Abstract
Background and Aim
TL1A (TNFSF15) is a major Crohn's disease (CD) susceptibility gene, especially in the East Asian population, and is also known to be associated with some clinical phenotypes, such as stricturing and penetrating behavior. This study aims to investigate the association between TL1A genotype and the long‐term therapeutic outcomes of infliximab and adalimumab in Japanese CD patients.
Methods
We investigated 119 biologic‐naïve CD patients treated with infliximab or adalimumab. TL1A ‐358C/T (rs6478109) was genotyped as a tag single nucleotide polymorphism (SNP) for CD risk or nonrisk haplotype of TL1A (the ‐358C allele is a risk allele for CD development). We compared the long‐term therapeutic outcomes of anti‐tumor necrosis factor (TNF) antibodies between the TL1A ‐358C/C group and the C/T+T/T group.
Results
Sixty‐nine cases (58.0%) were homozygous for the risk allele (TL1A ‐358C/C group), and 50 cases (42.0%) were heterozygous for the risk allele or homozygous for the protective allele (TL1A ‐358C/T+T/T group). No significant differences were found in the cumulative retention rates and the relapse‐free survival between the TL1A genotypes. However, the surgery‐free survival was significantly lower in the TL1A ‐358C/C group than in the C/T+T/T group (log‐rank test, P < 0.05). Multivariate analysis showed that TL1A ‐358C/C was identified as an independent risk factor for surgery (hazard ratio, 4.67; 95% confidence interval, 1.39–22.1; P = 0.025).
Conclusion
An association was found between the TL1A genotype and the therapeutic outcomes of anti‐TNF therapy. Our data indicate that the design of customized therapy with anti‐TNF antibodies using TL1A genomic information could be effective in the future.
Keywords: adalimumab, Crohn's disease, infliximab, TL1A (TNFSF15)
In this study, we investigated the association between TL1A genotype and the long‐term therapeutic outcomes of infliximab and adalimumab in Crohn's disease (CD) patients. We investigated 119 biologic‐naïve CD patients treated with infliximab or adalimumab and found an association between the TL1A genotype and surgery‐free survival during anti‐tumor necrosis factor (TNF) therapy. The patients whose genotypes are homozygous for the TL1A ‐358C (risk allele of CD development) show lower surgery‐free survival with anti‐TNF treatment.
Introduction
Crohn's disease (CD) is a chronic inflammatory bowel disease that involves the small and/or large intestine characterized by patchy transluminal inflammation, granuloma formation, and gut fibrosis. Quite a high number of CD patients experience complications during the disease course, such as gut stricture, fistula, perforation, abdominal abscess, and perianal fistula, which frequently require surgical treatments. The etiology of CD is still unclear, but the disorders of the gut mucosal immune systems caused by the various environmental factors and genetic factors are hypothesized as major pathogenesis. Concerning the genetic factors associated with the risk of CD development, various previous studies have identified the genes or loci, such as NOD2/CARD15, 1 TL1A (TNFSF15), 2 ATG16L1, 3 IL23R, 4 and RAP1A. 5
TL1A is one of the important members of the tumor necrosis factor (TNF)/TNF receptor superfamily that play a critical role in various immunological responses involved in several inflammatory diseases, including CD. 6 , 7 , 8 TL1A has been identified as a major CD susceptibility gene. 9 The first report by Yamazaki et al. described highly significant associations of single nucleotide polymorphism (SNPs) and haplotypes within the TL1A genes in Japanese patients based on the result of a genome‐wide association study. 2 Subsequent replication studies confirmed the similar significant association not only in Asian cohorts 10 , 11 but also in some Western cohorts. 12 , 13 TL1A has also been reported to be associated with some clinical phenotypes of CD. Kakuta et al. reported the association between TL1A SNPs and anal lesions. 10 Hirano et al. reported the association between TL1A rs3810936 C allele and ileocecal location, structuring, and penetrating behavior. 14 Yang et al. also reported that nonrisk allele homozygotes of some TL1A SNPs are risk factors for strictures/nonperianal penetrating complications and perianal fistula. 15 Thus, TL1A has susceptibility not only to CD development but also to disease phenotypes. However, little is known about the association between TL1A genotypes and the long‐term prognosis of CD. In recent decades, anti‐TNFα antibodies, such as infliximab (IFX) and adalimumab (ADA), have been the main therapeutic agents for refractory CD patients. Hence, it is quite important to clarify whether the TL1A genotype affects the long‐term prognosis of anti‐TNF therapy for CD.
In this study, we investigated the association between TL1A genotype and the long‐term therapeutic outcomes of IFX and ADA in Japanese CD patients.
Methods
Study design and patients
This was a retrospective cohort study at a single center. We enrolled a total of 228 Japanese CD patients treated with IFX or ADA as a first biologic at Tohoku University Hospital between 2003 and 2013. Of 228 patients, we excluded patients who received anti‐TNF therapy as a postoperative maintenance therapy (n = 47), patients who could not be followed up after 8 weeks due to either primary nonresponse or intolerance (n = 8), and patients for whom genetic analyses were not available (n = 54). A total of 119 patients were investigated in this study.
This study was approved by the institutional ethics committee, and written informed consent was obtained from all patients.
Protocol of anti‐TNF therapy
The anti‐TNF‐α antibodies were administered to CD patients with moderate to severe disease activities. IFX was administered at a 5 mg/kg infusion in weeks 0, 2, and 6 as an induction therapy. When a clinical response was observed, maintenance treatment with IFX (5 mg/kg every 8 weeks) was initiated. In some cases with IFX loss of response (LOR), IFX dose was increased to up to 10 mg/kg. ADA was injected subcutaneously at 160 mg/body in week 0 and 80 mg/body in week 2. When a clinical response was observed, 40 mg/body of ADA was administered subcutaneously every 2 weeks as maintenance. ADA dose escalation was not performed in the case of LOR because the dose escalation was not officially approved during our study period in Japan.
Genotyping of TL1A
In this study, we performed the genotyping of TL1A ‐358C/T (rs6478109) as a tag SNP for CD risk or nonrisk haplotype. In a previous study, it was reported that ‐358C/T had the highest odds ratios for CD development 10 and could be regarded as a tag SNP for CD risk or nonrisk haplotype. 2 The ‐358C allele is a risk allele for CD development.
DNA was extracted from samples collected from the patients' peripheral blood. TL1A ‐358C/T (rs6478109) was genotyped using the TaqMan SNP Genotyping Assay Kit (assay ID: C_1305297_10) and the ABI StepOnePlus Real‐Time Polymerase Chain Reaction System (Applied Biosystems, Foster City, CA, USA) per manufacture's protocol.
Overall long‐term outcomes
Kaplan–Meier methods were used to analyze the overall long‐term outcomes of anti‐TNF therapy by focusing on the following three end‐points: cumulative retention rate (end‐point: discontinuation of the agent due to any cause), cumulative relapse‐free survival (end‐point: clinical relapse), and cumulative surgery‐free survival (end‐point: surgical bowel resection). Clinical relapse was defined as the necessity of treatment stepup, including additional administration of steroids or thiopurine, dose escalation or switching of the anti‐TNF antibody, hospitalization, and surgery.
Comparisons of the long‐term outcomes among the TL1A genotypes
We classified the patients into two groups based on the genotyping results: TL1A ‐358C/C group, which is homozygous for the CD risk allele, and TL1A ‐358C/T+T/T group, which is heterozygous or homozygous for the protective allele.
The log‐rank test was used to compare the long‐term outcomes between TL1A ‐358C/C group and C/T+T/T group. In addition, a multivariate analysis was conducted using the Cox proportional hazard model to assess whether the TL1A genotype could be an independent risk factor for poor long‐term outcomes.
Statistical analysis
The Kaplan–Meier method was used for statistical analysis of long‐term outcomes. The log‐rank test was used to compare the long‐term outcomes between the two groups. Risk factors associated with the long‐term outcomes were examined using a Cox proportional hazard model. P < 0.05 was considered statistically significant for between‐group comparisons. JMP Pro 13.2.1 (SAS Institute Inc., Cary, NC, USA) was used for all statistical analyses.
Results
Baseline characteristics of the patients
Baseline characteristics of the patients are presented in Table 1. Patients included 77 males (64.7%). Thirty‐nine patients (32.8%) were diagnosed with CD at younger than 20 years of age. The disease duration was less than 3 years in 40 patients (33.6%) at anti‐TNF antibody induction. The disease locations were ileal, ileocolonic, and colonic in 16 (13.4%), 83 (69.7%), and 20 (16.8%) patients, respectively. The disease behaviors were inflammatory, stricture, and fistula in 38 (31.9%), 53 (44.5%), and 28 (23.5%) patients, respectively. Eighty‐six patients (72.3%) had anal lesion, and 74 (62.2%) patients had a history of intestinal resection. Smoking habit was confirmed in 39 (32.8%) patients; however, smoking history was unknown in 40 (33.6%) patients. Sixteen cases (13.4%) were treated with concomitant thiopurines. As for the genotyping results of TL1A ‐358C/T, the frequency of C allele, identified as the risk allele for CD, was 75.2%. Genotype frequency of TL1A ‐358C/T in the study population was C/C, C/T, and T/T in 69 (58.0%), 41 (34.5%), and 9 (7.5%) patients, respectively. Sixty‐nine cases (58.0%) were homozygous for the risk allele (TL1A ‐358C/C group), and 50 cases (42.0%) were heterozygous for the risk allele or homozygous for the protective allele (TL1A ‐358C/T+T/T group).
Table 1.
Clinical backgrounds of the study population
| n (%) | |
|---|---|
| Gender | |
| Male | 77 (64.7) |
| Female | 42 (35.3) |
| Age at diagnosis (year) | |
| <20 | 39 (32.8) |
| ≧20 | 80 (67.2) |
| Disease duration at the biologics induction (year) | |
| <3 | 40 (33.6) |
| ≧3 | 79 (66.4) |
| Disease location | |
| Ileal | 16 (13.4) |
| Ileocolonic | 83 (69.7) |
| Colonic | 20 (16.8) |
| Disease behavior | |
| Inflammatory | 38 (31.9) |
| Stricture | 53 (44.5) |
| Fistula | 28 (23.5) |
| Anal lesion | |
| No | 33 (27.7) |
| Yes | 86 (72.3) |
| Previous intestinal resection | |
| No | 45 (37.8) |
| Yes | 74 (62.2) |
| Smoking | |
| Yes | 39 (32.8) |
| No | 40 (33.6) |
| Unknown | 40 (33.6) |
| Concomitant thiopurine | |
| No | 103 (86.6) |
| Yes | 16 (13.4) |
| Anti‐TNF antibody | |
| Infliximab | 101 (84.9) |
| Adalimumab | 18 (15.1) |
| Genotype frequency of TL1A ‐358C/T (rs6478109) | |
| CC | 69 (58.0) |
| CT | 41 (34.5) |
| TT | 9 (7.5) |
| Frequency of TL1A ‐358 C/C group and C/T+T/T group | |
| TL1A ‐358C/C | 69 (58.0) |
| TL1A ‐358C/T+T/T | 50 (42.0) |
TNF, tumor necrosis factor.
Overall long‐term outcomes of anti‐TNF therapy
The Kaplan–Meier method was used to analyze the overall long‐term outcomes. The cumulative retention rates at 1, 3, and 5 years were 90.3, 78.9, and 73.3%, respectively (Fig. 1). The relapse‐free survival rates at 1, 3, and 5 years were 81.1, 54.3, and 36.8%, respectively (Fig. 2). The surgery‐free survival rates at 1, 3, and 5 years were 94.3, 86.9, and 80.3%, respectively (Fig. 3).
Figure 1.
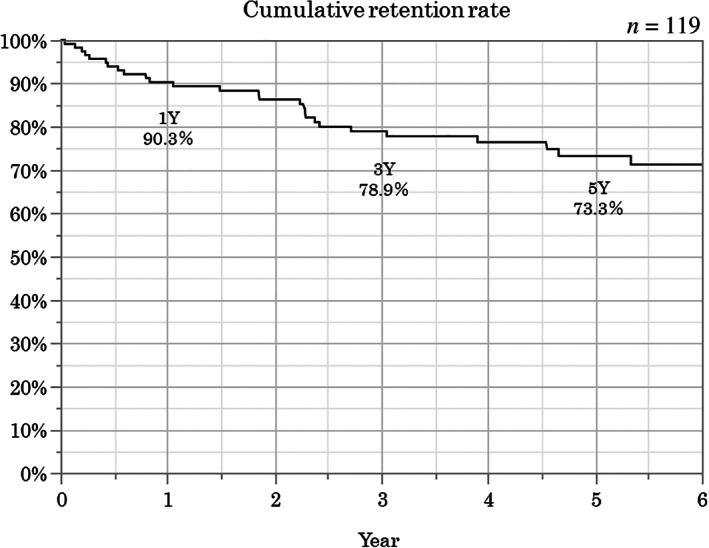
Overall cumulative retention rate. The cumulative retention rates at 1, 3, and 5 years were 90.3, 78.9, and 73.3%, respectively.
Figure 2.
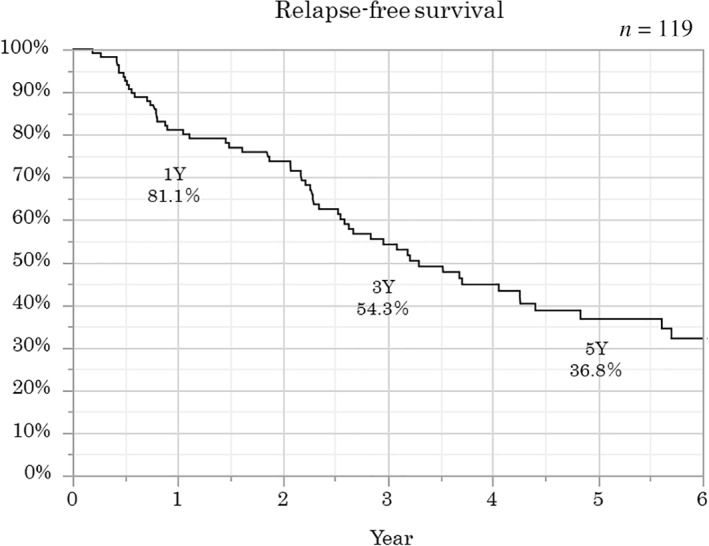
Overall relapse‐free survival. The relapse‐free survival rates at 1, 3, and 5 years were 81.1, 54.3, and 36.8%, respectively.
Figure 3.
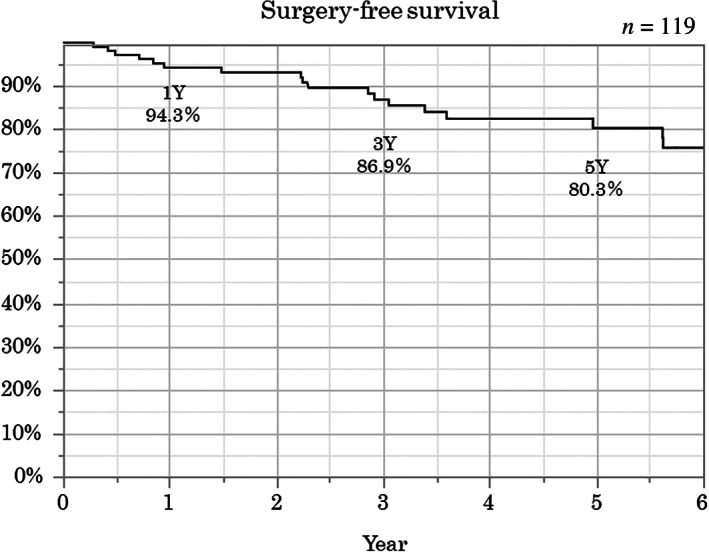
Overall surgery‐free survival. The surgery‐free survival rates at 1, 3, and 5 years were 94.3, 86.9, and 80.3%, respectively.
Impact of TL1A genotype for long‐term outcomes of anti‐TNF therapy
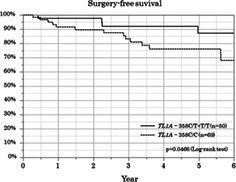
Associations between the clinical factors, including TL1A genotype, and the long‐term outcomes analyzed by univariate analysis (log‐rank test) are summarized in Table 2. The surgery‐free survival was significantly lower in the TL1A ‐358C/C group than in the C/T+T/T group (P < 0.05) (Fig. 4).
Table 2.
Association between clinical factors and long‐term outcomes (univariate analysis†)
| Clinical factor | n | Cumulative retention rate | Cumulative relapse‐free survival | Cumulative surgery free survival |
|---|---|---|---|---|
| P‐value† | ||||
| Gender | ||||
| Male | 77 | 0.1384 | 0.1604 | 0.8257 |
| Female | 42 | |||
| Age at diagnosis (year) | ||||
| <20 | 39 | 0.9087 | 0.4555 | 0.596 |
| ≧20 | 80 | |||
| Disease duration at the biologics induction (year) | ||||
| <3 | 40 | 0.9265 | 0.5584 | 0.733 |
| ≧3 | 79 | |||
| Disease location | ||||
| Ileal | 16 | 0.2981 | 0.9937 | 0.3793 |
| Ileocolonic | 83 | |||
| Colonic | 20 | |||
| Disease behavior | ||||
| Inflammatory | 38 | 0.2231 | 0.9948 | 0.6217 |
| Stricture | 53 | |||
| Fistula | 28 | |||
| Anal lesion | ||||
| No | 33 | 0.3662 | 0.261 | 0.5588 |
| Yes | 86 | |||
| Previous intestinal resection | ||||
| No | 45 | 0.9963 | 0.8593 | 0.4058 |
| Yes | 74 | |||
| Concomitant thiopurine | ||||
| No | 103 | 0.087 | 0.2323 | 0.8989 |
| Yes | 16 | |||
| TL1A ‐358C/T genotype | ||||
| ‐358C/T+T/T | 50 | 0.6799 | 0.2082 | 0.0466 †† |
| ‐358C/C | 69 | |||
Log‐rank test.
P‐value is less than 0.05.
Figure 4.
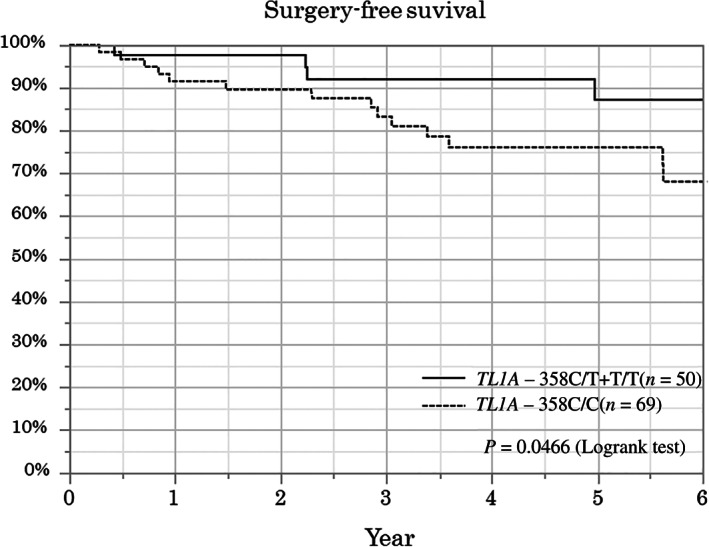
Comparison of surgery‐free survival between the TL1A ‐358C/C group and the C/T+T/T group. The surgery‐free survival rate was significantly lower in the TL1A ‐358C/C group than in the C/T+T/T group (log‐rank test, P < 0.05).
Risk factors associated with the surgery‐free survival analyzed by multivariate analysis (Cox proportional hazard model) are presented in Table 3. No clinical factors were identified as a risk for surgery except for the TL1A genotype. TL1A ‐358C/C was identified as an independent risk factor for surgery (hazard ratio [HR], 4.67; 95% confidence interval [CI], 1.39–22.1; P = 0.025).
Table 3.
Risk factors associated with the cumulative surgery‐free survivals (multivariate analysis†)
| Risk factor | n | Cumulative surgery‐free survival | |
|---|---|---|---|
| HR (95% CI) | P‐value | ||
| Gender | |||
| Male | 77 | 1 | 0.245 |
| Female | 42 | 1.96 (0.61–6.10) | |
| Age at diagnosis (year) | |||
| <20 | 39 | 1 | 0.222 |
| ≧20 | 80 | 2.20 (0.64–8.37) | |
| Disease duration at the biologics induction (year) | |||
| <3 | 40 | 1 | 0.7 |
| ≧3 | 79 | 0.806 (0.27–2.49) | |
| Disease location | |||
| Ileal | 16 | 1 | 0.308 |
| Ileocolonic | 83 | 0.467 (0.12–2.29) | |
| Colonic | 20 | 0.202 (0.02–1.55) | |
| Disease behavior | |||
| Inflammatory | 38 | 1 | 0.881 |
| Stricture | 53 | 0.982 (0.22–4.18) | |
| Fistula | 28 | 1.360 (0.24–7.91) | |
| Anal lesion | |||
| No | 33 | 1 | 0.329 |
| Yes | 86 | 0.571 (0.18–1.84) | |
| Previous intestinal resection | |||
| No | 45 | 1 | 0.874 |
| Yes | 74 | 1.119 (0.28–4.69) | |
| Concomitant thiopurine | |||
| No | 16 | 1 | 0.771 |
| Yes | 103 | 1.231 (0.25–4.48) | |
| TL1A ‐358C/T genotype | |||
| ‐358C/T+T/T | 50 | 1 | 0.025 †† |
| ‐358C/C | 69 | 4.674 (1.39–22.1) | |
Cox proportional hazard model.
CI, confidence interval; HR, hazard ratio.
P‐value is less than 0.05.
Discussion
This study showed that the TL1A genotype is associated with surgery‐free survival during anti‐TNF therapy in Japanese CD patients. Patients whose genotypes are homozygous for TL1A ‐358C (risk allele of CD development) showed lower surgery‐free survival. To our knowledge, this is the first report to describe the association between TL1A genotype and the therapeutic outcomes of anti‐TNF therapy for CD.
The results of overall long‐term outcomes of anti‐TNF therapy in this study were almost similar to the previous study. Our cumulative retention rates of the agents seemed to be consistent with the previous reports investigating the Japanese CD cohort. 16 , 17 As for the result of relapse‐free survival, approximately 20, 45, and 65% of our patients relapsed at 1, 3, and 5 years, respectively. These results indicated that LOR to the biologics gradually increased year by year. These results are not inconsistent with the recent systematic review by Qiu et al., which showed that the annual risk for LOR in anti‐TNF treatment was 20.9% per patient‐year. 18 The present outcome of surgery‐free survival seems to be satisfactory. Approximately more than 80% of the patients could avoid surgery at 5 years after anti‐TNF antibody inductions. Our results do not conflict with the recent observational cohort study reported by Eberhardson et al., which demonstrated that the cumulative rate of surgery exposed to TNF antibody at 5 years was 23%. 19
The most important finding of this study is the association between TL1A genotypes and surgery‐free survival during anti‐TNF therapy. Some previous studies reported the associations between the TL1A genotype and the disease behavior of CD. 10 , 14 , 15 However, there has been no report that describes the relationship between TL1A genotypes and the treatment outcomes of anti‐TNF agents. Basically, many studies have been performed on the role of TL1A on CD development. First, it was confirmed that increased TL1A expression was found in the inflamed gut or lamina propria T cells in CD patients. 20 , 21 , 22 Second, some studies using TL1A overexpression murine models revealed that TL1A overexpression in lymphoid or myeloid cells exhibited enhanced intestinal fibrosis or intestinal strictures. 23 , 24 These findings indicate that TL1A can play an important role in promoting not only mucosal inflammation but also fibrostenosis in CD development. Concerning the relationship between TL1A genotypes and their expression, the increased TL1A expressions in stimulated T cells or monocytes from risk haplotype for CD were reported. 25 , 26 Furthermore, a recent study revealed that SNP rs4263839 in TL1A showed the strongest association with disease progression in the 10‐year follow‐up of CD patients with inflammatory phenotype at diagnosis. 27 These previous findings lead to the hypothesis that patients homozygous for the TL1A risk haplotype could show higher TL1A expression, which could induce much severe disease progression and fibrostenosis than patients heterozygous or homozygous for nonrisk haplotype. Generally, the most common reasons for surgery are intestinal stenosis and fistula formation in the treatment course of CD. In this study, the most common reason for surgery was also intestinal stenosis. Thus, our results and previous evidence strongly suggest that the TL1A genotype affects the progression of the intestinal fibrostenosis even with anti‐TNF treatment.
According to aforementioned speculation, anti‐TL1A strategy could possibly be effective for the prevention of inflammation or fibrostenotic progression in CD treatment in the future. In fact, some recent in vivo studies have reported that the anti‐TL1A antibody reduces intestinal inflammation and fibrosis in murine colitis models. 28 , 29 , 30 This basic evidence indicates that anti‐TL1A antibody could be effective for CD, especially for patients homozygous for the TL1A risk haplotype.
The limitations of this study include a small number of the patients from a single center, the retrospective study design, lack of genetic data in some patients, missing data on smoking habits, lack of endoscopic or cross‐sectional imaging evaluations, and the imbalance in the number of patients between IFX and ADA. Thus, further large cohort studies would be ideal to confirm the obvious association between TL1A genotype and the long‐term treatment outcomes in anti‐TNF antibody.
In conclusion, we found an association between the TL1A genotype and the therapeutic outcomes of anti‐TNF therapy for CD. The patients whose genotypes are homozygous for the TL1A ‐358C (risk allele of CD development) show lower surgery‐free survival with anti‐TNF treatment. Our data indicate that the design of customized therapy with anti‐TNF antibodies using TL1A genomic information could be effective in the future.
Acknowledgments
The authors thank Prof. Tooru Shimosegawa for useful discussions and suggesting the topic studied in this paper.
Declaration of conflict of interest: The authors declare no conflict of interests for this article.
References
- 1. Hugot JP, Chamaillard M, Zouali H et al Association of NOD2 leucine‐rich repeat variants with susceptibility to Crohn's disease. Nature. 2001; 411: 599–603. [DOI] [PubMed] [Google Scholar]
- 2. Yamazaki K, McGovern D, Ragoussis J et al Single nucleotide polymorphisms in TNFSF15 confer susceptibility to Crohn's disease. Hum. Mol. Genet. 2005; 14: 3499–506. [DOI] [PubMed] [Google Scholar]
- 3. Hampe J, Franke A, Rosenstiel P et al A genome‐wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat. Genet. 2007; 39: 207–11. [DOI] [PubMed] [Google Scholar]
- 4. Duerr RH, Taylor KD, Brant SR et al A genome‐wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006; 314: 1461–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kakuta Y, Kawai Y, Naito T et al A genome‐wide association study identifying RAP1A as a novel susceptibility gene for Crohn's disease in Japanese individuals. J. Crohns Colitis. 2019; 13: 648–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001; 104: 487–501. [DOI] [PubMed] [Google Scholar]
- 7. Migone TS, Zhang J, Luo X et al TL1A is a TNF‐like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity. 2002; 16: 479–92. [DOI] [PubMed] [Google Scholar]
- 8. Endo K, Kinouchi Y, Kakuta Y, Ueki N, Takahashi S, Shimosegawa T. Involvement of NF‐kappa B pathway in TL1A gene expression induced by lipopolysaccharide. Cytokine. 2010; 49: 215–20. [DOI] [PubMed] [Google Scholar]
- 9. Zhang J, Zhang J, Wu D, Wang J, Dong W. Associations between TNFSF15 polymorphisms and susceptibility to ulcerative colitis and Crohn's disease: a meta‐analysis. Autoimmunity. 2014; 47: 512–8. [DOI] [PubMed] [Google Scholar]
- 10. Kakuta Y, Kinouchi Y, Negoro K, Takahashi S, Shimosegawa T. Association study of TNFSF15 polymorphisms in Japanese patients with inflammatory bowel disease. Gut. 2006; 55: 1527–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang SK, Lim J, Chang HS et al Association of TNFSF15 with Crohn's disease in Koreans. Am. J. Gastroenterol. 2008; 103: 1437–42. [DOI] [PubMed] [Google Scholar]
- 12. Thiebaut R, Kotti S, Jung C et al TNFSF15 polymorphisms are associated with susceptibility to inflammatory bowel disease in a new European cohort. Am. J. Gastroenterol. 2009; 104: 384–91. [DOI] [PubMed] [Google Scholar]
- 13. Franke A, McGovern DP, Barrett JC et al Genome‐wide meta‐analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat. Genet. 2010; 42: 1118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hirano A, Yamazaki K, Umeno J et al Association study of 71 European Crohn's disease susceptibility loci in a Japanese population. Inflamm. Bowel Dis. 2013; 19: 526–33. [DOI] [PubMed] [Google Scholar]
- 15. Yang DH, Yang SK, Song K et al TNFSF15 is an independent predictor for the development of Crohn's disease‐related complications in Koreans. J. Crohns Colitis. 2014; 8: 1315–26. [DOI] [PubMed] [Google Scholar]
- 16. Tanaka H, Kamata N, Yamada A et al Long‐term retention of adalimumab treatment and associated prognostic factors for 1189 patients with Crohn's disease. J. Gastroenterol. Hepatol. 2018; 33: 1031–8. [DOI] [PubMed] [Google Scholar]
- 17. Moroi R, Endo K, Yamamoto K et al Long‐term prognosis of Japanese patients with biologic‐naive Crohn's disease treated with anti‐tumor necrosis factor‐alpha antibodies. Intest. Res. 2019; 17: 94–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qiu Y, Chen BL, Mao R et al Systematic review with meta‐analysis: loss of response and requirement of anti‐TNFalpha dose intensification in Crohn's disease. J. Gastroenterol. 2017; 52: 535–54. [DOI] [PubMed] [Google Scholar]
- 19. Eberhardson M, Soderling JK, Neovius M et al Anti‐TNF treatment in Crohn's disease and risk of bowel resection‐a population based cohort study. Aliment. Pharmacol. Ther. 2017; 46: 589–98. [DOI] [PubMed] [Google Scholar]
- 20. Prehn JL, Mehdizadeh S, Landers CJ et al Potential role for TL1A, the new TNF‐family member and potent costimulator of IFN‐gamma, in mucosal inflammation. Clin. Immunol. 2004; 112: 66–77. [DOI] [PubMed] [Google Scholar]
- 21. Bamias G, Martin C 3rd, Marini M et al Expression, localization, and functional activity of TL1A, a novel Th1‐polarizing cytokine in inflammatory bowel disease. J. Immunol. 2003; 171: 4868–74. [DOI] [PubMed] [Google Scholar]
- 22. Bamias G, Kaltsa G, Siakavellas SI et al Differential expression of the TL1A/DcR3 system of TNF/TNFR‐like proteins in large vs. small intestinal Crohn's disease. Dig. Liver Dis. 2012; 44: 30–6. [DOI] [PubMed] [Google Scholar]
- 23. Shih DQ, Barrett R, Zhang X et al Constitutive TL1A (TNFSF15) expression on lymphoid or myeloid cells leads to mild intestinal inflammation and fibrosis. PLoS One. 2011; 6: e16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barrett R, Zhang X, Koon HW et al Constitutive TL1A expression under colitogenic conditions modulates the severity and location of gut mucosal inflammation and induces fibrostenosis. Am. J. Pathol. 2012; 180: 636–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kakuta Y, Ueki N, Kinouchi Y et al TNFSF15 transcripts from risk haplotype for Crohn's disease are overexpressed in stimulated T cells. Hum. Mol. Genet. 2009; 18: 1089–98. [DOI] [PubMed] [Google Scholar]
- 26. Michelsen KS, Thomas LS, Taylor KD et al IBD‐associated TL1A gene (TNFSF15) haplotypes determine increased expression of TL1A protein. PLoS One. 2009; 4: e4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pernat Drobez C, Ferkolj I, Potocnik U, Repnik K. Crohn's disease candidate gene alleles predict time to progression from inflammatory B1 to stricturing B2, or penetrating B3 phenotype. Genet. Test. Mol. Biomarkers. 2018; 22: 143–51. [DOI] [PubMed] [Google Scholar]
- 28. Shih DQ, Zheng L, Zhang X et al Inhibition of a novel fibrogenic factor Tl1a reverses established colonic fibrosis. Mucosal Immunol. 2014; 7: 1492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Clarke AW, Poulton L, Shim D et al An anti‐TL1A antibody for the treatment of asthma and inflammatory bowel disease. MAbs. 2018; 10: 664–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li H, Song J, Niu G et al TL1A blocking ameliorates intestinal fibrosis in the T cell transfer model of chronic colitis in mice. Pathol. Res. Pract. 2018; 214: 217–27. [DOI] [PubMed] [Google Scholar]


