovenous shunting, ii) did not have an intracranial AVM on DSA, or iii) did not consent to participate in research. We included only patients treated with microsurgery, either alone or in combination with stereotactic radiosurgery and/or endovascular embolization (Figure 1). We limited our analysis to patients who had undergone microsurgical resection of the AVM nidus, as this is the most definitive technique for complete obliteration of an AVM nidus. At our institution, if no nidus is found on the post-operative DSA, but nonspecific arteriovenous shunting is seen, then we perform an early follow-up DSA within 6 months. If there is no residual nidus or AV shunting on the immediate post-operative DSA in patients under age 25 years, and they have no signs or symptoms to suggest AVM recurrence, then we perform a follow-up DSA 5 years post-operatively to assess for late asymptomatic AVM recurrence.
Figure 1.
Cohort selection
All available DSAs for each patient were reviewed by two fellowship-trained neurointerventional radiologists. We reviewed baseline imaging to confirm the presence of an intracranial AVM. We assessed AVM characteristics including maximal dimension, eloquent location, presence of exclusive deep venous drainage and other angioarchitectural features according to a structured approach [4]. We reviewed follow-up imaging to confirm complete angiographic cure on post-treatment DSA, and to classify presence or absence of AVM recurrence on subsequent imaging. AVM recurrence was defined as the angiographic appearance of new arteriovenous shunting and/or nidus vessels not present on the post-treatment DSA; we classified the location of the recurrence (same as or remote from the baseline AVM). CT and MR imaging data were also assessed for current or prior intracranial hemorrhage. Demographic variables and clinical data were collected including patient age at the time of initial diagnosis, at the time of complete angiographic cure, and at subsequent follow up DSAs.
Statistical Analysis
Patients were dichotomized into those with versus those without AVM recurrence. Survival analyses were performed with angiographic recurrence considered as an event. We used the date of surgical resection as the initial starting point and censored observations at the last date of DSA imaging. We calculated the 5-year recurrence rate using the Kaplan-Meier estimator. We used a Mann-Whitney U test to test whether the time to angiographic recurrence differed between symptomatic and asymptomatic cases with recurrence. We tested whether patient characteristics and AVM imaging features were associated with recurrence using log rank tests and Cox proportional hazards models. Given that all AVM recurrences were in patients who initially presented with hemorrhage, we restricted Cox models to those who initially presented with hemorrhage. Due the small number of events, we only performed univariable Cox regression analyses. Data analysis was performed using Stata 15.1 software (College Station, TX: StataCorp, LLC).
RESULTS
Patient Demographics
A total of 115 patients met inclusion criteria and were included in the analysis. The patient age at the time of surgical resection was a mean of 14.9 years (median 14.8 years), with a range of 3.1 to 24.8 years; 51% of patients were female. One individual, who ultimately developed a recurrence, had a diagnosis of Hereditary Hemorrhagic Telangiectasia (HHT); however, a diagnosis of HHT was only inconsistently sought prior to establishment of an HHT Center of Excellence at UCSF in 2014. An initial presentation with intracranial hemorrhage was seen in 63% of patients, with seizures and headaches reported in 22% and 71% of patients, respectively. Patient and AVM characteristics stratified by recurrence versus no recurrence, as well as overall features are summarized in Table 1.
Table 1.
Summary of patient and AVM characteristics in those with versus without AVM recurrence and in the overall cohort.
| Characteristic | Recurrence | No recurrence | Overall |
|---|---|---|---|
| N=12 | N=103 | N=115 | |
| Follow-up (yrs) | 4.5 ± 2.5 | 2.1 ± 3.0 | 2.3 ± 3.1 |
| Age at resection (yrs) | 10.3 ± 1.2 | 15.4 ± 0.6 | 14.9 ± 0.5 |
| Female | 3 (25%) | 56 (54%) | 59 (51%) |
| HHT diagnosis | 1 (8%) | 0 (0%) | 1 (1%) |
| Initial hemorrhage | 12 (100%) | 60 (58%) | 72 (63%) |
| Initial seizures | 2 (17%) | 24 (24%) | 26 (23%) |
| Initial headaches | 11 (92%) | 68 (69%) | 79 (72%) |
| AVM largest diameter (mm) | 20.7 ± 14.6 | 26.4 ± 16.1 | 25.9 ± 15.9 |
| Exclusive deep venous drainage | 5/8 (63%) | 14/77 (18%) | 19/85 (22%) |
| Left hemisphere | 7/8 (88%) | 31/75 (41%) | 38/83 (46%) |
| Lobar location | 6/8 (75%) | 58/75 (77%) | 64/83 (77%) |
| Eloquent location | 2/8 (25%) | 41/74 (55%) | 43/82 (52%) |
Values are mean ± SD, N (%), or N/total non-missing (%)
AVM Characteristics
The mean size of the AVM nidus (in greatest dimension) was 25.9 mm (ranging from 4 to 100 mm), 78% were lobar, 46% were located in the left cerebral hemisphere, and 52% were in an eloquent location (Table 1). Deep venous drainage was present in 51% of cases.
Recurrence of AVM
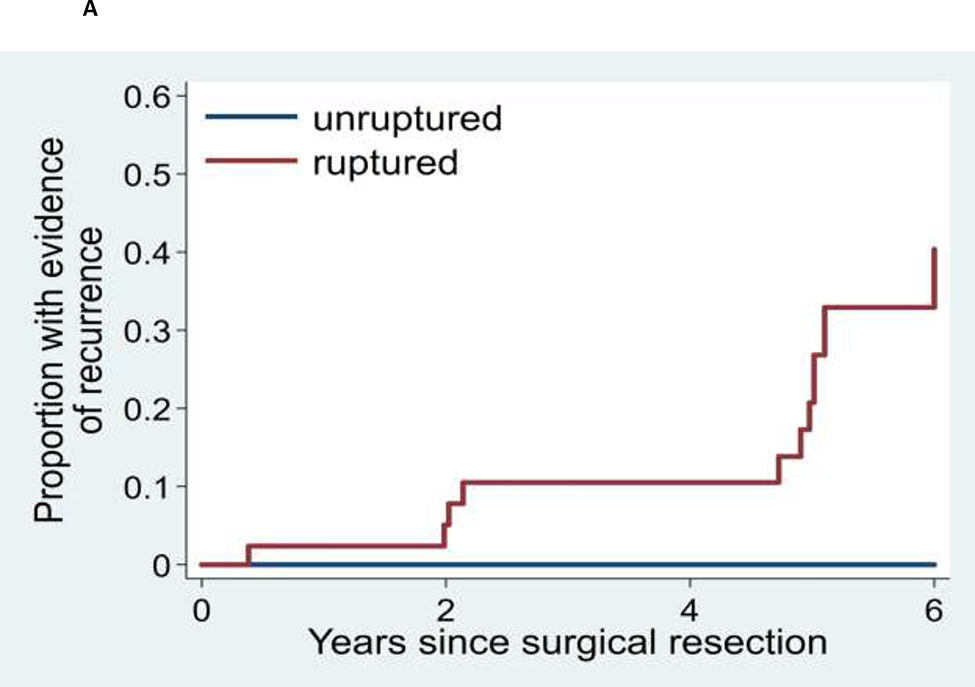
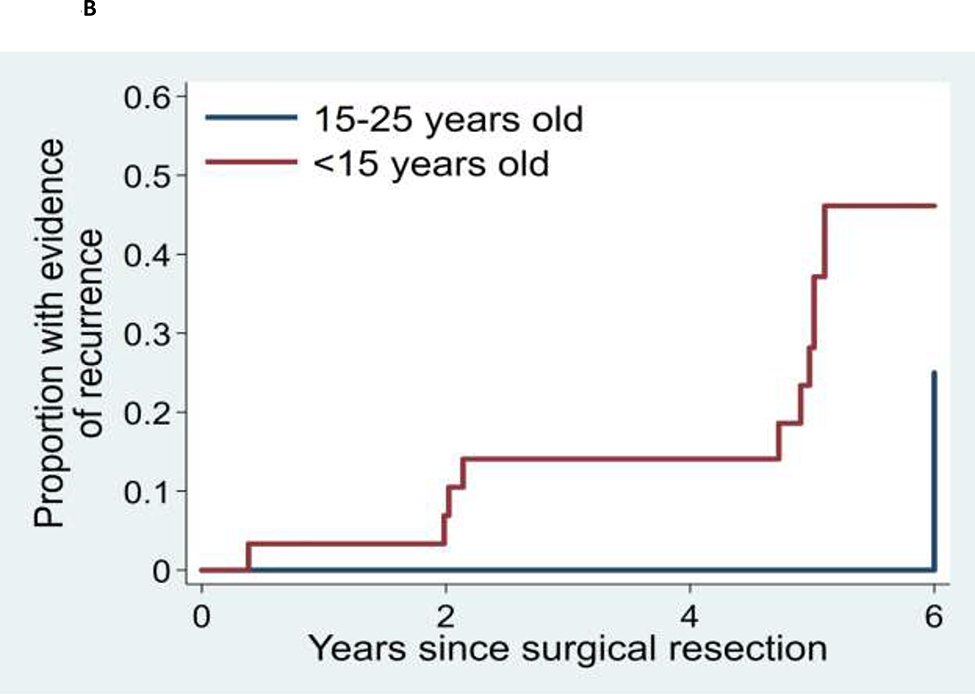
The mean time from baseline DSA to follow-up DSA was 2.3 years, ranging from 0 to 15 years. Twelve patients (10.4% of the entire 115 patient cohort and 16.7% of those 72 patients with hemorrhage at initial presentation) demonstrated AVM recurrence on follow up DSA. All patients with recurrence initially presented with intracranial hemorrhage, and intracranial hemorrhage was a significant predictor of recurrence (log rank p=0.037). The five-year Kaplan-Meier estimate of the recurrence rate among all patients under age 25 was 13.1% (95% CI: 6.0% - 27.0%). Among patients with initial hemorrhage, the five-year recurrence rate was 17.8% (95% CI: 8.3% – 35.7%) (Figures 2 and 3; Supplementary Figures I–III and Supplementary Table). All recurrences occurred in patients who were children at the time of their initial presentation; the oldest was 15 years of age at the time of the initial AVM surgery. The five-year recurrence rate for children (0 to 18 years of age) with an initial presentation of hemorrhage was 21.4% (95% CI: 10.1% - 41.9%). There were no appreciable inter-surgeon or inter-angiographer differences in AVM recurrence rates nor in angiographic detection of those recurrences.
Figure 2.
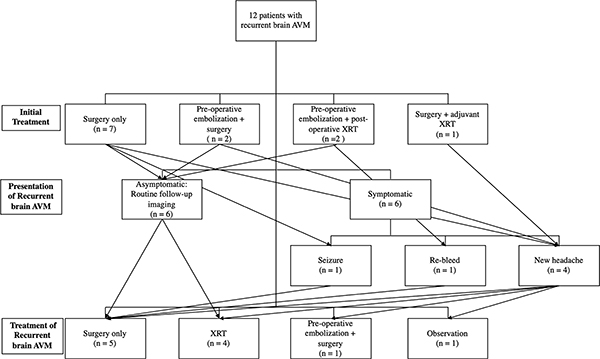
Treatments received by patients who ultimately had AVM recurrence
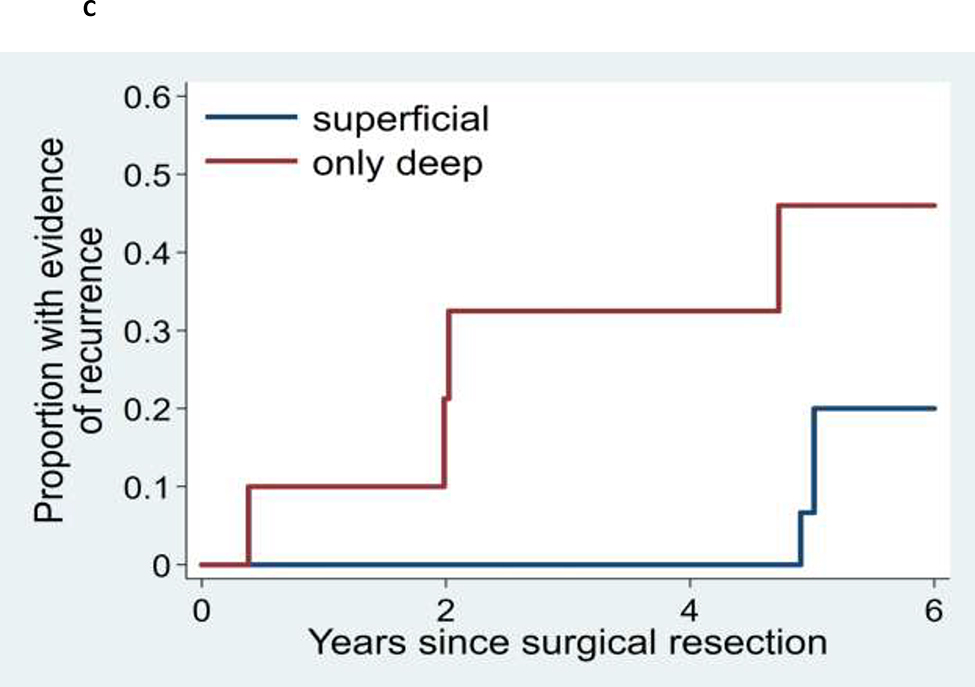
Figure 3.
Kaplan-Meier analyses of time to recurrence for: (A) AVM for patients who initially presented with or without AVM rupture, (B) AVM patients initially presenting between age 15 and 25 years versus patients presenting at less than age 15 years, and (C) AVMs with superficial versus deep venous drainage.
Among recurrent cases, 6/12 (50%) patients presented with new symptoms that prompted evaluation with DSA and 6/12 (50%) were asymptomatically diagnosed during scheduled DSA follow-up. The overall mean time to angiographic recurrence was 54.7 months. There was no difference in time-to-recurrence for asymptomatic (58.7 months) vs. symptomatic patients (50.7 months) (p = 0.69, Mann-Whitney U test). Characteristics of those patients with AVM recurrence are summarized in the Supplementary Table. Among the 12 recurrences, one AVM nidus came to the pial surface and the others ranged from 10.3 mm to 54.3 mm deep to the pial surface. The average depth of the most superficial aspect of the AVM nidus was 27.3 mm.
Using Cox regression, we found the risk of AVM recurrence decreased by 14% per each year increase in age at the time of initial surgical resection (HR=0.86, 95% CI: 0.75–0.99, p=0.031, Table 2). We also found that left-sided AVMs were more likely to recur (HR=5.22, 95% CI: 1.35–20.14, p=0.015), although this result would not survive a correction for multiple comparisons. No other predictor was nominally significant.
Table 2.
Cox regression analyses of predictors of AVM recurrence in a cohort of 115 patients with apparent angiographic cure of their initial AVM.
| Characteristic | Hazard Ratio | 95% CI | P-value |
|---|---|---|---|
| Age at resection (yrs) | 0.83 | (0.71-0.96) | 0.013 |
| Female | 0.53 | (0.14-2.12) | 0.373 |
| HHT diagnosis | 1.53 | (0.18-12.90) | 0.697 |
| Initial seizures | 3.88 | (0.77-19.45) | 0.099 |
| Initial headaches | 5.71 | (0.60-54.62) | 0.131 |
| AVM largest diam (mm) | 0.98 | (0.92-1.04) | 0.533 |
| Exclusive deep venous drainage | 4.36 | (0.82-23.34) | 0.085 |
| Left hemisphere | 5.20 | (0.60-44.67) | 0.133 |
| Lobar location | 3.16 | (0.38-26.47) | 0.290 |
| Eloquent location | 0.29 | (0.03-2.51) | 0.263 |
Analyses restricted to hemorrhagic cases and adjusted for patient age
DISCUSSION
We sought to understand which factors in children and young adults with brain AVMs predict increased risk for recurrence following treatment. We limited our analysis to patients who had undergone microsurgical resection of the AVM nidus, as this is the most definitive technique for complete obliteration of an AVM nidus. At our institution, we obtain a post-operative DSA between one to several days following surgery. If a residual AVM nidus is identified, it can be resected prior to the formation of scar tissue which could increase the complexity of a repeat surgical procedure. As outlined in the methods, depending on the results of the immediate postoperative DSA, we perform a follow-up DSA 6 months or 5 years later. Employing this general approach, the 5-year recurrence rate in our cohort of young patients initially presenting with hemorrhage was 17.8%. All recurrences occurred in patients who were children at the time of their initial hemorrhagic presentation; the oldest was 15 years of age at the time of the initial AVM surgery. The five-year recurrence rate for children (0 to 18 years of age) with an initial presentation of hemorrhage was 21.4%. This contrasts to 0% recurrence in patients under age 25 years initially presenting with unruptured AVMs.
Recurrence of cerebral AVMs following demonstration of obliteration on post-treatment angiogram is uncommon, mostly seen in children [1, 5, 6]. A review of the literature by Weil et al. revealed that 82% of AVM recurrences occurred in children with the average age of initial surgery in those patients reported to be 12 years[7]. Aboukais et al reviewed 139 consecutive patients of all ages who underwent microsurgery for a ruptured AVM: reappearance of an AVM was found in 7 patients between 12 and 42 months following treatment, all of whom were under the age of 18 [8]. The higher prevalence of AVM recurrence in children compared to adults may be related to intrinsic factors inherent to this patient population, such as a greater potential for cerebral blood vessel growth as compared to adults.
Current evidence suggests that formation of an AVM is a dynamic process associated with multiple inciting events. Developmentally expressed molecules, such as vascular endothelial growth factor (VEGF), may affect the development of new AVMs. Sonstein et al studied the relationship between the expression of VEGF and AVM recurrence in pediatric patients and showed a high rate of expression in recurrent AVMs in the pediatric population[9]. Other intrinsic vascular remodeling factors on the molecular level have been associated with AVMs in the brain. Nikolaev et al identified somatic activating KRAS mutations and increased extracellular signal-regulated kinase activity in the majority of tissues samples of AVMs that were analyzed[10]. Another possible explanation for AVM recurrence has been proposed by Pellettieri et al and has been described as the hidden compartment theory[11]. Vessels associated with an AVM that are unfilled initially due to an internal steal phenomenon into the AVM nidus may become recruited following resection of the nidus. These mature vessels may by located within, near, or remote from the nidus. Certain morphological factors have also been associated with AVM recurrence. There is a higher incidence of recurrence in AVMs demonstrating deep venous drainage in young patients with surgically resected brain AVMs[12]. Furthermore, exclusive deep venous drainage is reported to be more common in children than adults (28% versus 14%, p<0.001)[4].
There are other explanations that could account for the increased rate of recurrent AVMs in this cohort. In young patients, there may be intrinsic differences in the brain that would increase changes following hemorrhage and obscuration of components of the AVM nidus. If the residual components are thrombosed or compressed, they may not be visible on a post-operative DSA but could recanalize and be seen on delayed DSA. Alternatively, microscopic residual AVM nidus might elicit angiogenic factors particularly in young patients with the capacity to grow new blood vessels, leading to late recurrences.
In our cohort, AVM recurrence presented as hemorrhage, seizures, or radiologic recurrence on follow-up surveillance imaging 0.5 to 9 years following resection. Based on our results, long-term follow-up for treated AVMs with DSA is recommended to ensure stable treatment changes and to exclude recurrence. Detection of an AVM remnant may be especially challenging on post-treatment angiography obtained soon after treatment due to mass effect from surrounding parenchymal edema and/or hemorrhage. AVM location also appears to predict recurrence as 75% of recurrences in this cohort occurred in the left hemisphere. This supports previous work in both adult and pediatric patients, corroborating eloquent location may preclude more aggressive initial resection. It is unclear why this asymmetry persists even in the setting of angiographic “cure.” Additional factors, including mass effect from adjacent hematoma, vasospasm, or vessel thrombosis may decrease the sensitivity in detecting a small residual on early post-resection DSA.
This study is limited by the retrospective nature of the analysis and the fact that the patient population included is from a single institution, potentially affecting generalizability of results. Although this is true, our institution is a high-volume center for AVM treatment and a significant number of patients come from all over the country and some from other regions in the world. An additional limitation is that we can only infrequently assess for recurrence, as the definitive test—DSA—is invasive. Thus, with a 6 month post-surgical angiogram and a 5 year post-surgical angiogram, we can only rigorously assess 6 month and 5 year recurrence rates as opposed to annual recurrence rates.
For pediatric AVMs treated surgically, the majority of recurrences are detected within the first 15 months after the initial resection. Thus, some groups have recommended DSA at approximately 1 year following treatment to exclude recurrent disease[2, 13]. It is important to note, however, that AVM recurrences may occur outside the 12 month period: as late as 9 years following treatment in our study. Fifteen of the nineteen recurrences from our study were detected on imaging more than a year since last documented cure. Follow-up imaging should be tailored to each individual patient. Those patients with AVMs that demonstrate qualities associated with a higher likelihood of recurrence, such as deep venous drainage, should be followed closely and over a longer period of time. In addition, while it is necessary to document angiographic cure and to exclude recurrence in a patient with a previously treated AVM, the risks and potential benefits of routine imaging follow-up should be weighed. Frequent DSA especially in the pediatric population should be avoided to minimize risks associated with radiation and anesthesia. At a minimum, we recommend a 5-year follow-up DSA after the date of documented angiographic cure for those patients initially treated prior to 25 years of age.
Our findings indicate that brain AVMs in children and young adults require long-term follow-up. Although in our cohort only patients with initially ruptured brain AVMs had late recurrences, it is not certain that patients with initially unruptured brain AVMs are not at risk for late recurrences, particularly as many of these patients are not routinely followed with noninvasive or invasive imaging for an extended period after their AVM treatment. Recent reviews have, indeed, identified recurrences of AVMs following apparent angiographic cure after surgery or embolization, both in initially ruptured and unruptured lesions[2, 3]. In an era where endovascular cure of AVMs is increasingly being advocated—and thus, no operating microscope will have the opportunity to find tiny nidus residua that could lead to late recurrence—it will be particularly important to develop long-term imaging follow-up strategies and improved follow-up imaging techniques for both ruptured and unruptured brain AVMs.
Supplementary Material
Acknowledgments
Funding: R01EB012031 (SWH), R01NS099268 (HK, SWH), R01NS034949 (HK, SWH), U54065705 (MTL, HK, SWH)
Disclosures: AC (none), GD (none), MTC (none), ERS (none), DLC (research contracts with UCSF, Siemens Healthineers, unrelated), JN (none), AAA (none), CF (grants, Pediatric Epilepsy Research Foundation, unrelated), MRA (none), MTL (none), CFD (core imaging lab contracts with UCSF, Stryker, unrelated), VVH (none), RTH (none), HK (consulting fees, Recursion Pharmaceuticals, unrelated), HJF (none), NG (none), SWH (equity in ThrombX, unrelated; core imaging lab contracts with UCSF, Stryker and MicroVention, unrelated; research conracts with UCSF, Siemens Healthineers, unrelated; DSMB/CEC membership, Route 92 and Imperative, unrelated)
Abbreviations
- AVM
arteriovenous malformation
- CI
confidence interval
- CT
computed tomography
- DSA
digital subtraction angiography
- HR
hazard ratio
- HHT
hereditary hemorrhagic telangiectasia
- KRAS
Kirsten RAt Sarcoma gene
- MR
magnetic resonance
- UCSF
University of California San Francisco
- VEGF
vascular endothelial growth factor
Contributor Information
Alexander Copelan, Division of Neurointerventional Radiology, Department of Radiology and Biomedical Imaging, University of California, San Francisco..
Gerald Drocton, Division of Neurointerventional Radiology, Department of Radiology and Biomedical Imaging, University of California, San Francisco..
M. Travis Caton, Division of Neurointerventional Radiology, Department of Radiology and Biomedical Imaging, University of California, San Francisco..
Eric R. Smith, Division of Neurointerventional Radiology, Department of Radiology and Biomedical Imaging, University of California, San Francisco..
Daniel L. Cooke, Division of Neurointerventional Radiology, Department of Radiology and Biomedical Imaging, University of California, San Francisco.; Center for Cerebrovascular Research, University of California, San Francisco. Pediatric Brain Center, University of California, San Francisco.
Jeffrey Nelson, Center for Cerebrovascular Research, University of California, San Francisco..
Adib A. Abla, Center for Cerebrovascular Research, University of California, San Francisco.; Pediatric Brain Center, University of California, San Francisco. Division of Cerebrovascular Neurosurgery, Department of Neurological Surgery, University of California, San Francisco.
Christine Fox, Center for Cerebrovascular Research, University of California, San Francisco.; Departments of Neurology and Pediatrics, University of California, San Francisco.
Matthew R. Amans, Division of Neurointerventional Radiology, Department of Radiology and Biomedical Imaging, University of California, San Francisco.; Center for Cerebrovascular Research, University of California, San Francisco. Pediatric Brain Center, University of California, San Francisco.
Christopher F. Dowd, Division of Neurointerventional Radiology, Department of Radiology and Biomedical Imaging, University of California, San Francisco.; Center for Cerebrovascular Research, University of California, San Francisco. Pediatric Brain Center, University of California, San Francisco. Division of Cerebrovascular Neurosurgery, Department of Neurological Surgery, University of California, San Francisco. Department of Anesthesia and Perioperative Care, University of California, San Francisco.
Van V. Halbach, Division of Neurointerventional Radiology, Department of Radiology and Biomedical Imaging, University of California, San Francisco.; Center for Cerebrovascular Research, University of California, San Francisco. Pediatric Brain Center, University of California, San Francisco. Division of Cerebrovascular Neurosurgery, Department of Neurological Surgery, University of California, San Francisco. Department of Anesthesia and Perioperative Care, University of California, San Francisco.
Randall T. Higashida, Division of Neurointerventional Radiology, Department of Radiology and Biomedical Imaging, University of California, San Francisco.; Center for Cerebrovascular Research, University of California, San Francisco. Pediatric Brain Center, University of California, San Francisco. Division of Cerebrovascular Neurosurgery, Department of Neurological Surgery, University of California, San Francisco. Department of Anesthesia and Perioperative Care, University of California, San Francisco.
Michael T. Lawton, Division of Neurovascular Surgery, Department of Neurosurgery, Barrow, Neurological Institute, Phoenix, AZ.
Helen Kim, Center for Cerebrovascular Research, University of California, San Francisco.; Department of Anesthesia and Perioperative Care, University of California, San Francisco.
Heather J. Fullerton, Center for Cerebrovascular Research, University of California, San Francisco.; Pediatric Brain Center, University of California, San Francisco. Departments of Neurology and Pediatrics, University of California, San Francisco.
Nalin Gupta, Center for Cerebrovascular Research, University of California, San Francisco.; Division of Pediatric Neurosurgery, Department of Neurological Surgery, University of California, San Francisco.
Steven W. Hetts, Division of Neurointerventional Radiology, Department of Radiology and Biomedical Imaging, University of California, San Francisco.; Center for Cerebrovascular Research, University of California, San Francisco. Pediatric Brain Center, University of California, San Francisco.
REFERENCES
- 1.Ali MJ, Bendok BR, Rosenblatt S, Rose JE, Getch CC, and Batjer HH. Recurrence of pediatric cerebral arteriovenous malformations after angiographically documented resection. Pediatr Neurosurg, 2003. 39(1): p. 32–8. [DOI] [PubMed] [Google Scholar]
- 2.Jhaveri A, Amirabadi A, Dirks P, Kulkarni AV, Shroff MM, Shkumat N, Krings T, Pereira VM, Rea V, and Muthusami P. Predictive Value of MRI in Diagnosing Brain AVM Recurrence after Angiographically Documented Exclusion in Children. AJNR Am J Neuroradiol, 2019. 40(7): p. 1227–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorenson TJ, Brinjikji W, Bortolotti C, Kaufmann G, and Lanzino G. Recurrent Brain Arteriovenous Malformations (AVMs): A Systematic Review. World Neurosurg, 2018. 116: p. e856–e866. [DOI] [PubMed] [Google Scholar]
- 4.Hetts SW, et al. , Influence of patient age on angioarchitecture of brain arteriovenous malformations. AJNR Am J Neuroradiol, 2014. 35(7): p. 1376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andaluz N, Myseros JS, Sathi S, Crone KR, and Tew JM Jr. Recurrence of cerebral arteriovenous malformations in children: report of two cases and review of the literature. Surg Neurol, 2004. 62(4): p. 324–30; discussion 330–1. [DOI] [PubMed] [Google Scholar]
- 6.Hladky JP, Lejeune JP, Blond S, Pruvo JP, and Dhellemmes P. Cerebral arteriovenous malformations in children: report on 62 cases. Childs Nerv Syst, 1994. 10(5): p. 328–33. [DOI] [PubMed] [Google Scholar]
- 7.Weil AG, Li S, and Zhao JZ. Recurrence of a cerebral arteriovenous malformation following complete surgical resection: A case report and review of the literature. Surg Neurol Int, 2011. 2: p. 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aboukais R, Vinchon M, Quidet M, Bourgeois P, Leclerc X, and Lejeune JP. Reappearance of arteriovenous malformations after complete resection of ruptured arteriovenous malformations: true recurrence or false-negative early postoperative imaging result? J Neurosurg, 2017. 126(4): p. 1088–1093. [DOI] [PubMed] [Google Scholar]
- 9.Sonstein WJ, Kader A, Michelsen WJ, Llena JF, Hirano A, and Casper D. Expression of vascular endothelial growth factor in pediatric and adult cerebral arteriovenous malformations: an immunocytochemical study. J Neurosurg, 1996. 85(5): p. 838–45. [DOI] [PubMed] [Google Scholar]
- 10.Nikolaev SI, Vetiska S, Bonilla X, Boudreau E, Jauhiainen S, Reza Jahromi B, Khyzha N, DiStefano PV, Suutarinen S, Kiehl TR, et al. Somatic Activating KRAS Mutations in Arteriovenous Malformations of the Brain. N Engl J Med, 2018. 378(3): p. 250–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pellettieri L, Svendsen P, Wikholm G, and Carlsson CA. Hidden compartments in AVMs--a new concept. Acta Radiol, 1997. 38(1): p. 2–7. [DOI] [PubMed] [Google Scholar]
- 12.Brown RD Jr, Wiebers DO, Torner JC, and O’Fallon WM. Frequency of intracranial hemorrhage as a presenting symptom and subtype analysis: a population-based study of intracranial vascular malformations in Olmsted County, Minnesota. J Neurosurg, 1996. 85(1): p. 29–32. [DOI] [PubMed] [Google Scholar]
- 13.Lang SS, Beslow LA, Bailey RL, Vossough A, Ekstrom J, Heuer GG, and Storm PB. Follow-up imaging to detect recurrence of surgically treated pediatric arteriovenous malformations. J Neurosurg Pediatr, 2012. 9(5): p. 497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.