Abstract
Background and Aim
A self‐expandable metallic stent (SEMS) is commonly used for biliary stricture caused by pancreatic cancer. Covered SEMS may obstruct the cystic duct, causing acute cholecystitis. This study aimed to determine the outcomes of using a half‐covered SEMS with an offset covered portion for preventing cystic duct obstruction.
Methods
Among 80 patients with half‐covered SEMS placement for the treatment of pancreatic cancer‐induced distal biliary stricture, 74 were followed up. The half‐covered SEMS has a total length of 6 or 7 cm, and the offset covered part was 0.5–4.5 or 0.5–5.5 cm, respectively. Intraductal ultrasonography (IDUS) and endoscopic nasobiliary drainage (ENBD) were performed during the initial endoscopic retrograde cholangiopancreatography (ERCP). IDUS findings and ENBD tube cholangiogram confirmed the cystic duct confluence. SEMS placement was performed on the second ERCP or several weeks after the initial tube stent placement.
Results
Half‐covered SEMS placement was successful in all patients. However, four (5.4%) patients exhibited early complications, including acute cholecystitis in one patient and stent displacement in another. Over 30 days, cholangitis, tumor growth, and stent displacement occurred in nine (11.3%), five (6.3%), and two (2.5%) patients, respectively. The median stent patency was 71.1 weeks, and the median overall survival in patients with and without chemotherapy was 31.8 and 12.2 weeks, respectively.
Conclusions
With confirmation of the cystic duct confluence, half‐covered SEMS placement may become a treatment option for distal biliary stricture caused by pancreatic cancer to prevent acute cholecystitis. Half‐covered SEMS patency was comparable with that of covered SEMS.
Keywords: acute cholecystitis, covered metallic stent, pancreatic cancer, self‐expandable metallic stent
Half‐covered self‐expandable metallic stents (SEMS) were successfully placed for biliary stricture due to pancreatic cancer. With careful confirmation of the cystic duct confluence, half‐covered SEMS placement may become a treatment option for distal biliary stricture to prevent acute cholecystitis. Its stent patency was comparable with that of covered SEMS.

Introduction
Endoscopic biliary stenting for malignant biliary stricture has been widely performed for obstructive jaundice palliation. As an advantage, a self‐expandable metallic stent (SEMS) has a larger inner lumina than a plastic tube stent. 1 , 2 Histologically, SEMS placement is mainly indicated for alleviating jaundice related to unresectable malignant biliary stricture. Palliation of obstructive jaundice improves the quality of life (QOL) and allows patients with inoperative pancreatic cancer to receive chemotherapy and experience a prolonged survival time. 2 Appropriate biliary drainage and control are necessary for patients with pancreatic cancer to receive neoadjuvant chemotherapy preoperatively. Covered SEMS (CSEMS) have some advantages, including a longer stent patency for preventing tumor ingrowth through the mesh, compared with uncovered metallic stents (USEMS). 3 , 4 , 5
Although CSEMS is superior to USEMS, CSEMS placement may induce acute cholecystitis. CSEMS reportedly has a higher risk of acute cholecystitis than USEMS, with a rate ranging from 2.5 to 12%. 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 The development of acute cholecystitis generally requires some interventional procedures that may hinder chemotherapy; consequently, patients' QOL may decrease considerably. Thus, initial complications, including acute cholecystitis, should be minimized as much as possible. Preventing acute cholecystitis after CSEMS placement is an important clinical problem. Therefore, researchers need to find ways on how to prevent acute cholecystitis, which is a serious complication, after CSEMS placement for pancreatic cancer cases.
Acute cholecystitis after SEMS placement is reportedly caused by cystic duct occlusion. 7 Hence, we used a half‐covered metallic stent (half‐covered SEMS) in which the cover portion was adjusted to avoid cystic duct obstruction, which could result in cholecystitis. In this study, we examined the usefulness of this half‐covered SEMS for patients with distal biliary stricture caused by pancreatic cancer.
Methods
Patients
A total of 525 patients were admitted because of distal bile duct stricture caused by pancreatic cancer between May 2008 and December 2018. Among them, 334 patients were deemed suitable to undergo SEMS placement. All patients were diagnosed with pancreatic cancer pathologically by bile duct biopsy and/or biliary cytology using the bile juice from a nasobiliary drainage tube or fine‐needle aspiration cytology by endoscopic ultrasonography guidance. Of the 334 patients, 80 were considered fit to undergo half‐covered SEMS placement. These patients were thought to match the half‐covered SEMS both because of the range of biliary stricture and the position of the cystic duct confluence. In conventional CSEMS placement, the cystic duct confluence was close to the upper side of the biliary stricture (≤1 cm), possibly obstructing the cystic duct and progressing to cholecystitis. Moreover, the patients had a stenosis length consistent with the covered part of the half‐covered SEMS. Of the 80 patients, 74 were followed up clinically over 1 month in our institution or related institutions. The mean age of these patients was 70.2 years, with 33 women and 41 men. We examined acute cholecystitis development, stent‐related complications and stent patency, and overall survival after stent placement. The characteristics of the patients are shown in Table 1.
Table 1.
Patient characteristics in half‐covered SEMS
| Age | 52–84 (mean 70.2) years old |
| Gender (male: female) | 41:33 |
| Performance status score (0:1) | 61:13 |
| ALP before initial ERCP † | 1359 ± 849 U/L |
| ALP after SEMS placement † , ‡ | 799 ± 450 U/L |
| Total bilirubin before initial ERCP † | 7.6 ± 6.2 mg/dL |
| Total bilirubin after SEMS placement † , ‡ | 2.0 ± 1.7 mg/dL |
| Success rate of half‐covered SEMS placement | 74 (100%) |
| Clinical response rate | 74 (100%) |
| Early complication | 4 (5.4%) |
| Delayed complications | 16 (21.6%) |
| Received surgical resection | 6 (8.1%) |
| Received chemotherapy | 43 (58.1%) |
| Patients alive: death during observation period | 31:43 |
Data were expressed as mean ± SD.
Data were obtained 2–7 (mean3.9) days after half‐covered SEMS placement.
ALP, alkaline phosphatase; ERCP, endoscopic retrograde cholangiopancreatography; SEMS, self‐expandable metallic stent.
The institutional review board of our hospital approved this study, and all patients provided a written consent for the procedures.
Half‐covered metallic stent
The half‐covered SEMS was a Niti‐S partially covered metallic stent (Taewoong Medical Co., Ltd., Gimpo‐si South Korea) that is designed with an offset covered portion to avoid overlapping the cystic duct confluence. The stent measures 10 mm in diameter and 6 or 7 cm in total length, with the covered part at 0.5–4.5 or 0.5–5.5 cm from the duodenum side, respectively (Fig. 1).
Figure 1.
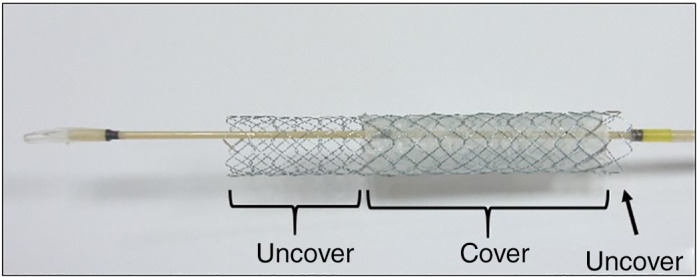
Half‐covered metallic stent. A partially covered stent having an offset covered portion. The stent measures 10 mm in diameter and 6 or 7 cm in total length, with the covered part at 0.5–4.5 and 0.5–5.5 cm from the duodenum side.
Stent insertion and deployment
For diagnosing biliary stricture and biliary drainage, all patients underwent endoscopic retrograde cholangiopancreatography (ERCP). To facilitate stent insertion and to preclude pancreatitis after successful biliary cannulation and guide wire insertion through the stricture, we performed endoscopic sphincterotomy. To diagnose a stricture and to determine the position of the cystic duct confluence, we used intraductal ultrasonography of the bile duct. For the histological diagnosis of a biliary stricture, bile duct biopsy was performed. Then, we inserted a nasobiliary drainage tube for biliary drainage and bile juice cytology. After 7–10 days of confirming biliary drainage, all patients underwent cholangiography from the nasobiliary tube and second ERCP for stent insertion. Using a measuring guide wire (Cheer Leader+M, Piolax, Tokyo, Japan), we determined the position of the cystic duct confluence, the length of biliary stricture, and the distance from the papilla Vateri to the upper site of the stricture. On the basis of these findings, we selected the appropriate half‐covered SEMS (6 or 7 cm in length) to be deployed for matched patients and then performed the stent placement (Fig. 2).
Figure 2.
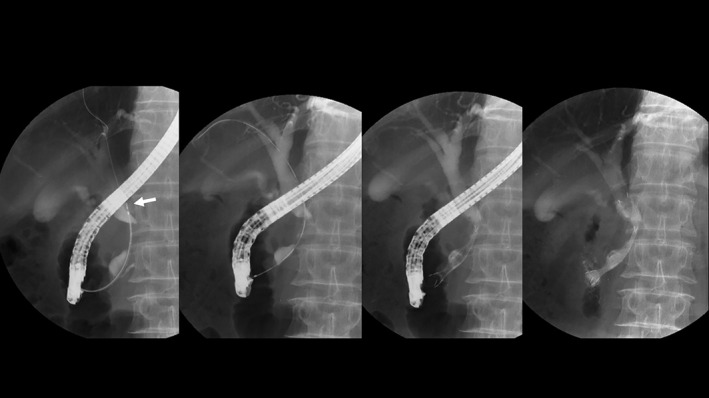
Half‐covered metallic stent placement. Endoscopic retrograde cholangiography and intraductal ultrasonography confirmed the position of the cystic duct confluence. At the second endoscopic retrograde cholangiography, the position of cystic duct confluence (arrow), the length of biliary stricture, and the distance from the papilla Vateri to the upper site of the stricture were determined using a measuring guide wire. The half‐covered stent was selected and deployed.
Definition of events
Stent insertion was considered successful when the half‐covered SEMS was placed across the stricture with appropriate radiographic positioning.
A complication was defined as early if it occurred within 30 days after stent placement and as delayed if it occurred over 30 days after the placement. Acute cholecystitis was diagnosed if right upper abdominal pain and fever of over 38.0 °C were manifested, together with supportive evidence on biochemical indication and ultrasonographic and computed tomography imaging studies. Stent patency was defined as the period of time between stent insertion and stent occlusion. Cholangitis was diagnosed if the patient experienced fever that persisted for 24 h without another discernible cause, together with biochemical evidence of cholestasis. Reintervention was defined as the need for further endoscopic procedure to improve biliary drainage after initial stent insertion.
Statistical analysis
Prevention of acute cholecystitis development after stent placement and stent patency was the primary endpoint, whereas patient survival was the secondary endpoint. Moreover, we compared stent patency and patient survival, with or without chemotherapy. The period of stent patency was estimated using the Kaplan–Meier method. Patients without recurrent biliary obstruction were censored at the date of last follow‐up (31 December 2019) or date of death. We also used the Kaplan–Meier method to evaluate patient survival, with living patients censored at the date of last follow‐up (31 December 2019). The same method was used in comparing cumulative stent patency and patient survival. Furthermore, data were compared between patients with and without chemotherapy using the log‐rank test, and a P‐value of <0.05 was considered statistically significant.
Results
Early complications and acute cholecystitis
All patients had successful half‐covered SEMS placement. However, four (5.4%) patients experienced early complications. For instance, one patient exhibited acute cholecystitis. In this case, the cystic duct confluence was found right above the upper edge of the stricture. The covered part of the half‐covered SEMS solely overlapped the cystic duct confluence. Three days after the stent placement, acute cholecystitis developed; hence, percutaneous transhepatic gallbladder aspiration using a 21‐gauge needle was performed. Consequently, the patient quickly recovered. Apparently, acute cholecystitis developed because the covered part of the stent obstructed the cystic duct confluence (Fig. 3). In one patient, stent dislocation toward the duodenum was observed 2 weeks after stent insertion. The patient was successfully treated by stent trimming using argon plasma coagulation (APC300, ERBE, Tübingen, Germany). Another early complication related to stent insertion included postsphincterotomy hemorrhage with hyperamylasemia in one patient. This complication was immediately and successfully managed by directly injecting hypertonic saline–epinephrine solution and providing conservative therapy. Meanwhile, the third patient manifested mild cholangitis, which was successfully managed by antibiotic administration.
Figure 3.
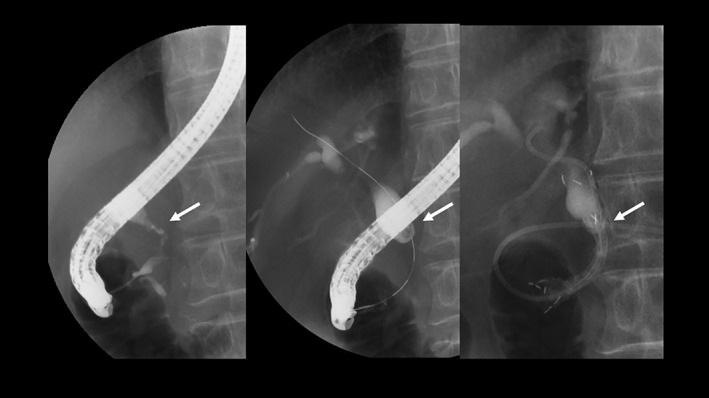
Acute cholecystitis after stent insertion. The cystic duct confluence was confirmed to be located right above the upper edge of the stricture (arrow). The covered part overlapped the cystic duct confluence only. Three days after the stent placement, acute cholecystitis developed.
Delayed complications and stent patency
A total of 16 (21.6%) patients experienced delayed complications, such as cholangitis caused by food impaction or sludge (nine patients), tumor ingrowth (five patients), and stent dislocation to the duodenum (two patients). Specifically, four patients had stent occlusion because of tumor ingrowth at the proximal side of the stent at 8.5, 11.7, 23.8, and 34 weeks, respectively, whereas one patient experienced it at the distal side at 20.8 weeks. The two remaining patients manifested stent dislocation toward the duodenum at 46.2, and 71.1 weeks, respectively (Table 2). These 16 (21.6%) patients required reintervention. For those nine patients with stent occlusion caused by food residue or sludge, their stents were cleaned using a stone retrieval balloon catheter. In the case of tumor ingrowth, three patients underwent additional metallic stent placement, and one underwent nasobiliary drainage only because of surgical treatment 1 week after. Meanwhile, the two patients with stent dislocation were successfully treated by stent trimming using argon plasma coagulation, but one of them underwent another SEMS placement.
Table 2.
Complications over 30 days and reintervention
| Reason for reintervention | No. of patients | Time to onset (weeks) | Reintervention |
|---|---|---|---|
| Cholangitis due to food impaction or sludge | 9 | 3.0, 4.1, 7.0, 7.1, 8.1, 8.2, 8.5, 14.0, 26.4 | Stent cleaning using a stone retrieval balloon catheter |
| Tumor ingrowth at proximal side of the stent | 4 | 8.5, 11.7, 23.8, 34.0 | Additional metallic stent placement (3), nasobiliary drainage (1) |
| Tumor ingrowth at distal side of the stent | 1 | 20.8 | Additional metallic stent placement |
| Stent dislocation | 2 | 46.2, 71.1 | Stent trimming using argon plasma coagulation |
The observation period after stent placement was 4.2–159 weeks (median: 22.7 weeks). During this period, 31 patients were alive, whereas 43 died. The median stent patency in all patients was 71.1 weeks, whereas that in patients undergoing chemotherapy was 71.1 weeks (Fig. 4).
Figure 4.
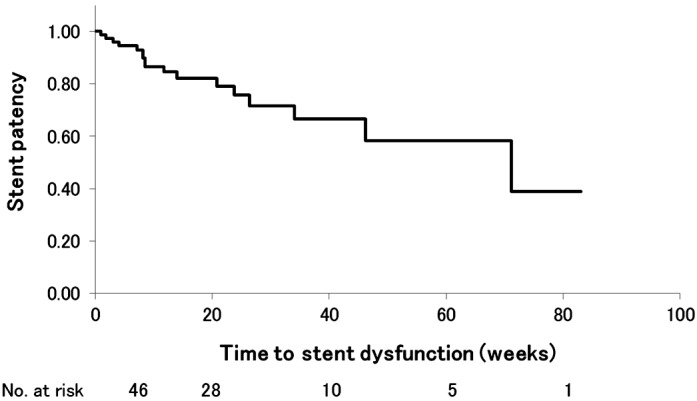
Kaplan–Meier curve showing cumulative stent patency. The median stent patency was 71.1 weeks, with no significant difference between patients with and those without chemotherapy.
Patient survival
Seven patients underwent surgical resection of the pancreatic cancer. Except for these 7 patients, 43 patients received ) at least one course of usual chemotherapy (gemcitabine or gemcitabine plus paclitaxel. The median survival in patients with and without chemotherapy was 31.8 and 12.2 weeks, respectively (P < 0.01) (Fig. 5).
Figure 5.
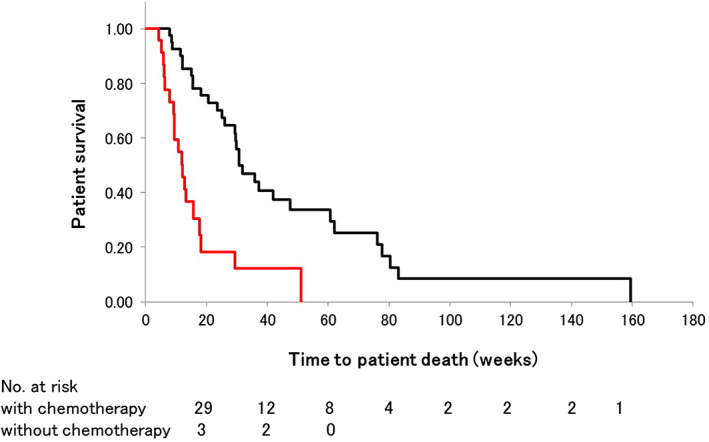
Kaplan–Meier curve showing cumulative patient survival. The median survival in patients with and without chemotherapy was 31.8 and 12.2 weeks, respectively (P < 0.01). ( ), With chemotherapy; (
), With chemotherapy; ( ), without chemotherapy.
), without chemotherapy.
Discussion
SEMS placement as a palliative treatment for obstructive jaundice caused by malignant biliary stricture improves patients' QOL. CSEMS is more advantageous than USEMS because of its longer stent patency for preventing tumor ingrowth through the stent mesh. For patients with pancreatic cancer, CSEMS is also reportedly more beneficial than USEMS. 3 , 4 , 5 In fact, the Clinical Practice Guidelines for Pancreatic Cancer 2019 from the Japan Pancreas Society recommends CSEMS for patients with biliary stricture.
However, CSEMS have some distinctive complications, such as acute cholecystitis and acute pancreatitis in the early period and stent migration and stent clogging in the late period. 3 , 4 , 5 , 6 , 7 , 8 , 9 In patients with malignant biliary stricture, CSEMS has a higher risk of acute cholecystitis than USEMS, obtaining a rate of 2.5–12%. 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 Takinami et al. reported that CSEMS is an important risk factor of acute cholecystitis. 18 Acute cholecystitis development needs some intervention procedures, such as cholecystectomy or percutaneous drainage, which could be fatal.
Isayama et al. previously described that cholecystitis development is not related to the type of metallic stent but the presence of tumor invasion into the cystic duct orifice. 11 Recently, they reported that SEMS with a high axial force has a high risk of cholecystitis; thus, tumor involvement to the cystic duct orifice and higher axial force are risk factors for cholecystitis development. 19
Moreover, fully covered SEMS has a higher rate of acute cholecystitis than USEMS, even in patients with pancreatic cancer. 20 , 21 In our study, one patient (1.3%) exhibited acute cholecystitis because of stent misalignment caused by the exact overlapping of the stent's covered part on the cystic duct confluence. The main mechanism of acute cholecystitis development after metallic stent placement could be cystic duct obstruction. We reported the results of using a half‐covered SEMS that is designed with an offset covered portion to cover the stenosis part and not to obstruct the cystic duct confluence.
The half‐covered SEMS is a braided‐type, partially covered stent and has a lower axial force than the other CSEMS. 22 It was used for selected patients to adjust the stent trait and the biliary anatomy and could prevent acute cholecystitis.
The usefulness of metallic stent for malignant biliary stricture has been extensively reported. However, studies focusing on the biliary structure caused by pancreatic carcinoma are limited. 2 , 3 , 20 , 21 , 23 In these reports, the median stent patency was 59.8 and 83.3 weeks 2 , 3 in patients with inoperable pancreatic cancer, and the time from stent placement to stent dysfunction was 13.1, 31.4, and 13.8 weeks 20 , 21 , 23 in patients with pancreatic cancer who received neoadjuvant chemotherapy. In our study, the median stent patency in all patients and those with chemotherapy was 71.1 weeks, and it was intermediate between these results.
Meanwhile, stent dysfunction occurred in 21.6% of our patients, similar to those in previous reports (34.6, 23.3, 22.2, 25.5, and 12%). 2 , 3 , 20 , 21 , 23 The stent patency and stent dysfunction rates of the half‐covered SEMS were comparable with those in previous reports. It is still a problem that the use of covered or half‐covered SEMS leads to higher rates of reintervention because of cholangitis due to sludge formation or food impaction compared with the use of uncovered SEMS.
For patients with inoperable pancreatic cancer, the median patient survival is 35.4 and 40.7 weeks. 2 , 3 These results are longer than those in patients who underwent half‐covered SEMS placement (31.8 and 12.2 weeks in patients with and without chemotherapy, respectively). The variability of the survival may be explained by the differences in the degree and progression of the cancer; however, the details are still unknown. In this study, more than half of the patients died during the observation period.
This report is a retrospective single‐center cohort study with a relatively small number of patients. Studies focusing on SEMS for malignant biliary stenosis are numerous, but those specializing in bile duct stricture caused by pancreatic cancer are few. Nonetheless, our study has a relatively large number of patients compared with previous reports with the same topic. Hence, we believe that one suggestion has been obtained regarding acute cholecystitis prevention, that is, the use of a half‐covered SEMS for malignant biliary stenosis caused by pancreatic cancer. Our study also revealed that cystic duct obstruction caused by the covered part of the SEMS could be one of the major causes of acute cholecystitis development after SEMS placement.
In conclusion, with appropriate position and careful confirmation of the cystic duct confluence, using a half‐covered SEMS may be suitable for patients with pancreatic cancer to prevent acute cholecystitis after CSEMS. The stent patency of the half‐covered SEMS was also comparable with that of the CSEMS. Furthermore, the half‐covered SEMS may become an option for distal biliary stricture caused by pancreatic cancer and may be useful for other malignant biliary strictures in matched patients.
Declaration of conflict of interest: All authors declare no potential conflicts of interest for this article.
References
- 1. Almadi MA, Barkun A, Martel M. Plastic vs. self‐expandable metal stents for palliation in malignant biliary obstruction: a series of meta‐analyses. Am. J. Gastroenterol. 2017; 112: 260–73. [DOI] [PubMed] [Google Scholar]
- 2. Isayama H, Yasuda I, Ryozawa S et al Results of a Japanese multicenter, randomized trial of endoscopic stenting for non‐resectable pancreatic head cancer (JM‐test): covered Wallstent versus DoubleLayer stent. Dig. Endosc. 2011; 23: 310–5. [DOI] [PubMed] [Google Scholar]
- 3. Kitano M, Yamashita Y, Tanaka K et al Covered self‐expandable metal stents with an anti‐migration system improve patency duration without increased complications compared with uncovered stents for distal biliary obstruction caused by pancreatic carcinoma: a randomized multicenter trial. Am. J. Gastroenterol. 2013; 108: 1713–22. [DOI] [PubMed] [Google Scholar]
- 4. Isayama H, Komatsu Y, Tsujino T et al A prospective randomized study of “covered” versus “uncovered” diamond stents for the management of distal malignant biliary obstruction. Gut. 2004; 53: 729–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krokidis M, Fanelli F, Orgera G et al Percutaneous palliation of pancreatic head cancer: randomized comparison of ePTFE/FEP‐covered versus uncovered nitinol biliary stents. Cardiovasc. Intervent. Radiol. 2011; 34: 352–61. [DOI] [PubMed] [Google Scholar]
- 6. Moole H, Bechtold ML, Cashman M et al Covered versus uncovered self‐expandable metal stents for malignant biliary strictures: a meta‐analysis and systematic review. Indian J. Gastroenterol. 2016; 35: 323–30. [DOI] [PubMed] [Google Scholar]
- 7. Yokota Y, Fukasawa M, Takano S et al Partially covered metal stents have longer patency than uncovered and fully covered metal stents in the management of distal malignant biliary obstruction: a retrospective study. BMC Gastroenterol. 2017; 17: 105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Conio M, Mangiavillano B, Caruso A et al Covered versus uncovered self‐expandable metal stent for palliation of primary malignant extrahepatic biliary strictures: a randomized multicenter study. Gastrointest. Endosc. 2018; 88: 283–91. [DOI] [PubMed] [Google Scholar]
- 9. Tringali A, Hassan C, Rota M, Rossi M, Mutignani M, Aabakken L. Covered vs. uncovered self‐expandable metal stents for malignant distal biliary strictures: a systematic review and meta‐analysis. Endoscopy. 2018; 50: 631–41. [DOI] [PubMed] [Google Scholar]
- 10. Kahaleh M, Tokar J, Conaway MR et al Efficacy and complications of covered Wallstents in malignant distal biliary obstruction. Gastrointest. Endosc. 2005; 61: 528–33. [DOI] [PubMed] [Google Scholar]
- 11. Isayama H, Kawabe T, Nakai Y et al Cholecystitis after metallic stent placement in patients with malignant distal biliary obstruction. Clin. Gastroenterol. Hepatol. 2006; 4: 1148–53. [DOI] [PubMed] [Google Scholar]
- 12. Fumex F, Coumaros D, Napoleon B et al Similar performance but higher cholecystitis rate with covered biliary stents: results from a prospective multicenter evaluation. Endoscopy. 2006; 38: 787–92. [DOI] [PubMed] [Google Scholar]
- 13. Isayama H, Nakai Y, Kawakubo K et al Covered metallic stenting for malignant distal biliary obstruction: clinical results according to stent type. J. Hepatobiliary Pancreat. Sci. 2011; 18: 673–7. [DOI] [PubMed] [Google Scholar]
- 14. Tsuchiya T, Itoi T, Gotoda T et al A multicenter prospective study of the short‐term outcome of a newly developed partially covered self‐expandable metallic biliary stent (WallFlex®). Dig. Dis. Sci. 2011; 56: 1889–95. [DOI] [PubMed] [Google Scholar]
- 15. Jang S, Stevens T, Parsi M et al Association of covered metallic stents with cholecystitis and stent migration in malignant biliary stricture. Gastrointest. Endosc. 2018; 87: 1061–70. [DOI] [PubMed] [Google Scholar]
- 16. Telford JJ, Carr‐Locke DL, Baron TH et al A randomized trial comparing uncovered and partially covered self‐expandable metal stents in the palliation of distal malignant biliary obstruction. Gastrointest. Endosc. 2010; 72: 907–14. [DOI] [PubMed] [Google Scholar]
- 17. Suk KT, Kim HS, Kim JW et al Risk factors for cholecystitis after metal stent placement in malignant biliary obstruction. Gastrointest. Endosc. 2006; 64: 522–9. [DOI] [PubMed] [Google Scholar]
- 18. Takinami M, Murohisa G, Yoshizawa Y, Shimizu E, Nagasawa M. Risk factors for cholecystitis after stent placement in patients with distal malignant biliary obstruction. J. Hepatobiliary Pancreat. Sci. 2020; 27: 470–6. [DOI] [PubMed] [Google Scholar]
- 19. Nakai Y, Isayama H, Kawakubo K et al Metallic stent with high axial force as a risk factor for cholecystitis in distal malignant biliary obstruction. J. Gastroenterol. Hepatol. 2014; 29: 1557–62. [DOI] [PubMed] [Google Scholar]
- 20. Seo DW, Sherman S, Dua KS et al Covered and uncovered biliary metal stents provide similar relief of biliary obstruction during neoadjuvant therapy in pancreatic cancer: a randomized trial. Gastrointest. Endosc. 2019; 90: 602–12. [DOI] [PubMed] [Google Scholar]
- 21. Gardner TB, Spangler CC, Byanova KL et al Cost‐effectiveness and clinical efficacy of biliary stents in patients undergoing neoadjuvant therapy for pancreatic adenocarcinoma in a randomized controlled trial. Gastrointest. Endosc. 2016; 84: 460–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Isayama H, Nakai Y, Toyokawa Y et al Measurement of radial and axial forces of biliary self‐expandable metallic stents. Gastrointest. Endosc. 2009; 70: 37–44. [DOI] [PubMed] [Google Scholar]
- 23. Kubota K, Sato T, Watanabe S et al Covered self‐expandable metal stent deployment promises safe neoadjuvant chemoradiation therapy in patients with orderline resectable pancreatic head cancer. Dig. Endosc. 2014; 26: 77–86. [DOI] [PubMed] [Google Scholar]


