Abstract
Purpose
Polydeoxyribonucleotide (PDRN) is a substance known to suppress inflammation and accelerate wound healing. In this experiment, the effect of PDRN treatment on carbon tetrachloride (CCl4)-evoked acute liver injury (ALI) was investigated using mice.
Methods
We analyzed the levels of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) and conducted hematoxylin and eosin staining in accompany with terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling staining. Western blot analysis was also conducted to assess the expressions of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, adenosine A2A receptor, Bcl-2-associated X protein (Bax), and B-cell lymphoma 2 (Bcl-2). The mice were received intraperitoneal injection of 10-mL/kg CCl4, 4 times, once every 2 days. The mice in the PDRN treatment groups received intraperitoneal injection of 200-μL distilled water comprising each concentration of PDRN for 7 days starting 1 day after first CCl4 injection.
Results
ALT and AST concentrations in the serum were reduced and TNF-α, IL-1β, and IL-6 expressions were decreased by PDRN injection in CCl4-evoked ALI mice. PDRN injection suppressed Bax versus Bcl-2 ratio and reduced the percentage of TUNE-positive cells in CCl4-evoked ALI mice. PDRN injection overexpressed adenosine A2A receptor in CCl4-evoked ALI mice.
Conclusions
The therapeutic efficacy of PDRN also can be expected for CCl4-evoked acute urogenital injury in addition to ALI. The current research suggests that PDRN may be used for the therapeutic agent of CCl4-evoked ALI.
Keywords: Acute liver injury, Carbon tetrachloride, Adenosine A2A receptor, Polydeoxyribonucleotide, Proinflammatory cytokines, Apoptosis
• HIGHLIGHTS
- PDRN injection reduced proinflammatory cytokine production, Bax to Bcl-2 ratio, and TUNE-positive cell percentage.
- PDRN injection overexpressed adenosine A2A receptor in CCl4-evoked ALI mice.
- The current research suggests that PDRN may be used in the treatment of CCl4-evoked ALI.
INTRODUCTION
Acute liver injury (ALI) is a short-term liver injury that causes jaundice, severe blood clotting disorders, and high mortality [1]. Cytokines are a large group of polypeptides produced by various cells, and T lymphocytes are the main source of cytokine production. When activated, T lymphocytes differentiate into 2 types of helper (Th) cells, Th1 cells and Th2 cells. Th1 cells produce tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-2, and interferon-γ, while Th2 cells secrete IL-4, IL-5, IL-6, IL-10, and IL-13 [2]. It is currently well known that TNF-α, IL-1β, and IL-6 aggravate the progression of ALI [3]. These cytokines contribute and amplify the process of liver damage [4].
While apoptosis exerts a critical effect on organ development and maintenance of homeostasis, abnormal apoptosis acts as a disease-causing mechanism. B-cell lymphoma 2 (Bcl-2), which exerts anti-apoptotic effect, suppresses the process of apoptotic cell death, while Bcl-2-associated X protein (Bax), which exerts pro-apoptotic effect, promotes the progress of apoptotic cell death [5]. An imbalance between increased Bax and decreased Bcl-2 initiates process of apoptosis and enhances susceptibility to damage [6,7]. Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining histologically detects fragmentation of DNA, a typical feature of apoptosisinduced cell death [8].
The action of adenosine occurs via the following G proteincoupled receptors which are diversely expressed in immune cells such as A1, A2A, A2B, A3 [9]. Among these adenosine receptors, the adenosine A2A receptor is present in many cells responsible for healing of wound [10]. Polydeoxyribonucleotide (PDRN) is adenosine A2A receptor agonist, extracted from salmon sperm, and promotes repair of tissue damage caused by burns and wounds [11]. PDRN promotes the expression of vascular endothelial growth factor through activation of the adenosine A2A receptor [12,13]. PDRN induces suppressive effect on inflammation through reducing the expressions TNF-α and IL-6, examples of proinflammatory cytokines, and increasing the expression of IL-10, example of anti-inflammatory cytokines [13,14].
It is well known that PDRN inhibits inflammation and facilitates wound healing, but the pharmacological actions of PDRN on ALI are not well elucidated. In the current experiment, the effect of PDRN application on carbon tetrachloride (CCl4)-evoked ALI was investigated using mice. In this experiment, we analyzed the levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) and conducted hematoxylin and eosin (H&E) staining in accompany with TUNEL staining. Western blot analysis was also used to assess the expressions of TNF-α, IL-1β, IL-6, adenosine A2A receptor, Bax, and Bcl-2.
MATERIALS AND METHODS
Animals and Classification
Six weeks old male adult ICR mice weighing 30±2 g were purchased for use in this experiment. We achieved approval number from the Institutional Care and Use Committee of Kyung Hee University (KHUASP[SE]-17-001). The mice were controlled to have free access to food and water in a laboratory that was temperature-controlled to 23°C ±2°C and daytime controlled from 08:00 AM to 20:00 PM. The mice were randomly classified into 5 groups: control group, ALI-caused group, ALIcaused and 2 mg/kg PDRN-injected group, ALI-caused and 4 mg/kg PDRN-injected group, and ALI-caused and 8 mg/kg PDRN-injected group (n=8 in each group).
Induction of ALI
The mouse model of ALI was constructed as shown in the description below [15]. The mice were intraperitoneally injected with 10-mL/kg CCl4 (0.4-mL CCl4 is solubilized in 100-mL corn oil), 4 times, once every 2 days. The mice in the PDRN-injected groups received intraperitoneally injection with 200-μL distilled water comprising each concentration of PDRN (Placentex, Pharmaresearch Products Co. Ltd., Pangyo, Korea) for 7 days starting 1 day after first CCl4 injection. On the contrary, intraperitoneally injection of distilled water without PDRN was applied into the control mice and ALI-caused mice for the same duration.
Concentrations of ALT and AST
The mice were sacrificed to obtain blood after the last PDRN administration (7 days after CCl4 injection). After anesthetizing mice by Zoletil 50 (10 mg/kg, intraperitoneally; Vibac Laboratories, Carros, Frances), blood was drawn through heart puncture. The collected blood was allowed to stand at room temperature for 1 hour and next centrifuged at 3,000 rpm for 20 minutes to separate the serum. The concentration levels of serum ALT and AST were analyzed by ALT and AST activity assay kits (AM101-K, Asan Pharm Co., Seoul, Korea), and serum levels of ALT and AST were detected at 510-nm absorbance of a microplate reader model 680 (Bio-Rad Laboratories, Hercules, CA, USA).
Live Tissue Preparation
After blood collection, normal saline was injected via portal vein, and left lobe of liver was separated from each mouse. The liver was fixed with 4% paraformaldehyde, then dehydrated using graded ethanol, incubated with xylene, finally penetrated and fixed in paraffin. Since then, coronal sections with 5 μm thick were prepared using a paraffin microtome (Thermo Co., Cheshire, UK). The sections were placed on the coated slides, the slides were dried overnight on a 37°C hot plate. An average of 6 sliced sections was obtained from each liver tissue.
H&E Staining
H&E staining was done as described below in order to observe the histological changes in the liver [16]. The slides were incubated with Mayer’s hematoxylin (DAKO, Glostrup, Denmark) for 30 seconds, washed by water, immersed in eosin (Sigma Chemical Co., St. Louis, MO, USA) for 10 seconds, and then washed again by water. After air drying the slides at room temperature, the slides were immersed 2 times in 95% ethanol, 100% ethanol, 50% ethanol with 50% xylene, next 100% xylene, respectively. Permount (Thermo Fisher Scientific, New Jersey, NJ, USA) was used to mount the slides on a coverslip.
Western Blot Analysis
Western blot analysis was done as shown in the description below [8,17]. The liver tissues were homogenized using lysis buffer comprising 150mM NaCl, 100mM NaF, 50mM Tris-HCl (pH 8.0), 1mM phenylmethylsulfonyl fluoride, 1mM Na2VO4, 1mM ethylene glycol tetraacetic acid, 1.5mM MgCl2·6H2O, 10% glycerol, and 1% Triton X-100, and next centrifuged at 14,000 rpm for 30 minutes. Antibodies for rabbit adenosine A2A receptor (1:1,000; Abcam, Cambridge, UK), rabbit IL-1β (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), goat IL-6 (1:1,000; Santa Cruz Biotechnology), goat TNF-α (1:1,000; Santa Cruz Biotechnology), mouse β-actin (1:1,000; Santa Cruz Biotechnology), mouse Bax (1:1,000; Santa Cruz Biotechnology), and mouse Bcl-2 (1:1,000; Santa Cruz Biotechnology) were chosen for the primary antibodies. For the secondary antibodies, horseradish peroxidase-conjugated antimouse antibody (1:2,000; Vector Laboratories, Burlingame, CA, USA) for β-actin, Bax, Bcl-2, horseradish peroxidase-conjugated anti-rabbit antibody (1:3,000; Vector Laboratories) for IL-1β, adenosine A2A receptor, and horseradish peroxidase-conjugated anti-goat antibody (1:2,000; Vector Laboratories) for IL-6, TNF-α were chosen. Membrane transfer was carried out at 4°C using a cold pack and precooled buffer. The enhanced chemiluminescence detection kit (Santa Cruz Biotechnology) detected the amount of band, and the detected bands were compared by measuring the relative expression of proteins with Molecular Analyst ver. 1.4.1 (Bio-Rad).
TUNEL Staining
TUNEL staining was conducted as shown in the description below [8,18] using the In Situ Cell Death Detection Kit (Roche, Mannheim, Germany). These sections were treated with a solution of 2:1 ethanol to acetic acid, washed, treated to proteinase K (100 μg/mL), and treated with 3% H2O2. Next, the sections were incubated with 0.5% Triton X-100, treated with the TUNEL-reaction solution, and finally visualized by treating with Converter-POD and 0.05% 3,3’-diaminobenzidine. After air drying the slides overnight at room temperature, Permount (Thermo Fisher Scientific) was used to mount the slides on a coverslip.
An Image-Pro Plus computer-aided image analysis system (Media Cyberbetics Inc., Silver Spring, MD, USA) coupled to an optical microscope (Olympus, Tokyo, Japan) was used to calculate the percentage of TUNEL-positive hepatocytes in each liver tissue slice. To identify TUNEL-positive hepatocytes, 4 fields of view were randomly selected from each slide. Over 100 cells per field were calculated under ×200 magnification, and the percentage of TUNEL-positive hepatocyte was counted by the following formula: TUNEL-positive cell number/total cell number×100 (%).
Data Analysis
In order to analysis the data, 1-way analysis of variance and Duncan post hoc test was used. The experimental data were indicated as the mean±standard error of the mean. From P<0.05, it represents statistical significance.
RESULTS
Serum AST and ALT Concentrations
Fig. 1 shows that concentration levels of serum AST and ALT were enhanced by ALI occurrence (P<0.05), whereas PDRN injection reduced the ALI-evoked AST and ALT concentrations (P<0.05).
Fig. 1.
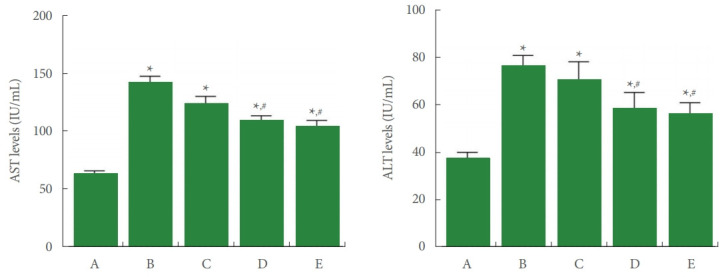
Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels in serum. Left panel: AST level in each group. Right panel: ALT level in each group. A, control group; B, ALI-caused group; C, ALI-caused and 2 mg/kg PDRN-injected group; D, ALI-caused and 4 mg/kg PDRN-injected group; E, ALI-caused and 8 mg/kg PDRN-injected group. ALI, acute liver injury; PDRN, polydeoxyribonucleotide. *P<0.05 when compared with the control group. #P<0.05 when compared with the ALI-caused group.
TNF-α Expressions
Fig. 2 shows that TNF-α expression in the liver tissue was enhanced by ALI occurrence (P<0.05), whereas PDRN injection inhibited ALI-evoked TNF-α expression (P<0.05).
Fig. 2.
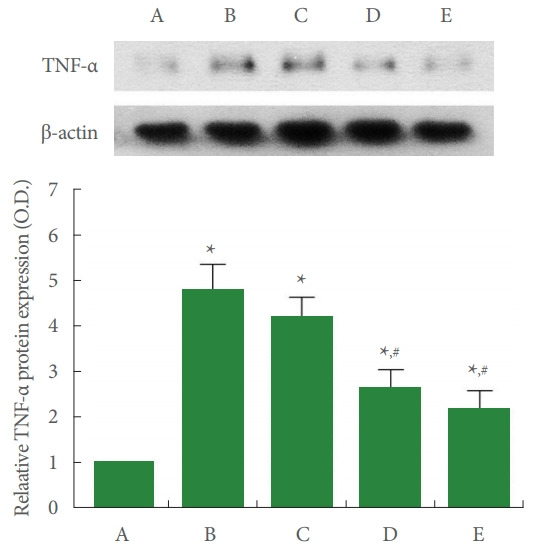
Tumor necrosis factor (TNF)-α expression in the liver tissue. Upper panel: representative TNF-α expression. Lower panel: data of TNF-α expression. A, control group; B, ALI-caused group; C, ALI-caused and 2 mg/kg PDRN-injected group; D, ALI-caused and 4 mg/kg PDRN-injected group; E, ALI-caused and 8 mg/kg PDRN-injected group. ALI, acute liver injury; PDRN, polydeoxyribonucleotide. *P<0.05 when compared with the control group. #P<0.05 when compared with the ALI-caused group.
IL-1β Expressions
Fig. 3 shows that IL-1β expression in the liver tissue was enhanced by ALI occurrence (P<0.05), in contrast, 8 mg/kg PDRN injection suppressed ALI-evoked IL-1β expression (P<0.05).
Fig. 3.
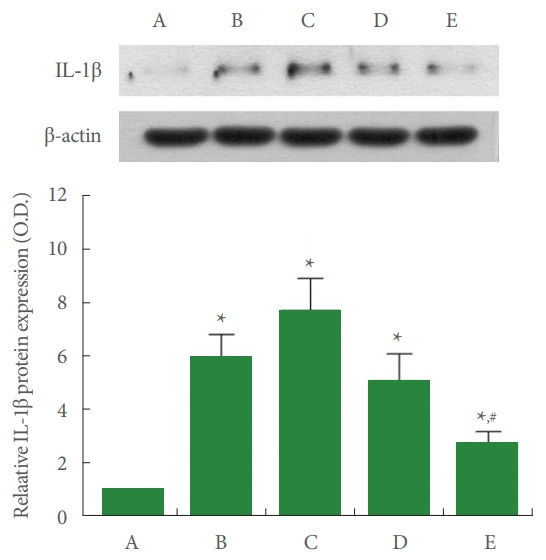
Interleukin (IL)-1β expression in the liver tissue. Upper panel: representative IL-1β expression. Lower panel: data of IL1β expression. A, control group; B, ALI-caused group; C, ALIcaused and 2 mg/kg PDRN-injected group; D, ALI-caused and 4 mg/kg PDRN-injected group; E, ALI-caused and 8 mg/kg PDRN-injected group. ALI, acute liver injury; PDRN, polydeoxyribonucleotide. *P<0.05 when compared with the control group. #P<0.05 when compared with the ALI-caused group.
IL-6 Expressions
Fig. 4 shows that IL-6 expression in the liver tissue was enhanced by ALI occurrence (P<0.05), whereas 4 mg/kg and 8 mg/kg PDRN injection decreased ALI-evoked IL-6 expression (P<0.05).
Fig. 4.
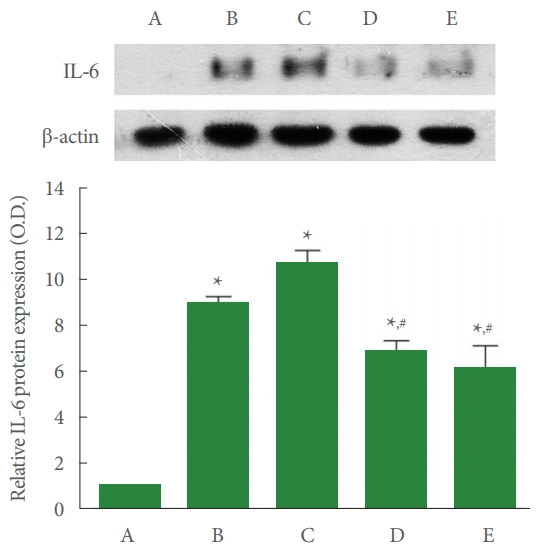
Interleukin (IL)-6 expression in the liver tissue. Upper panel: representative IL-6 expression. Lower panel: data of IL-6 expression. A, control group; B, ALI-caused group; C, ALIcaused and 2 mg/kg PDRN-injected group; D, ALI-caused and 4 mg/kg PDRN-injected group; E, ALI-caused and 8 mg/kg PDRN-injected group. ALI, acute liver injury; PDRN, polydeoxyribonucleotide. *P<0.05 when compared with the control group. #P<0.05 when compared with the ALI-caused group.
Adenosine A2A Receptor Expression
Fig. 5 shows that adenosine A2A receptor expression in the liver tissue was suppressed by ALI occurrence (P <0.05), whereas PDRN injection overexpressed ALI-evoked adenosine A2A receptor (P<0.05).
Fig. 5.
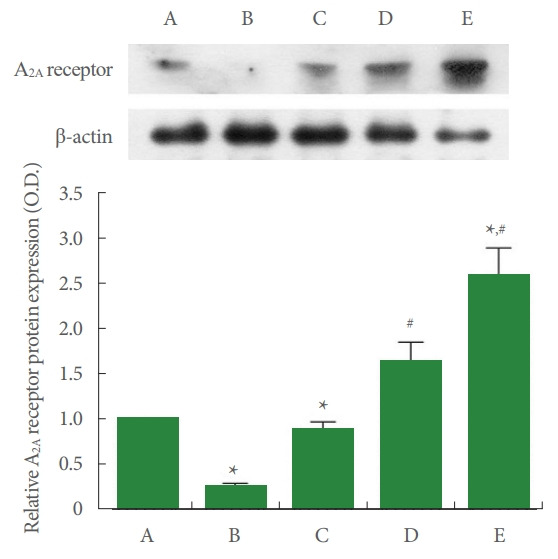
Adenosine A2A receptor expression in the liver tissue. Upper panel: representative adenosine A2A receptor expression. Lower panel: data of adenosine A2A receptor expression. A, control group; B, ALI-caused group; C, ALI-caused and 2 mg/kg PDRN-injected group; D, ALI-caused and 4 mg/kg PDRN-injected group; E, ALI-caused and 8 mg/kg PDRN-injected group. ALI, acute liver injury; PDRN, polydeoxyribonucleotide. *P<0.05 when compared with the control group. #P<0.05 when compared with the ALI-caused group.
Bax and Bcl-2 Expression
Fig. 6 shows that expression of Bax was increased and expression of Bcl-2 was suppressed in the liver tissue by ALI occurrence (P<0.05), thereby improved Bax vs Bcl-2 ratio (P<0.05). In contrast, PDRN injection inhibited expression of Bax and increased expression of Bcl-2 (P<0.05), thereby inhibited Bax vs Bcl-2 ratio (P<0.05).
Fig. 6.
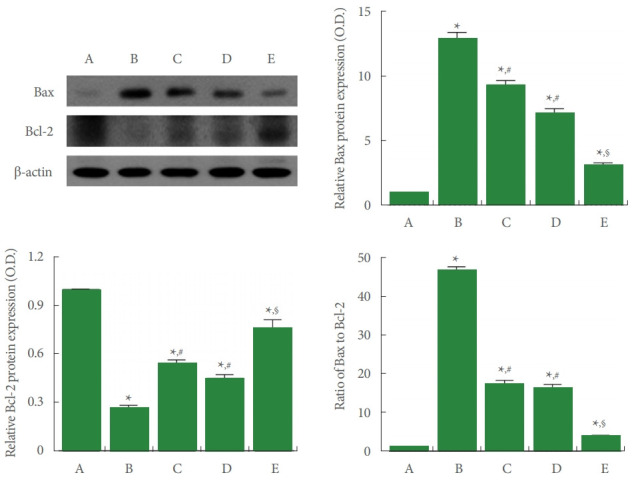
Bcl-2-associated X protein (Bax) and B-cell lymphoma 2 (Bcl-2) expressions in the liver tissue. Upper left panel: representative Bax and Bcl-2 expressions. Upper right and lower panels: data of Bax and Bcl-2 expressions. A, control group; B, ALI-caused group; C, ALI-caused and 2 mg/kg PDRN-injected group; D, ALI-caused and 4 mg/kg PDRN-injected group; E, ALI-caused and 8 mg/kg PDRN-injected group. ALI, acute liver injury; PDRN, polydeoxyribonucleotide. *P<0.05 when compared with the control group. #P<0.05 when compared with the ALI-caused group. §P<0.05 when compared with the ALI-caused and 2 mg/kg PDRN-injected group.
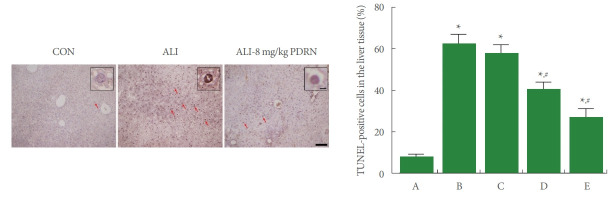
TUNEL-Positive Cells
Fig. 7 shows that the percentage of TUNEL-positive cells in the liver tissue was increased by ALI occurrence (P<0.05), whereas, PDRN injection suppressed the percentage of ALI-evoked TUNEL-positive cells (P<0.05).
Fig. 7.
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL)-positive cells in the liver tissue. Left panel: photomicrographs of TUNEL-positive cells. The scale bar means 100 μm. Insets are higher magnification (The scale bar means 250 μm). Red arrows indicate TUNEL-positive cells. Right panel: percentage of TUNEL-positive cells. A, control group; B, ALI-caused group; C, ALI-caused and 2 mg/kg PDRN-injected group; D, ALI-caused and 4 mg/kg PDRN-injected group; E, ALI-caused and 8 mg/kg PDRN-injected group. ALI, acute liver injury; PDRN, polydeoxyribonucleotide. *P<0.05 when compared with the control group. #P<0.05 when compared with the ALI-caused group.
DISCUSSION
CCl4 is a well-known hepatotoxic agent, and an animal model of CCl4-evoked liver damage has been widely used to assess the potential of agents to prevent hepatotoxicity [19]. CCl4 interferes with endogenous antioxidant enzymes and induces inflammatory response, leading to tissue damage and cell death [3]. Because anti-inflammatory effect can be produced by stimulating adenosine A2A receptor in immune cells [9,13], agonists on adenosine A2A receptor have been recommended as drugs to treat inflammation-related diseases [20]. It was demonstrated that adenosine A2A receptor agonist PDRN suppressed inflammation and exerted antiapoptotic effect against gastric ulcer that occurred in gerbils [13]. In the current results, adenosine A2A receptor expression was inhibited by CCl4 injection, meanwhile, treatment with PDRN overexpressed the adenosine A2A receptor in CCl4-induced ALI mice. The current study suggests that PDRN potently activated adenosine A2A receptor by enhancing the expression of adenosine A2A receptor.
Impaired liver function and/or accelerated activation of the innate immune system can cause liver stress and threaten liver cell survival. In this case, biomarkers of hepatocellular damage such as serum aminotransferases (ALT and AST) are increased [4]. Reduction in ALT and AST concentrations indicates that CCl4-evoked liver damage was alleviated [21]. In the current study, concentration levels of AST and ALT in the serum were increased by CCl4 injection, indicating that hepatocytes were damaged by CCl4 injection. In contrast, ALT and AST concentrations in the serum were suppressed by PDRN application in CCl4-evoked ALI mice. The current study suggests that PDRN has an alleviating effect on CCl4-evoked liver damage in mice.
The expression levels of TNF-α, IL-1, and IL-6 were enhanced by Toll-like receptor 4 in Kupffer cells [22]. Inflammation is an important pathological mechanism indicative of CCl4-evoked liver damage [23]. Inflammation accelerates the secretion of proinflammatory mediators, and excessive secretion of inflammation-causing mediators accelerates CCl4- evoked liver damage [19]. The expression levels of TNF-α, IL-1β, and IL-6 were profoundly enhanced by CCl4 injection [21]. Since TNF-α, IL-1β, and IL-6 are important precursors that induce inflammation and cause liver damage, inhibition on TNF-α, IL-1β, and IL-6 alleviated inflammatory damage to the liver [19]. In the current results, the expression levels of TNF-α, IL-1β, and IL-6 were enhanced by CCl4 injection, in contrast, the expression levels TNF-α, IL-1β, and IL-6 were suppressed by PDRN application in CCl4-evoked ALI mice. The current data indicates that overexpression of adenosine A2A receptor by PDRN inhibited the secretion of TNF-α, IL-1β, and IL-6 in the CCl4-evoked ALI mice.
Bcl-2 and Bcl-xL lose their protective function by forming heterodimers with the major pro-apoptotic member Bax [24]. The balance of Bcl-2 and Bax can be used as a decisive factor in determining whether cells die or live. In the epileptic rats or traumatic brain injury rats, Bcl-2 expression was suppressed and Bax expression was enhanced in the hippocampus, leading to an increment of Bax vs Bcl-2 ratio, which initiates the process of cell death of apoptosis [7,25]. Tendon injury increased apoptosis activity with degenerative changes [6]. Pilocarpine injection enhanced TUNEL-positive cell number in the dentate gyrus of hippocampus, suggesting that induction of epileptic rats initiated apoptotic process [25]. Intraventricular application of lipopolysaccharide enhanced TUNEL-positive cell number in the motor cortex, demonstrating that lipopolysaccharide potentiated progress of apoptosis [8].
The current results demonstrated that expression of Bax was enhnaced and expression of Bcl-2 was suppressed by CCl4 application, causing in increased Bax to Bcl-2 ratio. In addition, TUNEL-positive cell percentage was enhanced by CCl4 injection. Meanwhile, PDRN treatment inhibited Bax vs Bcl-2 ratio and reduced TUNE-positive cell percentage. The current study suggests that PDRN exerted suppressive effect on apoptotic process in the CCl4-evoked ALI mice.
In this study, PDRN treatment inhibited production of proinflammatory cytokine and then downregulated levels of AST and ALT, thereby attenuating CCl4-evoked ALI, leading to liver tissue regeneration. The strongest effect of PDRN was observed at 8-mg/kg PDRN, the highest concentration used in this experiment. Through this experiment, the therapeutic efficacy of PDRN also can be expected for CCl4-evoked acute urogenital injury in addition to ALI. The current research suggests that PDRN may be used in the treatment of CCl4-evoked ALI.
Footnotes
Research Ethics
This experimental procedure was approved by the Institutional Animal Care and Use Committee of Kyung Hee University (KHUASP[SE]-17-001).
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION STATEMENT
·Conceptualization: SL
·Data curation: SL, KYW
·Formal Analysis: SL, KYW
·Funding acquisition: SJ
·Methodology: SL, KYW
·Project Administration: SJ
·Visualization: SL, KYW
·Writing – Original Draft: SJ
·Writing – Review & Editing: SJ
REFERENCES
- 1.Bernal W, Auzinger G, Dhawan A, Wendon J. Acute liver failure. Lancet. 2010;376:190–201. doi: 10.1016/S0140-6736(10)60274-7. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez C, Buyse M, German-Fattal M, Gimenez F. Influence of the pro-inflammatory cytokines on P-glycoprotein expression and functionality. J Pharm Pharm Sci. 2004;7:359–71. [PubMed] [Google Scholar]
- 3.Zhang DG, Zhang C, Wang JX, Wang BW, Wang H, Zhang ZH, et al. Obeticholic acid protects against carbon tetrachloride-induced acute liver injury and inflammation. Toxicol Appl Pharmacol. 2017;314:39–47. doi: 10.1016/j.taap.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Malhi H, Guicciardi ME, Gores GJ. Hepatocyte death: a clear and present danger. Physiol Rev. 2010;90:1165–94. doi: 10.1152/physrev.00061.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim YM, Jin JJ, Lee SJ, Seo TB, Ji ES. Treadmill exercise with bone marrow stromal cells transplantation facilitates neuroprotective effect through BDNF-ERK1/2 pathway in spinal cord injury rats. J Exerc Rehabil. 2018;14:335–40. doi: 10.12965/jer.1836264.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell R, Robles-Harris MA, Anderson M, Laudier D, Schaffler MB, Flatow EL, et al. Inhibition of apoptosis exacerbates fatigue-damage tendon injuries in an in vivo rat model. Eur Cell Mater. 2018;36:44–56. doi: 10.22203/eCM.v036a04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ko IG, Kim SE, Hwang L, Jin JJ, Kim CJ, Kim BK, et al. Late starting treadmill exercise improves spatial leaning ability through suppressing CREP/BDNF/TrkB signaling pathway following traumatic brain injury in rats. J Exerc Rehabil. 2018;14:327–34. doi: 10.12965/jer.1836248.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song SH, Jee YS, Ko IG, Lee SW, Sim YJ, Kim DY, et al. Treadmill exercise and wheel exercise improve motor function by suppressing apoptotic neuronal cell death in brain inflammation rats. J Exerc Rehabil. 2018;14:911–9. doi: 10.12965/jer.1836508.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Odashima M, Otaka M, Jin M, Komatsu K, Wada I, Horikawa Y, et al. Attenuation of gastric mucosal inflammation induced by aspirin through activation of A2A adenosine receptor in rats. World J Gastroenterol. 2006;12:568–73. doi: 10.3748/wjg.v12.i4.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen DK, Montesinos MC, Williams AJ, Kelly M, Cronstein BN. Th1 cytokines regulate adenosine receptors and their downstream signaling elements in human microvascular endothelial cells. J Immunol. 2003;171:3991–8. doi: 10.4049/jimmunol.171.8.3991. [DOI] [PubMed] [Google Scholar]
- 11.Altavilla D, Bitto A, Polito F, Marini H, Minutoli L, Di Stefano V, et al. Polydeoxyribonucleotide (PDRN): a safe approach to induce therapeutic angiogenesis in peripheral artery occlusive disease and in diabetic foot ulcers. Cardiovasc Hematol Agents Med Chem. 2009;7:313–21. doi: 10.2174/187152509789541909. [DOI] [PubMed] [Google Scholar]
- 12.Minutoli L, Arena S, Bonvissuto G, Bitto A, Polito F, Irrera N, et al. Activation of adenosine A2A receptors by polydeoxyribonucleotide increases vascular endothelial growth factor and protects against testicular damage induced by experimental varicocele in rats. Fertil Steril. 2011;95:1510–3. doi: 10.1016/j.fertnstert.2010.07.1047. [DOI] [PubMed] [Google Scholar]
- 13.Jeon JW, Lee JI, Shin HP, Cha JM, Joo KR, Kim SH, et al. Adenosine A2A-receptor agonist polydeoxyribonucleotide promotes gastric ulcer healing in Mongolian gerbils. Animal Cells Syst. 2014;18:399–406. [Google Scholar]
- 14.Bitto A, Polito F, Irrera N, D’Ascola A, Avenoso A, Nastasi G, et al. Polydeoxyribonucleotide reduces cytokine production and the severity of collagen-induced arthritis by stimulation of adenosine A2A receptor. Arthritis Rheum. 2011;63:3364–71. doi: 10.1002/art.30538. [DOI] [PubMed] [Google Scholar]
- 15.Abdel-Hamid NM, Wahid A, Mohamed EM, Abdel-Aziz MA, Mohafez OM, Bakar S. New pathways driving the experimental hepatoprotective action of tempol (4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl) against acute hepatotoxicity. Biomed Pharmacother. 2016;79:215–21. doi: 10.1016/j.biopha.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 16.Ko IG, Kim SE, Jin JJ, Hwang L, Ji ES, Kim CJ, et al. Combination therapy with polydeoxyribonucleotide and proton pump inhibitor enhances therapeutic effectiveness for gastric ulcer in rats. Life Sci. 2018;203:12–9. doi: 10.1016/j.lfs.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Kim M, Kim TW, Kim CJ, Shin MS, Hong M, Park HS, et al. Berberine ameliorates brain inflammation in poloxamer 407-induced hyperlipidemic rats. Int Neurourol J. 2019;23(Suppl 2):S102–10. doi: 10.5213/inj.1938216.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho JW, Jung SY, Kim DY, Chung YR, Choi HH, Jeon JW, et al. PI3K-Akt-Wnt pathway is implicated in exercise-induced improvement of short-term memory in cerebral palsy rats. Int Neurourol J. 2018;22(Suppl 3):S156–64. doi: 10.5213/inj.1836224.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsai JC, Chiu CS, Chen YC, Lee MS, Hao XY, Hsieh MT, et al. Hepatoprotective effect of Coreopsis tinctoria flowers against carbon tetrachloride-induced liver damage in mice. BMC Complement Altern Med. 2017;17:139. doi: 10.1186/s12906-017-1604-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milne GR, Palmer TM. Anti-inflammatory and immunosuppressive effects of the A2A adenosine receptor. ScientificWorldJournal. 2011;11:320–39. doi: 10.1100/tsw.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang Q, Bai F, Nie J, Lu S, Lu C, Zhu X, et al. Didymin ameliorates hepatic injury through inhibition of MAPK and NF-κB pathways by up-regulating RKIP expression. Int Immunopharmacol. 2017;42:130–8. doi: 10.1016/j.intimp.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 22.Mandrekar P, Szabo G. Signalling pathways in alcohol-induced liver inflammation. J Hepatol. 2009;50:1258–66. doi: 10.1016/j.jhep.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ebaid H, Bashandy SA, Alhazza IM, Rady A, El-Shehry S. Folic acid and melatonin ameliorate carbon tetrachloride-induced hepatic injury, oxidative stress and inflammation in rats. Nutr Metab (Lond) 2013;10:20. doi: 10.1186/1743-7075-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuwana T, Newmeyer DD. Bcl-2-family proteins and the role of mito¬chondria in apoptosis. Curr Opin Cell Biol. 2003;15:691–9. doi: 10.1016/j.ceb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Lee JM, Ji ES, Kim TW, Kim CJ, Shin MS, Lim BV, et al. Treadmill exercise improves memory function by inhibiting hippocampal apoptosis in pilocarpine-induced epileptic rats. J Exerc Rehabil. 2018;14:713–23. doi: 10.12965/jer.36394.197. [DOI] [PMC free article] [PubMed] [Google Scholar]