Abstract
Although most COVID‐19 patients typically present with respiratory symptoms, many patients could experience digestive symptoms as the major complaint. We performed a systematic review and meta‐analysis to investigate the exact prevalence of digestive symptoms and liver injury in COVID‐19 patients and compare the difference between patients with and without digestive symptoms. PubMed, Embase, Ovid, Wanfang data, and CNKI were searched until 24 April 2020 to identify studies that reported digestive symptoms and liver injury in COVID‐19 patients. A random‐effect model was used to combine the data. Finally, 64 studies with 15 141 patients were included. The pooled rate of digestive symptoms and liver dysfunction was 31.8% (95 CI 21.0–42.5%, I 2 = 97.6%) and 27.4% (95 CI 16.9–37.9%, I 2 = 97.9%), respectively. Patients with digestive symptoms were more likely to present with fatigue (OR 2.28, 95 CI 1.66–3.14, P < 0.00001, I 2 = 31%), myalgia (OR 1.96, 95 CI 1.06–3.65, P = 0.03, I 2 = 69%), and acute respiratory disease syndrome (ARDS) (OR 2.94, 95 CI 1.17–7.40, P = 0.02, I 2 = 0) and had a trend to present as severe/critical type (OR 1.87, 95 CI 0.98–3.57, P = 0.06, I 2 = 58%). Severe/critical patients were more likely to present with diarrhea (OR 2.02, 95 CI 1.16–3.50, P = 0.01, I 2 = 64) and have high alanine aminotransferase (ALT) (OR 2.08, 95 CI 1.55–2.81, P < 0.00001, I 2 = 13%,) and aspartate aminotransferase (AST) (OR 3.53, 95 CI 2.76–4.51, P < 0.00001, I 2 = 0). The pooled rate of patients with digestive symptoms was 28.7% (95 CI 17.6–39.8%) and 42.8% (95 CI 23.4–62.3%) in studies from China and out of China, respectively. COVID‐19 patients had a high rate of digestive symptoms and liver injury. Patients with digestive symptoms had a trend to develop severe/critical illness.
Keywords: COVID‐19, digestive symptoms, liver injury, meta‐analysis
COVID‐19 patients had a high rate of digestive symptoms and liver injury. Patients with digestive symptoms had a trend to develop severe/critical illness.
Introduction
Currently, the pandemic of novel coronavirus disease (COVID‐19) has developed as a big threat to global health. Although the majority of COVID‐19 patients typically present with respiratory symptoms and signs, many patients could experience extrapulmonary symptoms such as digestive symptoms, including diarrhea, loss of appetite, nausea/vomiting, and abdominal pain, as the major complaints. 1 , 2 , 3 These features may be attributable to the following fact: 1) COVID‐19 is caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), and its receptor angiotensin converting enzyme 2 (ACE2) was found to be highly expressed in gastrointestinal (GI) epithelial cells, providing a prerequisite for SARS‐CoV‐2 infection and 2) SARS‐CoV‐2 viral RNA has been found in the stool specimens of infected patients, and 20% of patients showed prolonged presence of SARS‐CoV‐2 RNA in fecal samples after testing negative for the virus in the respiratory system . 4 There findings suggest that SARS‐CoV‐2 may be able to actively infect and replicate in the GI tract.
Moreover, GI infection could be the first manifestation antedating respiratory symptoms, 5 and six patients only suffering digestive symptoms but no respiratory symptoms as clinical manifestation were reported. 1 Thus, the implications of digestive symptoms in patients with COVID‐19 absolutely has significant importance. To date, there are increasing data showing that the GI tract and liver can be involved in COVID‐19 and that the infected patients could have corresponding organ damage and symptoms. 6 However, the prevalence of digestive symptoms and liver injury varied remarkably among studies. The percentage of patients with GI tract manifestations was reported to be 7.5–61.1%, 7 , 8 and liver injury was identified in 2.5–55.0% 9 , 10 of patients with COVID‐19. Thus, the exact prevalence of digestive symptoms and liver injury in COVID‐19 remains unclear. Furthermore, several studies reported that COVID‐19 patients with digestive symptoms tended to suffer a worse clinical outcome and higher risk of mortality compared to those without digestive symptoms, as well as a have a longer time from symptom onset to admission. 1 In addition, the prevalence of diarrhea and abdomen pain was significantly higher in severe patients than that in mild patients. 2 , 5 However, these studies are basically conducted in a single center from a single country involving patients from a single ethical background. In addition, the sample size of most studies was relatively small.
Therefore, we performed a systematic review and meta‐analysis using global‐wide data and aimed to comprehensively investigate 1) the exact prevalence of digestive symptoms and liver injury in COVID‐19 patients and 2) the relationship between digestive symptoms and clinical characteristics, especially the presence or absence of severe disease.
Materials and methods
Search strategy and studies selection
This study followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement. 11 PubMed, Ovid, Embase, Wanfang data, and China National Knowledge Infrastructure (CNKI) were searched for studies from December 2019 to March 27, 2020 to identify all case studies. Article language limit was not set. An updated search was performed on 24 April 2020. The search terms used were: 2019 novel coronavirus, 2019‐nCoV, COVID‐19, COVID19, SARS‐CoV‐2, SAR2, Coronavirus disease 2019, Coronavirus 2019, and Wuhan coronavirus. Studies meeting all the following criteria were included: (a) reported digestive manifestations in COVID‐19 patients; (b) the sample size of COVID‐19 patients was more than 10; (c) and full‐text articles that were peer reviewed. Exclusion criteria were as follows: (a) research data were missing; (b) duplicate reported data or paper; (c) case report, letters, editorials, reviews, and meta‐analyses not presenting original data; and (d) abstracts from conferences and commentary articles. We also reviewed the references of included articles to guarantee the comprehensiveness and accuracy of our research.
Data extraction
After performing the literature search independently, the two investigators (Jian Wan and Xuan Wang) used EndNote X 9.0 software to exclude duplicate records. After screening the title and abstract of the articles independently, two authors (Jian Wan and Xuan Wang) reviewed the full text to select potentially eligible studies and then, using predesigned standard forms, extracted data from the eligible studies independently. They captured the names of the authors, published year, type of study, country, study design, characteristics of the patients (including their number, the number of severe/critical type patients, age, gender, comorbidities, symptoms), digestive symptoms (such as diarrhea, nausea, vomiting, abdominal pain, and loss of appetite), and liver function [including alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin (TBil)]. Any disagreements were resolved by discussion with the third reviewer (Jie Liang).
Outcomes of interest
The primary outcome was the rate of various digestive symptoms and liver function in COVID‐19 patients. Secondary outcomes included the difference between patients with and without digestive symptoms and between normal/mild and severe/critical patients with COVID‐19.
Quality assessment
Two reviewers (Jian Wan and Xuan Wang) used an 11‐item checklist that was recommended by the Agency for Healthcare Research and Quality (AHRQ). 12 Article quality was assessed as follows: low quality (0–3), moderate quality (4–7), and high quality (8–11).
Statistical analysis
The data on all outcomes of interest were analyzed using Review Manager version 5.3 (Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) and Stata software version 12.0 (Stata Corporation, College Station, TX, USA). Heterogeneity among studies was tested using the Cochran Chi‐square test and I 2. A random‐effect model was used to combine the outcomes of interest because of the heterogeneity within and between studies. A random‐effect model would give a more conservative estimate of the 95% confidence interval (CI). Data were presented with odds ratio (OR) and 95% CI using forest plots. I 2 statistic and Cochran's Q test were used to assess statistical heterogeneity. The statistical significance level was set at P < 0.05. Statistical heterogeneity was set at P < 0.10 for the Q test and I 2 > 50% for the I 2 value. 13
Results
Characteristics of included studies
Based on the previous search strategy, a total of 22 493 studies were obtained from the databases. After deleting duplicate records and screening the abstract and title, 150 articles were selected for full‐text assessment. Finally, 64 studies 1 , 2 , 5 , 7 , 8 , 9 , 10 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 were included in the meta‐analysis, including data from 15 141 patients. A flow diagram of the search results and study selection is shown in Figure 1. The main characteristics of the included studies and patients are shown in Tables 1 and 2, respectively. Fifty‐five studies were from China, and nine were from out of China (Austria:1, America: 5, Spain: 1, and Singapore:2). Twenty‐five studies were published in Chinese, and the other 39 were published in English. The quality assessment of the studies is summarized in Table 1.
Figure 1.
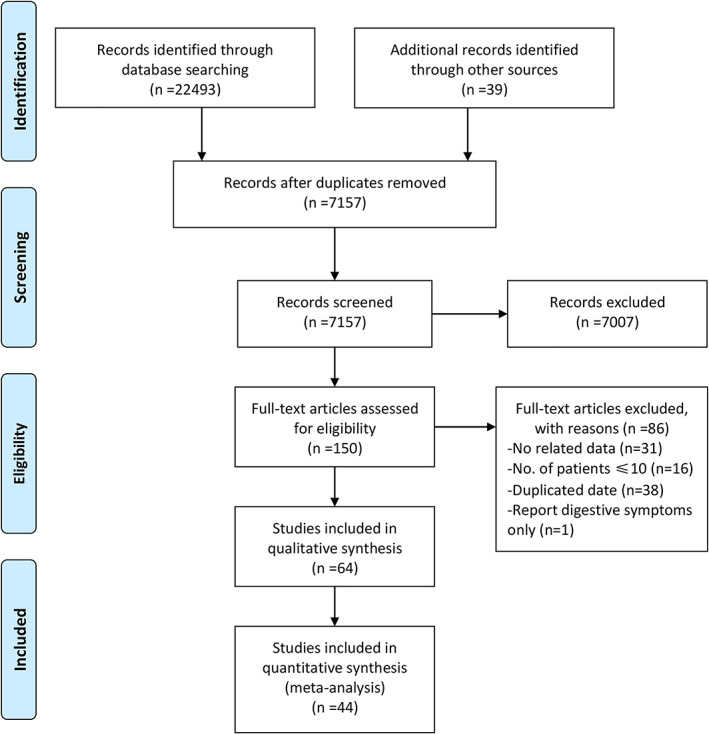
Flow diagram of the search results and study selection.
Table 1.
Characteristics of studies
| Author | Published language | Areas, center | Cases (severe) | Female | Age | Digestive comorbidities † | Liver comorbidities | Quality score |
|---|---|---|---|---|---|---|---|---|
| Min Liu 35 | Chinese | Hubei, 1 | 30 (4) | 20 | 35 ± 8 | — | — | 6 |
| Hansheng Xie 56 | English | Hubei, 1 | 79 (28) | 35 | 60 (range 48–66) | — | — | 6 |
| Fei Zhou 69 | English | Hubei, 2 | 191 (119) | 72 | 56 (IQR 46–67) | — | — | 9 |
| Xiaobo Yang 60 | English | Hubei, 1 | 52 (52) | 17 | 59.7 ± 13.3 | — | — | 8 |
| Heshui Shi 45 | English | Hubei, 2 | 81 | 39 | 49.5 ± 11.0 | — | 7 | 8 |
| Chaomin Wu 54 | English | Hubei, 1 | 201 | 73 | 51 (IQR 43–60) | — | 7 | 8 |
| Tao Chen 21 | English | Hubei, 1 | 274 | 103 | 62 (IQR 44–70) | 14 | 11 | 9 |
| Ling Mao 37 | English | Hubei, 3 | 214 (88) | 127 | 52.7 ± 15.5 | — | — | 7 |
| Qian Li 32 | Chinese | Hubei, 2 | 30 (1) | 12 | 6 (range 0–14) | — | — | 7 |
| Kui Liu 34 | English | Hubei, 9 | 137 | 76 | 57 (range 20–83) | — | — | 7 |
| Dan Fang 25 | Chinese | Hubei, 1 | 305 (46) | 159 | 57(range 18–95) | — | — | 6 |
| Lei Pan 1 | English | Hubei, 3 | 204 | 97 | 52.91 ± 15.98 | 7 | — | 8 |
| Yuanmei Guo 27 | Chinese | Hubei, 1 | 663 (409) | 342 | 58 (IQR 44–69) | 31 | — | 7 |
| Jin‐jin Zhang 64 | English | Hubei, 1 | 140 (58) | 69 | 57 (range 25–87) | 21 | 14 | 7 |
| Xiaoxia Lu 36 | English | Hubei, 1 | 171 | 67 | 6.7 (range 1 day–15) | — | — | 6 |
| Min Bai 17 | Chinese | Hubei, 1 | 472 | 257 | 50.7 ± 11.6 | — | — | 6 |
| Shi Chen 20 | Chinese | Hubei, 1 | 109 (44) | 61 | 52.5 ± 10.8 | — | — | 7 |
| Gemin Zhang 63 | English | Hubei, 1 | 95 (32) | 42 | 49 (range 39–58) | — | — | 8 |
| Zili Zhou 70 | English | Hubei, 1 | 254 | 139 | 50.6 (range 15–87) | — | 3 | 7 |
| K. Wang 51 | English | Hubei, 1 | 114 | 56 | 53 (range 23–78) | 5 | — | 6 |
| Zhongliang Wang 52 | English | Hubei, 1 | 69 | 37 | 42 (IQR 35–62) | — | 1 | 6 |
| Fenghua Xu 57 | Chinese | Hubei, 1 | 251 (175) | 119 | 59.9 ± 15.7 | — | 4 | 6 |
| Rong Wang 44 | Chinese | Hubei, 1 | 96 (54) | 50 | NA | 7 | 2 | 5 |
| Pingzheng Mo 38 | English | Hubei, 1 | 155 (92) | 69 | 54 (IQR 42–66) | — | 7 | 7 |
| Dawei Wang 5 | English | Hubei, 1 | 138 | 63 | 56 (IQR 42–68) | — | 4 | 8 |
| Wei‐jie Guan 2 | English | China, 522 | 1099 (173) | 459 | 47 (IQR 35–58) | — | 23 | 9 |
| Dahai Zhao 66 | English | Anhui, 2 | 19 | 8 | 48 (IQR 27 ~ 56) | — | 1 | 7 |
| Yalin Li 14 | Chinese | Anhui, 1 | 49 (27) | 21 | 45 (range 14 ~ 82) | — | 3 | 5 |
| De Chang 18 | English | Beijing, 3 | 13 | 3 | 34 (IQR 34–48) | — | — | 6 |
| Ke Wen 53 | Chinese | Beijing, 1 | 46 (11) | 19 | 41.8 ± 16.3 | — | — | 6 |
| Xi Xu 59 | English | Guangdong, 1 | 90 | 51 | 50 (range 18–86) | — | — | 7 |
| Jing Yuan 62 | English | Guangdong, 1 | 94 (11) | 52 | 40 (range 1–78) | — | — | 8 |
| Lu Lin 7 | English | Guangdong, 1 | 95 (20) | 50 | 45.3 ± 18.3 | — | — | 8 |
| Rui Zhao 67 | Chinese | Guangxi, 1 | 28(2) | 17 | 44.5 (range 11–68) | — | — | 5 |
| Chan Sun 46 | Chinese | Henan, 16 | 150 (39) | 83 | 45 ± 16 | — | 1 | 6 |
| Ye Zhao 8 | Chinese | Henan, 1 | 106 (15) | 40 | 48.9 ± 13.1 | — | 2 | 8 |
| Fang Zheng 68 | English | Hunan, 1 | 161 (30) | 81 | 45 (range 33.5–57) | — | 4 | 6 |
| Xin Tan 47 | Chinese | Hunan, 1 | 13 (1) | 9 | 8 (range 1–17) | — | — | 6 |
| Dan Li 31 | Chinese | Hunan, NA | 80 (17) | 40 | 47.5 (range 3–90) | — | 3 | 6 |
| Tianmin Xu 58 | English | Jiangsu, 1 | 51 | 26 |
35 (IQR 29–51) (n = 15) 37(IQR24–47.5) (n = 17) 53 (IQR35–65) (n = 19) |
— | 1 | 6 |
| Miao Zhu 15 | Chinese | Jiangsu, 1 | 23 | 13 | 50.0 ± 13.0 | — | — | 5 |
| Jian Wu 10 | English | Jiangsu and Anhui, 4 | 280 (83) | 129 | 43.12 ± 19.02 | 16 | 7 | 7 |
| Tianxin Xiang 55 | Chinese | Jiangxi, 1 | 49 (9) | 16 | 42.9 (range 18–78) | — | 6 | 6 |
| Shuxiang Zhang 65 | Chinese | Ningxia, 1 | 34 (5) | 14 | 41 ± 17 | — | — | 7 |
| Na Yao 9 | Chinese | Shaanxi, 1 | 40 (22) | 15 | 53.9 ± 15.8 | — | — | 6 |
| Jun Chen 19 | English | Shanghai, 1 | 249 | 123 | 51(IQR 36–64) | — | 2 | 6 |
| Dan Li 2 30 | Chinese | Liaoning, 1 | 30 (9) | 12 | 43 (range 21–72) | — | — | 6 |
| Xiaochun Dong 23 | Chinese | Tianjin, NA | 135 (62) | 63 | 48.6 ± 16.8 | — | — | 5 |
| Kelvin Kai‐Wang 29 | English | Hongkong, 2 | 23 (10) | 10 | 62 (range 37–75) | — | — | 8 |
| Xi Jin 28 | English | Zhejiang, NA | 651 | 320 | 46.1 ± 14.2 (n = 74) 45.1 ± 14.5 (n = 577) | — | 25 | 9 |
| Xiaolong Qi 41 | English | China, 9 | 70 (3) | 31 |
41 (IQR 27.5–50) (n = 32) 38.5 (IQR 26–47.3) (n = 38) |
— | — | 6 |
| Duan Wang 50 | Chinese | China, 21 | 31 (0) | NA | 7.1 (range 0.5–17) | — | — | 5 |
| Chuan Liu 33 | Chinese | China, 7 | 32 (4) | 12 | 38.5 (IQR 26.3–45.8) | — | 1 | 7 |
| Suxin Wan 49 | Chinese | Chongqing, 1 | 135 (40) | 63 | 47 (IQR 36–55) | — | 2 | 6 |
| Jing Yuan 16 | Chinese | Chongqing, 1 | 223 (31) | 117 | 46.5 ± 16.1 | — | 8 | 5 |
| Maria Effenberger 24 | English | Austria,1 | 40 | 16 |
58.4 ± 17.1 (n = 18) 66.3 (±13.1) (n = 13) 78.3 (±13.8) (n = 9) |
— | — | 5 |
| The COVID‐19 Investigation Team 48 | English | America, 6 | 12 | 4 | 53 (range 21–68) | — | 2 | 5 |
| Safiya Richardson 43 | English | America, 12 | 5700 | 2263 | 63 (IQR 52–75) | — | 30 | 6 |
| George Cholankeril 22 | English | America, 1 | 116 | 54 | 50 (IQR 35–67) | — | 3 | 5 |
| Walker D. Redd 42 | English | America, 9 | 318 | 144 | 63.4 ± 16.6 | 122 | — | 5 |
| Yael R. Nobel 39 | English | America, 1 | 278 | 133 | Range 18–30 (n = 31); 31–50 (n = 69); 51–70 (n = 103); >70 (n = 75) | — | — | 5 |
| Mario Fernández‐Ruiz 26 | English | Spain, 1 | 18 | 4 | 72 (range 39–80) | 7 | 7 | 5 |
| Rachael Pung 40 | English | Singapore, 1 | 17 | 10 | 40 (IQR 36–51) | — | — | 6 |
| BE Young 61 | English | Singapore, 4 | 18 | 9 | 47 (range 31–73) | — | — | 7 |
Including the patients with liver comorbidities.
IQR, interquartile range; NA, not available;
Table 2.
Characteristics of digestive symptoms and liver function of the study patients
| Author, year | Cases | Diarrhea | Nausea and/or Vomiting | Abdominal pain | Loss of appetite | Digestive symptoms | Elevated ALT | Elevated AST | Elevated TBil | Abnormal liver functions |
|---|---|---|---|---|---|---|---|---|---|---|
| Min Liu 35 | 30 | — | — | — | — | 9/30 | — | — | — | 7/30 |
| Hansheng Xie 56 | 79 | 7 | — | — | — | — | 25/79 | 28/79 | 4/79 | — |
| Fei Zhou 69 | 191 | 9 | 7 | — | — | — | 59/189 | — | — | — |
| Xiaobo Yang 60 | 52 | — | 2 | — | — | — | — | — | — | 15/52 |
| Heshui Shi 45 | 81 | 3 | 4 | — | 1 | — | — | 43/81 | — | — |
| Chaomin Wu 54 | 201 | — | — | — | — | — | 43/198 | 59/198 | 10/198 | — |
| Tao Chen 21 | 274 | 77 | 24,16 † | 19 | 66 | — | 60/274 | 84/274 | — | — |
| Ling Mao 37 | 214 | 41 | — | 10 | 68 | — | — | — | — | — |
| Qian Li 32 | 30 | 2 | 2 | — | — | — | — | — | — | — |
| Kui Liu 34 | 137 | 11 | — | — | — | — | — | — | — | — |
| Dan Fang 25 | 201 | 146/295 ‡ | 59,32 | 12 | 101 | 159/201 ‡ | 76/304 | 97/304 | 6/304 | 119/304 |
| Lei Pan 1 | 204 | 35 | 4 | 2 | 81 | 103/204 | 27/204 | 22/204 | ||
| Yuanmei Guo 27 | 663 | 70 | — | — | — | — | 151/617 | 171/617 | — | — |
| Jin‐jin Zhang 64 | 139 | 18 | 24,7 | 8 | 17 | 55/139 | — | — | — | — |
| Xiaoxia Lu 36 | 171 | 15 | 11 | — | — | — | — | — | — | — |
| Min Bai 17 | 472 | 38 | 10 | — | — | — | — | — | — | — |
| Shi Chen 20 | 109 | 23 | — | — | — | — | 25/109 | 23/109 | — | — |
| Gemin Zhang 63 | 95 | — | — | — | — | — | 52/95 | 45/95 | — | — |
| Zili Zhou 70 | 254 | 46 | 21,15 | 3 | — | 66/254 | — | — | — | — |
| K. Wang 51 | 114 | 3 | — | — | — | — | — | — | — | — |
| Zhongliang Wang 52 | 69 | 10 | 3 | — | 7 | 23/69 | 19/69 | — | — | |
| Fenghua Xu 57 | 251 | 30 | 19 | 3 | 85 | 116/251 | 106/251 | 110/251 | 31/251 | 143/251 |
| Rong Wang 44 | 96 | 11 | — | — | — | — | — | — | — | — |
| Pingzheng Mo 38 | 155 | 7 | 3,3 | 3 | 26 | — | — | — | — | — |
| Dawei Wang 5 | 138 | 14 | 14,5 | 3 | 55 | — | — | — | — | — |
| Wei‐jie Guan 2 | 1099 | 42 | 55 | — | — | — | 158/741 | 168/757 | 76/722 | — |
| Dahai Zhao 66 | 19 | 1 | — | — | — | — | 5/18 | 5/18 | — | — |
| Yalin Li 14 | 49 | 31 | — | — | — | — | 13/49 | 11/49 | — | — |
| De Chang 18 | 13 | 1 | — | — | — | — | — | — | — | — |
| Ke Wen 53 | 46 | 1 | — | — | — | — | 8/46 | 5/46 | — | — |
| Xi Xu 59 | 90 | 5 | 5,2 | — | — | — | — | — | — | — |
| Jing Yuan 62 | 94 | 8 | — | — | — | — | — | — | — | — |
| Lu Lin 7 | 95 | 23 | 17,4 | — | 17 | 58/95 | — | — | — | — |
| Rui Zhao 67 | 28 | — | — | — | — | — | 6/28 | 3/28 | — | — |
| Chan Sun 46 | 150 | 2 | — | — | 20 | — | 24/150 | 15/150 | 3/150 | — |
| Ye Zhao 8 | 106 | 7 | 1 | 0 | — | 8/106 | — | — | — | — |
| Fang Zheng 68 | 161 | 17 | 6 | — | — | — | 13/161 | 22/161 | 9/161 | — |
| Xin Tan 47 | 13 | 2 | 1 | 1 | — | — | — | — | — | — |
| Dan Li 31 | 80 | 13 | — | — | — | — | — | — | — | — |
| Tianmin Xu 58 | 51 | 5 | — | — | — | — | — | — | — | — |
| Miao Zhu 15 | 23 | — | — | — | — | 2/23 | — | — | — | 2/23 |
| Jian Wu 10 | 280 | 7 | 3 | — | — | — | — | — | — | 7/280 |
| Tianxin Xiang 55 | 49 | 2 | — | — | — | — | — | — | — | — |
| Shuxiang Zhang 65 | 34 | 1 | — | 1 | — | — | — | — | — | — |
| Na Yao 9 | 40 | — | — | 1 | — | 7/40 | 21/40 | — | 10/40 | 22/40 |
| Jun Chen 19 | 249 | 8 | — | — | — | — | — | — | — | — |
| Dan Li 2 30 | 30 | — | — | — | — | 5/30 | — | — | — | 6/30 |
| Xiaochun Dong 23 | 135 | 7 | — | — | — | — | — | — | — | — |
| Kelvin Kai‐Wang 29 | 23 | 2 | 1 | — | — | — | 4/23 | — | — | — |
| Xi Jin 28 | 651 | 53 | 10,11 | — | — | 74/651 | — | — | — | 64/651 |
| Xiaolong Qi 41 | 70 | 7 | — | — | — | — | 15/70 | 5/70 | 25/70 | 32/70 |
| Duan Wang 50 | 31 | 3 | 2 | — | — | — | — | — | — | 6/27 |
| Chuan Liu 33 | 32 | — | — | — | — | — | 9/32 | 2/32 | — | — |
| Suxin Wan 49 | 135 | 18 | 4 | — | 6 | — | — | 30/135 | — | — |
| Jing Yuan 16 | 223 | 12 | — | — | — | — | 42/223 | 32/223 | — | — |
| Maria Effenberger 24 | 40 | 22 | 11,5 | — | — | — | — | — | — | — |
| The COVID‐19 Investigation Team 48 | 12 | 1 | 1 | — | — | — | — | — | — | — |
| Safiya Richardson 43 | 5700 | — | — | — | — | — | — | 3263/5700 | — | — |
| George Cholankeril 22 | 116 | 12 | 12 | 10 | 22 | 37/116 | — | — | — | 26/65 |
| Walker D. Redd 42 | 318 | 107 | 84,49 | 46 | 110 | 195/318 | — | — | — | — |
| Yael R. Nobel 39 | 278 | 56 | 63 | — | — | 97/278 | — | — | — | — |
| Mario Fernández‐Ruiz 26 | 18 | 4 | 1 | — | — | — | — | — | — | — |
| Rachael Pung 40 | 17 | 4 | 1 | — | — | — | — | — | — | — |
| BE Young 61 | 18 | 3 | — | — | — | — | — | — | — | — |
No. of patients with nausea, No. of patients with vomiting.
Excluded from the meta‐analysis (many patients presented with diarrhea after using oseltamivir and/or abidor).
AST, aspartate aminotransferase; ALT, alanine aminotransaminase; TBil, total bilirubin;
Digestive symptoms
The pooled results of 14 studies (2535 patients) showed that the rate of patients with digestive symptoms was 31.8% (95 CI 21.0–42.5%, I 2 = 97.6%, heterogeneity P = 0.000) (Fig. S1a). The main digestive symptoms were diarrhea (53 studies, 8604 patients: 11.2%, 95 CI 9.3–13.1%, I 2 = 90.7%, heterogeneity P = 0.000) (Fig. S1c), nausea and/or vomiting (33 studies, 6165 patients: 10.0%, 95 CI 7.6–12.3%, I 2 = 94.4%, heterogeneity P = 0.000) (Fig. S1f), loss of appetite (15 studies, 2540 patients: 21.3%, 95 CI 14.0–28.7%, I 2 = 97%, heterogeneity P = 0.000) (Fig. S1d), and abdominal pain (14 studies, 2203 patients: 4.6%, 95 CI 2.7–6.5%, I 2 = 82.5%, heterogeneity P = 0.000) (Fig. S1e). The pooled estimate of digestive disease comorbidities was 11.2% (95 CI 6.1–16.3%, I 2 = 95.2%, heterogeneity P = 0.000, 9 studies, 2107 patients) (Fig. S1b) (Table 3).
Table 3.
Results of meta‐analysis (random‐effect model)
| Characteristics | Studies | Patients | I 2 (%) | Heterogeneity P | Pooled rate (%) | 95% CI (%) |
|---|---|---|---|---|---|---|
| Digestive symptoms | 14 | 2535 | 97.6 | 0.000 | 31.8 | 21.0–42.5 |
| Diarrhea | 53 | 8604 | 90.7 | 0.000 | 11.2 | 9.3–13.1 |
| Nausea and/or vomiting | 33 | 6165 | 94.4 | 0.000 | 10.0 | 7.6–12.3 |
| Loss of appetite | 15 | 2540 | 97 | 0.000 | 21.3 | 14.0–28.7 |
| Abdominal pain | 14 | 2203 | 82.5 | 0.000 | 4.6 | 2.7–6.5 |
| Digestive diseases | 9 | 2107 | 95.2 | 0.000 | 11.2 | 6.1–16.3 |
| Abnormal liver function | 12 | 1878 | 97.9 | 0.000 | 27.4 | 16.9–37.9 |
| ALT | 23 | 3973 | 87.8 | 0.000 | 25.3 | 21.3–29.2 |
| AST | 23 | 9650 | 98.8 | 0.000 | 25.4 | 16.1–34.6 |
| TBil | 9 | 1975 | 91.5 | 0.000 | 8.8 | 5.1–12.5 |
| Liver diseases | 29 | 10 839 | 75 | 0.000 | 2.5 | 1.8–3.3 |
ALT, alanine aminotransaminase; AST, aspartate aminotransferase; CI, confidence level; TBil, total bilirubin.
Liver injury
The pooled results of 12 studies (1878 patients) showed that the rate of patients with abnormal liver function was 27.4% (95 CI 16.9–37.9%, I 2 = 97.9%, heterogeneity P = 0.000) (Fig. S2a). The pooled results demonstrated that the rate of high ALT was 25.3% (95 CI 21.3–29.2%, I 2 = 87.8%, heterogeneity P = 0.000, 23 studies, 3973 patients) (Fig. S2c), the rate of high AST was 25.4% (95 CI 16.1–34.6%, I 2 = 98.8%, heterogeneity P = 0.000, 23 studies, 9650 patients) (Fig. S2d), and the rate of high TBil was 8.8% (95 CI 5.1–12.5%, I 2 = 91.5%, heterogeneity P = 0.000, 9 studies, 1975 patients) (Fig. S2e). The pooled rate of liver diseases comorbidities was 2.5% (95 CI 1.8–3.3%, I 2 = 75%, heterogeneity P = 0.000, 29 studies, 10 839 patients) (Fig. S2b) (Table 3).
Subgroup analysis of comparing COVID‐19 patients with and without digestive symptoms
There were eight studies 1 , 7 , 8 , 27 , 28 , 42 , 57 , 70 including 2542 patients focusing on the differences between patients with and without digestive symptoms. Patients with digestive symptoms were more likely to present with fatigue (OR 2.28, 95 CI 1.66–3.14, P < 0.00001, I 2 = 31%, heterogeneity P = 0.21, 5 studies, 1992 patients) and myalgia (OR 1.96, 95 CI 1.06–3.65, P = 0.03, I 2 = 69%, heterogeneity P = 0.04, 3 studies, 1223 patients) (Fig. S3). There was no significance between patients with and without digestive symptoms in age, gender, fever, sore throat, cough, sputum production, chest tightness, dyspnea, headache, dizziness, hemoptysis, and comorbidities. When comparing the difference in complications, patients with digestive symptoms were more likely to present with ARDS (OR 2.94, 95 CI 1.17–7.40, P = 0.02, I 2 = 0, heterogeneity P = 0.59, 2 studies, 905 patients) (Fig. 2). No difference was found in shock, acute heart failure, arrhythmia, pneumonia, and liver injury. Patients with digestive symptoms had a trend to present as severe/critical type (OR 1.87, 95 CI 0.98–3.57, P = 0.06, I 2 = 58%, heterogeneity P = 0.07, 4 study, 1515 patients) (Fig. 2). When comparing the difference in treatments, patients with digestive symptoms were more likely to be treated with immunoglobulins (OR 2.39, 95 CI 1.53–3.72, P = 0.0001, I 2 = 0, heterogeneity P = 0.34, 2 study, 458 patients). No difference was found in mechanical ventilation, antibiotics, glucocorticoids, antivirals, extracorporeal membrane oxygenation (ECMO), and intensive care unit admission (Fig. S3).
Figure 2.
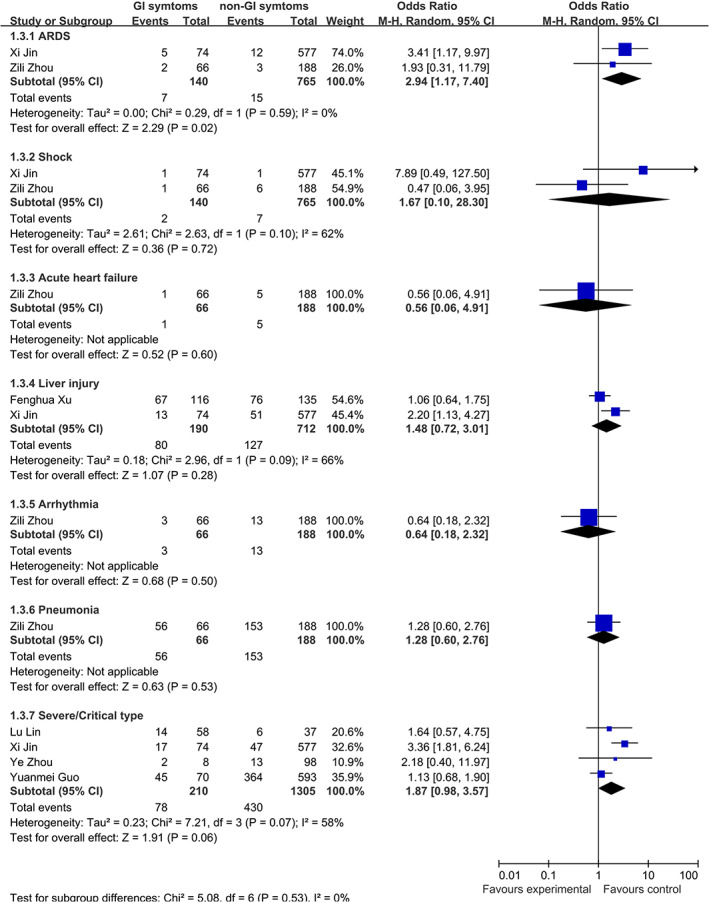
Comparison of complications between COVID‐19 patients with and without digestive symptoms.
Subgroup analysis: Severe/critical versus mild/normal type
Patients with severe/critical type were more likely to present with diarrhea (OR 2.02, 95 CI 1.16–3.50, P = 0.01, I 2 = 64, heterogeneity P = 0.0003, 16 studies, 3849 patients) and have high ALT (OR 2.08, 95 CI 1.55–2.81, P < 0.00001, I2 = 13%, heterogeneity P = 0.33, 8 studies, 1830 patients) and AST (OR 3.53, 95 CI 2.76–4.51, P < 0.00001, I 2 = 0, heterogeneity P = 0.57, 8 studies, 1959 patients) (Fig. 3). No difference was found in nausea and/or vomiting, abdominal pain, loss of appetite, and TBil (Fig. 3).
Figure 3.
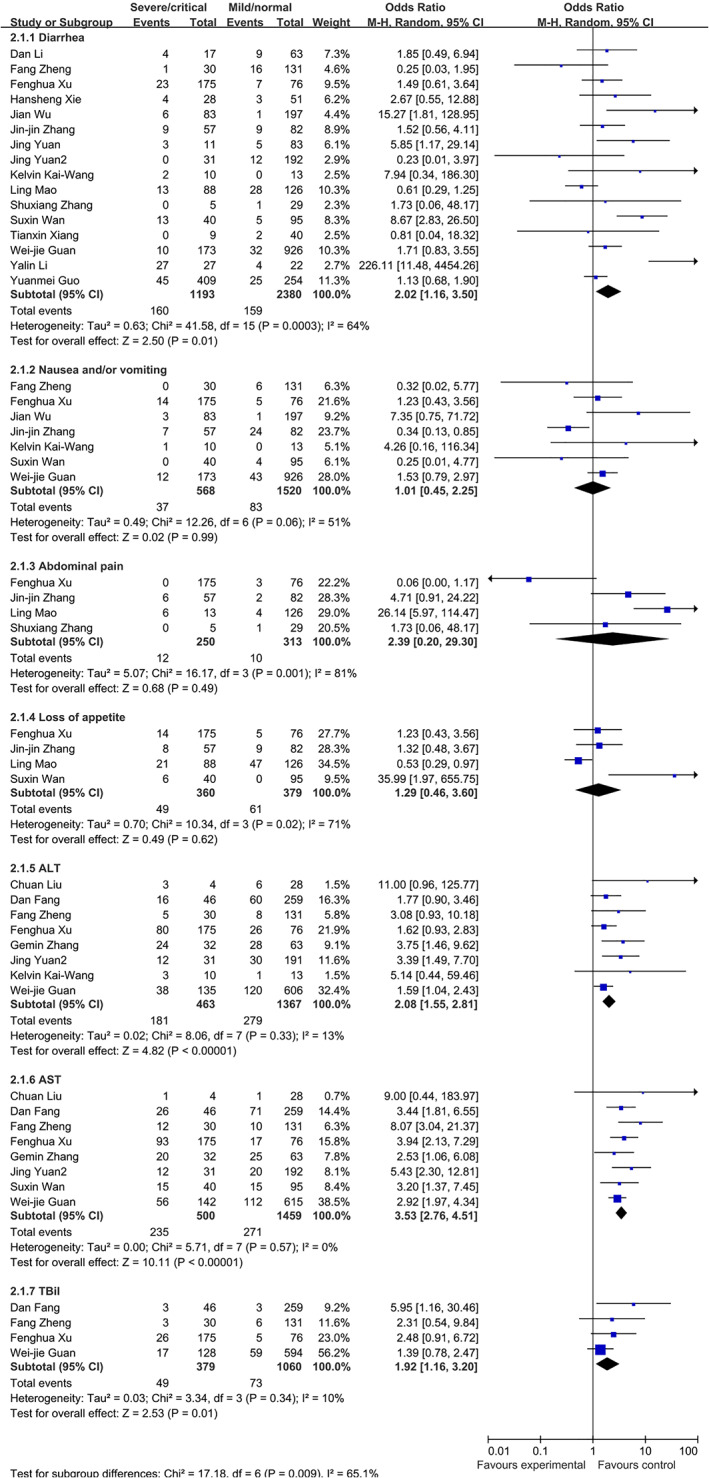
Comparison of normal/mild and severe/critical patients with COVID‐19.
Subgroup analysis: China versus out of China
The pooled rate of patients with digestive symptoms was 28.7% (95 CI 17.6–39.8%) and 42.8% (95 CI 23.4–62.3%) in studies from China and out of China, respectively (Fig. S1a). The pooled rate of patients with diarrhea was 9.6% (95 CI 7.9–11.4%) and 23.5% (95 CI 14.2–32.9%) in studies from China and out of China, respectively (Fig. S1c). The pooled rate of patients with loss of appetite was 20.5% (95 CI 12.8–28.2%) and 27.0% (95 CI 11.7–42.3%) in studies from China and out of China, respectively (Fig. S1d). The pooled rate of patients with abdominal pain was 3.2% (95 CI 1.8–4.6%) and 11.8% (95 CI 6.1–17.5%) in studies from China and out of China, respectively (Fig. S1e). The pooled rate of patients with nausea and/or vomiting was 7.6% (95 CI 5.6–9.5%) and 19.4% (95 CI 7.6–31.7%) in studies from China and out of China, respectively (Fig. S1f). The pooled rate of patients with high AST was 23.6% (95 CI 18.9–28.3%) and 57.2% (95 CI 56.0–58.5%) in studies from China and out of China, respectively (Fig. S2d). The pooled rate of patients with liver dysfunction was 27.9% (95 CI 16.7–39.1%) and 22.4% (95 CI 14.8–30.0%) in studies from China and out of China, respectively (Fig. S2a).
Publication bias
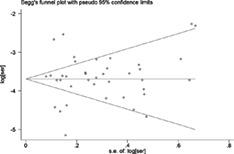
The Begg funnel plot for the rate of diarrhea in COVID‐19 patients is shown in Figure S4. There was no publication bias for Begg's test (P = 1.000) and Egger's test (P = 0.945). Publication bias was also analyzed in the digestive symptom‐related outcomes, which included more than 10 studies. No publication bias was found in the rate of nausea and/or vomiting (Begg's test P = 0.215, Egger's test P = 0.254), loss of appetite (Begg's test P = 0.274, Egger's test P = 0.429), abdominal pain (Begg's test P = 1.000, Egger's test P = 0.752), and digestive symptoms (Begg's test P = 0.669, Egger's test P = 0.411).
Discussion
In this meta‐analysis, we demonstrated that the pooled rate of digestive symptoms and abnormal liver function was 31.8 and 27.4%, respectively. The most common digestive symptom was loss of appetite and diarrhea. COVID‐19 patients with digestive symptoms are more likely to present with fatigue, myalgia, and ARDS when compared with patients without digestive symptoms. Furthermore, severe/critical patients are more likely to present with diarrhea and liver dysfunction.
A previous meta‐analysis 71 summarizing the clinical, laboratory, and imaging features of COVID‐19 showed that the pooled rate of diarrhea, elevated AST, and liver diseases in COVID‐19 patients was 6, 33, and 3%, respectively. This meta‐analysis included only 19 studies, and only 6 studies had reported diarrhea in COVID‐19 patients. None of other digestive symptoms were analyzed, such as nausea, vomiting, and loss of appetite, which were also common symptoms in COVID‐19 patients. After the meta‐analysis was published, several large sample studies focusing on the clinical features, especially the digestive features, were published. So, a further meta‐analysis with more concise results was needed to obtain a deeper understanding of the digestive symptoms in the COVID‐19 patients.
When we performed the presented meta‐analysis, a similar study from Hong Kong was published. 72 We included liver injury in our study and excluded studies from the same department of the same hospital, which differed from the Hong Kong study. For example, many patients in Chen et al.'s study 73 and Huang et al.'s study 74 were also included in Zhou et al.'s study 69 and Wu et al.'s study. 54 We excluded Chen et al.'s study 73 and Huang et al.'s study 73 , 74 to make our results more precise, and when calculating the rate of digestive symptoms, we included the studies that mentioned the studied patients reporting digestive symptoms. However, in Cheung et al.'s meta‐analysis, they also included the studies that did not mention patients having definite digestive symptoms and then counted the number of the most frequent single digestive symptom (like diarrhea, vomiting…) to calculate the prevalence of digestive symptoms. This significantly reduced the rate of digestive symptoms, which can explain why the rate of digestive symptoms in our study (28.8%) was much higher than Cheung et al.'s meta‐analysis (17.6%). 72
Fever and cough were the most emphasized symptoms, and the screening of patients with SAR‐COV‐2 infection started by measuring body temperature. Patients with uncommon symptoms might be misdiagnosed, which could pose a great potential danger to the whole society. Overlooking the digestive symptoms by the public or the physicians might contribute to transmission as some patients only presented with digestive symptoms. Among the 204 COVID‐19 patients in Pan et al.'s study, 1 6 patients presented with only digestive symptoms in the absence of respiratory symptoms (one patient even without fever). Luo et al.'s 75 study reported that 183 (16%) patients only presented with GI symptoms of the 1141 confirmed COVID‐19 cases. Jin et al.'s study 28 demonstrated that 11.4% patients presented with at least one GI tract symptom (nausea, vomiting, and diarrhea), and diarrhea was the most common GI symptom. In the present meta‐analysis, we confirmed that a large number of COVID‐19 patients could present with digestive symptoms. Patients with digestive symptoms seemed to have a high rate of fatigue, liver injury, and ARDS. This might be of great significance to the treatment of COVID‐19. Currently, few studies focused on the difference between COVIP‐19 patients with and without digestive symptoms. Our results need to be confirmed by a large‐sample, well‐designed study.
Patients with digestive symptoms had a tendency to develop severe/critical illness in our study (P = 0.06). When comparing with the normal/mild patients, severe/critical patients had a higher rate of diarrhea. Patients with digestive symptoms had a longer time from onset to admission (8.95 days vs 7.26 days). 1 This may be because uncommon digestive symptoms led to a delay in diagnosis and treatment for COVID‐19. These results highlight the importance of recognition of the digestive symptoms associated with COVID‐19.
COVID‐19 patients had a high rate of liver injury, especially the severe patients. Huang et al.'s study 74 demonstrated that the rate of elevated AST was 37%, and the rate was up to 62% in severe patients. In a national multicenter study, 2 the rate of elevated ALT, AST, and TBil was 21.3, 22.2, and 10.5%, respectively. The rate of elevated ALT, AST, and TBil was higher in severe patients than in nonsevere patients. 2 Our study also showed that COVID‐19 patients had a high rate of liver dysfunction, and severe/critical patients had a higher rate of liver dysfunction than normal/mild patients, which was consistent with previous studies. Monitoring and assessment of liver function should be strengthened when treating COVID‐19 patients, especially in critically ill patients.
The major strengths of this study are listed as follow: First, we excluded studies from the same department of the same hospital to make our results more reliable. Furthermore, COVID‐19 patients reported at the time were mainly Chinese, and a considerable number of case was summarized and published in Chinese journals; in this study, we specifically included 18 studies published in Chinese, with a total of 2008 patients, to demonstrate a more precise prevalence and impact of GI involvement in patients with COVID‐19. Moreover, this is the first meta‐analysis comparing the difference between COVID‐19 patients with and without digestive symptoms. In addition, there were some limitations in our study. First, all studies included in this meta‐analysis were retrospective studies with large heterogeneity. Second, most patients in our meta‐analysis were Chinese, and whether our results were applicable to patients in other countries was unknown.
Conclusions
In summary, digestive symptoms are common, with a prevalence of about 30%, in patients with COVID‐19. Patients with digestive symptoms are more likely to present with fatigue, myalgia, and ARDS and have a tendency to develop severe/critical illness. Furthermore, severe/critical patients are more likely to present with diarrhea and liver dysfunction.
Ethics approval
Ethical approval was not required as this study is a meta‐analysis of published studies.
Supporting information
Figure S1 Meta‐analysis of the rate of (a) digestive symptoms, (b) digestive comorbidities, (c) diarrhea, (d) loss of appetite, (e) abdominal pain, and (f) nausea and/or vomiting in COVID‐19 patients.
Figure S2 Meta‐analysis of the rate of (a) abnormal liver function, (b) liver comorbidities, (c) high ALT, (d) high AST, and (e) high TBil in COVID‐19 patients.
Figure S3 Comparison of COVID‐19 patients with and without digestive symptoms.
Figure S4 Begg funnel plot for the rate of diarrhea in COVID‐19 patients
Acknowledgements
Our study was supported by the National Natural Science Foundation of China (81421003, 81627807, 81772650, 81322037, and 81572302) and the National Key Research and Development Plan of China (2017YFC0908300).
Declaration of conflict of interest: None.
Author contribution: Jian Wan and Jie Liang formulated the research questions and designed the study. Jian Wan, Xuan Wang, and Jie Liang developed the search strategy. Jian Wan and Xuan Wang collected and analyzed the data. Yujie Zhang, Yirong Jin, and Yanting Shi verified the data. Jian Wan, Xuan Wang, and Song Su drafted the manuscript. Yujie Zhang, Kaichun Wu, and Jie Liang revised the paper. All authors critically reviewed the manuscript for relevant intellectual content. All authors have read and approved the final version of the manuscript.
Guarantor of the article: Jie Liang.
References
- 1. Pan L, Mu M, Yang P et al Clinical characteristics of COVID‐19 patients with digestive symptoms in Hubei, China: a descriptive, cross‐sectional, multicenter study. Am. J. Gastroenterol. 2020; 115: 766–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guan WJ, Ni ZY, Hu Y et al Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020; 382: 1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wong SH, Lui RN, Sung JJ. Covid‐19 and the digestive system. J. Gastroenterol. Hepatol. 2020; 35: 744–8. [DOI] [PubMed] [Google Scholar]
- 4. Holshue ML, DeBolt C, Lindquist S et al First Case of 2019 Novel Coronavirus in the United States. N. Engl. J. Med. 2020; 382: 929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang D, Hu B, Hu C et al Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus‐Infected Pneumonia in Wuhan, China. JAMA. 2020; 323: 1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mao R, Liang J, Wu K‐C, Chen M‐H. Responding to COVID‐19: Perspectives from the Chinese Society of Gastroenterology. Gastroenterology. 2020; 158: 2024–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lin L, Jiang X, Zhang Z et al Gastrointestinal symptoms of 95 cases with SARS‐CoV‐2 infection. Gut. 2020; 69: 997–1001. [DOI] [PubMed] [Google Scholar]
- 8. Zhao Y, Zhong SP, Li F et al Clinical characteristics and risk factors of gastrointestinal symptoms in patients with novel coronavirus pneumonia in Xinyang, Henan province. Chin. J. Dig. 2020; 40: E011–11. [Google Scholar]
- 9. Yao N, Wang SN, Lian JQ et al Clinical characteristics and influencing factors of patients with novel coronavirus pneumonia combined with liver injury in Shaanxi region. Zhonghua Gan Zang Bing Za Zhi. 2020; 28: E003 10.3760/cma.j.cn501113-20200226-00070. [DOI] [PubMed] [Google Scholar]
- 10. Wu J, Li W, Shi X et al Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID‐19). J. Intern. Med. 2020; 288: 128–38. 10.1111/joim.13063. [DOI] [PubMed] [Google Scholar]
- 11. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rostom A, Dubé C, Cranney A, et al. Celiac Disease. Rockville (MD): Agency for Healthcare Research and Quality (US); 2004 Sep. (Evidence Reports/Technology Assessments, No. 104.) Appendix D. Quality Assessment Forms. Available from URL: https://www.ncbi.nlm.nih.gov/books/NBK35156/
- 13. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ (Clin. Res. Ed.). 2003; 327: 557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li YL, Shan NB, Sun W et al Comparative study for clinical features between COVID‐19 patients with conventional type and heavy/critical type. Pract. J. Card. Cerebral Pneumal Vasc. Dis. 2020; 28: 14–19. 10.3969/j.issn.1008-5971.2020.03.004. [DOI] [Google Scholar]
- 15. Zhu M, Lyu Q, Gu M et al Clinical diagnosis and integrated treatment of traditional Chinese and western medicine for confirmed patients with coronavirus disease 2019 in Yangzhou area of Jiangsu Province. J. Clin. Med. Pract. 2020; 24: 1–5. [Google Scholar]
- 16. Yuan J, Sun YY, Zuo YJ et al A retrospective analysis of the clinical characteristics of 223 NCP patients in Chongqing. J. Southwest Univ. (Nat. Sci.). 2020; 42: 55–60. [Google Scholar]
- 17. Bai M, Liu X, Wu W, Sun K, Huang X, Jin H. Clinical characteristics of 472 cases of novel coronavirus pneumonia in Wuhan Jiangan Makeshift (Fangcang) Hospital. Clin. Focus. 2020; 35: 297–301. 10.3969/j.issn.1004-583X.2020.04.002. [DOI] [Google Scholar]
- 18. Chang D, Lin M, Wei L et al Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA. 2020; 323: 1092–3. 10.1001/jama.2020.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen J, Qi T, Liu L et al Clinical progression of patients with COVID‐19 in Shanghai, China. J. Infect. 2020; 80: e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen S, Wu JJ, Li ZM et al Clinical features of 109 cases of novel coronavirus pneumonia. Chin. J. Infect. Dis. 2020; 38: E015–E015. Epub ahead of print. [Google Scholar]
- 21. Chen T, Wu D, Chen H et al Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ (Clin. Res. Ed.). 2020; 368: m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cholankeril G, Podboy A, Aivaliotis VI et al High prevalence of concurrent gastrointestinal manifestations in patients with SARS‐CoV‐2: early experience from California. Gastroenterology. 2020; 159: 775–7. 10.1053/j.gastro.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dong XC, Li JM, Bai JY et al Epidemiological characteristics of confirmed COVID‐19 cases in Tianjin. Zhonghua Liu Xing Bing Xue Za Zhi. 2020; 41: 638–42. 10.3760/cma.j.cn112338-20200221-00146. [DOI] [PubMed] [Google Scholar]
- 24. Effenberger M, Grabherr F, Mayr L et al Faecal calprotectin indicates intestinal inflammation in COVID‐19. Gut. 2020; 69: 1543–4. 10.1136/gutjnl-2020-321388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fang D, Ma J, Guan J et al Manifestations of Digestive system in hospitalized patients with novel coronavirus pneumonia in Wuhan, China: a single‐center, descriptive study. Chin. J. Dig. 2020; 40:Epub ahead of print. 10.3760/cma.j.issn.0254-1432.2020.0005. [DOI] [Google Scholar]
- 26. Fernandez‐Ruiz M, Andres A, Loinaz C et al COVID‐19 in solid organ transplant recipients: a single‐center case series from Spain. Am. J. Transplant. 2020; 20: 1849–58. 10.1111/ajt.15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guo Y, Zhang J, Xiong Q et al Clinical characteristics of 70 patients with coronavirus disease 2019 accompanied with diarrhea. Chin. J. Dig. 2020; 40: 244–48. [Google Scholar]
- 28. Jin X, Lian JS, Hu JH et al Epidemiological, clinical and virological characteristics of 74 cases of coronavirus‐infected disease 2019 (COVID‐19) with gastrointestinal symptoms. Gut. 2020; 69: 1002–9. 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kai‐Wang K, Tsang OT‐Y, Leung W‐S et al Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS‐CoV‐2: an observational cohort study. Lancet Infect. Dis. 2020; 20: 565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li D, Liu H, Wang Y, Guo H, Wang Y, Wang K. Clinical features of 30 cases with novel coronavirus pneumonia. Chin. J. Infect. Dis. 2020; 38: 204–10. Epub ahead of print. [Google Scholar]
- 31. Li D, Long Y‐Z, Huang P et al Clinical characteristics of 80 patients with COVID‐19 in Zhuzhou City. Chin. J. Infect. Control. 2020; 19: 227–33. 10.12138/j.issn.1671-9638.20206514. [DOI] [Google Scholar]
- 32. Li Q, Peng X‐H, Sun Z‐Y, Shao J‐B. Clinical and imaging characteristics of children with coronavirus disease 2019 (COVID‐19). Radiol Practice. 2020;35:277–80. DOI: 10.13609/j.cnki.1000-0313.2020.03.007. [DOI] [Google Scholar]
- 33. Liu C, Jiang ZC, Shao CX et al Preliminary study of the relationship between novel coronavirus pneumonia and liver function damage: a multicenter study. Zhonghua Gan Zang Bing Za Zhi. 2020; 28: 148–52. 10.3760/cma.j.issn.1007-3418.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 34. Liu K, Fang Y‐Y, Deng Y et al Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin. Med. J. (Engl.). 2020; 133: 1025–31. 10.1097/cm9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu M, He P, Liu HG et al Clinical characteristics of 30 medical workers infected with new coronavirus pneumonia. Chin. J. Tuberculosis Respir. Dis. 2020; 43: 209–14. 10.3760/cma.j.issn.1001-0939.2020.03.014. [DOI] [PubMed] [Google Scholar]
- 36. Lu X, Zhang L, Du H et al SARS‐CoV‐2 infection in Children. N. Engl. J. Med. 2020; 382: 1663–5. 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mao L, Jin H, Wang M et al Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020; 77: 683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mo P, Xing Y, Xiao Y et al Clinical characteristics of refractory COVID‐19 pneumonia in Wuhan, China. Clin. Infect. Dis. 2020. Mar 16 [Online ahead of print]. 10.1093/cid/ciaa270. [DOI] [Google Scholar]
- 39. Nobel YR, Phipps M, Zucker J et al Gastrointestinal Symptoms and COVID‐19: Case‐Control Study from the United States. Gastroenterology. 2020; 159: 373–375.e2. 10.1053/j.gastro.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pung R, Chiew CJ, Young BE et al Investigation of three clusters of COVID‐19 in Singapore: implications for surveillance and response measures. Lancet. 2020; 395: 1039–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Qi X, Liu C, Jiang Z et al Multicenter analysis of clinical characteristics and outcome of COVID‐19 patients with liver injury. J. Hepatol. 2020; 73: 455–8. 10.1016/j.jhep.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Redd WD, Zhou JC, Hathorn KE et al Prevalence and characteristics of gastrointestinal symptoms in patients with SARS‐CoV‐2 infection in the United States: a multicenter Cohort Study. Gastroenterology. 2020; 159: 765–767.e2. 10.1053/j.gastro.2020.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Richardson S, Hirsch JS, Narasimhan M et al Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York city area. JAMA. 2020; 323: 2052–9. 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. WANG R, Xie L, Du P, Fan H, Song M. Clinical characteristics of 96 hospitalized patients with coronavirus disease 2019. Chin. J. Respir. Crit. Care Med. 2020; 19: 144–7. 10.7507/1671-6205.202002066. [DOI] [Google Scholar]
- 45. Shi H, Han X, Jiang N et al Radiological findings from 81 patients with COVID‐19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect. Dis. 2020; 20: 425–34. 10.1016/s473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sun C, Zhang XB, Dai Y, Xu XZ, Zhao J. Clinical analysis of 150 cases of 2019 novel coronavirus infection in Nanyang City, Henan Province. Chin. J. Tuberculosis Respir. Dis. 2020; 43: E042. [DOI] [PubMed] [Google Scholar]
- 47. Tan X, Huang J, Zhao F, Zhou Y, Li JQ, Wang XY. Clinical features of children with SARS‐CoV‐2 infection: an analysis of 13 cases from Changsha, China. Chin. J. Contempor. Pediatr. 2020; 22: 294–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Team C‐I. Clinical and virologic characteristics of the first 12 patients with coronavirus disease 2019 (COVID‐19) in the United States. Nat. Med. 2020; 26: 861–8. 10.1038/s41591-020-0877-5. [DOI] [PubMed] [Google Scholar]
- 49. Wan S, Xiang Y, Fang W et al Clinical features and treatment of COVID‐19 patients in northeast Chongqing. J. Med. Virol. 2020; 92: 797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang D, Ju XL, Xie F et al Clinical analysis of 31 cases of 2019 novel coronavirus infection in children from six provinces (autonomous region) of northern China. Zhonghua Erke Zazhi. 2020; 58: 2. [DOI] [PubMed] [Google Scholar]
- 51. Wang K, Kang S, Tian R, Zhang X, Zhang X, Wang Y. Imaging manifestations and diagnostic value of chest CT of coronavirus disease 2019 (COVID‐19) in the Xiaogan area. Clin. Radiol. 2020; 75: 341–7. 10.1016/j.crad.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China. Clin. Infect. Dis. 2020; 71: 769–77. 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wen K, Li W, Zhang D et al Epidemiological and clinical characteristics of 46 newly‐admitted coronavirus disease 2019 cases in Beijing. Chin. J. Infect. Dis. 2020; 38: E011–E011. [Google Scholar]
- 54. Wu C, Chen X, Cai Y et al Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020. Published online March 13, 2020; 180: 934–43. 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xiang T, Liu J, Xu F et al Analysis of clinical characteristics of 49 patients with novel coronavirus pneumonia in Jiangxi province. Chin. J. Respir. Crit. Care Med. 2020; 19: 154–60. 10.7507/1671-6205.202002070. [DOI] [Google Scholar]
- 56. Xie H, Zhao J, Lian N, Lin S, Xie Q, Zhuo H. Clinical characteristics of non‐ICU hospitalized patients with coronavirus disease 2019 and liver injury: a retrospective study. Liver Int. 2020; 40: 1321–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Xu F, Qin X, Zhang L et al Clinical characteristics of gastrointestinal symptoms and liver function injury in patients with coronavirus disease 2019. Chin. J. Dig. 2020; 40: 69–73. [Google Scholar]
- 58. Xu T, Chen C, Zhu Z et al Clinical features and dynamics of viral load in imported and non‐imported patients with COVID‐19. Int. J Infect. Dis. 2020; 94: 68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xu X, Yu C, Qu J et al Imaging and clinical features of patients with 2019 novel coronavirus SARS‐CoV‐2. Eur. J. Nucl. Med. Mol. Imaging. 2020; 47: 1275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yang X, Yu Y, Xu J et al Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir. Med. 2020; 8: 475–81. 10.1016/s2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Young BE, Ong SWX, Kalimuddin S et al Epidemiologic features and clinical course of patients infected with SARS‐CoV‐2 in Singapore. JAMA. 2020; 323: 1488–94. 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yuan J, Zou R, Zeng L et al The correlation between viral clearance and biochemical outcomes of 94 COVID‐19 infected discharged patients. Inflamm. Res. 2020; 69: 599–606. 10.1007/s00011-020-01342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang G, Zhang J, Wang B, Zhu X, Wang Q, Qiu S. Analysis of clinical characteristics and laboratory findings of 95 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a retrospective analysis. Respir. Res. 2020; 21: 74 10.1186/s12931-020-01338-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang JJ, Dong X, Cao YY et al Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020; 75: 1730–41. 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 65. Zhang S, Li J, Zhou W et al The analysis of clinical characteristics of 34 novel coronavirus pneumonia cases in Ningxia Hui autonomous region. Chin. J. Tuberc. Respir. Dis. 2020; 43: 431–6. [DOI] [PubMed] [Google Scholar]
- 66. Zhao D, Yao F, Wang L et al A comparative study on the clinical features of COVID‐19 pneumonia to other pneumonias. Clin. Infect. Dis. 2020; 71: 756–61. 10.1093/cid/ciaa247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhao R, Liang Y, Lin Y, Lu N, Li Q, Li Y. Clinical characteristics of 28 patients with novel coronavirus pneumonia. Chin. J. Infect. Dis. 2020; 38: E006–E006. [Google Scholar]
- 68. Zheng F, Tang W, Li H, Huang YX, Xie YL, Zhou ZG. Clinical characteristics of 161 cases of corona virus disease 2019 (COVID‐19) in Changsha. Eur. Rev. Med. Pharmacol. Sci. 2020; 24: 3404–10. [DOI] [PubMed] [Google Scholar]
- 69. Zhou F, Yu T, Du R et al Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020; 395: 1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhou Z, Zhao N, Shu Y, Han S, Chen B, Shu X. Effect of gastrointestinal symptoms on patients infected with COVID‐19. Gastroenterology. 2020; 158: 2294–7. 10.1053/j.gastro.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rodriguez‐Morales AJ, Cardona‐Ospina JA, Gutiérrez‐Ocampo E et al Clinical, laboratory and imaging features of COVID‐19: a systematic review and meta‐analysis. Travel Med. Infect. Dis. 2020; 34: 101623 10.1016/j.tmaid.2020.101623:101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cheung KS, Hung IF, Chan PP et al Gastrointestinal manifestations of SARS‐CoV‐2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta‐analysis. Gastroenterology. 2020; 159: 81–95. 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chen N, Zhou M, Dong X et al Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020; 395: 507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Huang C, Wang Y, Li X et al Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Luo S, Zhang X, Xu H. Don't overlook digestive symptoms in patients with 2019 novel coronavirus disease (COVID‐19). Clin. Gastroenterol. Hepatol. 2020; 18: 1636–7. 10.1016/j.cgh.2020.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Meta‐analysis of the rate of (a) digestive symptoms, (b) digestive comorbidities, (c) diarrhea, (d) loss of appetite, (e) abdominal pain, and (f) nausea and/or vomiting in COVID‐19 patients.
Figure S2 Meta‐analysis of the rate of (a) abnormal liver function, (b) liver comorbidities, (c) high ALT, (d) high AST, and (e) high TBil in COVID‐19 patients.
Figure S3 Comparison of COVID‐19 patients with and without digestive symptoms.
Figure S4 Begg funnel plot for the rate of diarrhea in COVID‐19 patients


