Abstract
Background and Aim
Lenvatinib (LEN) has an antitumor effect with an early reduction in contrast enhancement for unresectable hepatocellular carcinoma (HCC). The aim of this study was to reveal the most useful radiological response evaluation for overall survival (OS) in patients treated with LEN.
Methods
Patients receiving LEN therapy (n = 80) were retrospectively recruited from April 2018 to January 2020. Enhanced computed tomography scans were performed at baseline and every 4–8 weeks. OS and radiological response were evaluated using response evaluation criteria in solid tumors (RECIST 1.1), modified RECIST (mRECIST), and Choi criteria. To be eligible for study, a minimal cumulative duration of LEN was 4 weeks. A total of 62 patients were included in the analysis.
Results
The median OS was 469 days. The RECIST 1.1, mRECIST, and Choi criteria identified 14 (22.5%), 30 (48.3%), and 33 (53.2%) patients with an objective response, respectively. In the univariate analysis, Child–Pugh class B, major vascular invasion, and high alpha‐fetoprotein (>200) were statistically significant poor prognostic factors. Radiological response was a significantly better prognostic factor in each criterion (RECIST, mRECIST, and Choi). In the multivariate analysis, radiological response evaluated by RECIST (hazard ratio, 0.259; 95% confidence interval, 0.0723–0.928; P = 0.038) was an independent factor. Furthermore, only RECIST significantly stratified prognosis (P = 0.041) when limited to the first evaluation.
Conclusion
RECIST 1.1 was useful even as early therapeutic evaluation for HCC patients treated with LEN. Understanding the characteristics of radiological response over time may contribute to improving the prognosis of patients with HCC.
Keywords: Choi criteria, hepatocellular carcinoma, lenvatinib, modified response evaluation criteria in solid tumors, response evaluation criteria in solid tumors 1.1
The RECIST 1.1 was a useful, even early, therapeutic evaluation for hepatocellular carcinoma patients treated with lenvatinib. Maintaining relative dose intensity of lenvatinib is crucial after the first evaluation, especially with the mRECIST and Choi criteria, which evaluate staining, in Barcelona Clinic Liver Cancer stage B patients.
Introduction
Lenvatinib (LEN) is a newly approved, multikinase inhibitor for unresectable hepatocellular carcinoma (HCC) 1 , 2 , 3 , 4 and is a first‐line agent in addition to sorafenib 5 (SOR). Several reports have revealed the clinical characteristics of patients treated with LEN in a real‐world setting. LEN was favorable for not only tyrosine kinase inhibitor (TKI)‐experienced 6 and elder 7 patients but also for those with portal vein tumor thrombosis. 8 Prognostic factors such as liver function, 9 , 10 relative dose intensity, 11 neutrophil‐to‐lymphocyte ratio, 12 and nutritional index 13 were also reported.
While we previously conducted a radiological response study, 14 some studies reported on radiological objective response (OR) and its predictors (alpha‐fetoprotein [AFP], 15 albumin–bilirubin grade, 16 and relative dose intensity [RDI] 17 ).
The RECIST 1.1 is considered the standard evaluation method for tumor radiological response in clinical trials. This criterion established the impact on survival outcomes in patients with solid tumors. 18 The development and validation of systemic therapies necessitated new criteria to assess tumor responses, which led to the introduction of the mRECIST. 19 , 20 Previous studies demonstrated that OR evaluated by mRECIST is an independent predictor of overall survival (OS) in HCC patients. 20 , 21 , 22 , 23 Llovet et al. reviewed the performance and novel refinements of the mRECIST 24 10 years prior to this publication. Choi et al. developed a composite end‐point for gastrointestinal stromal tumors (GISTs) treated with imatinib, which included tumor size and enhancement. 25 Some reports suggested that such an evaluation tool was also useful for HCC. 14 , 26 , 27 , 28
While there have been reports on the response criteria for SOR treatment, which radiological method is suitable for predicting the prognosis of patients treated with LEN is unknown. Although OR was obtained at early time points, progressive disease (PD) often followed (due to LEN discontinuation and other factors). Therefore, additional studies are needed to further validate the predictive radiological response on survival following LEN administration and to identify the optimal time point to assess a radiological response.
The aims of this study were to reveal the most useful radiological response evaluation and prognostic factors in patients receiving LEN. Moreover, we assessed the relationship between the time course of each radiological response and prognosis.
Methods
Patients
Consent to LEN therapy was obtained from 80 patients at the Musashino Red Cross Hospital from April 2018 to January 2020. There was a total of 334 treatment cases with advanced HCC in the same period, as described in Table S1, Supporting information. The diagnosis of HCC was based on pathologically proven HCC or radiologic findings, such as typical arterial enhancement of the tumor followed by a washout pattern in the images in the portal venous phase or the equilibrium phase on dynamic computed tomography (CT) imaging or magnetic resonance imaging (MRI) in accordance with the criteria of practice guidelines. 29 , 30 , 31 To support the diagnosis of HCC, we also used the Liver Imaging Reporting and Data System, 32 , 33 , 34 which was created to standardize the reporting and collection of CT and MRI data for HCC.
The inclusion criteria for LEN treatment were as follows: metastatic or locally advanced HCC that was unresectable or refractory to transarterial chemoembolization (TACE), Barcelona Clinic Liver Cancer (BCLC) stage B or C, 35 and an Eastern Cooperative Oncology Group performance status of 0–1. 36 An abdominal CT scan was performed within 8 weeks after LEN administration. The exclusion criteria were as follows: patients with missing data due to early discontinuation (less than 4 weeks) because of LEN intolerance or loss to follow‐up.
Written informed consent was obtained from all study participants. The ethics committee of the Musashino Red Cross Hospital approved the study. The investigation was conducted in accordance with the Declaration of Helsinki. A total of 62 patients were finally included in this cohort.
LEN treatment
LEN was orally administered at 8 mg/day to patients weighing <60 kg and 12 mg/day to those ≥60 kg. Treatment was discontinued due to any unacceptable or serious adverse events (AEs) or tumor progression. LEN was reduced or interrupted if a patient developed any unacceptable grade 2 or any grade 3 drug‐related AEs. If a patient developed AEs, dose reduction or temporary interruption was maintained until the symptoms resolved to lower than grade 2. AEs were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. 37 , 38
CT scan procedures
Chest, abdomen, and pelvis CT scans were performed with a 64‐section multidetector CT scanner (LightSpeed VCT or Optima CT660 or Revolution GSI; GE Healthcare, Milwaukee, WI, USA, http://www.gehealthcare.com). First, a precontrast abdominal scan was performed. Next, 300 mgI/mL of a nonionic iodinated contrast agent was administered intravenously. Its volume was 750 mgI/kg, and it was administered through a 20–22‐gauge catheter via an antecubital vein over 35 s. The arterial, portal venous, and equilibrium phases were obtained 40, 70, and 150 s after contrast medium administration, respectively. No oral contrast medium was administered.
For each target lesion, arterial phase acquisition was used to measure the maximum diameter, the maximum unidimensional enhanced diameter, the product of the bidimensional enhanced diameters, and tumor density. Based on these parameters, we evaluated the response according to the RECIST 1.1, mRECIST, and Choi criteria, as previously reported. 14 Due to the absence of specific guidelines on the use of the Choi criteria in HCC, we adapted the original Choi criteria to fit specific HCC patterns, as previously reported. 26 Because HCC is a hypervascular tumor, the density was measured during the arterial phase instead of the portal phase, as originally described for GISTs. 25 , 26 , 27
Study end‐point
The end‐point for this analysis was OS, which was calculated from the date of initiation of lenvatinib treatment until death by any cause or the last follow‐up. Baseline factors associated with OS were analyzed. The factors analyzed for prognostic significance included the following: age, gender, serum data (albumin, bilirubin, prothrombin time, and AFP), Child–Pugh score before LEN administration, and HCC conditions (major portal vein, vessel invasion, distant metastasis, and BCLC stage). Furthermore, we analyzed radiological response and OS.
Statistical analyses
The Chi‐square and Fisher's exact tests were used to compare categorical variables, and the Student t test or the Mann–Whitney U test was used to compare continuous variables. Cumulative patient survival percentage curves were prepared using the Kaplan–Meier method. The cumulative survival curves were compared using the log‐rank test. The factors associated with patient prognosis were analyzed using a Cox proportional hazards model. Significance was set at P < 0.05. The GraphPad Prism software (GraphPad Software, San Diego, CA, USA) and EZR (Saitama Medical Center, Jichi Medical University, Shimotsuke, Japan) were used to analyze statistical significance.
Results
Baseline characteristics
To be eligible for this study, 62 patients received a minimal cumulative duration of 4 weeks of LEN. The patient characteristics are shown in Table 1. The median age was 74 (49–93) years old. There were 30 (48%) patients older than 75 years old. The number of patients with Child–Pugh class A was 54 (87%), and the number with grade B was 8 (13%). There were 25 (40%) and 37 (60%) patients with BCLC stages B (intermediate) and C (advanced), respectively. A total of 26 (42%) patients had extrahepatic metastasis, and 17 (27%) had major vascular invasion. The number of TKI‐naive patients was 39 (63%), while the number of TKI‐experienced patients was 23 (37%). The median LEN treatment duration was 156.5 (30–662) days.
Table 1.
Baseline clinical characteristics of patients treated with lenvatinib
| Lenvatinib (n=62) | |
|---|---|
| Age (years), median (range) | 74 (49–93) |
| Age group (years) | |
| <65 | 10 (16%) |
| ≥65 to <75 | 22 (36%) |
| ≥75 | 30 (48%) |
| Gender: male/female (%) | 53 (85%)/9 (15%) |
| Bodyweight (kg) | |
| <60 | 30 (48%) |
| ≥60 | 32 (52%) |
| Etiology of chronic liver disease | |
| HBV | 12 (19%) |
| HCV | 30 (48%) |
| Alcohol | 8 (13%) |
| Others | 12 (19%) |
| Child–Pugh class | |
| A | 54 (87%) |
| B | 8 (13%) |
| ECOG PS | |
| 0 | 42 (68%) |
| 1 | 20 (32%) |
| BCLC | |
| B (intermediate stage) | 25 (40%) |
| C (advanced stage) | 37 (60%) |
| Extrahepatic spread | |
| Yes | 26 (42%) |
| No | 36 (58%) |
| Macroscopic vascular invasion | |
| Yes | 17 (27%) |
| No | 45 (73%) |
| Baseline AFP concentration | |
| Median | 113.1 (1.6–70 000) |
| Baseline AFP concentration group (ng/mL) | |
| <200 | 34 (55%) |
| ≥200 | 27 (44%) |
| Missing | 1 (1%) |
| Concomitant systemic antiviral therapy for HBV or HCV | 19 (31%) |
| Clinical course (systemic therapy) | |
| First line | 39 (63%) |
| Second line | 6 (10%) |
| Third line | 17 (27%) |
| Median LEN treatment duration (days) | 156.5 (30–662) |
AFP, alpha‐fetoprotein; BCLC, Barcelona Clinic Liver Cancer; HBV, hepatitis B virus; HCV, hepatitis C virus; LEN, lenvatinib; PS, performance status.
OS and radiological response
The median OS of patients treated with LEN was 469 days (Fig. 1a).
Figure 1.
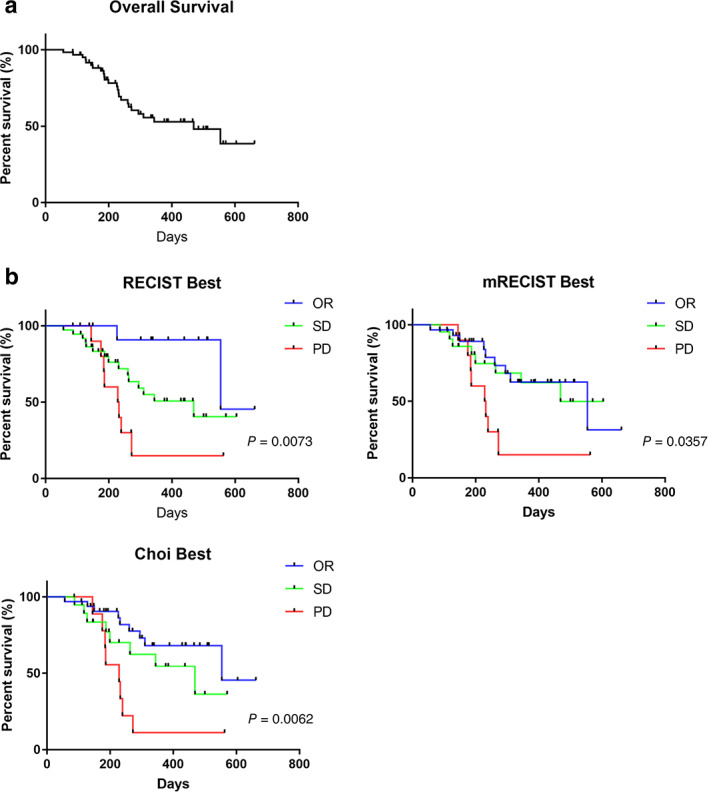
Survival and radiological best objective response assessed by the RECIST 1.1, mRECIST, and Choi criteria. (a) Overall survival (OS) of patients with hepatocellular carcinoma treated with lenvatinib. (b) Patients with the best objective response (OR, blue), stable disease (SD, green), and progressive disease (PD, red) assessed by the RECIST 1.1, mRECIST, and Choi criteria. The P‐values are from log‐rank tests.
The best radiological response to LEN treatment was evaluated with the RECIST 1.1, mRECIST, and Choi criteria. According to RECIST 1.1, 14 (22.5%) and 11 (17.7%) patients had OR and PD, respectively. A total of 37 patients (61.7%) had stable disease (SD). The mRECIST and Choi criteria identified 30 (48.3%) and 33 (53.2%) patients with OR, respectively. As shown in Table 2, the objective response rate (ORR) with the RECIST 1.1 was significantly lower than that with the mRECIST and Choi criteria (P = 0.001, Chi‐square test).
Table 2.
Best radiological response
| Best radiological response | RECIST | mRECIST | Choi |
|---|---|---|---|
| CR (n) | 1 | 5 | 1 |
| PR (n) | 13 | 25 | 32 |
| SD (n) | 37 | 22 | 19 |
| PD (n) | 11 | 10 | 9 |
| Total (n) | 62 | 62 | 62 |
| ORR (%) | 22.5 | 48.3 | 53.2 |
| DCR (%) | 82.2 | 83.8 | 85.4 |
CR, complete response; DCR, disease control rate; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease.
The number of patients with PD and the disease control rate were similar among the three groups. The time to progression (TTP) was 124 days, 127 days, and 143 days for each criterion (RECIST, mRECIST, and Choi, Figure S1A, Supporting information). The median OS of patients who received additional therapy after PD assessment for LEN treatment was longer than those who did not receive additional therapy (Fig. S1B and Table S2).
Survival curves for the best response according to the RECIST 1.1, mRECIST, and Choi criteria are shown in Figure 1b. For each method, OS in patients with OR and SD was significantly longer than that in patients with PD (RECIST P = 0.0073, mRECIST P = 0.0357, Choi criteria P = 0.0062). The OS with OR for the RECIST, mRECIST, and Choi criteria was the same: 554 days.
Prognostic factors
Next, we investigated the prognostic factors of OS in advanced HCC patients treated with LEN using univariate and multivariate analyses. In the univariate analysis, Child–Pugh class B, major vascular invasion, and high AFP (>200) were significant poor prognosis factors for patients treated with LEN. Radiological response according to each criterion was a significant positive prognostic factor. In the multivariate analysis, only the best radiological response according to the RECIST (hazard ratio [HR], 0.259; 95% confidence interval [CI] 0.0723–0.928; P = 0.038) was an independent factor of OS in advanced HCC patients treated with LEN (Table 3).
Table 3.
Cox proportional hazards regression analysis for factors associated with prognosis of patients treated with lenvatinib
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age | 1.03 | 0.989–1.082 | 0.139 | |||
| Gender male | 0.901 | 0.268–3.036 | 0.867 | |||
| Child Pugh ≥ 7 (B) | 5.507 | 1.598–18.97 | 0.00687 | 2.348 | 0.644–8.559 | 0.196 |
| MVI | 2.815 | 1.276–6.207 | 0.0103 | 1.984 | 0.878–4.482 | 0.995 |
| EHS | 0.947 | 0.429–2.088 | 0.892 | |||
| AFP > 200 | 2.736 | 1.219–6.14 | 0.0147 | 2.388 | 0.997–5.720 | 0.0508 |
| Past history of TKI | 1.137 | 0.499–2.586 | 0.76 | |||
| RECIST best (OR/SD/PD) | 0.377 | 0.201–0.708 | 0.0024 | 0.259 | 0.0723–0.928 | 0.038 |
| mRECIST best (OR/SD/PD) | 0.581 | 0.344–0.981 | 0.0422 | 0.0646 | 0.00282–1.481 | 0.863 |
| Choi best (OR/SD/PD) | 0.482 | 0.293–0.792 | 0.00395 | 0.0893 | 0.00343–2.323 | 0.146 |
Boldface means P value are <0.05.
AFP, alpha‐fetoprotein; EHS, extrahepatic spread; HR, hazards ratios; MVI, major vascular invasion; OR, objective response; PD, progressive disease; SD, stable disease; TKI, tyrosine kinase inhibitor.
Prognosis and timing of radiological evaluation
We focused on the first and second radiological evaluations. Survival curves for the first response according to the RECIST 1.1, mRECIST, and Choi criteria are shown in Figure 2a. Among the three methods, the RECIST significantly stratified OS of patients (P = 0.0412). Radiological evaluation with the contrast effect (mRECIST and Choi) had no significant difference in OS (P = 0.182 and 0.0928, respectively).
Figure 2.
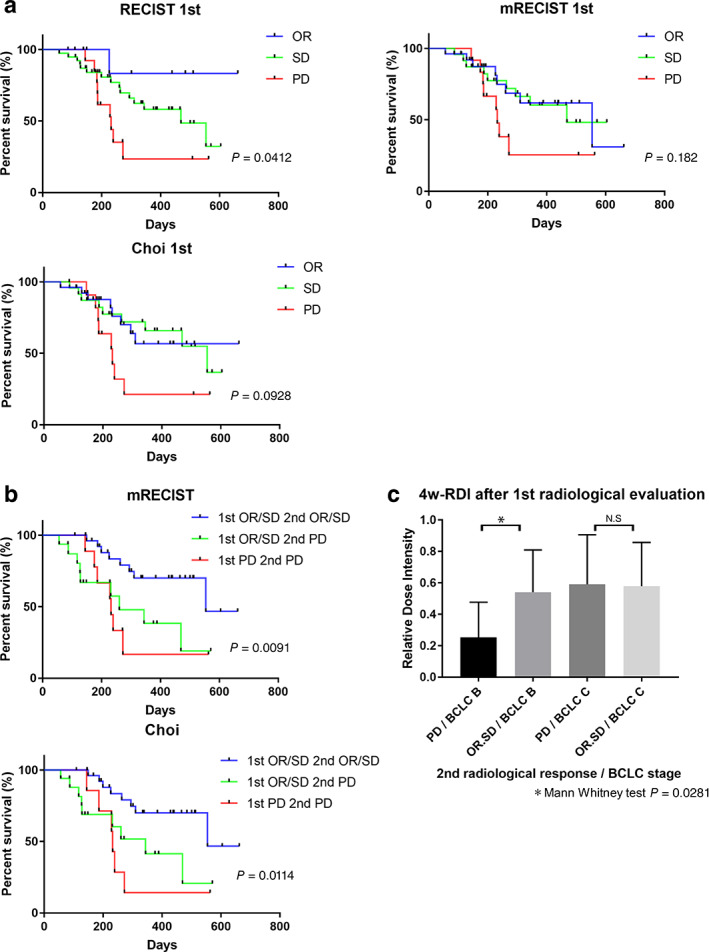
Survival and radiological objective response assessed by the first and second evaluations with the RECIST 1.1, mRECIST, and Choi criteria. (a) Patients with objective response (OR, blue), stable disease (SD, green), and progressive disease (PD, red) in the first evaluation. The P‐values are from log‐rank tests. (b) Survival curve of patients grouped by the first and second mRECIST and Choi criteria evaluations. OR or SD in the first and second evaluations (blue), OR or SD in the first evaluation and PD in the second evaluation (green), and PD in the first and second evaluations (red). The P‐values are from log‐rank tests. (c) The 4‐week relative dose intensity (RDI) after the first radiological evaluation and the second radiological response classified as BCLC stages B and C.
To clarify the gap, we assessed the first and second radiological response according to the mRECIST and Choi criteria. A total of 54 patients underwent two consecutive radiological evaluations. As described in Table 4, the first and second evaluations had diversity. With the RECIST, OR was generally achieved in the first evaluation, and OR and SD were noted in the second evaluation. However, with the mRECIST and Choi, the OR was widely evaluated in the first evaluation, and OR, SD, and PD were noted in the second evaluation.
Table 4.
First and second radiological response
| RECIST | |||
|---|---|---|---|
| First | n | Second | n (%) |
| OR | 8 | OR | 5 (62.5) |
| SD | 2 (25.0) | ||
| PD | 1 (12.5) | ||
| SD | 35 | OR | 3 (8.6) |
| SD | 18 (51.4) | ||
| PD | 14 (40.0) | ||
| mRECIST | |||
|---|---|---|---|
| First | n | Second | n (%) |
| OR | 23 | OR | 11 (47.8) |
| SD | 4 (17.4) | ||
| PD | 8 (34.8) | ||
| SD | 22 | OR | 3 (13.6) |
| SD | 11 (50.0) | ||
| PD | 8 (36.4) | ||
| Choi | |||
|---|---|---|---|
| First | n | Second | n (%) |
| OR | 23 | OR | 11 (47.8) |
| SD | 4 (17.4) | ||
| PD | 8 (34.8) | ||
| SD | 23 | OR | 5 (21.8) |
| SD | 9 (39.1) | ||
| PD | 9 (39.1) | ||
OR, objective response; PD, progressive disease; SD, stable disease.
Patients who were evaluated to have OR and SD twice consecutively with the mRECIST and Choi criteria had significantly better prognosis than those who were evaluated to have OR and SD in the first evaluation and PD in the second evaluation (mRECIST: HR, 0.324; 95% CI, 0.111–0.949; log‐rank test, P = 0.0141; Choi criteria: HR, 0.293; 95% CI, 0.101–0.845; log‐rank test, P = 0.0231, Fig. 2b). There are some reports on the initial RDI and effects of LEN. 11 , 17 Maintaining a higher RDI led to a better prognosis in patients treated with LEN. Thus, we analyzed the relationship between a 4‐week RDI after the first radiological evaluation and radiological response in the second evaluation classified by the BCLC stage. The mRECIST and Choi criteria evaluation results were the same in the second evaluation. In patients with BCLC stage B, the RDI of patients with OR and SD in the second evaluation was significantly higher than those with PD in the second evaluation (0.566 vs 0.25; Mann–Whitney U test, P = 0.0281; Fig. 2c). Thus, it was difficult to stratify prognosis, especially in the first radiological evaluation with the contrast effect (mRECIST and Choi criteria), because the time course RDI affected the radiological response.
Discussion
This study provided the initial evidence that the RECIST was useful for stratifying prognosis (OR, SD, and PD) even in an early evaluation for HCC patients treated with LEN. This study also validated that LEN showed high ORR in each criterion (Table 2) as previously reported. 39
The RECIST has high accuracy and precision for the evaluation of cancer treatment, including immuno‐oncology, and it is commonly used not only in clinical trials but in clinical practice for other carcinomas. 40 In this study, the RECIST showed high accuracy, as demonstrated by the best radiological response (Fig. 1), and was an independent prognostic factor (Table 3) and limited to the first radiological response (Fig. 2).
Previous studies demonstrated that OR evaluated by the mRECIST is an independent predictor of OS in HCC patients. 20 , 21 , 22 , 23 The mRECIST will become a more useful HCC treatment evaluation tool if ideal evaluation is possible, as shown in a review. 24
In trials, the ORR, progression‐free survival, and time to progression evaluated by the mRECIST targeting early or intermediate HCC cases and both the mRECIST and RECIST in advanced cases is recommended by American and European guidelines. 29 , 30 , 41 , 42 The Choi criteria have the characteristics of both the RECIST and mRECIST, and they can be objective. As there are still only a few reports, 14 , 26 , 28 further studies are expected to prove the usefulness of the Choi criteria.
However, it is often difficult to distinguish tumor necrosis and reduced arterial perfusion caused by changes in local hemodynamics with images only. As some cases showed tumor reperfusion when LEN was discontinued, staining criteria (mRECIST and Choi) should be carefully used in LEN treatment. Although a good response was obtained in the first evaluation, PD was often found in the subsequent evaluation. In the present study, patients evaluated to have OR or SD in the first evaluation and PD in the second evaluation had a significantly poorer prognosis than those evaluated to have OR or SD in two consecutive evaluations (Fig. 2b). Furthermore, the RDI of BCLC stage B patients evaluated to have PD in the second evaluation was significantly lower than those who were evaluated to have OR or SD in the second evaluation. There were more elderly and lower‐weight patients in our cohort than in clinical trials. 4 Therefore, it was difficult to maintain a high RDI due to dose reduction and discontinuation rates. The RECIST may be more useful than the mRECIST or Choi criteria for the first evaluation in such a patient population. Continuous management of adverse effects and LEN doses is also crucial after the first evaluation, especially with the mRECIST and Choi criteria, which evaluate staining. Patients who cannot maintain the RDI during BCLC stage B may be considered for additional TACE therapy. 43 , 44
Necrosis evaluation is an important issue when anticancer drugs that can dynamically change the staining pattern, such as LEN, are used. The evaluation of staining methods (mRECIST and Choi criteria) was suitable for the effect of local control therapies, such as TACE and radiofrequency ablation. Currently, objective tumor shrinkage evaluations, such as the RECIST, remain useful for chemotherapy, with which it was difficult to achieve complete necrosis. In the future, evaluation methods with staining will become the gold standard if convenient tools for such qualitative diagnosis other than biopsy are invented. We emphasize that these results are particularly applicable in early to mid‐term assessment of LEN therapy because of dynamic changes in the staining pattern. This study suggested the necessity of considering the evaluation method depending on the anti‐HCC drug.
The present study had several limitations. First, although the study showed the usefulness of radiological assessment in a cohort that included TKI‐experienced patients, real‐world reports on LEN, including our study, have mostly been made on Japanese populations. They may not be able to adapt to racial differences. LEN has been approved in many other countries, and worldwide reports and validation studies are expected. Second, the observation period and number of patients may be insufficient for long‐term survival evaluations and solid comparisons. Third, although the CT scans were performed at fixed times, corrections, such as bolus tracking, might lead to a more accurate image evaluation for staining. 45 , 46
In conclusion, the OR and PD evaluation with the RECIST 1.1, mRECIST, and Choi criteria was useful for observing LEN effects and HCC prognosis. Among these criteria, the RECIST 1.1 were useful for therapeutic evaluation even in early HCC patients treated with LEN. Understanding not only the characteristics of the radiological response but also patient characteristics and RDI over time may contribute to improving HCC patients' prognosis.
Supporting information
Figure S1. The progressive disease and additional therapy. (A) Time to progression (TTP) of patients with hepatocellular carcinoma treated with lenvatinib assessed by the RECIST 1.1, mRECIST, and Choi criteria. (B) Survival curve of patients grouped by additional therapies after progressive disease (PD) assessed by the RECIST 1.1, mRECIST, and Choi criteria.
Table S1. Total number of treatment cases with advanced hepatocellular carcinoma between April 2018 and January 2020.
Table S2. Characteristics of patients evaluated progression disease.
Acknowledgments
This study was funded by a grant‐in‐aid from the Japan Agency for Medical Research and Development (grant no. JP19fk0210025h0003). The authors appreciate the cooperation of Ms S. Kobori, Musashino Red Cross Hospital office.
Declaration of conflict of interest: Kaoru Tsuchiya, Masayuki Kurosaki, and Namiki Izumi received advisory board fees and honoraria for speakers’ bureau from Bayer and Eisai.
Author contribution: Shun Kaneko, Kaoru Tsuchiya, Masayuki Kurosaki, and Namiki Izumi contributed to the study concept and design. Yutaka Yasui, Kento Inada, Sakura Kirino, Koji Yamashita, Leona Osawa, Yuka Hayakawa, Shuhei Sekiguchi, Mayu Higuchi, Kenta Takaura, Chiaki Maeyashiki, Nobuharu Tamaki, Hiroyuki Nakanishi, Jun Itakura, and Yuka Takahashi contributed to the acquisition of data. Takaya Takeguchi and Yuko Takeguchi contributed to the independent radiological review. Shun Kaneko, Kaoru Tsuchiya, Yutaka Yasui, and Masayuki Kurosaki contributed to the analysis and interpretation of data. Shun Kaneko, Kaoru Tsuchiya, and Masayuki Kurosaki contributed to the drafting of the manuscript. Shun Kaneko, Kaoru Tsuchiya, and Masayuki Kurosaki contributed to statistical analysis. Yoshiro Himeno and Namiki Izumi contributed to study supervision. All of the authors gave final approval of the manuscript. All of the authors agreed to be accountable for all aspects of the work.
References
- 1. Matsui J, Yamamoto Y, Funahashi Y et al E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int. J. Cancer. 2008; 122: 664–71. [DOI] [PubMed] [Google Scholar]
- 2. Matsui J, Funahashi Y, Uenaka T, Watanabe T, Tsuruoka A, Asada M. Multi‐kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA‐MB‐231 via inhibition of vascular endothelial growth factor‐receptor (VEGF‐R) 2 and VEGF‐R3 kinase. Clin. Cancer Res. 2008; 14: 5459–65. [DOI] [PubMed] [Google Scholar]
- 3. Yamamoto Y, Matsui J, Matsushima T et al Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc. Cell. 2014; 6: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kudo M, Finn RS, Qin S et al Lenvatinib versus sorafenib in first‐line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non‐inferiority trial. Lancet. 2018; 391: 1163–73. [DOI] [PubMed] [Google Scholar]
- 5. Llovet JM, Ricci S, Mazzaferro V et al Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008; 359: 378–90. [DOI] [PubMed] [Google Scholar]
- 6. Hiraoka A, Kumada T, Kariyama K et al Therapeutic potential of lenvatinib for unresectable hepatocellular carcinoma in clinical practice: multicenter analysis. Hepatol. Res. 2019; 49: 111–7. [DOI] [PubMed] [Google Scholar]
- 7. Tada T, Kumada T, Hiraoka A et al Safety and efficacy of lenvatinib in elderly patients with unresectable hepatocellular carcinoma: a multicenter analysis with propensity score matching. Hepatol. Res. 2020; 50: 75–83. [DOI] [PubMed] [Google Scholar]
- 8. Kuzuya T, Ishigami M, Ito T et al Sorafenib vs. lenvatinib as first‐line therapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Anticancer Res. 2020; 40: 2283–90. [DOI] [PubMed] [Google Scholar]
- 9. Hiraoka A, Kumada T, Atsukawa M et al Prognostic factor of lenvatinib for unresectable hepatocellular carcinoma in real‐world conditions‐Multicenter analysis. Cancer Med. 2019; 8: 3719–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hiraoka A, Kumada T, Fukunishi S et al Post‐Progression Treatment Eligibility of Unresectable Hepatocellular Carcinoma Patients Treated with Lenvatinib. Liver Cancer. 2020; 9: 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kirino S, Tsuchiya K, Kurosaki M et al Relative dose intensity over the first four weeks of lenvatinib therapy is a factor of favorable response and overall survival in patients with unresectable hepatocellular carcinoma. PLoS One. 2020; 15: e0231828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tada T, Kumada T, Hiraoka A et al Neutrophil‐to‐lymphocyte ratio is associated with survival in patients with unresectable hepatocellular carcinoma treated with lenvatinib. Liver Int. 2020; 40: 968–76. [DOI] [PubMed] [Google Scholar]
- 13. Hiraoka A, Kumada T, Tada T et al Nutritional index as prognostic indicator in patients receiving lenvatinib treatment for unresectable hepatocellular carcinoma. Oncology. 2020; 98: 295–302. [DOI] [PubMed] [Google Scholar]
- 14. Kaneko S, Tsuchiya K, Kurosaki M et al Three criteria for radiological response on survival in patients with hepatocellular carcinoma treated with lenvatinib. Hepatol. Res. 2020; 50: 137–43. [DOI] [PubMed] [Google Scholar]
- 15. Kodama K, Kawaoka T, Namba M et al Correlation between early tumor marker response and imaging response in patients with advanced hepatocellular carcinoma treated with lenvatinib. Oncology. 2019; 97: 75–81. [DOI] [PubMed] [Google Scholar]
- 16. Saeki I, Yamasaki T, Yamashita S et al Early predictors of objective response in patients with hepatocellular carcinoma undergoing lenvatinib treatment. Cancers. 2020; 12: 779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Takahashi A, Moriguchi M, Seko Y et al Impact of relative dose intensity of early‐phase lenvatinib treatment on therapeutic response in hepatocellular carcinoma. Anticancer Res. 2019; 39: 5149–56. [DOI] [PubMed] [Google Scholar]
- 18. Eisenhauer EA, Therasse P, Bogaerts J et al New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer. 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 19. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010; 30: 52–60. [DOI] [PubMed] [Google Scholar]
- 20. Lencioni R, Montal R, Torres F et al Objective response by mRECIST as a predictor and potential surrogate end‐point of overall survival in advanced HCC. J. Hepatol. 2017; 66: 1166–72. [DOI] [PubMed] [Google Scholar]
- 21. Meyer T, Palmer DH, Cheng AL, Hocke J, Loembe AB, Yen CJ. mRECIST to predict survival in advanced hepatocellular carcinoma: analysis of two randomised phase II trials comparing nintedanib vs sorafenib. Liver Int. 2017; 37: 1047–55. [DOI] [PubMed] [Google Scholar]
- 22. Kudo M, Ueshima K, Yokosuka O et al Sorafenib plus low‐dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): a randomised, open label, phase 3 trial. Lancet Gastroenterol. Hepatol. 2018; 3: 424–32. [DOI] [PubMed] [Google Scholar]
- 23. Vincenzi B, Di Maio M, Silletta M et al Prognostic relevance of objective response according to EASL criteria and mRECIST criteria in hepatocellular carcinoma patients treated with loco‐regional therapies: a literature‐based meta‐analysis. PLoS One. 2015; 10: e0133488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Llovet JM, Lencioni R. mRECIST for HCC: performance and novel refinements. J. Hepatol. 2020; 72: 288–306. [DOI] [PubMed] [Google Scholar]
- 25. Choi H, Charnsangavej C, Faria SC et al Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J. Clin. Oncol. 2007; 25: 1753–9. [DOI] [PubMed] [Google Scholar]
- 26. Ronot M, Bouattour M, Wassermann J et al Alternative response criteria (Choi, European association for the study of the liver, and modified Response Evaluation Criteria in Solid Tumors [RECIST]) Versus RECIST 1.1 in patients with advanced hepatocellular carcinoma treated with sorafenib. Oncologist. 2014; 19: 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gavanier M, Ayav A, Sellal C et al CT imaging findings in patients with advanced hepatocellular carcinoma treated with sorafenib: alternative response criteria (Choi, European Association for the Study of the Liver, and modified Response Evaluation Criteria in Solid Tumor (mRECIST)) versus RECIST 1.1. Eur. J. Radiol. 2016; 85: 103–12. [DOI] [PubMed] [Google Scholar]
- 28. Weng Z, Ertle J, Zheng S et al Choi criteria are superior in evaluating tumor response in patients treated with transarterial radioembolization for hepatocellular carcinoma. Oncol. Lett. 2013; 6: 1707–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heimbach JK, Kulik LM, Finn RS et al AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018; 67: 358–80. [DOI] [PubMed] [Google Scholar]
- 30. EASL clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 2018; 69: 182–236. [DOI] [PubMed] [Google Scholar]
- 31. Kokudo N, Takemura N, Hasegawa K et al Clinical practice guidelines for hepatocellular carcinoma: the Japan Society of Hepatology 2017 (4th JSH‐HCC guidelines) 2019 update. Hepatol. Res. 2019; 49: 1109–13. [DOI] [PubMed] [Google Scholar]
- 32. Kim BR, Lee JM, Lee DH et al Diagnostic performance of gadoxetic acid‐enhanced Liver MR imaging versus multidetector CT in the detection of dysplastic nodules and early hepatocellular carcinoma. Radiology. 2017; 285: 134–46. [DOI] [PubMed] [Google Scholar]
- 33. Santillan C, Chernyak V, Sirlin C. LI‐RADS categories: concepts, definitions, and criteria. Abdom. Radiol. 2018; 43: 101–10. [DOI] [PubMed] [Google Scholar]
- 34. Motosugi U, Murakami T, Lee JM, Fowler KJ, Heiken JP, Sirlin CB. Recommendation for terminology: nodules without arterial phase hyperenhancement and with hepatobiliary phase hypointensity in chronic liver disease. J. Magn. Reson. Imaging. 2018; 48: 1169–71. [DOI] [PubMed] [Google Scholar]
- 35. Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin. Liver Dis. 1999; 19: 329–38. [DOI] [PubMed] [Google Scholar]
- 36. Oken MM, Creech RH, Tormey DC et al Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982; 5: 649–55. [PubMed] [Google Scholar]
- 37. Ikeda M, Kobayashi M, Tahara M, Kaneko S. Optimal management of patients with hepatocellular carcinoma treated with lenvatinib. Expert Opin. Drug Saf. 2018; 17: 1095–105. [DOI] [PubMed] [Google Scholar]
- 38. National Cancer Institute . Protocol Development Cancer Therapy Available from URL: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40
- 39. Kudo M. Extremely high objective response rate of lenvatinib: its clinical relevance and changing the treatment paradigm in hepatocellular carcinoma. Liver Cancer. 2018; 7: 215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wolchok JD, Hoos A, O'Day S et al Guidelines for the evaluation of immune therapy activity in solid tumors: immune‐related response criteria. Clin. Cancer Res. 2009; 15: 7412–20. [DOI] [PubMed] [Google Scholar]
- 41. Marrero JA, Kulik LM, Sirlin CB et al Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018; 68: 723–50. [DOI] [PubMed] [Google Scholar]
- 42. Vogel A, Cervantes A, Chau I et al Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann. Oncol. 2019; 30: 871–3. [DOI] [PubMed] [Google Scholar]
- 43. Kosaka Y, Kawaoka T, Aikata H et al A case of advanced HCC treated with lenvatinib after hepatic arterial infusion chemotherapy combined with radiation therapy treatment for portal vein tumor thrombosis in the main trunk. Clin. J. Gastroenterol. 2020; 13: 839–843. [DOI] [PubMed] [Google Scholar]
- 44. Kudo M. A new treatment option for intermediate‐stage hepatocellular carcinoma with high tumor burden: initial lenvatinib therapy with subsequent selective TACE. Liver Cancer. 2019; 8: 299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mehnert F, Pereira PL, Trubenbach J, Kopp AF, Claussen CD. Automatic bolus tracking in monophasic spiral CT of the liver: liver‐to‐lesion conspicuity. Eur. Radiol. 2001; 11: 580–4. [DOI] [PubMed] [Google Scholar]
- 46. Gordic S, Puippe GD, Krauss B et al Correlation between dual‐energy and perfusion CT in patients with hepatocellular carcinoma. Radiology. 2016; 280: 78–87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The progressive disease and additional therapy. (A) Time to progression (TTP) of patients with hepatocellular carcinoma treated with lenvatinib assessed by the RECIST 1.1, mRECIST, and Choi criteria. (B) Survival curve of patients grouped by additional therapies after progressive disease (PD) assessed by the RECIST 1.1, mRECIST, and Choi criteria.
Table S1. Total number of treatment cases with advanced hepatocellular carcinoma between April 2018 and January 2020.
Table S2. Characteristics of patients evaluated progression disease.


