Abstract
The current review provides a quantitative synthesis of the empirical literature on sleep disturbance as a risk factor for suicidal thoughts and behaviors (STBs). A systematic search of PsycINFO, MEDLINE, and the references of prior reviews resulted in 41 eligible studies included in this meta-analysis. Sleep disturbance, including insomnia, prospectively predicted STBs, yielding small-to-medium to medium effect sizes for these associations. Complicating interpretation of these findings however, is that few studies of suicidal ideation and suicide attempts, as well as none of suicide deaths, assessed short-term risk (i.e., employed follow-up assessments of under a month). Such studies are needed to evaluate current conceptualizations of sleep dysregulation as being involved in acute risk for suicidal behavior. This want of short-term risk studies also suggests that current clinical recommendations to monitor sleep as a potential warning sign of suicide risk has a relatively modest empirical basis, being largely driven by cross-sectional or retrospective research. The current review ends with recommendations for generating future research on short-term risk and greater differentiation between acute and chronic aspects of sleep disturbance, and by providing a model of how sleep disturbance may confer risk for STBs through neuroinflammatory and stress processes and associated impairments in executive control.
Keywords: insomnia, meta-analysis, sleep, suicidal ideation, suicide
Introduction
Come Sleep! O Sleep, the certain knot of peace,
The baiting-place of wit, the balm of woe,
The poor man’s wealth, the prisoner’s release,
Th’ indifferent judge between the high and low.
With shield of proof shield me from out the prease
Of those fierce darts despair at me doth throw
– from Sir Philip Sidney, Astrophil and Stella Sonnet XXXIX
Suicide is one of the leading causes of death worldwide and is therefore a major public health concern (World Health Organization, 2019). Imperative to the development of prevention and intervention tools for suicidal thoughts and behaviors (STBs; i.e., suicidal ideation, attempts, and death) is the identification of mutable risk factors. Sleep disturbance has received growing attention as a potentially salient risk factor for suicide. Indeed, a decrease of even just one hour of sleep may be associated with significantly greater likelihood of these outcomes (Winsler et al., 2014). Furthermore, sleep disturbance is prominently featured as a warning sign (i.e., precipitating risk factor) for STBs by the American Association of Suicidology (American Association of Suicidology, 2019), the American Foundation for Suicide Prevention (American Foundation for Suicide Prevention, 2019), the National Institute of Mental Health (National Institute of Mental Health, 2019), and the Substance Abuse and Mental Health Services Administration (Substance Abuse and Mental Health Services Administration, 2019). Consistent with this position, several current theoretical perspectives hold that acute suicide risk occurs during relatively short-lived periods of heightened arousal or agitation (Deisenhammer et al., 2009; Miller & Prinstein, 2019; Tucker et al., 2016; Wolfe-Clark & Bryan, 2017), and sleep disruption has been conceptualized as a manifestation of the over-arousal characteristic of this period of acute suicide risk (Ribeiro et al., 2015; Rogers et al., 2016; Tucker et al., 2016).
When evaluating existing evidence for the role of sleep disturbances in suicide risk, it is important to observe the distinction between risk factors and correlates. A longitudinal design is necessary to assess the extent to which a variable may be considered a risk factor, temporally preceding the outcome of interest (Kraemer et al., 1997). In contrast to risk factors, correlates are determined with cross-sectional designs and therefore lack evidence of temporal precedence; correlates cannot establish prospective prediction and cannot be ruled out as concomitants or epiphenomena of the mental health outcome of interest. Correlates are thus limited in the degree to which they may inform treatment development.
To date, there have been six systematic reviews (Bernert et al., 2015; Chiu et al., 2018; Kearns et al., 2020; Liu, Tu, et al., 2019; Malik et al., 2014; Pigeon et al., 2012), all providing evidence suggesting that a range of sleep disturbances are indeed correlates of STBs. Although four of these prior systematic reviews were meta-analyses (Chiu et al., 2018; Liu, Tu, et al., 2019; Malik et al., 2014; Pigeon et al., 2012), which offer a unique advantage over narrative reviews in that they allow for quantitative estimates of the magnitude of the association between sleep disturbance and STBs, most of the studies included in these meta-analyses employed cross-sectional designs or analyses. Significantly less empirical research has focused on the relationship between sleep indices and suicide outcomes utilizing longitudinal designs, leaving unclear the strength of sleep disturbance as a true risk factor for STBs. Moreover, it is possible that the greater representation of cross-sectional studies in these prior meta-analyses may have led to an overestimate of the strength of the relation between sleep disturbance indices and suicide risk, especially given evidence that one of these prior meta-analyses has found that cross-sectional designs may produce larger effect sizes (RR = 3.14) than longitudinal designs (RR = 1.94) in this relation (Pigeon et al., 2012). Finally, there has been only one systematic review focusing specifically on longitudinal associations between sleep and suicide, a narrative review of adolescent studies. The majority of the studies in this review provided evidence of a prospective relation between sleep problems and suicidal thoughts and behaviors (Kearns et al., 2020).
The present review aimed to accomplish several goals. The current study aimed to provide the first systematic review and meta-analysis of studies examining the prospective relationships between sleep disturbance and STBs. It also intended to identify potential moderators of the strength of these associations. Additionally, an earlier meta-analysis (Pigeon et al., 2012) that evaluated whether the strength of association between sleep disturbance and suicidality was moderated by the adoption of a cross-sectional versus longitudinal design featured only seven studies that were eligible for the current review.1 The body of literature has expanded considerably since then such that the small number of available longitudinal studies at the time necessitated collapsing across STBs in evaluating the association between sleep disturbance and suicide, whereas there are now a sufficient number of longitudinal studies to observe the important distinction between each form of STB in separate meta-analyses. A more recent meta-analysis, focusing exclusively on psychiatric patient samples (Malik et al., 2014) categorized 11 studies as employing a longitudinal design, and just six of these featured longitudinal analyses. The two remaining meta-analyses focused exclusively on sleep behavior in adolescents, with one featuring only two studies with longitudinal analyses included in the current review (Liu, Tu, et al., 2019), and the other none (Chiu et al., 2018). The current quantitative synthesis of the literature therefore builds substantially upon prior meta-analytic work in this area with the intention of clarifying the temporal nature of the association between sleep disturbance and STBs. This review then follows with a discussion of methodological and conceptual issues relevant to the study of sleep disturbance as a risk factor for STBs, and ends by outlining a mechanistic model wherein disturbed sleep may lead to heightened risk for suicide, thereby to provide a guide for future research in this area.
Method
Search strategy and eligibility criteria
A systematic search of the literature was conducted in PsycINFO and MEDLINE to identify studies of potential relevance to the current review published from inception to August 1, 2019. The following search string was applied: (suicid* OR ―self-harm‖ OR parasuicid* OR self-injur*) AND (sleep* OR insomnia* OR hypersomnia* OR nightmare* OR polysomnogra* OR ―REM‖ OR actigraph*) AND (―longitudinal* OR future OR later OR “follow-up” OR “follow up” OR predict* OR prospective* OR risk OR “multi-wave” OR ―multiwave‖). The search results were limited to: (i) English-language publications and (ii) peer-reviewed journals. This search was supplemented by screening the references of prior systematic reviews of studies of sleep and suicide (Bernert et al., 2015; Chiu et al., 2018; Kearns et al., 2020; Liu, Tu, et al., 2019; Malik et al., 2014; Pigeon et al., 2012), which yielded three new publications. This search strategy yielded a total of 2,521 articles, of which 1,720 were unique reports. First, the eligibility of an article was determined based on the title and abstract. If the eligibly could not be determined solely from the title and abstract, the full text was also examined. Each search result was independently reviewed for eligibility by two authors. In the event of a discrepancy between authors, it was resolved independently by a third author.
The study inclusion criteria were: (i) sleep disturbance was assessed distinctly from other constructs (e.g., not as a compound variable of health conditions or behaviors); (ii) STBs were analyzed separately from other constructs (e.g., non-suicidal self-injury or suicide risk composites); (iii) each STB was distinguished from other aspects of suicidality (i.e., suicidal ideation, suicide attempts, and suicide deaths, were analyzed separately); (iv) sleep disturbance and STBs were assessed systematically; (v) quantitative data were presented on the association between sleep disturbance and STB(s); and (vi) studies included a longitudinal component, with sleep disturbance analysed in relation to a future STB (i.e., longitudinal studies that conducted analyses with temporal overlap between sleep disturbance and STBs were not eligible). In some cases, it was not possible to determine study eligibility based on the information provided in the study. When this occurred, the research team made every effort to obtain additional needed information by contacting the corresponding author or obtaining details from other publications that used the same dataset and/or provided a description of the relevant measure(s).
Data extraction
We extracted data for nine study characteristics. These included four sample characteristics: (i) sample age group (adolescent or adult); (ii) mean age of sample; (iii) sample type (i.e., epidemiological, community, at-risk/mixed, and clinical); and (iv) percentage of female participants in the study sample. Data for five study design characteristics were also extracted: (i) method of measuring sleep disturbance (i.e., self-report versus interview); (ii) method of measuring STB(s) (i.e., self-report versus interview); (iii) time-frame of sleep disturbance measure; (iv) time-frame of STB(s) measure; and (v) length of study assessment follow-up period.
Data analysis
Analyses were conducted using Comprehensive Meta-Analysis, Version 3.3.070 (Biostat, 2014). The standardized mean difference (SMD; Cohen’s d) was used to calculate effect sizes for analyses of potential associations with STBs. Standard guidelines for Cohen’s d were used to determine the size of the effect (i.e., .20 = small effect size, .50 = medium, and .80 = large; Kraemer et al., 2003). Pooled effects were calculated so that values greater than zero reflected a positive association between the sleep disturbance index of interest and STB (i.e., sleep disturbance is associated with greater likelihood of the STB). Weighted effect sizes were calculated by pooling effects across all relevant studies. In studies that presented data on associations between sleep disturbance and STBs for multiple time-points, data for the association for the shortest available time-point was selected for analysis, given the growing recognition of the need for identifying short-term risk for these outcomes (Glenn & Nock, 2014).
Random-effects models were generated for all analyses to account for the expected high heterogeneity across studies due to differences in design, measures, and samples. Random-effects models are more fitting than fixed-effects models in this case because they are able to account for this high heterogeneity by including sampling and study-level errors; the pooled effect size represents the mean of a distribution of true effect sizes instead of one true effect size. Fixed-effects models estimate within-study variance only, assuming that a single true effect size exists across studies. In fixed-effects models, variance is attributed to sampling error.
The I2 statistic was used to examine heterogeneity across studies. I2 represents the percentage of the variance in an effect estimate that is due to heterogeneity across studies rather than sampling error (i.e., chance). Low heterogeneity is indicated by I2 values of approximately 25%, and moderate heterogeneity by I2 values of 50%. Substantial heterogeneity attributed to real differences in study samples and methodology is indicated by an I2 value of 75%, suggesting that the observed heterogeneity is more than would be expected with random error (Higgins et al., 2003). Significant heterogeneity suggests the need to conduct moderator analyses to identify potential sources of this heterogeneity. In the current study, each potential moderator was assessed individually, with an estimated effect size at each level of the moderator.
Publication bias is an important consideration when conducting meta-analyses. As studies with small effect sizes or non-significant findings are less likely to be published, these studies may be more likely to be excluded from meta-analyses, resulting in a potentially inflated estimate of the overall effect size. To account for the presence of potential publication bias, Duval and Tweedie’s trim-and-fill analysis (Duval & Tweedie, 2000) and Egger’s regression intercept (Egger et al., 1997) were used. Duval and Tweedie’s trim-and-fill analysis generates an estimate of the number of missing studies based on asymmetry in a funnel plot of the standard error of each study in a meta-analysis (based on the study’s sample size) against the study’s effect size. This analysis also calculates an effect size estimate and confidence interval, adjusting for missing studies. Of note, this procedure assumes homogeneity of effect sizes, so the results must be interpreted with caution in the context of significant heterogeneity. Egger’s regression intercept also provides an estimate of potential publication bias through use of a linear regression approach to assess study effect sizes relative to their standard error.
Results
Out of the 1,720 unique publications identified with our search parameters, we excluded 1,106 based on their titles and abstracts. We excluded an additional 614 publications based on full-text review, resulting in 44 articles meeting the eligibility criteria. In cases where an article did not provide sufficient data for meta-analysis, every effort was made to contact the study authors (i.e., the first author, corresponding author, and senior author) to obtain the necessary data. This resulted in additional data required for meta-analysis being obtained from the authors of three studies (Kivelä et al., 2019; Littlewood et al., 2019; Wang et al., 2019). In the cases of studies meeting eligibility criteria but featuring overlapping samples, determination of which study to include in the meta-analysis was based, in descending order, on: (i) briefer follow-up period, given the aforementioned growing focus in the field on determining predictors of short-term risk for STBs (Glenn & Nock, 2014); and (ii) more recent date of publication. In cases where two studies featured overlapping samples but examined sleep disturbance in relation to different STBs, however, both studies were retained for the relevant analyses. Whenever it remained unclear after full-text inspection whether two studies reported on overlapping samples, the study authors were contacted to seek clarity on this issue. Nine eligible articles featured overlapping samples, three of which were excluded at this stage. This resulted in a final set of 41 articles included in the current review (see Figure 1 and Table 1). These studies included samples drawn from 11 countries (China, Finland, France, Japan, the Netherlands, Norway, South Korea, Sweden, Taiwan, United Kingdom, and United States). Among the studies with samples from the United States (n = 20), 70% reported data on race and 50% on ethnicity. Among these studies, more recent publications were more likely to report sample ethnicity (r = .52, p = .02), with a large effect for this association, but not sample race (r = .42, p = .06), although a medium-to-large effect was found in this case.
Fig. 1.
PRISMA flow chart of literature search.
Table 1.
Study characteristics
| Study Author(s) (year) | Na | % Femalea | Mean Agea | Sample | Sleep |
Suicidal thoughts and behaviors |
Length of Follow-upb | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictor(s) | Measure(s) | Format | Time Frame | Outcome(s) | Measure(s) | Format | Time Frame | ||||||
| Allan et al. (2017) | 252 | — | — | clinical | general insomnia | ISI | Q | current | SI | C-SSRS | I | 1 year | 1 year |
| Berglund (1986) | 87 | 45.00 | — | clinical | sleep disturbance | SSM | I | not provided | suicide | death records | N/A | 24 years | 24 years |
| Bernert et al. (2014) | 420 | — | 74.90 | community | sleep disturbance | SQI | Q | current | suicide | death records | N/A | 10 years | 10 years |
| Bjørngaard et al. (2011) | 41,758 | — | — | community | sleep disturbance | SSM | Q | 1 month | suicide | death records | N/A | 20 years | 20 years |
| Bryan et al. (2015) Sample 2c | 158 | 13.10 | 27.32 | clinical | general insomnia | ISI | Q | current | SI, SA | BSS, SASII | Q, I | 1 week, 2 years | 6 months, 2 years |
| Bryan et al. (2015) Sample 3c | 54 | 16.70 | 25.51 | clinical | general insomnia | ISI | Q | current | SI | BSS | Q | 1 week | 3 months |
| Eikelenboom et al. (2019)1 | 1,713 | 68.65 | 42.16 | clinical | general insomnia | WHI-IRS | Q | 1 month | SA | SSM | Q | 6 years | 6 years |
| Fawcett et al. (1990) | 954 | 57.97 | 38.10 | clinical | general insomnia | SADS | I | current | suicide | not provided | — | 10 years | 10 years |
| Fujino et al. (2005) | 13,259 | 55.34 | 52.96 | community | initial insomnia, middle insomnia, terminal insomnia, non-restorative sleep | SSM | Q | not provided | suicide | death records | N/A | 13 years | 13 years |
| Gunnell et al (2013) | 393,983 | 50.53 | 40.20 | community | general insomnia | SSM | Q | 1 month | suicide | death records | N/A | 7.4 years | 7.4 years |
| Hartwig et al. (2019) | 187 | — | — | clinical | sleep disturbance | PHQ-9 | Q | 2 weeks | SI | C-SSRS | I | 1 month | 6 months |
| Hogstedt et al. (2018) | 17,219 | — | — | community | initial insomnia | SSM | Q | current | SA, suicide | medical records, death records | N/A | 36 years | 36 years |
| Hom et al. (2019a) | 624 | 21.10 | 25.24 | clinical | general insomnia | ISI | Q | current | SI | ASIQ | Q | 1 month | 3 months |
| Hom et al. (2019b) | 226 | 89.00 | 19.42 | community | general insomnia | ISI | Q | current | SI | DSI-SS | Q | 2 weeks | 2 months |
| Hung et al. (2015) | 101,764 | 49.10 | community | general insomnia | BSRS | Q | 1 week | suicide | death records | N/A | 3.8 years | 3.8 years | |
| Kang et al. (2014) | 909 | 58.00 | 72.40 | community | general insomnia | SSM | I | 1 month | SI | GMS | I | 1 month | 2 years |
| Kivelä et al. (2019)1 | 230 | 66.52 | 41.40 | clinical | general insomnia | WHI-IRS | Q | 1 month | SI | BSS | Q | 1 week | 2 years |
| Lau et al. (2020) | 206 | 79.13 | 14.86 | clinical | general insomnia | QIDS-A-SR-17 | Q | current | SI, SA | CHRT-SR, SSM | Q, I | 1 month, 6 months | 1 month, 6 months |
| Li et al. (2010)2 | 1,231 | 68.20 | 42.50 | clinical | general insomnia, nightmares | SSM | Q | 1 year | SA | medical records | N/A | 1 year | 1 year |
| Li et al. (2012)2 | 362 | 81.80 | 44.60 | clinical | general insomnia, nightmares | SSM | Q | 1 year | SI | medical records | N/A | 4 years | 4 years |
| Littlewood et al. (2019) | 51 | 66.67 | 35.47 | clinical | general insomnia | actigraphy | N/A | 1 day | SI | EMA | N/A | 1 day | 1 week |
| Liu et al. (2019) | 7,072 | 50 | 14.59 | community | general insomnia; hyposomnia; hypersomnia nightmares | AHQ | Q | 1 month | SI, SA | AHQ | Q | 1 year | 1 year |
| Mars et al. (2019) | 380 | 80.00 | 16.00 | clinical | general insomnia | SSM | Q | current | SA | SSM | Q | 5 years | 5 years |
| Mirsu-Paun et al. (2017) | 68 | 64.70 | 37.80 | clinical | general insomnia, hypersomnia, non-restorative sleep | ESS, ISI, SSM | Q | current | SI | C-SSRS | I | 1 month | 1 month |
| Montgomery et al. (1983) | 38 | 68.42 | 35.71 | clinical | general insomnia | MADRS | I | current | SA | not provided | — | 6 months | 6 months |
| Na et al. (2019) | 59,596 | 32.77 | — | community | sleep disturbance | medical records | N/A | current | suicide | death records | N/A | variable | variable |
| Nadorff et al. (2014) | 1,529 | 51.34 | 35.55 | clinical | sleep disturbance | BDI-II | Q | 2 weeks | SI | BDI-II | Q | 2 weeks | 6 weeks |
| Nrugham et al. (2008) | 265 | 77.00 | 14.90 | mixed | initial insomnia, middle insomnia, terminal insomnia, hypersomnia, non-restorative sleep | K-SADS-PL | I | lifetime | SA | K-SADS-PL, SSM | I, Q | 5 years | 5 years |
| Paffenbarger et al. (1994) | 12,078 | 0 | — | community | general insomnia | SSM | Q | not provided | suicide | death records | N/A | 27 years | 27 years |
| Ribeiro et al. (2012) | 239 | 18.01 | 22.19 | clinical | general insomnia | SSM | Q | current | SI, SA | MSSI, SSM | Q I |
1 month | 1 month |
| Ribeiro et al. (2019) | 1,021 | 66.40 | 26.54 | at-risk | general insomnia | ISI | Q | current | SI, SA | BSS, SITBI-SR | Q | 1 week 3 days |
2 weeks 3 days |
| Roane & Taylor (2008) | 3,582 | 54.40 | 15.83 | community | general insomnia | SSM | Q | 1 year | SI, SA | SSM | Q | 1 year | 7 years |
| Ross et al. (1990) | 247 | 50.00 | — | community | hypersomnia | SSM | Q | current | suicide | death records | N/A | 5 years | 5 years |
| Roy (1993) | 44 | — | — | clinical | general insomnia, sleep disturbance | HDRS, BDI-II | I, Q | 2 weeks | suicide | not provided | — | 1 year | 1 year |
| Shim & Jeong (2018) | 3,362 | 18.95 | 22.66 | community | sleep disturbance | PSQI | Q | 1 month | SI | BDI-II, KSI | Q | 1 week 2 weeks |
13 months |
| Sjöström et al. (2009) | 165 | 78.18 | 35.27 | clinical | initial insomnia, middle insomnia, terminal insomnia, nightmares | USI | Q | current | SA | medical records | N/A | 2 years | 2 years |
| Stange et al. (2016) | 2,741 | 58.00 | 40.10 | clinical | hyposomnia, hypersomnia | CMF | I | current | SI | CMF | I | current | 20.4 months |
| Strandheim et al. (2014) | 2,399 | 53.52 | — | community | initial insomnia | SSM | Q | 1 month | SI | SSM | Q | current | 4 years |
| Tanskanen et al. (2001) | 22,732 | 48.37 | 43.50 | community | nightmares | SSM | Q | 1 month | suicide | death records | N/A | 24 years | 24 years |
| Wang et al. (2019) | 4,645 | — | — | at-risk | general insomnia | BIQ | Q | 1 month | SI | C-SSRS-SR | Q | 1 month | 9 months |
| Wong et al. (2011) | 392 | 28.57 | — | mixed | general insomnia, nightmares | YSR | Q | 6 months | SI | YSR | Q | 6 months | 3 years |
| Zuromski et al. (2017) | 589 | 72.33 | 34.00 | clinical | general insomnia | ISI | Q | 3 days | SI | DSI-SS | Q | 3 days | 3 days |
Note. AHQ = Adolescent Health Questionnaire; ASIQ = Adult Suicidal Ideation Questionnaire; BDI-II = Beck Depression Inventory (sleep and/or suicidal ideation items); BIQ = Brief Insomnia Questionnaire (adaptation); BSRS = Brief Symptoms Rating Scale; BSS = Beck Scale for Suicidal Ideation; CHRT-SR = Concise Health Risk Tracking Self-Report; CMF = Clinical Monitoring Form; C-SSRS = Columbia Suicide Severity Rating Scale; C-SSRS-SR = Columbia Suicide Severity Rating Scale-Self-Report; DSI-SS = Depressive Symptom Inventory – Suicidality Subscale; ESS = Epworth Sleepiness Scale; GMS = Geriatric Mental State diagnostic schedule; HDRS = Hamilton Depression Rating Scale (insomnia items); ISI = Insomnia Severity Index; K-SADS-PL = Kiddie-Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version; KSI = Korea Advanced Institute of Science and Technology Scale for Suicidal Ideation; MADRS = Montgomery-Åsberg Depression Rating Scale; MSSI = Modified Scale for Suicidal Ideation; PHQ-9 = Patient Health Questionnaire-9 (sleep item); PSQI = Pittsburgh Sleep Quality Index; QIDS-A-SR-17 = Quick Inventory of Depressive Symptomatology-Adolescent Version Self-Report (insomnia items); SADS = Schedule for Affective Disorders and Schizophrenia; SASII = Suicide Attempt Self-Injury Interview; SITBI-SR = Self-Injurious Thoughts and Behaviors Interview – Self-Report version; SQI = Sleep Quality Index; SSM = study-specific measure; USI = Uppsala Sleep Inventory; WHI-IRS = Women’s Health Initiative Insomnia Rating Scale; YSR = Youth Self-Report
EMA = ecological momentary assessment; I = interview; N/A = not applicable; Q = questionnaire; SI = suicidal ideation; SA = suicide attempts
Studies with identical superscripts were drawn from same or overlapping samples but presented unique data included in this review.
The sample size, mean age, and percentage female for participants included in relevant analyses, rather than of the entire study sample, are presented and were incorporated in moderator analyses whenever available. For ease of presentation, whenever the sample size varied across multiple relevant analyses within a study, the largest cumulative sample size across these analyses is presented here, and the sample size used in each analysis was retained in the relevant meta-analysis for purposes of obtaining weighted effect sizes.
For studies that had variable follow-up lengths between participants (e.g., studies that recruited over multiple years but used the same end-date for all participants), the longest possible duration was presented in this table. Whenever data from multi-wave analyses were presented, the longest follow-up between consecutive waves, rather than across all waves combined, was presented. In cases where data across all waves were collapsed for analysis, the time interval covered by the relevant waves combined is presented. Whenever data across all waves were not combined for analysis, the shortest time interval was selected for analysis and presented here.
The first sample in this study was not eligible for inclusion because it did not assess the association between sleep disturbance and prospective suicidal ideation or behavior.
Overall sleep disturbance
A total of 26 unique effects were obtained for the association between sleep disturbance and prospective suicidal ideation, 13 for future suicide attempts, and 14 for associations with suicide deaths (see Table 2). The pooled effect size for sleep disturbances in relation to suicidal ideation was small-to-medium (d = .41, 95% CI = .30 – .51), with follow-ups ranging from three days to seven years (median follow-up length = 10.5 months). In the case of sleep disturbance prospectively predicting suicide attempts, the pooled effect size was small-to-medium (d = .39, 95% CI = .16 – .63), and follow-ups ranged from three days to 36 years (median follow-up length = two years). As for the relation between sleep disturbance and suicide deaths, a medium pooled effect size was obtained (d = .50, 95% CI = .14 – .85), follow-ups ranging from one to 36 years (median follow-up length = 10 years).
Table 2.
Sleep disturbance and insomnia as prospective predictors of suicidal ideation and behaviors.
| Effect Size Analyses |
Heterogeneity Analyses |
Publication Bias Analyses |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| k | N | d | 95% CI | p | I2 | p | Egger’s regression test p | Trim-and-fill |
||
| d | 95% CI | |||||||||
| Overall Sleep Disturbance | ||||||||||
| Suicidal Ideation | 26 | 30,862 | .41 | .30 – .51 | <.001 | 84.75% | <.001 | <.001 | .35 | .25 – .45 |
| Suicide Attempts | 13 | 33,288 | .39 | .16 – .63 | <.001 | 94.64% | <.001 | .04 | .39 | .16 – .63 |
| Suicide Deaths | 14 | 664,141 | .50 | .14 – .85 | <.01 | 97.44% | <.001 | .07 | .58 | .31 – .86 |
| Insomnia | ||||||||||
| Suicidal Ideation | 22 | 25,784 | .45 | .32 – .58 | <.001 | 86.96% | <.001 | <.01 | .37 | .25 – .49 |
| Suicide Attempts | 13 | 33,286 | .38 | .15 – .61 | <.01 | 94.61% | <.001 | <.05 | .44 | .15 – .73 |
| Suicide Deaths | 8 | 539,301 | .30 | .20 – .41 | <.001 | 32.83% | .17 | .28 | .30 | .20 – .41 |
| Hypersomnia | ||||||||||
| Suicidal Ideation | 3 | 4,917 | .09 | −.07 – .25 | .28 | 56.83% | .10 | — | — | — |
| Suicide Attempts | 2 | 2,373 | .31 | −.56 – 1.17 | .49 | 87.17% | <.01 | — | — | — |
| Suicide Deaths | 1 | 247 | .84 | .25 – 1.42 | <.01 | — | — | — | — | — |
| Nightmares | ||||||||||
| Suicidal Ideation | 3 | 3,526 | .40 | .06 – .74 | .02 | 81.00% | <.01 | — | — | — |
| Suicide Attempts | 3 | 4,168 | .64 | .43 – .84 | <.001 | 0% | .96 | — | — | — |
| Suicide Deaths | 1 | 22,732 | .50 | .18 – .83 | <.01 | — | — | — | — | — |
Note: k = number of unique effects; CI = confidence interval
Effect size estimates where k < 3 should be considered unstable and interpreted with a degree of caution.
Significant heterogeneity was present for suicidal ideation, suicide attempt, and suicide death, and thus moderator analyses were indicated. Age as a categorical and continuous variable, respectively, percentage of female participants in each sample, sample type (clinical/at-risk versus community), sleep measure type (interview versus self-report), time-frame covered by the sleep measure (≤ one month versus > one month), suicide measure type (interview versus self-report), time-frame covered by the suicide measure (< one year versus ≥ one year), and length of follow-up assessment (< one year versus ≥ one year)2 were evaluated as candidate moderators (see Table 3). In the case of suicide deaths, it was not possible to conduct moderator analyses for age as a categorical variable (i.e., all but one sample was with adults), time-frame covered by the sleep measure (i.e., all studies either had time-frames of less than a month or provided no information on this design feature), suicide measure type (i.e., all studies used death records), time-frame covered by the suicide measure (all but one study had a time-frame of at least a year), and length of follow-up assessment (i.e., all studies featured follow-ups of at least one year).
Table 3.
Univariate and multivariate moderator analyses for overall sleep disturbance predicting suicide-related outcomes.
| Univariate Moderator Analyses |
Multivariate Meta-Regression Analyses |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| k | b | SE | d | 95% CI | p | b | SE | p | R2 | |
| Suicidal Ideation | .45g .67h |
|||||||||
| Age (Categorical) | 26 | .83 | ||||||||
| Age (Continuous) | 19 | <.01 | <.01 | .68 | ||||||
| Percentage Female | 22 | <.01 | <.01 | .49 | ||||||
| Sample Type | 25 | .08 | ||||||||
| Sleep Measure Type | 25 | <.01 | ||||||||
| Interviewa | 3 | .10 | −.11 – .32 | .36 | ||||||
| Questionnaire | 22 | .43 | .33 – .52 | <.001 | .20g .26h |
.14g .11h |
.15g .02h |
|||
| Sleep Measure Time Frame | 26 | .64 | ||||||||
| Suicide Measure Type | 24 | .35 | ||||||||
| Suicide Measure Time Frame | 26 | .13 | ||||||||
| Follow-Up Time Frame | 26 | <.01 | ||||||||
| < 1 Year | 13 | .54 | .39 – .68 | <.001 | .20g | .09g | .03g | |||
| ≥ 1 Yeara | 13 | .27 | .15 – .39 | <.001 | ||||||
| ≤ 1 Month | 6 | .61 | .49 – .73 | <.001 | .26h | .09h | <.01h | |||
| > 1 Montha | 20 | .34 | .23 – .44 | <.001 | ||||||
| Suicide Attempts | ||||||||||
| Age (Categorical) | 13 | .44 | ||||||||
| Age (Continuous) | 12 | .01 | .01 | .34 | ||||||
| Percentage Female | 12 | <.01 | <.01 | .36 | ||||||
| Sample Type | 12 | .81 | ||||||||
| Sleep Measure Type | 13 | .98 | ||||||||
| Sleep Measure Time Frame | 13 | .70 | ||||||||
| Suicide Measure Type | 8 | .02 | ||||||||
| Interview | 3 | .12 | −.08 – .32 | .25 | ||||||
| Questionnaire | 5 | .53 | .27 – .79 | <.001 | ||||||
| Suicide Measure Time Frame | 13 | .66 | ||||||||
| Follow-Up Time Frame | 13 | .66 | ||||||||
| Suicide Deaths | ||||||||||
| Age (Categorical)b | — | — | ||||||||
| Age (Continuous) | 7 | <.01 | <.01 | .86 | ||||||
| Percentage Female | 11 | <.01 | <.01 | .88 | ||||||
| Sample Type | .09 | |||||||||
| Sleep Measure Type | 13 | .01 | ||||||||
| Interview | 2 | .18 | .05 – .30 | <.01 | ||||||
| Questionnaire | 11 | .36 | .28 – .44 | <.001 | ||||||
| Sleep Measure Time Framec | — | — | ||||||||
| Suicide Measure Typed | — | — | ||||||||
| Suicide Measure Time Framee | — | — | ||||||||
| Follow-Up Time Framef | — | — | ||||||||
Note: k = number of unique effects; CI = confidence interval
In analyses of sample type, at-risk and clinical samples were combined and compared to community samples.
The category with the smallest effect size in univariate moderator analysis served as the reference group in the corresponding meta-regression analysis.
Moderator analysis was not conducted, as all but one study featured adult samples.
Moderator analysis was not conducted, as all studies had time frames of under one month or provided no information on the time frame used.
Moderator analysis was not conducted, as all data were drawn from death records.
Moderator analysis was not conducted, as all studies featured time frames of at least one year, with the exception of one study featuring a variable time frame.
Moderator analysis was not conducted, as only one study featured a follow-up of under one year.
Values reflect meta-regression analysis with follow-up time frames of under one year versus a year or over included as a moderator.
Values reflect meta-regression analysis with follow-up time frames of a month or briefer versus over a month included as a moderator.
In univariate moderator analyses, sleep measure type and length of study follow-up assessment moderated the association between sleep disturbance and prospective suicidal ideation. Specifically, a larger weighted effect was found for questionnaire measures of sleep disturbance (d = .43, 95% CI = .33 – .52) than for interview-based measures (d = .10, 95% CI = −.11 – .32), as well as for study follow-up assessments of under a year (d = .54, 95% CI = .39 – .68) than for follow-up assessments of at least a year (d = .27, 95% CI = .15 – .39). As only two studies evaluated sleep disturbance in relation to suicide attempts over a follow-up period of one month or briefer, and none in the case of sleep disturbance predicting suicide deaths, moderator analysis of was only conducted for this follow-up length in the case of suicidal ideation. A larger pooled effect was obtained for study follow-up assessments of a month or briefer (d = .61, 95% CI = −.49 – .73) than for follow-up lengths of over a month (d = .34, 95% CI = −.23 – .44). In a meta-regression analysis including sleep measure type and length of follow-up assessment, only the latter variable remained a significant moderator of the strength of the association between sleep disturbance and subsequent suicidal ideation (b = .20, p =. 03) when follow-up lengths of under a year versus a year or longer were considered. This multivariate model accounted for 45% of the variance in the effect sizes for sleep disturbance in relation to future suicidal ideation. When follow-up lengths of a month or briefer versus over a month were considered, both length of follow-assessment (b = .26, p < .01) and sleep measure type (b = .26, p = .02) remained significant moderators, with this multivariate model account for 67% of the variance in effect sizes for the association between sleep disturbance and suicidal ideation. In the case of sleep disturbance in relation to later suicide attempts, only suicide measure type emerged as a significant moderator, with a larger pooled effect observed for questionnaire measures (d = .53, 95% CI = .27 – .79) than for interview-based ones (d = .12, 95% CI = −.08 – .32). Finally, the strength of the association between sleep disturbance and suicide deaths varied as a function of the sleep measure used, with larger effects evident for questionnaire measures (d = .36, 95% CI = .28 – .44) than interview-based ones (d = .18, 95% CI = .05 – .30).
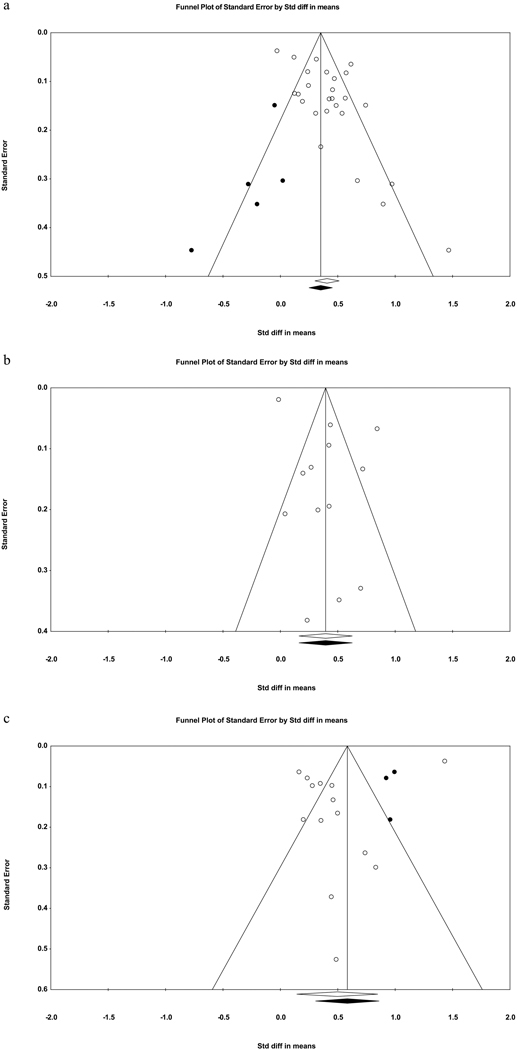
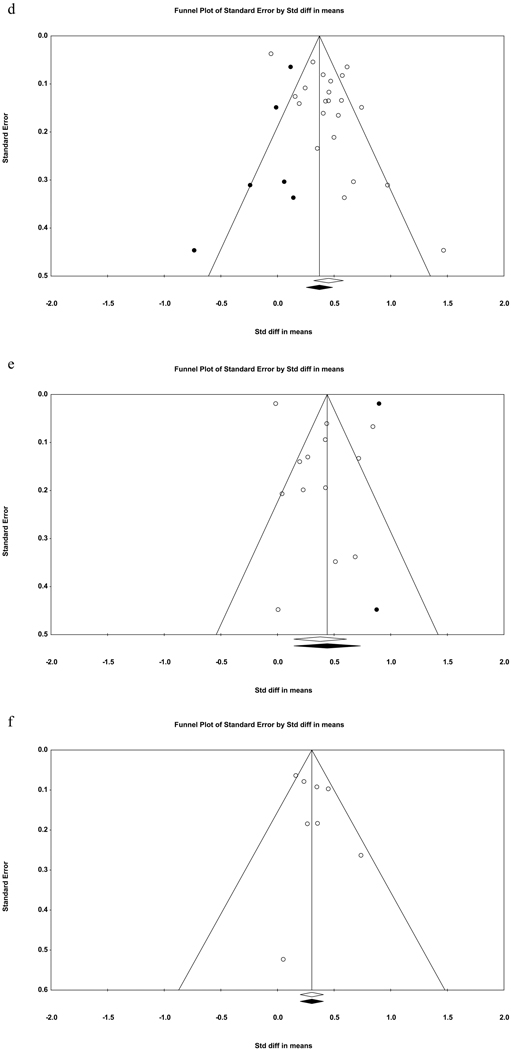
Evidence of publication bias was found for the association between sleep disturbance and future suicidal ideation (see Table 2), based on Egger’s regression test, Trim-and-fill analysis, and an asymmetrical funnel plot (Figure 2a). After adjusting for publication bias in the trim-and-fill analysis, the weighted effect remained small-to-medium (d = .35, 95% CI = .25 – .45). For the relation between sleep disturbance and prospective suicide attempts, only Egger’s regression test indicated the presence of publication bias. As for sleep disturbance in relation to prospective suicide deaths, Egger’s regression test yielded no evidence of publication bias whereas both the trim-and-fill analysis and the corresponding funnel plot (Figure 2c) suggested that the existing literature may underestimate the strength of this association (adjusted d = .58, 95% CI = .31 – .86).
Fig. 2.
Funnel plots for effect sizes in the meta-analyses. The vertical line indicates the weighted mean effect. Open circles indicate observed effects for actual studies, and closed circles indicate imputed effects for studies believed to be missing due to publication bias. The clear diamond reflects the unadjusted weighted mean effect size, whereas the black diamond reflects the weighted mean effect size after adjusting for publication bias.
2a. Overall sleep disturbance and suicidal ideation.
2b. Overall sleep disturbance and suicide attempts.
2c. Overall sleep disturbance and suicide deaths.
2d. General insomnia and suicidal ideation.
2e. General insomnia and suicide attempts.
2f. General insomnia and suicide deaths.
Insomnia
In total, 22 unique effects were obtained for the association between insomnia and subsequent suicidal ideation, 13 for later suicide attempts, and 8 for suicide deaths (see Table 2). A small-to-medium pooled effect was observed for insomnia in relation to suicidal ideation (d = .45, 95% CI = .32 – .58), with follow-ups ranging from three days to seven years (median follow-up length = 10.5 months). With regards to the association between insomnia and subsequent suicide attempts, a small-to-medium weighted effect was also found (d = .38, 95% CI = .15 – .61), with follow-ups ranging from three days to 36 years (median follow-up length = two years). As for insomnia as a predictor of suicide deaths, a small-to-medium pooled effect was found (d = .30, 95% CI = .20 – .41), with follow-ups ranging from one to 36 years (median follow-up length = 8.7 years).
Evidence of publication bias was found for insomnia in relation to prospective suicidal ideation and suicide attempts, respectively. In both cases, Egger’s regression test indicated the existence of significant publication bias. Trim-and-fill analyses and corresponding funnel plots (Figures 2d and 2e), however, indicated whereas correcting for publication bias would yield a smaller pooled effect for suicidal ideation (adjusted d = .37, 95% CI = .25 – .49), it would produce a larger pooled effect in the case of suicide attempts (adjusted d = .44, 95% CI = .15 – .73). No evidence of publication bias was found for insomnia as a prospective predictor of suicide deaths.
Hypersomnia and nightmares
There were relatively few studies of hypersomnia and nightmares, respectively, predicting later STBs, and thus the associated meta-analytic findings should be regarded as preliminary (see Table 2). Overall, evidence for an association between hypersomnia and STBs appeared modest, with non-significant pooled effects that were trivial for suicidal ideation (d = .09, 95% CI = −.07 – .25) and small-to-medium for suicide attempts (d = .31, 95% CI = −.56 – 1.17). There was only one effect for hypersomnia and suicide deaths (d = .84, 95% CI = .25 – 1.42). In the case of nightmares, the current evidence, although preliminary, was stronger for an association with STBs, with a small-to-medium weighted effect for prospective suicidal ideation (d = .40, 95% CI = .06 – .74) and medium weighted effect for prospective suicide attempts (d = .64, 95% CI = .43 – .84). Only one effect was available for the relation between nightmares and suicide deaths (d = .50, 95% CI = .18 – .83).
Discussion
The current study provided the first systematic review and meta-analysis of prospective associations between sleep disturbance and STBs. With the exception of a preliminary finding of a non-significant relation between hypersomnia and subsequent suicidal ideation, the results of analyses across several manifestations of sleep disturbance (i.e., overall disturbance, insomnia, hypersomnia, and nightmares) were consistent in yielding positive associations with all subsequent STBs (i.e., suicidal ideation, suicide attempts, and suicide deaths). For overall sleep disturbance and insomnia, both having a sizeable number of unique effects for quantitative analyses, the pooled effect sizes were all within the small-to-medium to medium range (ds = .30 to .50), which remained largely unchanged after adjusting for potential publication bias.
At first glance, these findings may appear to suggest that sleep disturbance and insomnia are generally significant but not necessarily potent longitudinal predictors of STBs. Important contextual information for interpreting the size of their weighted effects, however, is the length of follow-up employed by the individual studies. For overall sleep disturbance, median follow-ups were quite substantial, increasing in duration with increasing severity of STB, from 10.5 months for suicidal ideation to two years for suicide attempts to 10 years for suicide deaths. A nearly identical pattern was observed for insomnia, with median follow-ups increasing from 10.5 months for suicidal ideation to two years for suicide attempts to 8.7 years for suicide deaths. That sleep disturbance and insomnia have sufficient predictive power to produce pooled effect sizes of ds ≥ .39 and .30, respectively, for these outcomes over such lengthy stretches of time speaks to their value in understanding suicide risk. As additional context for interpreting these pooled effects, it may be noted that they are generally comparable in size to those found in meta-analyses of psychological and psychopharmacology therapies for a variety of mental health conditions, often over much briefer periods of time (e.g., Cuijpers et al., 2010, 2014; Weisz et al., 2017).
Moreover, one could make the case that the data are suggestive of the possibility that the relation between sleep disturbance and STBs may increase in strength with severity of outcome. That is, given that the absolute value of the weighted effects for overall sleep disturbance as a prospective predictor increases from suicidal ideation to suicide deaths, even as length of follow-up significantly increases, it is reasonable to suspect that the weighted effect sizes for suicide attempts and suicide deaths may increase substantially if evaluated on the same temporal scale as featured in studies of suicidal ideation. A similar pattern of increases in pooled effect sizes from suicidal ideation, to suicide attempts, to suicide deaths may also reasonably be hypothesized for insomnia as a risk factor if studies for these outcomes were restricted to the same length of follow-up. It was not possible, however, directly to test this possibility in the current meta-analysis, and it thus awaits evaluation in future research.
Supportive of an influence of length of follow-up on weighted effect sizes, studies of overall sleep disturbance prospectively predicting suicidal ideation over a period of under a year yielded significantly larger effects than did those with follow-ups of a year or more, and the moderator effect of follow-up length remained significant in a meta-regression analysis. This finding held when follow-ups of a month or briefer were compared to longer follow-ups, with a medium-to-large pooled effect observed for follow-ups of up to one month compared to a small-to-medium pooled effect in the case of longer follow-ups. Although follow-up length did not also significantly moderate the strength of the association between overall sleep disturbance and suicide attempts, only four of the included studies featured follow-ups of under a year, in contrast to 13 in the case of suicidal ideation. Furthermore, it may be that differences in the results of these moderator analyses are due to even shorter temporal intervals than a year being required to detect significantly larger effects. Six studies of overall sleep disturbance and suicidal ideation included follow-ups of one month or briefer, whereas only two studies with suicide attempts as the outcome featured follow-up intervals of comparable length. Of note, the study with the briefest follow-up period for suicide attempts (i.e., three days; Ribeiro, Huang, Fox, Walsh, & Linthicum, 2019) yielded the largest effect (d = .85) among studies with this outcome. Incidentally, none of the studies with suicide deaths included follow-ups briefer than a year. Future research evaluating sleep disturbance as a short-term risk factor (e.g., within a month) for suicidal behaviors is required to provide clarity to this issue.
This dearth of studies on sleep disturbance as a short-term risk factor for STBs is also a concern from a clinical standpoint; although the studies to date are of undoubted importance for establishing a longitudinal association between sleep irregularity and STBs, their generally long-term follow-ups limit their translational impact in clinical contexts, where the challenge for frontline clinicians is to determine short-term risk and to respond accordingly (e.g., determining severity of risk and appropriate handling of a suicidal patient, from increased monitoring to inpatient hospitalization and potential psychiatric residential placement). The want of short-term risk studies is all the more concerning when considered together with the fact that sleep disturbance, as mentioned above, is highlighted by several prominent suicide prevention organizations as a warning sign (i.e., precipitant) for suicidal behavior. This means that the evidence base for this conceptualization of sleep disturbance as a warning sign is relatively modest, being based essentially on cross-sectional or retrospective studies.
A question that naturally follows, given the perceived importance of sleep disturbance in triggering risk for STBs, is why such a marked paucity of studies exists of sleep and short-term risk for these outcomes. The reason for the want of research on short-term risk is that, although suicide is a leading cause of death (Centers for Disease Control and Prevention, 2019; World Health Organization, 2019), it is nevertheless a low-base-rate event; suicide attempts occur with relatively low frequency in the general population, and suicide deaths are considerably rarer still. This introduces the not insignificant methodological challenge in the study of suicide of achieving sufficient statistical power for meaningful analysis (American Psychiatric Association, 2004; Borges et al., 2006; Klonsky et al., 2016; Nock et al., 2008; Prinstein, 2008; Prinstein et al., 2008), an obstacle most often addressed by including longer follow-up periods so as to maximize the number of STBs available for analysis (Bredemeier & Miller, 2015; Franklin et al., 2017; Wenzel et al., 2011), thus the pattern found in the current review of median follow-up lengths increasing substantially with severity of STB.
This strategy is inherently impossible, however, in the case of studying short-term risk, necessitating the adoption of other methods in future studies to overcome the challenge of studying low-base-rate clinical phenomena over relatively brief periods of time. In particular, one approach for increasing the number of observations of events occurring at a low base rate is to include very large samples, although this strategy has feasibility challenges of its own (Klonsky et al., 2016; Prinstein, 2008). Another approach that may offer some promise is to recruit from high-risk or treatment-seeking populations, in which the base rate of prospective events would naturally be higher. This strategy has added clinical value in that individuals within such populations are much more representative of the ones clinicians encounter when conducting risk assessments and determining the appropriate course of action. This is an important consideration because even among traditionally high-risk populations, the majority of individuals do not engage in suicidal behavior (American Psychiatric Association, 2004; Lau et al., 2004; Liu et al., 2012; Nock et al., 2008; Sokero et al., 2006). It therefore remains a significant challenge for clinicians to assess suicide risk in high-risk individuals (Yen et al., 2013), and a better understanding of the determinants of short-term risk in such individuals is needed. Furthermore, risk factors identified in non-clinical or community samples may be of limited generalizability to the clinical or high-risk individuals more often encountered by mental health care providers (for examples, see King, Berona, Czyz, Horwitz, & Gipson, 2015; Yen et al., 2013; for a related discussion, see Millman, Gold, Mittal, & Schiffman, 2019).
We also found that interview-based measures of sleep disturbance produced smaller effects than did self-report measures of this construct. It is possible that this finding emerged given that the majority of the interview measures used were not specifically designed to measure sleep, and instead used items pulled from diagnostic interview depression modules (e.g., Hamilton Depression Rating Scale, the Schedule for Affective Disorders and Schizophrenia). Future research may consider employing both questionnaire and interview measures specifically designed to assess sleep when assessing the relationship between sleep and STBs to better understand the impact of method of assessment.
In addition to these clinical and methodological considerations, several critical conceptual issues remain to be addressed by future research in this area. First, a notable proportion of studies did not report data on the race of their samples, and even fewer on ethnicity, although there appears to be improvement in reporting practices in more recent years. These findings are generally consistent with those in a recent systematic review of reporting practices of sample characteristics in the suicide literature (Cha et al., 2018). Thorough reporting of sample sociodemographic characteristics is important for evaluating the generalizability of findings to traditionally underrepresented racial and ethnic groups, especially as risk for suicide has been found to be elevated or increasing in several of these populations (Sheftall et al., 2016).
Second, the studies included in the current review, with few exceptions (e.g., Littlewood et al., 2019), focused on evaluating whether sleep assessed at one time-point in isolation predicts STBs at a subsequent time-point. Meaningful contextual data in the measurement of sleep are therefore missing. Specifically, without the reference point of repeated assessments taken before or after, it is unclear to what degree such measures of sleep index irregularities that are chronic versus acute (i.e., deviations from the individual’s baseline). This is an important consideration because chronic or relatively stable processes and characteristics are likely more relevant to understanding long-term or general risk (Glenn et al., 2017), and their relatively low temporal variance means they lack the temporal sensitivity required to be robust predictors of short-term risk. Contrastingly, state-sensitive phenomena are more promising candidates for short-term risk (Glenn & Nock, 2014) and likely poor indicators of long-term risk. Clarifying which aspect of sleep disturbance, chronic or acute, is being assessed (e.g., through actigraphy and ecological momentary assessment, along with statistical techniques leveraging the resulting data) is important for accurately interpreting the strength of its prospective association with proximally and distally occurring STBs. It may be reasonably expected, for example, that chronic sleep irregularity may have a weaker association than acute sleep disturbance with proximal suicide attempts but a stronger relation with this outcome over the long term.
As a next step beyond establishing a temporal relation between sleep disturbance and STBs, there is a need for a conceptual model outlining the mechanistic pathways underlying this association, so as to serve as a roadmap for future research in this area and ultimately to inform efforts to intervene with individuals for whom disordered sleep may prove to be relevant to suicide risk. Implicit in mechanistic models connecting sleep disturbance to STBs is the view that this relation is a causal one. It is therefore noteworthy that in addition to demonstrating temporal precedence in the relation between sleep disturbance and STBs, a few studies have found evidence that this association is unidirectional; not only does sleep temporally predict STBs, at least in the case of suicidal ideation, but suicidal ideation, in turn, does not predict subsequent sleep behavior (Littlewood et al., 2019; Ribeiro et al., 2012). Even more implicative of a possible causal role for sleep disturbance in suicide risk, again in terms of suicidal ideation, there is some experimental evidence that ketamine may exert an anti-suicidal effect through its effect on normalizing sleep (Vande Voort et al., 2017).
As for precisely how sleep disturbance may increase suicide risk, this is currently unclear. One potential mechanistic pathway in the case of acute sleep disruption and short-term suicide risk, however, could involve inflammation and compromised executive functioning. That disrupted sleep may lead to activation of inflammatory processes (e.g., elevations in interleukin-6 [IL-6] and C-reactive protein [CRP]) has received substantial support from observational and experimental studies (Irwin et al., 2016; Irwin & Piber, 2018). Additionally, therapeutic interventions for insomnia (i.e., cognitive behavioral therapy for insomnia [CBT-I]) have been found to produce reductions in some markers of inflammation (Irwin & Piber, 2018). Several markers of inflammation (e.g., IL-6), in turn, have been implicated in suicide risk (Black & Miller, 2015). Importantly, these findings were not limited to peripheral biomarkers of inflammation. Indeed, peripheral cytokines may produce neuroinflammatory responses through several pathways and processes, including communicating through the vagus nerve and microglial activation (Debnath et al., 2011), and abnormal elevations in these pro-inflammatory cytokines have been identified in the cerebrospinal fluid and post-mortem brain samples of suicidal individuals (Black & Miller, 2015).
These aberrations in neuroinflammation are consistent with the possibility that the inflammatory response produced by sleep disruption may increase suicide risk through impairing executive control. More directly supportive of this hypothesis, heightened expression of several pro-inflammatory cytokines (e.g., IL-6) has been found in the prefrontal cortex of suicide decedents (Pandey et al., 2012; also see Lutz, Mechawar, & Turecki, 2017). Also relevant are findings that executive functions are the cognitive systems to be first, and most severely, impaired by sleep deprivation (Diamond, 2013). Finally, different facets of executive control have, in turn, been associated with suicide risk (Liu et al., 2017; Miranda et al., 2012).
Also promising as a focus for future study is whether sleep disturbance may serve as an observable mediating mechanism between other potential proximal risk factors and STBs. There is accumulating evidence that life stress, particularly interpersonal stressors, may play a prominent role in risk for STB (Liu & Miller, 2014), especially short-term risk (Bagge et al., 2013). Interestingly, theoretical support for interpersonal stress leading to sleep dysregulation relevant to suicide risk may be found in the social zeitgeber theory (Ehlers et al., 1988). This theory holds that psychosocial stress may increase risk for depression by disrupting an individual’s social routines, and thereby their biological circadian rhythms. Although it has been proposed that this theory may apply to suicide (Bernert & Joiner, 2007) and psychosocial stress does indeed impair sleep (Kim & Dimsdale, 2007), this possibility remains untested. Furthermore, sleep dysregulation, in turn, may also contribute to interpersonal stress. In line with this possibility, a study using a sleep-deprivation paradigm found it to lead adolescents to behave in ways characterized by negative affect, conflict, withdrawal, and dominance during a social conflict task (McMakin et al., 2016).
In summary, sleep disturbance, including insomnia, appears to be prospectively predictive of STBs, with small-to-medium to medium effect sizes for these associations. The vast majority of studies to date, however, have evaluated sleep disturbance as a long-term risk factor for STBs, leaving unclear the degree to which disrupted sleep predicts short-term risk, a question of considerable clinical relevance, given that clinical risk assessments are more focused on determining whether an individual will engage in suicidal behavior in the near future. Similarly awaiting future investigation is greater precision in the aspect of sleep disturbance measured in association with risk for STBs over several time scales. In particular, there is a need for studies employing multi-wave measures of sleep to disambiguate acute from chronic sleep disturbance in assessing short-term suicide risk. Finally, as a potential framework for future work in this area, a conceptual model is outlined, wherein sleep disturbance is involved in stress processes to confer suicide risk through neuroinflammation-induced impairments in executive control.
Acknowledgements
Preparation of this manuscript was supported in part by the National Institute of Mental Health of the National Institutes of Health under Award Numbers RF1MH120830, R01MH101138, R01MH115905, and R21MH112055. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agency.
Footnotes
An eighth study appears in both the earlier meta-analysis and the current one, but only cross-sectional data from the study were included in the prior review. In addition, although several other studies in this prior meta-analysis featured a longitudinal design, they nonetheless employed cross-sectional analyses of sleep in relation to STBs and thus were not eligible for inclusion in the present meta-analysis.
One-year follow-ups were chosen because there was an insufficient number of studies to conduct moderator analyses with briefer follow-up lengths in the case of suicide attempts and suicide deaths. Given the clinical and conceptual importance of understanding the role of sleep disturbance in short-term risk for suicide, however, we have conducted additional moderator analyses with shorter follow-up periods where feasible (i.e., in the case of suicidal ideation).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
* Articles marked with an asterisk were included in the meta-analysis.
- *Allan NP, Conner KR, Pigeon WR, Gros DF, Salami TK, & Stecker T. (2017). Insomnia and suicidal ideation and behaviors in former and current U.S. service members: Does depression mediate the relations? Psychiatry Research, 252, 296–302. 10.1016/j.psychres.2017.03.009 [DOI] [PubMed] [Google Scholar]
- American Association of Suicidology. (2019). Warning signs. https://suicidology.org/resources/warning-signs/
- American Foundation for Suicide Prevention. (2019). Risk factors and warning signs. https://afsp.org/about-suicide/risk-factors-and-warning-signs/
- American Psychiatric Association. (2004). American Psychiatric Association Practice Guideline for the Assessment and Treatment of Suicidal Behaviors. American Psychiatric Publishing. [Google Scholar]
- Bagge CL, Glenn CR, & Lee H-J (2013). Quantifying the impact of recent negative life events on suicide attempts. Journal of Abnormal Psychology, 122, 359–368. 10.1037/a0030371 [DOI] [PubMed] [Google Scholar]
- *Berglund M. (1986). Suicide in male alcoholics with peptic ulcers. Alcoholism: Clinical and Experimental Research, 10, 631–634. 10.1111/j.1530-0277.1986.tb05158.x [DOI] [PubMed] [Google Scholar]
- Bernert RA, & Joiner TE (2007). Sleep disturbances and suicide risk: A review of the literature. Neuropsychiatric Disease and Treatment, 3, 735–743. 10.1016/j.jsmc.2014.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernert RA, Kim JS, Iwata NG, & Perlis ML (2015). Sleep disturbances as an evidence-based suicide risk factor. Current Psychiatry Reports, 17, 1–9. 10.1007/s11920-015-0554-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Bernert RA, Turvey CL, Conwell Y, & Joiner TEJ (2014). Association of poor subjective sleep quality with risk for death by suicide during a 10-year period: A longitudinal, population-based study of late life. JAMA Psychiatry, 71, 1129–1137. 10.1001/jamapsychiatry.2014.1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biostat. (2014). Comprehensive Meta-Analysis Version 3. Biostat. [Google Scholar]
- *Bjørngaard JH, Bjerkeset O, Romundstad P, & Gunnell D. (2011). Sleeping problems and suicide in 75,000 Norwegian adults: A 20 year follow-up of the HUNT I Study. Sleep, 34, 1155–1159. 10.5665/sleep.1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black C, & Miller BJ (2015). Meta-analysis of cytokines and chemokines in suicidality: Distinguishing suicidal versus nonsuicidal patients. Biological Psychiatry, 78, 28–37. 10.1016/j.biopsych.2014.10.014 [DOI] [PubMed] [Google Scholar]
- Borges G, Angst J, Nock MK, Ruscio AM, Walters EE, & Kessler RC (2006). A risk index for 12-month suicide attempts in the National Comorbidity Survey Replication (NCS-R). Psychological Medicine, 36, 1747–1757. 10.1017/S0033291706008786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredemeier K, & Miller IW (2015). Executive function and suicidality: A systematic qualitative review. Clinical Psychology Review, 40, 170–183. 10.1016/j.cpr.2015.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Bryan CJ, Gonzales J, Rudd MD, Bryan AO, Clemans TA, Ray-Sannerud B, Wertenberger E, Leeson B, Heron EA, Morrow CE, & Etienne N. (2015). Depression mediates the relation of insomnia severity with suicide risk in three clinical samples of U.S. military personnel. Depression and Anxiety, 32, 647–655. 10.1002/da.22383 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2019). Web-based Injury Statistics Query and Reporting System (WISQARS). Centers for Disease Control and Prevention; http://www.cdc.gov/injury/wisqars/LeadingCauses.html [Google Scholar]
- Cha CB, Tezanos KM, Peros OM, Ng MY, Ribeiro JD, Nock MK, & Franklin JC (2018). Accounting for diversity in suicide research: Sampling and sample reporting practices in the United States. Suicide and Life-Threatening Behavior, 48, 131–139. 10.1111/sltb.12344 [DOI] [PubMed] [Google Scholar]
- Chiu HY, Lee HC, Chen PY, Lai YF, & Tu YK (2018). Associations between sleep duration and suicidality in adolescents: A systematic review and dose–response meta-analysis. Sleep Medicine Reviews, 42, 119–126. 10.1016/j.smrv.2018.07.003 [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Sijbrandij M, Koole SL, Andersson G, Beekman AT, & Reynolds CF (2014). Adding psychotherapy to antidepressant medication in depression and anxiety disorders: A meta-analysis. World Psychiatry, 13, 56–67. 10.1002/wps.20089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuijpers P, van Straten A, Schuurmans J, van Oppen P, Hollon SD, & Andersson G. (2010). Psychotherapy for chronic major depression and dysthymia: A meta-analysis. Clinical Psychology Review, 30, 51–62. 10.1016/j.cpr.2009.09.003 [DOI] [PubMed] [Google Scholar]
- Debnath M, Doyle KM, Langan C, McDonald C, Leonard B, & Cannon DM (2011). Recent advances in psychoneuroimmunology: Inflammation in psychiatric disorders. Translational Neuroscience, 2, 121–137. 10.2478/s13380-011-0019-0 [DOI] [Google Scholar]
- Deisenhammer EA, Ing C-M, Strauss R, Kemmler G, Hinterhuber H, & Weiss EM (2009). The duration of the suicidal process: How much time is left for intervention between consideration and accomplishment of a suicide attempt? Journal of Clinical Psychiatry, 70, 19–24. 10.4088/JCP.07m03904 [DOI] [PubMed] [Google Scholar]
- Diamond A. (2013). Executive functions. Annual Review of Psychology, 64, 135–168. 10.1146/annurev-psych-113011-143750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval S, & Tweedie R. (2000). Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics, 56, 455–463. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, & Minder C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ, 315, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Frank E, & Kupfer DJ (1988). Social zeitgebers and biological rhythms: A unified approach to understanding the etiology of depression. Archives of General Psychiatry, 45, 948–952. 10.1001/archpsyc.1988.01800340076012 [DOI] [PubMed] [Google Scholar]
- *Eikelenboom M, Beekman ATF, Penninx BWJH, & Smit JH (2019). A 6-year longitudinal study of predictors for suicide attempts in major depressive disorder. Psychological Medicine, 49, 911–921. 10.1017/S0033291718001423 [DOI] [PubMed] [Google Scholar]
- *Fawcett J, Scheftner WA, Fogg L, Clark DC, Young MA, Hedeker D, & Gibbons R. (1990). Time-related predictors of suicide in major affective disorder. American Journal of Psychiatry, 147, 1189–1194. 10.1176/ajp.147.9.1189 [DOI] [PubMed] [Google Scholar]
- Franklin JC, Ribeiro JD, Fox KR, Bentley KH, Kleiman EM, Huang X, Musacchio KM, Jaroszewski AC, Chang BP, & Nock MK (2017). Risk factors for suicidal thoughts and behaviors: A meta-analysis of 50 years of research. Psychological Bulletin, 143, 187–232. 10.1037/bul0000084 [DOI] [PubMed] [Google Scholar]
- *Fujino Y, Mizoue T, Tokui N, & Yoshimura T. (2005). Prospective cohort study of stress, life satisfaction, self-rated health, insomnia, and suicide death in Japan. Suicide and Life-Threatening Behavior, 35, 227–237. 10.1521/suli.35.2.227.62876 [DOI] [PubMed] [Google Scholar]
- Glenn CR, Cha CB, Kleiman EM, & Nock MK (2017). Understanding suicide risk within the Research Domain Criteria (RDoC) framework: Insights, challenges, and future research considerations. Clinical Psychological Science, 5, 568–592. 10.1177/2167702616686854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn CR, & Nock MK (2014). Improving the short-term prediction of suicidal behavior. American Journal of Preventive Medicine, 47, S176–180. 10.1016/j.amepre.2014.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Gunnell D, Chang S. Sen, Tsai MK, Tsao CK, & Wen CP (2013). Sleep and suicide: an analysis of a cohort of 394,000 Taiwanese adults. Social Psychiatry and Psychiatric Epidemiology, 48, 1457–1465. 10.1007/s00127-013-0675-1 [DOI] [PubMed] [Google Scholar]
- *Hartwig EM, Rufino KA, Palmer CA, Shepard C, Alfano CA, Schanzer B, Mathew SJ, & Patriquin MA (2019). Trajectories of self-reported sleep disturbance across inpatient psychiatric treatment predict clinical outcome in comorbid major depressive disorder and generalized anxiety disorder. Journal of Affective Disorders, 251, 248–255. 10.1016/j.jad.2019.03.069 [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, & Altman DG (2003). Measuring inconsistency in meta-analyses. BMJ, 327, 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Hogstedt C, Forsell Y, Hemmingsson T, Lundberg I, & Lundin A. (2018). Psychological symptoms in late adolescence and long-term risk of suicide and suicide attempt. Suicide and Life-Threatening Behavior, 48, 315–327. 10.1111/sltb.12362 [DOI] [PubMed] [Google Scholar]
- *Hom MA, Duffy ME, Rogers ML, Hanson JE, Gutierrez PM, & Joiner TE (2019). Examining the link between prior suicidality and subsequent suicidal ideation among high-risk US military service members. Psychological Medicine, 49, 2237–2246. 10.1017/S0033291718003124 [DOI] [PubMed] [Google Scholar]
- *Hom MA, Stanley IH, Chu C, Sanabria MM, Christensen K, Albury EA, Rogers ML, & Joiner TE (2019). A longitudinal study of psychological factors as mediators of the relationship between insomnia symptoms and suicidal ideation among young adults. Journal of Clinical Sleep Medicine, 15, 55–63. 10.5664/jcsm.7570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Hung GC-L, Kwok C-L, Yip PS, Gunnell D, & Chen Y-Y (2015). Predicting suicide in older adults - A community-based cohort study in Taipei City, Taiwan. Journal of Affective Disorders, 172, 165–170. 10.1016/j.jad.2014.09.037 [DOI] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, & Carroll JE (2016). Sleep disturbance, sleep duration, and inflammation: A systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biological Psychiatry, 80, 40–52. 10.1016/j.biopsych.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, & Piber D. (2018). Insomnia and inflammation: A two hit model of depression risk and prevention. World Psychiatry, 17, 359–361. 10.1002/wps.20556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Kang H-J, Stewart R, Jeong B-O, Kim S-Y, Bae K-Y, Kim S-W, Kim J-M, Shin I-S, & Yoon J-S (2014). Suicidal ideation in elderly Korean population: A two-year longitudinal study. International Psychogeriatrics, 26, 59–67. 10.1017/S1041610213001634 [DOI] [PubMed] [Google Scholar]
- Kearns JC, Coppersmith DDL, Santee AC, Insel C, Pigeon WR, & Glenn CR (2020). Sleep problems and suicide risk in youth: A systematic review, developmental framework, and implications for hospital treatment. General Hospital Psychiatry, 63, 141–151. 10.1016/j.genhosppsych.2018.09.011 [DOI] [PubMed] [Google Scholar]
- Kim EJ, & Dimsdale JE (2007). The effect of psychosocial stress on sleep: A review of polysomnographic evidence. Behavioral Sleep Medicine, 5, 256–278. 10.1080/15402000701557383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CA, Berona J, Czyz E, Horwitz AG, & Gipson PY (2015). Identifying adolescents at highly elevated risk for suicidal behavior in the emergency department. Journal of Child and Adolescent Psychopharmacology, 25, 100–108. 10.1089/cap.2014.0049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Kivelä L, Krause-Utz A, Mouthaan J, Schoorl M, de Kleine R, Elzinga B, Eikelenboom M, Penninx BWJH, van der Does W, & Antypa N. (2019). Longitudinal course of suicidal ideation and predictors of its persistence – A NESDA study. Journal of Affective Disorders, 257, 365–375. 10.1016/j.jad.2019.07.042 [DOI] [PubMed] [Google Scholar]
- Klonsky ED, May AM, & Saffer BY (2016). Suicide, suicide attempts, and suicidal ideation. Annual Review of Clinical Psychology, 12, 307–330. 10.1146/annurev-clinpsy-021815-093204 [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Kazdin AE, Offord DR, Kessler RC, Jensen PS, & Kupfer DJ (1997). Coming to terms with the terms of risk. Archives of General Psychiatry, 54, 337–343. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Morgan GA, Leech NL, Gliner JA, Vaske JJ, & Harmon RJ (2003). Measures of clinical significance. Journal of the American Academy of Child and Adolescent Psychiatry, 42, 1524–1529. 10.1097/00004583-200312000-00022 [DOI] [PubMed] [Google Scholar]
- *Lau JW, Stewart SM, King JD, Kennard BD, & Emslie GJ (2020). The association between baseline insomnia symptoms and future suicide attempts within an intensive outpatient treatment program for suicide. Psychiatry Research, 287, 112527. 10.1016/j.psychres.2019.112527 [DOI] [PubMed] [Google Scholar]
- Lau MA, Segal ZV, & Williams JMG (2004). Teasdale’s differential activation hypothesis: implications for mechanisms of depressive relapse and suicidal behaviour. Behaviour Research and Therapy, 42, 1001–1017. 10.1016/j.brat.2004.03.003 [DOI] [PubMed] [Google Scholar]
- *Li SX, Lam SP, Chan JWY, Yu MWM, & Wing Y-K (2012). Residual sleep disturbances in patients remitted from major depressive disorder: A 4-year naturalistic follow-up study. Sleep, 35, 1153–1161. 10.5665/sleep.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Li SX, Lam SP, Yu MWM, Zhang J, & Wing YK (2010). Nocturnal sleep disturbances as a predictor of suicide attempts among psychiatric outpatients. Journal of Clinical Psychiatry, 71, 1440–1446. 10.4088/jcp.09m05661gry [DOI] [PubMed] [Google Scholar]
- *Littlewood DL, Kyle SD, Carter L-AA, Peters S, Pratt D, & Gooding P. (2019). Short sleep duration and poor sleep quality predict next-day suicidal ideation: an ecological momentary assessment study. Psychological Medicine, 49, 403–411. 10.1017/S0033291718001009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JW, Tu YK, Lai YF, Lee HC, Tsai PS, Chen TJ, Huang HC, Chen YT, & Chiu HY (2019). Associations between sleep disturbances and suicidal ideation, plans, and attempts in adolescents: a systematic review and meta-analysis. Sleep, 42, zsz054. 10.1093/sleep/zsz054 [DOI] [PubMed] [Google Scholar]
- Liu RT, & Miller I. (2014). Life events and suicidal ideation and behavior: A systematic review. Clinical Psychology Review, 34, 181–192. 10.1016/j.cpr.2014.01.006 [DOI] [PubMed] [Google Scholar]
- Liu RT, Trout ZM, Hernandez EM, Cheek SM, & Gerlus N. (2017). A behavioral and cognitive neuroscience perspective on impulsivity, suicide, and non-suicidal self-injury: Meta-analysis and recommendations for future research. Neuroscience and Biobehavioral Reviews, 83, 440–450. 10.1016/J.NEUBIOREV.2017.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RT, Vassileva J, Gonzalez R, & Martin EM (2012). A comparison of delay discounting among substance users with and without suicide attempt history. Psychology of Addictive Behaviors, 26, 980–985. 10.1037/a0027384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Liu X, Liu Z-Z, Chen R-H, Cheng X-Z, Bo Z-G, Wang Z-Y, Yang Y, Fan F, & Jia C-X (2019). Nightmares are associated with future suicide attempt and non-suicidal self-injury in adolescents. Journal of Clinical Psychiatry, 80, 18m12181. 10.4088/JCP.18m12181 [DOI] [PubMed] [Google Scholar]
- Lutz P-E, Mechawar N, & Turecki G. (2017). Neuropathology of suicide: recent findings and future directions. Molecular Psychiatry, 22, 1395–1412. 10.1038/mp.2017.141 [DOI] [PubMed] [Google Scholar]
- Malik S, Kanwar A, Sim LA, Prokop LJ, Wang Z, Benkhadra K, & Murad MH (2014). The association between sleep disturbances and suicidal behaviors in patients with psychiatric diagnoses: A systematic review and meta-analysis. Systematic Reviews, 3, 1–9. 10.1186/2046-4053-3-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Mars B, Heron J, Klonsky ED, Moran P, O’Connor RC, Tilling K, Wilkinson P, & Gunnell D. (2019). Predictors of future suicide attempt among adolescents with suicidal thoughts or non-suicidal self-harm: a population-based birth cohort study. Lancet Psychiatry, 6, 327–337. 10.1016/S2215-0366(19)30030-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMakin DL, Dahl RE, Buysse DJ, Cousins JC, Forbes EE, Silk JS, Siegle GJ, & Franzen PL (2016). The impact of experimental sleep restriction on affective functioning in social and nonsocial contexts among adolescents. Journal of Child Psychology and Psychiatry, 57, 1027–1037. 10.1111/jcpp.12568 [DOI] [PubMed] [Google Scholar]
- Miller AB, & Prinstein MJ (2019). Adolescent Suicide as a Failure of Acute Stress-Response Systems. Annual Review of Clinical Psychology, 15, 425–450. 10.1146/annurev-clinpsy-050718-095625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millman ZB, Gold JM, Mittal VA, & Schiffman J. (2019). The critical need for help-seeking controls in clinical high-risk research. Clinical Psychological Science, 7, 1171–1189. 10.1177/2167702619855660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R, Gallagher M, Bauchner B, Vaysman R, & Marroquín B. (2012). Cognitive inflexibility as a prospective predictor of suicidal ideation among young adults with a suicide attempt history. Depression and Anxiety, 29, 180–186. 10.1002/da.20915 [DOI] [PubMed] [Google Scholar]
- *Mirsu-Paun A, Jaussent I, Komar G, Courtet P, & Lopez-Castroman J. (2017). Sleep complaints associated with wish to die after a suicide crisis—an exploratory study. Journal of Sleep Research, 26, 726–731. 10.1111/jsr.12537 [DOI] [PubMed] [Google Scholar]
- *Montgomery SA, Roy D, & Montgomery DB (1983). The prevention of recurrent suicidal acts. British Journal of Clinical Pharmacology, 15, 183S–188S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Na EJ, Lee H, Myung W, Fava M, Mischoulon D, Paik JW, Hong JP, Choi KW, Kim H, & Jeon HJ (2019). Risks of completed suicide of community individuals with ICD-10 disorders across age groups: A nationwide population-based nested case-control study in South Korea. Psychiatry Investigation, 16, 314–324. 10.30773/pi.2019.02.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Nadorff MR, Ellis TE, Allen JG, Winer ES, & Herrera S. (2014). Presence and persistence of sleep-related symptoms and suicidal ideation in psychiatric inpatients. Crisis, 35, 398–405. 10.1027/0227-5910/a000279 [DOI] [PubMed] [Google Scholar]
- National Institute of Mental Health. (2019). Suicide prevention. https://www.nimh.nih.gov/health/topics/suicide-prevention/index.shtml
- Nock MK, Borges G, Bromet EJ, Cha CB, Kessler RC, & Lee S. (2008). Suicide and suicidal behavior. Epidemiologic Reviews, 30, 133–154. 10.1093/epirev/mxn002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Nrugham L, Larsson B, & Sund AM (2008). Specific depressive symptoms and disorders as associates and predictors of suicidal acts across adolescence. Journal of Affective Disorders, 111, 83–93. 10.1016/j.jad.2008.02.010 [DOI] [PubMed] [Google Scholar]
- *Paffenbarger RS Jr, Lee I-M, & Leung R. (1994). Physical activity and personal characteristics associated with depression and suicide in American college men. Acta Psychiatrica Scandinavica, 377, 16–22. 10.1093/ehr/LXXXVIII.CCCXLIX.751 [DOI] [PubMed] [Google Scholar]
- Pandey GN, Rizavi HS, Ren X, Fareed J, Hoppensteadt DA, Roberts RC, Conley RR, & Dwivedi Y. (2012). Proinflammatory cytokines in the prefrontal cortex of teenage suicide victims. Journal of Psychiatric Research, 46, 57–63. 10.1016/j.jpsychires.2011.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigeon WR, Pinquart M, & Conner K. (2012). Meta-analysis of sleep disturbance and suicidal thoughts and behaviors. Journal of Clinical Psychiatry, 73, e1160–1167. 10.4088/JCP.11r07586 [DOI] [PubMed] [Google Scholar]
- Prinstein MJ (2008). Introduction to the special section on suicide and nonsuicidal self-injury: A review of unique challenges and important directions for self-injury science. Journal of Consulting and Clinical Psychology, 76, 1–8. 10.1037/0022-006X.76.1.1 [DOI] [PubMed] [Google Scholar]
- Prinstein MJ, Nock MK, Simon V, Aikins JW, Cheah CSL, & Spirito A. (2008). Longitudinal trajectories and predictors of adolescent suicidal ideation and attempts following inpatient hospitalization. Journal of Consulting and Clinical Psychology, 76, 92–103. 10.1037/0022-006X.76.1.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Ribeiro JD, Huang X, Fox KR, Walsh CG, & Linthicum KP (2019). Predicting imminent suicidal thoughts and nonfatal attempts: The role of complexity. Clinical Psychological Science, 7, 941–957. 10.1177/2167702619838464 [DOI] [Google Scholar]
- *Ribeiro JD, Pease JL, Gutierrez PM, Silva C, Bernert RA, Rudd MD, & Joiner TEJ (2012). Sleep problems outperform depression and hopelessness as cross-sectional and longitudinal predictors of suicidal ideation and behavior in young adults in the military. Journal of Affective Disorders, 136, 743–750. 10.1016/j.jad.2011.09.049 [DOI] [PubMed] [Google Scholar]
- Ribeiro JD, Yen S, Joiner T, & Siegler IC (2015). Capability for suicide interacts with states of heightened arousal to predict death by suicide beyond the effects of depression and hopelessness. Journal of Affective Disorders, 188, 53–59. 10.1016/j.jad.2015.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Roane BM, & Taylor DJ (2008). Adolescent insomnia as a risk factor for early adult depression and substance abuse. Sleep, 31, 1351–1356. 10.5665/sleep/31.10.1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers ML, Tucker RP, Law KC, Michaels MS, Anestis MD, & Joiner TE (2016). Manifestations of overarousal account for the association between cognitive anxiety sensitivity and suicidal ideation. Journal of Affective Disorders, 192, 116–124. 10.1016/j.jad.2015.12.014 [DOI] [PubMed] [Google Scholar]
- *Ross RK, Bernstein L, Trent L, Henderson BE, & Paganini-Hill A. (1990). A prospective study of risk factors for traumatic deaths in a retirement community. Preventive Medicine, 19, 323–334. 10.1016/0091-7435(90)90032-F [DOI] [PubMed] [Google Scholar]
- *Roy A. (1993). Anxiety and suicide in depression. Canadian Journal of Psychiatry, 38, 694–695. [DOI] [PubMed] [Google Scholar]
- Sheftall AH, Asti L, Horowitz LM, Felts A, Fontanella CA, Campo JV, & Bridge JA (2016). Suicide in elementary school-aged children and early adolescents. Pediatrics, 138, e20160436. 10.1542/peds.2016-0436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Shim G, & Jeong B. (2018). Predicting suicidal ideation in college students with mental health screening questionnaires. Psychiatry Investigation, 15, 1037–1045. 10.30773/pi.2018.08.21.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Sjöström N, Hetta J, & Waern M. (2009). Persistent nightmares are associated with repeat suicide attempt. A prospective study. Psychiatry Research, 170, 208–211. 10.1016/j.psychres.2008.09.006 [DOI] [PubMed] [Google Scholar]
- Sokero P, Eerola M, Rytsälä H, Melartin T, Leskelä U, Lestelä-Mielonen P, & Isometsä E. (2006). Decline in suicidal ideation among patients with MDD is preceded by decline in depression and hopelessness. Journal of Affective Disorders, 95, 95–102. 10.1016/j.jad.2006.04.028 [DOI] [PubMed] [Google Scholar]
- *Stange JP, Kleiman EM, Sylvia LG, da Silva Magalhães PV, Berk M, Nierenberg AA, & Deckersbach T. (2016). Specific mood symptoms confer risk for subsequent suicidal ideation in bipolar disorder with and without suicide attempt history: Multi-wave data from STEP-BD. Depression and Anxiety, 33, 464–472. 10.1002/da.22464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Strandheim A, Bjerkeset O, Gunnell D, Bjørnelv S, Holmen TL, & Bentzen N. (2014). Risk factors for suicidal thoughts in adolescence-a prospective cohort study: The Young-HUNT study. BMJ Open, 4, e005867. 10.1136/bmjopen-2014-005867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2019). Suicide prevention. https://www.samhsa.gov/find-help/suicide-prevention
- *Tanskanen A, Tuomilehto J, Vinamäki H, Vartiainen E, Lehtonen J, & Puska P. (2001). Nightmares as predictors of suicide. Sleep, 24, 845–848. [PubMed] [Google Scholar]
- Tucker RP, Michaels MS, Rogers ML, Wingate LRR, & Joiner TE (2016). Construct validity of a proposed new diagnostic entity: Acute Suicidal Affective Disturbance (ASAD). Journal of Affective Disorders, 189, 365–378. 10.1016/j.jad.2015.07.049 [DOI] [PubMed] [Google Scholar]
- Vande Voort JL, Ballard ED, Luckenbaugh DA, Bernert RA, Richards EM, Niciu MJ, Park LT, Machado-Vieira R, Duncan WC, & Zarate CA (2017). Antisuicidal response following ketamine infusion is associated with decreased nighttime wakefulness in major depressive disorder and bipolar disorder. Journal of Clinical Psychiatry, 78, 1068–1074. 10.4088/JCP.15m10440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Wang HE, Campbell-Sills L, Kessler RC, Sun X, Heeringa SG, Nock MK, Ursano RJ, Jain S, & Stein MB (2019). Pre-deployment insomnia is associated with post-deployment post-traumatic stress disorder and suicidal ideation in US Army soldiers. Sleep, 42, 1–9. 10.1093/sleep/zsy229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz JR, Kuppens S, Ng MY, Eckshtain D, Ugueto AM, Vaughn-Coaxum R, Jensen-Doss A, Hawley KM, Krumholz Marchette LS, Chu BC, Robin Weersing V, & Fordwood SR (2017). What five decades of research tells us about the effects of youth psychological therapy: A multilevel meta-analysis and implications for science and practice. American Psychologist, 72, 79–117. 10.1037/a0040360 [DOI] [PubMed] [Google Scholar]
- Wenzel A, Berchick ER, Tenhave T, Halberstadt S, Brown GK, & Beck AT (2011). Predictors of suicide relative to other deaths in patients with suicide attempts and suicide ideation: A 30-year prospective study. Journal of Affective Disorders, 132, 375–382. 10.1016/j.jad.2011.03.006 [DOI] [PubMed] [Google Scholar]
- Winsler A, Deutsch A, Vorona RD, Payne PA, & Szklo-Coxe M. (2014). Sleepless in Fairfax: The difference one more hour of sleep can make for teen hopelessness, suicidal ideation, and substance use. Journal of Youth and Adolescence, 44, 362–378. 10.1007/s10964-014-0170-3 [DOI] [PubMed] [Google Scholar]
- Wolfe-Clark AL, & Bryan CJ (2017). Integrating two theoretical models to understand and prevent military and veteran suicide. Armed Forces and Society, 43, 478–499. 10.1177/0095327X16646645 [DOI] [Google Scholar]
- *Wong MM, Brower KJ, & Zucker RA (2011). Sleep problems, suicidal ideation, and self-harm behaviors in adolescence. Journal of Psychiatric Research, 45, 505–511. 10.1016/j.jpsychires.2010.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2019). Suicide. https://www.who.int/en/news-room/fact-sheets/detail/suicide
- Yen S, Weinstock LM, Andover MS, Sheets ES, Selby EA, & Spirito A. (2013). Prospective predictors of adolescent suicidality: 6-month post-hospitalization follow-up. Psychological Medicine, 43, 983–993. 10.1017/S0033291712001912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Zuromski KL, Cero I, & Witte TK (2017). Insomnia symptoms drive changes in suicide ideation: A latent difference score model of community adults over a brief interval. Journal of Abnormal Psychology, 126, 739–749. 10.1037/abn0000282 [DOI] [PubMed] [Google Scholar]