Abstract
Purpose of review:
This review aims to summarize and critically evaluate the current literature on the associations between individual and socio-cultural factors that increase risk for cannabis use disorder (CUD), and policy change.
Recent findings:
Epidemiological studies show that areas with permissive legal cannabis climates are associated with greater individual risk factors for CUD. This includes: (1) higher rates of edible consumption and vaping, (2) higher delta-9-tetrahydrocannabinol (THC) potency and lower cannabidiol (CBD) levels, and, (3) younger age of initiation of use.
Summary:
A change in the socio-cultural level, such as shifts in the legalization of cannabis, could interact with individual-level factors in their associations with CUD. There is currently a lack of empirical studies that evaluate this interaction. We propose that future research consider a bioecological framework for CUD to allow for a comprehensive understanding of the effects of legal climate that could inform policy and clinical practice.
Keywords: cannabis use disorder, risk, public health, legalization, bioecological model, cultural
Introduction
The recent shift towards legalizing cannabis in the U.S. and worldwide has been met with some preliminary evidence of increased rates of cannabis use disorder (CUD; [1,2]). However, increased rates of CUD in historically cannabis legalized regions have generally been inconsistent [3–5]. While the mechanisms that mediate the association between cannabis-legal climates and rates of CUD are still unknown, epidemiological studies describe a potentially elevated role of certain risk factors in a cannabis-legal climate. For example, attitudes such as perception of harm coincides with an increased number of individuals seeking treatment for CUD. Take for instance a ~13% increase in CUD-related treatment from 2003 to 2016 globally that co-occurred with a ~24% decrease in perceived harm from cannabis from 2010 to 2016 [3]. Further, countries with the highest rates of cannabis dependence, such as the United States, Canada, Australia, New Zealand, and those in Western Europe, have legalized cannabis to some degree in the majority but not all have. For example, New Zealand is currently discussing legalizing cannabis in 2020, yet has a high rate of cannabis dependence currently [3–5] (see Table 1). This suggests that there may not be a direct relationship between cannabis legalization and CUD rates. Instead, the relationship between these two variables is likely due to underlying changes to risk factors for CUD, which modulate the effect of legalization on CUD. For instance, the average age of users appears to be the primary difference between cannabis legal and illegal climates, with decriminalized regions having an earlier age of initiation [1,4–8].
Table 1. Compendium of studies in multiple regions on factors related to the development of CUD across different levels of cannabis permissiveness (in terms of legal status).
*At the time of the study, parentheses () indicate the year of policy change, if any. Data obtained primarily from EMCDDA reports.
**Countries with missing data on the provided variables were excluded from this table.
| Region | Cannabis Legal status* | Study | N | Age of Sample | Individual Factors | Use Variables | Micro-environment Factors | ||
|---|---|---|---|---|---|---|---|---|---|
| Study done across 31 countries** | Bogt et al. (2006) | 50816 | 15 | -- | Perceived availability (Percent easiness) & Peer Drug use (Percent believed to use) | ||||
| Croatia |
Illegal (2013) |
19.0 | |||||||
| Czech Rep | Illegal | 11.0 | |||||||
| Denmark | Possession Illegal | 23.0 | |||||||
| Estonia | Personal possession is Depenalized | 12.0 | |||||||
| Finland | Personal possession is Depenalized | - | |||||||
| France | Illegal | 34.0 | |||||||
| Greenland | Illegal | 11.0 | |||||||
| Hungary | Legal from 2003–2013 | 2.0 | |||||||
| Ireland | Illegal | 24.0 | |||||||
| Italy |
Illegal (2014) |
44.0 | |||||||
| Greece |
Illegal (2017) |
10.0 | |||||||
| Latvia | Personal possession is Depenalized | 12.0 | |||||||
| Lithuania | Personal possession is Depenalized | 7.0 | |||||||
| Malta |
Illegal (2015) |
3.0 | |||||||
| Netherlands | Depenalized | 17.0 | |||||||
| Poland | Illegal | 8.0 | |||||||
| Portugal | Decriminalized | 16.0 | |||||||
| Russia | Personal possession is Depenalized | 4.0 | |||||||
| Slovenia | Personal possession is Depenalized | 26.0 | |||||||
| Sweden | Mixed (Illegal in majority, personal possession is depenalized in minority) | 6.0 | |||||||
| Ukraine | Illegal | 12.0 | |||||||
| UK | Illegal | 34.0 | |||||||
| USA | Mixed (Majority illegal/decrimi nalized, legal in minority) | 45.0 | |||||||
| Study done across 17 countries** | Degenhardt et al. (2008) | 85052 | 16+ | -- | Percent incidence of drug use by age 15 & 21 | ||||
| Columbia | Illegal | 10.2 | |||||||
| Mexico |
Illegal (Personal possession decriminalize d in 2009, medical legalization in 2017) |
8.0 | |||||||
| USA |
Mixed (Majority illegal/decrimi nalized, legal in minority) |
54.0 | |||||||
| Belgium | Personal possession is Depenalized | 22.0 | |||||||
| France | Illegal | 44.1 | |||||||
| Germany |
Illegal (2017) |
41.0 | |||||||
| Italy |
Illegal (2014) |
13.7 | |||||||
| Netherlands | Depenalized | 34.6 | |||||||
| Spain | Personal possession is decriminalized | 27.7 | |||||||
| Ukraine | Illegal | 12.3 | |||||||
| Israel | Legal | 13.7 | |||||||
| Lebanon | Illegal | 5.7 | |||||||
| Nigeria | Illegal | 1.9 | |||||||
| South Africa | Illegal | 11.0 | |||||||
| New Zealand | Illegal | 61.8 | |||||||
The question then becomes, how do changes in legal climates impact risk towards CUD that lead to these reportedly higher rates of CUD? Addressing this question relies on the assumption that these factors interact, such as in a bioecological model.
A bioecological model conceptualizes the presumed interactions between an individual’s biological makeup and the social environment [9]. Bronfenbrenner first introduced a bioecological interaction between biological maturation, the immediate family/community environment (microsystem), and the societal landscape (macrosystem) that shapes human development [9]. In this view, changes in any given system could lead to a cascade of effects in all systems. Bronfenbrenner’s bioecological model suggested that nature and nurture interact as opposed to the notion that one of these factors predominate the effects (e.g., environment, over and above personality, is the key factor in the development of resilience [10]). Applications of this model have informed the development of education and treatment strategies in childhood adversity [10]. Several disease models have also adopted bioecological models, including mental health disorders such as depression, anxiety, PTSD, schizophrenia, and ADHD to understand the onset and progression of symptoms as well as treatment outcomes. A study by Atzaba-Poria et al. (2004) found that microsystem interventions are better suited to prevent aggressive behavior in children, while macrosystem interventions are best to forestall anxiety and depression. Thus, a bioecological framework for CUD could facilitate more effective intervention.
Towards the goal of understanding how legal cannabis climates impact risk towards CUD, in this report we summarize known risk factors for CUD, describe studies that examine the relationship between legalization and CUD, and interpret how legalization might interact with risk factors such as in a bioecological framework.
Individual/biological factors
Individual/biological factors have been widely recognized to influence the risk for CUD. These factors range from genetics and biological variables such as biological sex, to personality/mood characteristics, psychological status, behavior, and neurocognitive functioning as well as demographic characteristics. In terms of CUD, individual/bioecological factors include being male [11–15], Native American, and being widowed, separated, or divorced [12]. In addition, poor school performance and low educational attainment have been linked to CUD [5,16]. On the other hand, being Black, Asian, or Hispanic have been shown to be associated with decreased risk for CUD [12], Mood- as well as personality-related variables also play a significant role in the development and maintenance of CUD, such as low levels of self-esteem, poor coping skills, and increased responsivity to stress [13,17]. High levels of discomfort intolerance (inability to tolerate discomfort) is associated with reduced vulnerability to cannabis-related problems, although this effect is lessened in depressed individuals [18]. Moreover, individual differences in neurocognitive functioning have been found predictive, such that motivational processes like craving and functioning of the underlying brain networks seem to be a risk factor, whereas cognitive control-related processes seem to be protective [19–21].
Genetic risk similarly contributes to the development of CUD. The results of family, twin, and adoption studies provide evidence that there are heritable influences on cannabis use, abuse, and dependence [22]. For example, acute and long-term response to cannabis has been associated with variants in the catechol-O-methyltransferase (COMT), AKT serine/threonine kinase 1 (AKT1), dopamine β-hydroxylase (DBH), cannabinoid receptor (CNR1), and serotonin transporter (5-HTTPLR) genes [23], Depending on the allele an individual may carry, these specific genetic polymorphisms seem to provide protection through the mediation of the relationship between cannabis use severity and symptom manifestation [23–26]. On the other hand, carrying another allele of these genetic polymorphisms can increase the likelihood of a psychiatric comorbidity such as: schizophrenia spectrum disorders, major depressive disorder, social anxiety, conduct disorder, bipolar disorder, ADHD, anti-social personality disorder, panic disorder, sleep disorders, and PTSD [4,11,12,22–24,27–41].
Finally, drug use patterns have been associated with progression to CUD. Initiation early in childhood or adolescence has been consistently associated with increases in the risk for CUD [42,43].
Furthermore, using cannabis in an increasingly frequent manner has been shown to predict the development of CUD [16,44]. Additionally, previous studies have shown that using tobacco and alcohol is correlated with using cannabis, and early initiation of tobacco use and alcohol consumption is predictive of early initiation of cannabis use [17,45,46].
Microsystem factors for CUD
One level beyond the individual/biological factors are microsystem factors. Microsystem factors are influences from the immediate social environment such as family and peer relationships, childhood experiences, and exposure to illicit activities [13,17,44–46]. For instance, family history of substance use disorders, immediate availability of drugs, and peer drug use predicts progression to cannabis use [13,17,44,45]. Similarly, a more positive attitude towards cannabis use or low perception of harm has been associated with increased cannabis use [13]. Many studies have found an association between life stressors and increased risk of CUD [47]. Experiencing trauma and stress early on, whether through adverse life events or childhood maltreatment, is predictive of early-onset use and later progression to addiction [47]. Dysfunctional family environments may not only disrupt the maturation of self-regulatory systems involved in stress response but also spearhead maladaptive coping skills, which can encourage risky patterns of cannabis use [46]. Other facets of an individual’s home environment have been associated with the development of CUD, including low socioeconomic status and less time spent with parents [13,45]. Of the microsystem factors linked to CUD, home environment and peer group are the most consistent contributors to cannabis use [7,13,17,45,48].
Although less research has focused on protective factors outside of genetic interplay, several microsystem factors have been recognized as protective for CUD. Just as a negative family environment in which a child may be subject to abuse and maltreatment can encourage use, a family environment composed of strong support among all members along with an individual’s positive perception of parental care can serve to decrease the risk for CUD [44]. Additionally, perceived disapproval of cannabis use by parents and close friends as well as the individual’s disapproval is associated with decreased risk for CUD [49].
Macrosystem factors for CUD
Macrosystem factors compose the larger societal environment of the individual beyond the microsystem. These factors encompass the legal/political environment, economy, predominant societal views, overarching cultural influences of the region, and cultural environment of the individual. Unfortunately, empirical studies on macrosystem factors contributing to CUD risk are limited. We are only aware of one study on macrosystem factors and cannabis use. This study found that as per-capita personal consumer expenditure (PCE) increases so does adolescent cannabis use [7]. Due to the scant literature on this topic, insight from studies examining related factors may be useful to understand these effects. Indeed, one study in Norway found that cannabis dealers were typically committed to either street culture or cannabis culture prior to dealing. Therefore, the culture an individual was exposed to early in life predicted initiation of cannabis dealing [50]. A study by Sznitman and Bretteville-Jensen (2015) found that perceived medicinal benefits of cannabis predicted an individual’s support for cannabis legalization. A recent review found that willingness to acknowledge CUD-related symptoms vary depending on the region [51]. However, participating in cannabis dealing, supporting cannabis legalization, and willingness to acknowledge CUD symptoms do not directly equate to increased/decreased risk for CUD. Thus far, precursory evidence suggests that macrosystem factors, specifically those at the socio-cultural level, can influence an individuals’ level of involvement with cannabis, public opinion of its use, and acknowledgement of CUD symptoms.
Cannabis legalization and CUD
Given the role that macrosystem factors may play as described in the previous section, it is equally important to shed light on the potential influence of shifts at the socio-cultural level for CUD. Changes in legalization are likely to have social and economic impacts [52–55]. To date, the literature has not yet directly examined the interaction between legal climate and risk/protective factors for CUD; however, hypotheses on the effect of macrosystem factors can be drawn from comparisons across different cannabis legal climates.
Tables 1 and 2 provide a summary of findings from different levels of cannabis permissiveness based on cannabis legalization status. This table highlights how regions with drug policies have variability in perceived availability of cannabis and levels of peer drug use. This finding from the table is concordant with a study by Bogt et al. (2006) that found that increased perceived availability of cannabis is a better predictor of frequent and lifetime use than the level of cannabis use in older individuals in the community, p = 0.007 and p = 0.040 respectively [7]. In contrast to the variability observed in perceived availability of cannabis, psychosis symptoms are associated with cannabis use across the board [56,57]. In terms of substance use factors, the association between early initiation of cannabis use, other substance use, and CUD development is consistent worldwide, regardless of legal status (Table 1). A similar study by Degenhardt et al (2008) in 17 countries found that New Zealand, the United States, France, Germany, and the Netherlands had the highest percent of users initiating use before age 21 despite the fact that at the time of the study, the U.S. and the Netherlands were the only regions with legalization or structured decriminalization, suggesting a null effect of permissiveness on early-onset use (i.e., <21 years old) [4]. This result is discordant with other studies demonstrating that the primary difference between cannabis legal and illegal climates was the onset of use, with decriminalized regions having an earlier age of initiation [1,4–8]. For the majority of countries with legalized cannabis use, the average age of onset is in adolescent-to-early adulthood. Specifically, in a study in a sample of over 80,000 individuals worldwide, the median age of onset of cannabis use was found to be between 18 to 22 years of age [4]. Given that studies have found that early initiation is a risk factor for CUD-related problems later on [13,45,58], this difference should be acknowledged. Additionally, studies have suggested an upward trend of cannabis abuse and dependence in regions where cannabis is legal or decriminalized compared to those in which it is illegal. However, these results were either only trending towards significance or influenced by confounding factors [59,60]. Furthermore, legality appears to influence the quantity of use, as well as methods of use. For example, in the U.S., increased odds of vaping and edible methods of cannabis use are predicted by medical legalization status and concentration of dispensaries [61]. Additionally, THC and CBD levels may be dependent upon the region and legal climate, although this needs to be confirmed through further empirical research. Initial studies have shown a relative increase in THC potency in areas with partial legalization, such as the US, but this is not consistent across studies nor all countries with cannabis legalization [62–65].
Table 2. Compendium of studies in a single region on factors related to the development of CUD across different levels of cannabis permissiveness (in terms of legal status).
*At the time of the study, parentheses () indicate the year of policy change, if any. Data obtained primarily from EMCDDA reports.
**Countries with missing data on the provided variables were excluded from this table.
| Region | Cannabis Legal status* | Study | N | Age of Sample | Individual Factors | Use Variables | Micro-environment Factors |
|---|---|---|---|---|---|---|---|
| Australia |
Illegal (up until medical legalization in 2016) |
Coffey et al. (2000) | 2032 | 14–15 | -- | Peer cannabis use (OR 2.1) and nicotine use (OR 2.0) associated with cannabis use. | -- |
| Degenhardt et al. (2001) | 10641 | 18+ | Participants who were cannabis dependent had a OR 2.84 for developing psychosis. | -- | -- | ||
| Clough et al. (2004) | 336 | 13–36 | -- | Alcohol use (OR 10.4) and tobacco use (OR 19.0) associated with current cannabis use. | -- | ||
| Lynskey et al. (2004) | 156 twin pairs discordant for cannabis dependence | 24–36 | Early MDD (OR 3.40 in dizygotic pairs, n.s. in monozygotic pairs) associated with subsequent cannabis use. | -- | -- | ||
| Teesson et al. (2012) | 8841 | 16–85 | Strong association between CUD and affective disorders (OR 3.0). | Prevalence of lifetime and past 12-month CUD 6% and 1% respectively. Strong association between CUD and alcohol use disorder (OR 3.6) Cannabis use more common in males (OR 2.0) and younger users (OR 4.6). |
-- | ||
| Germany |
Illegal (up until medical legalization in 2017) |
Hofler et al. (1999) | 1228 | 14–17 | Affective disorders (COR 2.9) and low self-esteem (COR 1.72) associated with cannabis use. | Baseline history of alcohol use (COR 5.2) predictive of cannabis use. | Family history of substance use disorder with a cumulative odds ratio (COR) of 1.43 predictive of cannabis use. |
| Sydow et al. (2002) | 2446 | 14–24 | Baseline use of other illicit drugs predicted cannabis dependence. | Parental death before age 15 and deprived socioeconomic status predicted cannabis dependence. Availability of drugs, peers’ drug use, and a more positive attitude of the individual towards drug use predicted cannabis use. |
|||
| Netherlands | Depenalized | Veen et al. (2004) | 133 | 15–54 | Cannabis users had earlier first symptomatic episodes of psychosis compared to nonusers. | -- | -- |
| New Zealand | Illegal | Poulton et al. (1997) | 641 | 15–21 | -- | Males were more likely to use and be dependent on cannabis than females. Early use substantially increased the risk for the development of cannabis dependence in young adulthood. | -- |
| Norway | Illegal | Pedersen (1990) | 1311 | 13–19 | Poor mental health (determined by number of depression and anxiety symptoms) correlated with heavy use. | -- | Parental divorce correlated with heavy cannabis use (having ever used the drug 50 times or more). |
| Norway & Israel | Illegal Legal | Sznitman and Bretteville-Jensen (2015) | 2175 648 |
18+ | -- | Past year cannabis use higher in Israel (13%) compared to Norway (5%), (p<.001). | -- |
| United Kingdom |
Illegal (up until medical legalization in 2010) |
Best et al. (2005) | 2078 | 14–16 | -- | -- | Lifetime cannabis users less likely to spend time regularly with both their mothers and fathers, but more likely to spend free time with friends who smoked, drank alcohol and used illicit drugs, and with friends involved in criminal activities. |
| USA |
Mixed Illegal/decriminalized: Majority of states Legal: Alaska, California, Colorado, District of Columbia (D.C.), Hawaii, Maine, Massachusetts, Montana, Nevada, Oregon, Vermont, Washington |
Stinson et al. (2006) | 43093 | 18+ | Greater odds of dependence for those widowed/separated/divorced. In those who were cannabis dependent, the prevalence for any mood disorder was 48.2%, any anxiety disorder was 43.5%, and any personality disorder was 76.7% |
Sample had an OR of 0.3 for past 12-month cannabis dependence. In those who were cannabis dependent, the prevalence for alcohol use disorder was 54.7% and 48.7% for nicotine dependence. | -- |
| Winters & Lee (2008) | 55230 | 18–26 | -- | Increased odds ratio of initiation of use in teenage years (12–18) compared to years 22–26; ORs 3.9–7.2. | -- | ||
| Buckner et al. (2008) | 1709 | 16.6 (Average age at Time 1- start of study) 30 (Time 4- end of study) |
14-year longitudinal study. Social anxiety disorder (SAD) at study entry was associated with 6.5 greater odds of cannabis dependence. | -- | -- | ||
| Cerda et al. (2012) | 34653 | 18+ | -- | States with medical marijuana laws (MMLs) had a higher OR (1.92) for marijuana use and marijuana abuse/dependence (OR 1.81) than states without MMLs. | -- | ||
| Hasin et al. (2015) | Time 1: 43093 Time 2: 36309 |
4.1% to 9.5% increase in use prevalence, 1.5% to 2.9% increase in DSM-IV cannabis use disorder in total sample, and 35.6% to 30.5% decrease in DSM-IV cannabis use disorder in previous users from 2001 to 2013. |
|||||
| Reed (2016) | Not listed | 21% to 31% increase in use prevalence in 18–25 year-olds from 2006 to 2014. | Increased THC-only and THC-positive motor vehicle fatalities, and decreased perception of health risk of use in adolescents from 2006 to 2014. |
||||
| Borodovsky et al. (2016) | 2838 | 32.5 (Average at time of study) | Individuals in MML states had a significantly higher likelihood of ever use of vaping (OR 2.04) and edibles (OR 1.78) than those in states without MMLs. In the vaping model, MML status was a significant predictor (OR: 1.77, p < 0.001), but neither recreational legalization status nor dispensary status were significant predictors (OR: 1.17–1.71, p= .27−.02). In the edible model both MML status and dispensary status were significant predictors (OR: 1.33, p = .007; OR: 1.88, p < .001) while recreational legalization status was not (OR: 1.39, p = .18). |
-- | |||
| Maier et al. (2017) | Not listed | No significant differences in property and violent crime rates from 2010 to 2014 |
Another empirical approach to the determination of the impacts of cannabis policies on CUD would be a prospective design. However, there have only been few such studies that directly compare CUD risk pre- versus post-policy change thus far. In two of the studies, prevalence rates of cannabis use and cannabis dependence were higher in states with legalization compared to those in which cannabis is not legalized (Table 2). Additionally, one study in Colorado found that the perception of risk of use declined following policy change [1]. These studies also examined crime statistics pre/post-policy change, but results were mixed as to whether any significant changes did occur [1,66]. Ongoing studies investigating pre- to post-legal changes in Canada will hopefully shed more light on these issues [67–69].
A bioecological framework for CUD towards understanding cannabis policy impacts
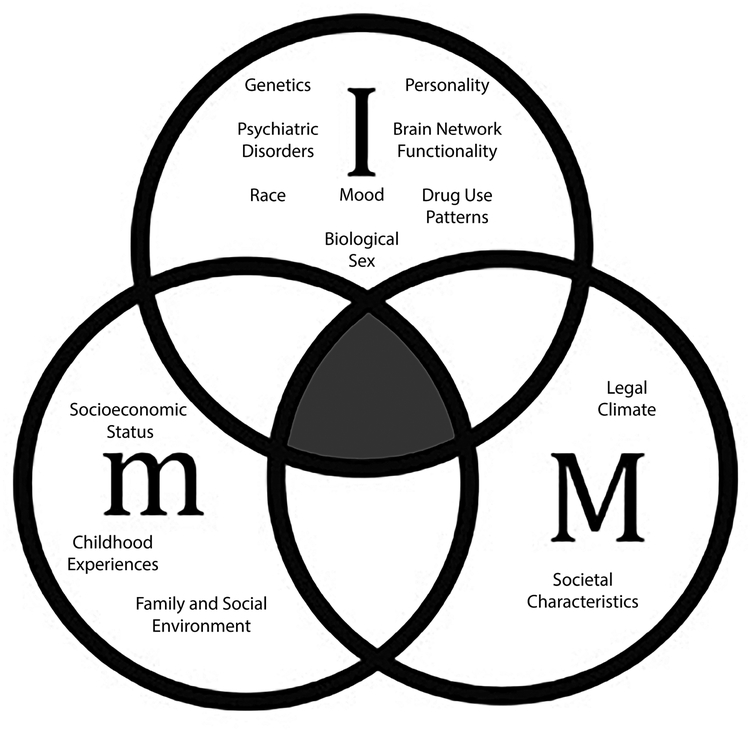
The literature highlights large individual variabilities in the development of CUD, which suggests that specific interactions between the factor levels are essential to determine the potential effects of cannabis policy changes. As depicted in Figure 1, we propose a model where all of the factor levels that contribute towards CUD are considered in a bioecological framework. In this model, individual/biological, micro- and macro-level factors converge towards a combined CUD vulnerability.
Fig.1. Bioecological systems contributing to CUD manifestation.
Proposed bioecological framework for determining CUD development where ‘I’ refers to individual factors such as personality, mood, genetics, psychiatric disorders, and use factors; ‘m’ refers to microsystem factors such as family and peer relationships; ‘M’ refers to macrosystem factors such as drug policy, society, and culture. The shaded area highlights the overlap between I, m and M that is critical in understanding development and treatment of CUD.
There is sufficient evidence in the current literature demonstrating some of these bioecological interactions. For instance, genetic and micro-environmental factors have been reported to increase one’s vulnerability to CUD. Dopamine availability mediated by genes for dopamine receptors (DRD4, DRD2, CNR1) has been shown to affect an individual’s susceptibility to environmental influences. For example, carriers of the DRD4 7-repeat allele are more/less likely to use cannabis during adolescence when levels of parental monitoring are low and high, respectively [70]. Additionally, exposure to early-life stress or a stressful home environment has been shown to interact with endocannabinoid genetics to influence levels of susceptibility to CUD. Specifically, endocannabinoid genes mediate the predictive effect of early-life stress and stress response (i.e., HPA axis activity or cortisol levels) on early-initiation of cannabis use [71]. Due to this evidence and many similar findings, it is evident that variations in the endocannabinoid system (measured via genetics or biological markers) can have a cumulative effect on risk factors identified in all ecological levels [72–79]. Other factor level interactions have shown that individual and macro-environmental factors can increase one’s vulnerability to CUD. Studies on this interaction have found that sex differences (higher use rates in males compared to females) are smaller in wealthier countries [7]. Additionally, the relationship between cannabis liberalization and increased cannabis use is lower in males compared to females [80]. These studies suggest that factors specific to the region can attenuate or amplify the effect of sex on CUD risk.
Taken together, the endocannabinoid genetic contribution to the influences of the stress response and home environment especially during adolescence (where early initiation of use is likely to occur) and early childhood should contribute to a bioecological model of CUD to thoroughly understand the impacts of policy. Additionally, the model should encompass the influence of macro-environment factors (e.g., the economy of the region and societal views of cannabis) on sex differences in cannabis use risk. By accounting for these factors in the bioecological model of CUD, we can better anticipate the effects of policy change and develop novel prevention efforts to address any cumulative impact it may have on vulnerability for CUD.
Studying the influence of macrosystem level factors such as legal climate poses substantial hurdles. For example, cannabis legalization as a dichotomous variable is too crude to measure underlying mechanisms that are a result of the culmination of an individual’s various macro-environments. If we only examine policy across regions, full legalization is rare [81–83]. With few exceptions, even the areas which are known for open cannabis consumption have low degrees of legalization termed depenalization or decriminalization [82]. These low levels of legalization decrease the penalties associated with cannabis use but do not mean that the government does not consider the substance illicit. Furthermore, regardless of legal policy, a population’s level of acceptance of cannabis use in a particular region can contribute to individual use patterns and attitudes, which may contribute to increased CUD risk [84–86]. Addressing other macrosystem factors such as healthcare system, support for addiction treatment and mental health, and societal attitude towards substance use and other psychiatric disorders could provide a better picture of this interaction.
What may be as important as the legal climate is a society’s cultural views on cannabis use [84–86]. Culture, however, must be operationalized. Cultural influences in CUD development can be defined as the normative culture of the region that is a collection of behaviors considered appropriate, but culture can also be described as a dynamic interaction of environmental, biological, and psychological factors that contribute to behavior [50,51]. Cultural influences are an essential component in the development of addiction and must be recognized in research. In sum, multidisciplinary studies are warranted that take into account social, anthropological, behavioral, clinical, genetic, and neurobiological aspects of CUD. These macro- and micro-system factors can exert influences on the interaction of risk/protective factors contributing to CUD and must be included as part of the conceptual framework.
f. Conclusions
Current research has provided extensive evidence for the unique impacts of biological, environmental, and societal influences on the development of CUD. While still relatively few, emergent studies such as those examining the effect of genetic influence and societal characteristics on microsystem risk factors demonstrate interactions between these different factor levels that result in modulated effects [7,71]. It is, therefore, important to examine the interactions between biological, micro- and macro-level factors within a bioecological framework to better (1) determine whether effects of these variables are in isolation or as a culmination of factor-level effects, and, (2) define protective and/or risk factors for CUD [87] that could inform research approaches, clinical practice, intervention development as well as cannabis policies (see Figure 1).
Funding:
This work was supported by the National Institutes of Health under Grant 1R01DA042490-01A1
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest Mackenzie Taylor, Janna Cousijn, and Francesca M. Filbey declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Reed J. Marijuana Legalization in Colorado: Early Findings: A Report Pursuant to Senate Bill 13–283 (March 2016). 2016;147. [Google Scholar]
- ••2.Hasin DS, Kerridge BT, Saha TD, Huang B, Pickering R, Smith SM, et al. Prevalence and Correlates of DSM-5 Cannabis Use Disorder, 2012–2013: Findings from the National Epidemiologic Survey on Alcohol and Related Conditions - III. Am J Psychiatry. 2016;173:588–99. [DOI] [PMC free article] [PubMed] [Google Scholar]; This epidemiology study examines prevalence of cannabis use disorder and related risk factors in the United States.
- 3.United Nations, Office on Drugs and Crime. World drug report 2016. 2016.
- 4.Degenhardt L, Chiu W-T, Sampson N, Kessler RC, Anthony JC, Angermeyer M, et al. Toward a Global View of Alcohol, Tobacco, Cannabis, and Cocaine Use: Findings from the WHO World Mental Health Surveys. PLOS Med. 2008;5:e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall W, Degenhardt L. Prevalence and correlates of cannabis use in developed and developing countries. Curr Opin Psychiatry. 2007;20:3. [DOI] [PubMed] [Google Scholar]
- 6.Poulton RG, Brooke M, Moffitt TE, Stanton WR, Silva PA. Prevalence and correlates of cannabis use and dependence in young New Zealanders. N Z Med J. 1997;110:68–70. [PubMed] [Google Scholar]
- 7.Bogt TT, Schmid H, Gabhainn SN, Fotiou A, Vollebergh W. Economic and cultural correlates of cannabis use among mid-adolescents in 31 countries. Addiction. 2006;101:241–51. [DOI] [PubMed] [Google Scholar]
- 8.Acuda SW, Eide AH. Epidemiological study of drug use in urban and rural secondary schools in Zimbabwe. 1984. [cited 2018 Jul 11]; Available from: https://opendocs.ids.ac.uk/opendocs/handle/123456789/7039 [PubMed]
- 9.Bronfenbrenner U. Ecological systems theory (1992). Mak Hum Hum Bioecological Perspect Hum Dev. Thousand Oaks, CA: Sage Publications Ltd; 2005. p. 106–73. [Google Scholar]
- 10.Ungar M, Ghazinour M, Richter J. Annual Research Review: What is resilience within the social ecology of human development? J Child Psychol Psychiatry. 2013;54:348–66. [DOI] [PubMed] [Google Scholar]
- 11.Teesson M, Slade T, Swift W, Mills K, Memedovic S, Mewton L, et al. Prevalence, correlates and comorbidity of DSM-IV Cannabis Use and Cannabis Use Disorders in Australia. Aust N Z J Psychiatry. 2012;46:1182–92. [DOI] [PubMed] [Google Scholar]
- 12.Stinson FS, Ruan WJ, Pickering R, Grant BF. Cannabis use disorders in the USA: prevalence, correlates and co-morbidity. Psychol Med. 2006;36:1447–60. [DOI] [PubMed] [Google Scholar]
- 13.von Sydow K, Lieb R, Pfister H, Höfler M, Wittchen H-U. What predicts incident use of cannabis and progression to abuse and dependence?: A 4-year prospective examination of risk factors in a community sample of adolescents and young adults. Drug Alcohol Depend. 2002;68:49–64. [DOI] [PubMed] [Google Scholar]
- 14.Clough A, D’Abbs P, Cairney S, Gray D, Maruff P, Parker R, et al. Emerging patterns of cannabis and other substance use in Aboriginal communities in Arnhem Land, Northern Territory: a study of two communities. Drug Alcohol Rev. 2004;23:381–90. [DOI] [PubMed] [Google Scholar]
- 15.Coffey C, Lynskey M, Wolfe R, Patton GC. Initiation and progression of cannabis use in a population-based Australian adolescent longitudinal study. Addiction. 95:1679–90. [DOI] [PubMed] [Google Scholar]
- •16.Castellanos-Ryan N, Pingault J-B, Parent S, Vitaro F, Tremblay RE, Séguin JR. Adolescent cannabis use, change in neurocognitive function, and high-school graduation: A longitudinal study from early adolescence to young adulthood. Dev Psychopathol. 2017;29:1253–66. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper is novel in that it employs a longitudinal approach to explore correlates of early cannabis initiation and predicts educational attainment based on cannabis use.
- 17.Hofler M, Lieb R, Perkonigg A, Schuster P, Sonntag H, Wittchen H-U. Covariates of cannabis use progression in a representative population sample of adolescents: a prospective examination of vulnerability and risk factors. Addiction. 1999;94:1679–94. [DOI] [PubMed] [Google Scholar]
- 18.Buckner JD, Keough ME, Schmidt NB. Problematic alcohol and cannabis use among young adults: The roles of depression and discomfort and distress tolerance. Addict Behav. 2007;32:1957–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verdejo-Garcia A, Benbrook A, Funderburk F, David P, Cadet J-L, Bolla KI.The differential relationship between cocaine use and marijuana use on decision-making performance over repeat testing with the Iowa Gambling Task. Drug Alcohol Depend. 2007;90:2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •20.Vingerhoets W, Koenders L, van den Brink W, Wiers R, Goudriaan A, van Amelsvoort T, et al. Cue-induced striatal activity in frequent cannabis users independently predicts cannabis problem severity three years later. J Psychopharmacol (Oxf). 2016;30:152–8. [DOI] [PubMed] [Google Scholar]; This paper utilizes a longitudinal approach to predict CUD symptom severity based on neural activity.
- 21.Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Goudriaan AE. Effect of baseline cannabis use and working-memory network function on changes in cannabis use in heavy cannabis users: a prospective fMRI study. Hum Brain Mapp. 2014;35:2470–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agrawal A, Lynskey MT. The genetic epidemiology of cannabis use, abuse and dependence. Addiction. 2006;101:801–12. [DOI] [PubMed] [Google Scholar]
- •23.Cosker E, Schwitzer T, Ramoz N, Ligier F, Lalanne L, Gorwood P, et al. The effect of interactions between genetics and cannabis use on neurocognition. A review. Prog Neuropsychopharmacol Biol Psychiatry. 2018;82:95–106. [DOI] [PubMed] [Google Scholar]; This review summaries how factors at the individual level (genetics and cognitive ability) can influence cannabis use.
- 24.Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H, et al. Moderation of the Effect of Adolescent-Onset Cannabis Use on Adult Psychosis by a Functional Polymorphism in the Catechol-O-Methyltransferase Gene: Longitudinal Evidence of a Gene X Environment Interaction. Biol Psychiatry. 2005;57:1117–27. [DOI] [PubMed] [Google Scholar]
- 25.Tunbridge EM, Dunn G, Murray RM, Evans N, Lister R, Stumpenhorst K, et al. Genetic moderation of the effects of cannabis: Catechol-O-methyltransferase (COMT) affects the impact of Δ9-tetrahydrocannabinol (THC) on working memory performance but not on the occurrence of psychotic experiences. J Psychopharmacol (Oxf). 2015;29:1146–51. [DOI] [PubMed] [Google Scholar]
- 26.Henquet C, Rosa A, Krabbendam L, Papiol S, Faňanás L, Drukker M, et al. An Experimental Study of Catechol-O-Methyltransferase Val158Met Moderation of Δ−9-Tetrahydrocannabinol-lnduced Effects on Psychosis and Cognition. Neuropsychopharmacology. 2006;31:2748–57. [DOI] [PubMed] [Google Scholar]
- 27.Manseau MW, Goff DC. Cannabinoids and Schizophrenia: Risks and Therapeutic Potential. Neurotherapeutics. 2015;12:816–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Estrada G, Fatjó-Vilas M, Muñoz MJ, Pulido G, Miñano MJ, Toledo E, et al. Cannabis use and age at onset of psychosis: further evidence of interaction with COMT Vall58Met polymorphism. Acta Psychiatr Scand. 123:485–92. [DOI] [PubMed] [Google Scholar]
- 29.Hambrecht M, Häfner H. Cannabis, vulnerability, and the onset of schizophrenia: An epidemiological perspective. Aust N Z J Psychiatry. 2000;34:468–75. [DOI] [PubMed] [Google Scholar]
- 30.Fu Q, Heath AC, Bucholz KK, Nelson E, Goldberg J, Lyons MJ, et al. Shared Genetic Risk of Major Depression, Alcohol Dependence, and Marijuana Dependence: Contribution of Antisocial Personality Disorder in Men. Arch Gen Psychiatry. 2002;59:1125–32. [DOI] [PubMed] [Google Scholar]
- 31.Biederman J, Wilens T, Mick E, Faraone SV, Weber W, Curtis S, et al. Is ADHD a Risk Factor for Psychoactive Substance Use Disorders? Findings From a Four-Year Prospective Follow-up Study. J Am Acad Child Adolesc Psychiatry. 1997;36:21–9. [DOI] [PubMed] [Google Scholar]
- 32.Lee SS, Humphreys KL, Flory K, Liu R, Glass K. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: A meta-analytic review. Clin Psychol Rev. 2011;31:328–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Angarita GA, Emadi N, Hodges S, Morgan PT. Sleep abnormalities associated with alcohol, cannabis, cocaine, and opiate use: a comprehensive review. Addict Sci Clin Pract. 2016;11:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Babson KA, Boden MT, Harris AH, Stickle TR, Bonn-Miller MO. Poor sleep quality as a risk factor for lapse following a cannabis quit attempt. J Subst Abuse Treat. 2013;44:438–43. [DOI] [PubMed] [Google Scholar]
- 35.Bonn-Miller MO, Harris AHS, Trafton JA. Prevalence of cannabis use disorder diagnoses among veterans in 2002, 2008, and 2009. Psychol Serv. 2012;9:404–16. [DOI] [PubMed] [Google Scholar]
- 36.Bonn-Miller MO, Babson KA, Vandrey R. Using cannabis to help you sleep: Heightened frequency of medical cannabis use among those with PTSD. Drug Alcohol Depend. 2014;136:162–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cornelius JR, Kirisci L, Reynolds M, Clark DB, Hayes J, Tarter R. PTSD contributes to teen and young adult cannabis use disorders. Addict Behav. 2010;35:91–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feingold D, Weiser M, Rehm J, Lev-Ran S. The association between cannabis use and anxiety disorders: Results from a population-based representative sample. Eur Neuropsychopharmacol J Eur Coll Neuropsychopharmacol. 2016;26:493–505. [DOI] [PubMed] [Google Scholar]
- 39.Kevorkian S, Bonn-Miller MO, Belendiuk K, Carney DM, Roberson-Nay R, Berenz EC. Associations among trauma, posttraumatic stress disorder, cannabis use, and cannabis use disorder in a nationally representative epidemiologic sample. Psychol Addict Behav J Soc Psychol Addict Behav. 2015;29:633–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruglass LM, Shevorykin A, Brezing C, Hu M-C, Hien DA. Demographic and clinical characteristics of treatment seeking women with full and subthreshold PTSD and concurrent cannabis and cocaine use disorders. J Subst Abuse Treat. 2017;80:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wittchen H-U, Fröhlich C, Behrendt S, Günther A, Rehm J, Zimmermann P, et al. Cannabis use and cannabis use disorders and their relationship to mental disorders: A 10-year prospective-longitudinal community study in adolescents. Drug Alcohol Depend. 2007;88:S60–70. [DOI] [PubMed] [Google Scholar]
- 42.Wagner FA, Anthony JC. From First Drug Use to Drug Dependence: Developmental Periods of Risk for Dependence upon Marijuana, Cocaine, and Alcohol. Neuropsychopharmacology. 2002;26:479–88. [DOI] [PubMed] [Google Scholar]
- 43.Winters KC, Lee C-YS. Likelihood of developing an alcohol and cannabis use disorder during youth: Association with recent use and age. Drug Alcohol Depend. 2008;92:239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.The Health and Social Effects of Nonmedical Cannabis Use. [Internet]. 2016. [cited 2018 Jun 12], Available from: http://www.deslibris.ca/ID/10090267
- 45.Best D, Best D, Gross S, Best D, Gross S, Manning V, et al. Cannabis use in adolescents: the impact of risk and protective factors and social functioning. Drug Alcohol Rev. 2005;24:483–8. [DOI] [PubMed] [Google Scholar]
- 46.Hayatbakhsh MR, Najman JM, Bor W, O’Callaghan MJ, Williams GM. Multiple Risk Factor Model Predicting Cannabis Use and Use Disorders: A Longitudinal Study. Am J Drug Alcohol Abuse. 2009;35:399–407. [DOI] [PubMed] [Google Scholar]
- 47.Hyman SM, Sinha R. Stress-Related Factors in Cannabis Use and Misuse: Implications for Prevention and Treatment. J Subst Abuse Treat. 2009;36:400–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pedersen W. Adolescents initiating cannabis use: cultural opposition or poor mental health? J Adolesc. 1990;13:327–39. [DOI] [PubMed] [Google Scholar]
- 49.Wu L-T, Swartz MS, Brady KT, Hoyle RH. Perceived Cannabis Use Norms and Cannabis Use among Adolescents in the United States. J Psychiatr Res. 2015;64:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sandberg S. The Importance of Culture for Cannabis MarketsTowards an Economic Sociology of Illegal Drug Markets. Br J Criminol. 2012;52:1133–51. [Google Scholar]
- 51.Prashad S, Milligan AL, Cousijn J, Filbey FM. Cross-Cultural Effects of Cannabis Use Disorder: Evidence to Support a Cultural Neuroscience Approach. Curr Addict Rep. 2017;4:100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Copeland J. Developments in the treatment of cannabis use disorder. Curr Opin Psychiatry. 2004;17:161. [Google Scholar]
- 53.Hopfer C. Implications of Marijuana Legalization for Adolescent Substance Use. Subst Abuse Off Publ Assoc Med Educ Res Subst Abuse. 2014;35:331–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hall W, Lynskey M. Evaluating the public health impacts of legalizing recreational cannabis use in the United States. Addiction. 111:1764–73. [DOI] [PubMed] [Google Scholar]
- 55.Joffe A, Yancy WS. Legalization of Marijuana: Potential Impact on Youth. Pediatrics. 2004;113:e632–8. [PubMed] [Google Scholar]
- 56.Veen ND, Selten J-P, van der Tweel I, Feller WG, Hoek HW, Kahn RS. Cannabis Use and Age at Onset of Schizophrenia. Am J Psychiatry. 2004;161:501–6. [DOI] [PubMed] [Google Scholar]
- 57.Degenhardt L, Hall W, Lynskey M. Alcohol, cannabis and tobacco use among Australians: a comparison of their associations with other drug use and use disorders, affective and anxiety disorders, and psychosis. Addiction. 2001;96:1603–14. [DOI] [PubMed] [Google Scholar]
- 58.Lynskey MT, Heath AC, Bucholz KK, Slutske WS, Madden PAF, Nelson EC, et al. Escalation of Drug Use in Early-Onset Cannabis Users vs Co-twin Controls. JAMA. 2003;289:427–33. [DOI] [PubMed] [Google Scholar]
- 59.Cerda M, Wall M, Keyes KM, Galea S, Hasin D. Medical marijuana laws in 50 states: Investigating the relationship between state legalization of medical marijuana and marijuana use, abuse and dependence. Drug Alcohol Depend. 2012;120:22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sznitman SR, Bretteville-Jensen AL. Public opinion and medical cannabis policies: examining the role of underlying beliefs and national medical cannabis policies. Harm Reduct J [internet]. 2015. [cited 2018 Sep 13];12 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4606899/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••61.Borodovsky JT, Crosier BS, Lee DC, Sargent JD, Budney AJ. Smoking, vaping, eating: Is legalization impacting the way people use cannabis? Int J Drug Policy. 2016;36:141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper provides an important example of how legalization may affect other components of cannabis use, without increasing CUD rates.
- 62.ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, Church JC. Changes in Cannabis Potency over the Last Two Decades (1995–2014) - Analysis of Current Data in the United States. Biol Psychiatry. 2016;79:613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sevigny EL, Pacula RL, Heaton P. The Effects of Medical Marijuana Laws on Potency. Int J Drug Policy. 2014;25:308–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••64.Chandra S, Radwan MM, Majumdar CG, Church JC, Freeman TP, ElSohly MA. New trends in cannabis potency in USA and Europe during the last decade (2008–2017). Eur Arch Psychiatry Clin Neurosci. 2019;269:5–15. [DOI] [PubMed] [Google Scholar]; This recently published paper provides evidence for a gradual increase in THC potency over more than a decade in the United States and Europe.
- 65.Niesink RJM, Rigter S, Koeter MW, Brunt TM. Potency trends of Δ9-tetrahydrocannabinol, cannabidiol and cannabinol in cannabis in the Netherlands: 2005–15. Addiction. 2015;110:1941–50. [DOI] [PubMed] [Google Scholar]
- 66.Maier SL, Mannes S, Koppenhofer EL. The Implications of Marijuana Decriminalization and Legalization on Crime in the United States. Contemp Drug Probl. 2017;44:125–46. [Google Scholar]
- •67.Rotermann M. Analysis of trends in the prevalence of cannabis use and related metrics in Canada. Health Rep. 2019;30:3–13. [DOI] [PubMed] [Google Scholar]; This paper provides preliminary evidence for an increase in cannabis use in Canada.
- 68.Mader J, Smith JM, Afzal AR, Szeto ACH, Winters KC. Correlates of lifetime cannabis use and cannabis use severity in a Canadian university sample. Addict Behav. 2019;98:106015. [DOI] [PubMed] [Google Scholar]
- 69.Sandhu HS, Anderson LN, Busse JW. Characteristics of Canadians likely to try or increase cannabis use following legalization for nonmedical purposes: a cross-sectional study. CMAJ Open. 2019;7:E399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Otten R, Barker ED, Huizink AC, Engels RCME. The Interplay between Parental Monitoring and the Dopamine D4 Receptor Gene in Adolescent Cannabis Use. PLOS ONE. 2012;7:e49432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Enoch M-A. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology (Berl). 2011;214:17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jutras-Aswad D, Jacobs MM, Yiannoulos G, Roussos P, Bitsios P, Nomura Y, et al. Cannabis-Dependence Risk Relates to Synergism between Neuroticism and Proenkephalin SNPs Associated with Amygdala Gene Expression: Case-Control Study. PLOS ONE. 2012;7:e39243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Verweij KJH, Zietsch BP, Lynskey MT, Medland SE, Neale MC, Martin NG, et al. Genetic and environmental influences on cannabis use initiation and problematic use: a meta-analysis of twin studies. Addict Abingdon Engl. 2010;105:417–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fatjó-Vilas M, Prats C, Fananâs L. Chapter e4 - COMT Genotypes, Cannabis Use, and Psychosis: Gene-Environment Interaction Evidence from Human Populations, and Its Methodological Concerns In: Preedy VR, editor. Handb Cannabis Relat Pathol [Internet]. San Diego: Academic Press; 2017. [cited 2019 Mar 6]. p. e29–41. Available from: http://www.sciencedirect.com/science/article/pii/B9780128007563000314 [Google Scholar]
- 75.Gerra MC, Manfredini M, Cortese E, Antonioni MC, Leonardi C, Magnelli F, et al. Genetic and Environmental Risk Factors for Cannabis Use: Preliminary Results for the Role of Parental Care Perception. Subst Use Misuse. 2019;0:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Segal-Gavish H, Gazit N, Barhum Y, Ben-Zur T, Taler M, Hornfeld SH, et al. BDNF overexpression prevents cognitive deficit elicited by adolescent cannabis exposure and host susceptibility interaction. Hum Mol Genet. 2017;26:2462–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Morgan CJA, Freeman TP, Powell J, Curran HV. AKT1 genotype moderates the acute psychotomimetic effects of naturalistically smoked cannabis in young cannabis smokers. Transl Psychiatry. 2016;6:e738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vink JM. Genetics of Addiction: Future Focus on Gene × Environment Interaction? J Stud Alcohol Drugs. 2016;77:684–7. [DOI] [PubMed] [Google Scholar]
- 79.Colizzi M, lyegbe C, Powell J, Ursini G, Porcelli A, Bonvino A, et al. Interaction Between Functional Genetic Variation of DRD2 and Cannabis Use on Risk of Psychosis. Schizophr Bull. 2015;41:1171–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shi Y, Lenzi M, An R. Cannabis Liberalization and Adolescent Cannabis Use: A Cross-National Study in 38 Countries. PLOS ONE. 2015;10:e0143562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reinarman C, Cohen PDA, Kaal HL. The Limited Relevance of Drug Policy: Cannabis in Amsterdam and in San Francisco. Am J Public Health. 2004;94:836–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wouters M, Korf DJ. Access to Licensed Cannabis Supply and the Separation of Markets Policy in the Netherlands. J Drug Issues. 2009;39:627–51. [Google Scholar]
- 83.Cabral TS. The 15th anniversary of the Portuguese drug policy: Its history, its success and its future. Drug Sci Policy Law. 2017;3:2050324516683640. [Google Scholar]
- 84.Sandberg S. Cannabis culture: A stable subculture in a changing world, Cannabis culture: A stable subculture in a changing world. Criminol Crim Justice. 2013;13:63–79. [Google Scholar]
- 85.General (US) O of the S, Services (US) C for MH, Health (US) Nl of M. Chapter 2 Culture Counts: The Influence of Culture and Society on Mental Health [Internet]. Substance Abuse and Mental Health Services Administration (US); 2001. [cited 2018 Aug 31]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK44249/ [Google Scholar]
- 86.Rubin V. Cannabis and Culture. Walter de Gruyter; 1975. [Google Scholar]
- ••87.Eriksson M, Ghazinour M, Hammarström A. Different uses of Bronfenbrenner’s ecological theory in public mental health research: what is their value for guiding public mental health policy and practice? Soc Theory Health. 2018;16:414–33. [Google Scholar]; This systematic review explores how aspects of Bronfenbrenner’s ecological theory has been and will continue to be applied in a mental health context.
- 88.Atzaba-Poria N, Pike A, Deater-Deckard K. Do risk factors for problem behaviour act in a cumulative manner? An examination of ethnic minority and majority children through an ecological perspective. J Child Psychol Psychiatry. 2004;45:707–18. [DOI] [PubMed] [Google Scholar]