Abstract
The inhibitory Smad7 acts as a critical suppressor of hepcidin, the major regulator of systemic iron homeostasis. In this study we define the mRNA expression of the two functionally related Smad proteins, Smad6 and Smad7, within pathways known to regulate hepcidin levels. Using mouse models for hereditary hemochromatosis (Hfe-, TfR2-, Hfe/TfR2-, Hjv- and hepcidin1-deficient mice) we show that hepcidin, Smad6 and Smad7 mRNA expression is coordinated in such a way that it correlates with the activity of the Bmp/Smad signaling pathway rather than with liver iron levels. This regulatory circuitry is disconnected by iron treatment of Hfe −/− and Hfe/TfR2 mice that significantly increases hepatic iron levels as well as hepcidin, Smad6 and Smad7 mRNA expression but fails to augment pSmad1/5/8 levels. This suggests that additional pathways contribute to the regulation of hepcidin, Smad6 and Smad7 under these conditions which do not require Hfe.
Keywords: Smad6, Smad7, Hepcidin, Hemochromatosis, Iron, Bmp/Smad
1. Introduction
Systemic iron homeostasis is disrupted in the common iron overload disorder Hereditary Hemochromatosis (HH) that is hallmarked by inappropriately low expression of hepcidin, a peptide hormone that controls plasma iron levels. Low hepcidin fails to inhibit iron export by ferroportin [1] and as a consequence, duodenal iron uptake and macrophage iron release is increased and excess iron accumulates especially in the liver. HH is caused by mutations in hepcidin itself [2] or hepcidin activators like Hfe (MHC class I-like protein) [3,4], transferrin receptor 2 (TfR2) [5–7], or the bone morphogenetic protein (Bmp) co-receptor hemojuvelin (Hjv) [8,9]. Recent findings highlight the critical importance of the iron-regulated Bmp/Smad signaling pathway in HH and respective disease models, in which the activity of the signaling pathway is attenuated [5,10–15].Thefinding that Bmp6 [16,17] and hepatocytic Smad4 knockout mice [18] are also hallmarked by low hepcidin expression and iron overload, further emphasized the important role for Bmp/Smad signaling for maintaining physiological hepcidin mRNA expression.
The Bmp/Smad signaling pathway is activated by binding of Bmp cytokines to cell surface type I and type II serine/threonine kinase receptors, which then induce phosphorylation of receptor-regulated Smads (Smad1, 5, and 8) [19,20]. Phosphorylated Smad1/5/8 proteins (pSmad1/5/8) form heteromeric complexes with Smad4 and translocate into the nucleus to regulate the transcription of target genes, such as hepcidin, Smad6 and Smad7, among others [19,20]. The inhibitory Smads, Smad6 and Smad7, block the Tgf-β/Bmp/Smad signaling pathways in a negative feed-back loop: Smad6 effectively inhibits Bmp/Smad signaling and only weakly affects the response to Tgf-β, contrasting the role of Smad7, which efficiently inhibits both signaling pathways [21].
Smad6 and Smad7 transcription is also stimulated by various non-Bmp/Tgf-β ligands, including epidermal growth factor [20], interferon-γ [22] or tumor necrosis factor-α [23]. More recently, Smad7 mRNA expression was shown to be regulated by dietary iron levels in mice [24,25]. Additional work revealed that Smad7 functions as a potent inhibitor of hepcidin mRNA expression by interfering with both Tgf-β and Bmp/Smad-mediated signaling pathways [26]. So far, a role for Smad6 in iron metabolism has not been addressed. In this study we define the expression of Smad6 and Smad7 within the pathways known to regulate hepcidin expression in murine models for HH.
2. Material and methods
2.1. Mice
2.1.1. Hfe −/− mice
Mice carrying an Hfe null allele in the C57BL/6J genetic background were described previously [27]. We housed Hfe −/− and Wild type (Wt) mice in the barrier facility of the EMBL, Heidelberg. Twelve Wt (C57BL/6J) and nineteen Hfe −/− female mice were fed standard chow (Harlan Teklad, containing 336 ppm iron) and were sacrificed for analysis between 8 and 12 weeks of age.
Systemic iron overload was induced in 5 Wt (C57BL/6J) and 5 Hfe −/− female mice by i.p. administration of 5 mg iron dextran solution (100 μl of a 50 mg/ml solution, containing 50,000 ppm iron, Sigma Aldrich, Schnelldorf) twice a week beginning from week 5 until week 7 of age (six doses in total) [28]. As a control, 4 Wt (C57BL/6J) and 4 Hfe −/− female mice received an injection of 0.5 mg of Dextran 5 solution (100 μl of 5mg/ml solution, Chemos Gm Regenstauf). Mice were sacrificed for analyses at 8 weeks of age. All mouse breeding and animal experiments were approved by and conducted in compliance with the guidelines of the Institutional Animal Care and User Committee of the EMBL.
2.1.2. Hfe −/−, Tfr2 −/− and compound Hfe/TfR2 mutant mice
Hfe −/−, Tfr2 −/− and compound Hfe/TfR2 mutant homozygous mice were raised on the FVB genetic background and maintained as previously described [13]. Briefly, Wt and mutant mice were fed standard chow (Purina 5001, containing 250 ppm iron) ad libitum after weaning. Dietary iron loading was achieved by weaning mice onto a diet containing an additional 25,000 ppm of carbonyl iron until the age of 5 weeks. Mice were sacrificed for analyses at 5 weeks of age. Each group comprised 3–5 male mice.
2.1.3. Wt and Hepcidin1 −/− mice
Six male Wt mice raised on C57BL/6N or Sv129 genetic background were fed standard chow (AO3, UAR; containing 200 ppm iron). Dietary iron loading of C57BL/6N (n=5) and Sv129 (n=4) was induced by adding 3% carbonyl iron (i.e. 30,000 ppm iron) as reduced pentacarbonyl iron to the diet for 3 weeks, as previously described [2]. Mice were sacrificed for analyses at 11 weeks of age.
Six Hepcidin1 −/− mutant males and six littermate control mice (Hepcidin1 +/+), raised on C57BL/6N [2], were fed standard chow (AO3, UAR; containing 200 ppm iron) and were sacrificed for the analysis at the age of 40 weeks.
2.1.4. Hjv −/− mice
Hjv −/− mice were kindly provided by Dr. Arber (Biozentrum, Department of Cell Biology, University of Basel, Switzerland) [8]. Mice were maintained on a mixed C57/Sv129 genetic background at the Animal Facility of the University of Heidelberg. Female mice (6 Hjv −/− mutant and 8 littermate Hjv +/+ control mice), age between 40 and 48 weeks were analyzed in this study.
2.2. Iron assays
Nonheme iron content of liver tissues was analyzed based on the principles of the method initially described by Torrence and Bothwell. The method was modified and scaled to a 96-well plate format as previously described [27].
2.3. RNA extraction, reverse transcription and real-time PCR
Total RNA was isolated from primary mouse hepatocytes, tissues and human hepatoma cells using Tri-Reagent (MRC Inc.). Two micrograms of total RNA was reverse transcribed using the Revertaid H Minus M-MuLV Reverse Transcriptase (Fermentas) following the manufacturer’s instructions. Quantitative real-time PCR was carried out in 20 μl reaction volumes using SYBR Green I dye on ABI Prism 7500 (Applied Biosystems, Applera Deutschland GmbH). The mRNA abundance of the investigated genes was calculated relative to the expression of the reference gene Gapdh and β-actin. Data were analyzed using a mathematical model based on the correction for exact PCR efficiencies. Relative mRNA expression normalized to the reference genes, Gapdh or β-actin, was compared across the mouse strains analyzed using the delta Ct method and the REST software [29]. Primers used in the study are listed:
mmHepcidin1 (5′-ATACCAATGCAGAAGAGAAGG-3′ and 5′-AACAG ATACCACACTGGGAA-3′)
mmGapdh (5′-GTGGAGATTGTTGCCATCAACGA-3′ and 5′-CCCATTC TCGGCCTTGACTGT-3′)
mmβ-actin (5′-GCTTCTTTGCAGCTCCTTCGT-3′ and 5′-ACCAGCGCA GCGATATCG-3′)
mmBmp6 (5′-ATGGCAGGACTGGATCATTGC-3′ and 5′-CCATCACAG TAGTTGGCAGCG-3′)
mmSmad6 (5′-GTTGCAACCCCTACCACTTC-3′ and 5′-GGAGGAGAC AGCCGAGAATA-3′)
mmSmad7 (5′-GCAGGCTGTCCAGATGCTGT-3′ and 5′-GATCCCCAG GCTCCAGAAGA-3′)
mmId1 (5′-ACCCTGAACGGCGAGATCA-3′ and 5′-TCGTCGGCTGGA ACACATG-3′).
2.4. Western blot analysis
Flash-frozen liver tissues were disrupted manually in RIPA lysis buffer (50 mM Tris–Cl pH 8.0/150 mM NaCl/1% NP-40/0.5% DOC/ 0.1% SDS) complemented with protease (Complete Mini (25) ROC 11836153001, Roche Diagnostics, Mannheim, Germany) and phosphatase (1 mM Na3O4Va/25 mM NaF/1 mM PMSF, Sigma Aldrich, Germany) inhibitors. Lysates were incubated on ice for 30 min and debris was removed by repeated centrifugation at 10,000 g (4 °C). Protein concentration of the supernatant was determined using the Pierce 660 nm Protein Assay Kit (Thermo Scientific Rockford USA) based on the method of Bradford. Protein extracts were diluted in 6× Laemmli buffer (0.35 Tris–HCl pH 6.8/1.0% SDS/0.6 M DTT/34% glycerol/bromphenol blue), incubated for 5 min (95 °C) and subjected to 10–12% SDS-polyacrylamide gel electrophoreses. Proteins were transferred to Westran S PVDF blotting membranes (Whatman Inc., UK). Membranes were blocked with 5% non-fat milk (Carl Roth T145.1) in TBST buffer (20 mM Tris–Cl pH 7.5/137.5 mM NaCl/0.1% Tween20, Sigma Aldrich, Germany) for 90 min at room temperature and hybridized with rabbit polyclonal antibodies to phosphorylated-Smad1/5/8, total Smad1, Smad4 and total Stat3 (all at 1:500; Cell Signaling Technology, Danvers, MA), and with mouse monoclonal antibody to phosphorylated-Stat3 (1:500; Cell Signaling Technology, Danvers, MA) and β-actin (clone AC-15; 1:10,000; 15 min RT, Sigma Aldrich, Germany) at 4 °C overnight and washed with TBST buffer. Following incubation with an anti-rabbit (1:5000, Sigma Aldrich, Germany) or anti-mouse IgG antibody (1:10,000, Sigma Aldrich, Germany) conjugated to horseradish peroxidase, enzyme activity was visualized by treating the membranes with the ECL kit (Perkin Elmer, Waltham USA), according to the manufacturer’s instructions and exposing to film (Amersham Hyperfilm ECL, Buckinghamshire, UK).
For Western-blot analysis of proteins prepared from primary hepatocytes, we used 30 °g of protein extracts which were separated on 4–12% gradient gels (Invitrogen, Germany) and transferred to nitrocellulose membranes by tank-blot procedure. For detection of phosphorylated Smad1/3 and Smad2 rabbit polyclonal antibodies were used (1:500 each, Cell Signaling Technology, Danvers, MA).
Quantitation was performed by densitometry analysis of the immunoblot staining intensity (ImageJ; www://rsb.info.nih.gov/ij/).
2.5. Smad6 overexpression in primary murine hepatocytes
Murine primary hepatocytes were isolated from C57BL/6 mice according to an optimized protocol described previously [30].Primary hepatocytes were transduced with adenoviral vector constructs generously provided by Prof. Heldin and Dr. Moustakas (Uppsala University) expressing the full-length murine Smad6 gene under the control of the CMV promoter (AdSmad6) and the β-galactosidase gene as a control (AdLacZ) in doses ranging between 5 × 106 and 6 × 107 infectious particles/ml. Forty-eight hours later the primary cultures were treated with TGFβ (5 ng/ml; for 6 h; Peprotech) and then harvested for the isolation of total RNA.
2.6. Statistical analysis
Data are shown as mean values±standard deviation (SD). Statistical analysis was performed using Student t-test (two-tailed; unequal variance). Statistically significant changes are marked by * (p valuesb.05), ** (p valuesb.005), *** (p valuesb.0005), and ns (no significant difference).
3. Results
3.1. Iron overload in wild-type mice triggers the coordinated increase of hepcidin, Smad6 and Smad7 mRNA expression
Recently, a role for Smad7 as an iron-responsive gene and a negative regulator of hepcidin expression has been reported [24–26].By contrast, a role for Smad6 in this process has not been investigated so far.
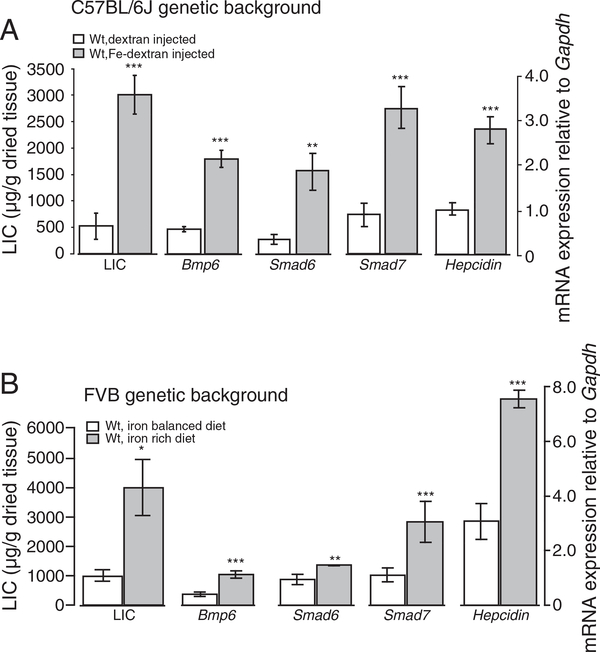
We show that systemic iron injections in C57BL/6J Wt mice and dietary iron supplementation into Wt mice raised on the FVB genetic background associate with increased liver iron deposition (LIC) and increased Bmp6, Smad6, Smad7 and hepcidin mRNA expression (Fig. 1A, Suppl. Fig. 2A; Suppl. Fig. 1A). Likewise, Wt mice raised on the C57BL/6N and Sv129 genetic background and maintained on iron-rich diet show a marked increase in LIC (7.3-fold, p<0.000637 and 10.2-fold, p<0.000689, respectively) [24,25,31] and increased mRNA expression of Bmp6, Smad6, Smad7 and hepcidin (Suppl. Fig. 1B,C). We conclude that Smad6 and Smad7 are co-regulated with hepcidin and Bmp6 in response to iron overload in Wt mice.
Fig. 1.
Iron supplementation increases Bmp6, Smad6, Smad7 and hepcidin mRNA expression in Wt mice maintained on different genetic backgrounds. Wt mice raised on C57BL/6J genetic background were injected with iron-dextran (n=5) or dextran as control (n=4) (A). In addition, Wt mice maintained on FVB genetic background were fed an iron-rich (4 mice/group) or iron-balanced diet (6 mice/group) (B). Hepatic non-heme iron concentrations (LIC) were measured and expressed as μg of iron per gram of dried liver tissue. Bmp6, Smad6, Smad7 and hepcidin mRNA was quantified by real-time PCR and normalized to the expression of Gapdh. Results are shown as the mean ± S.D.
3.2. Loss of Hfe, TfR2, Hfe and Tfr2 or Hjv impairs Smad6, Smad7 and hepcidin mRNA response to iron
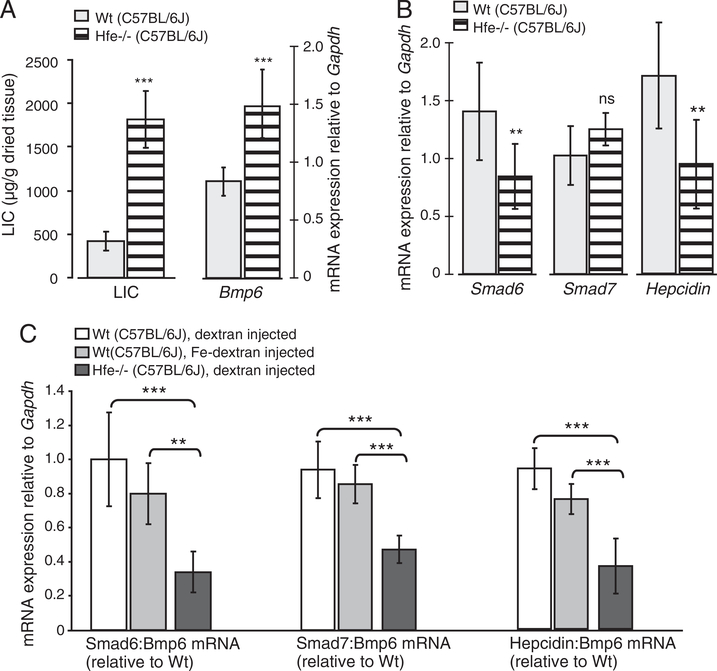
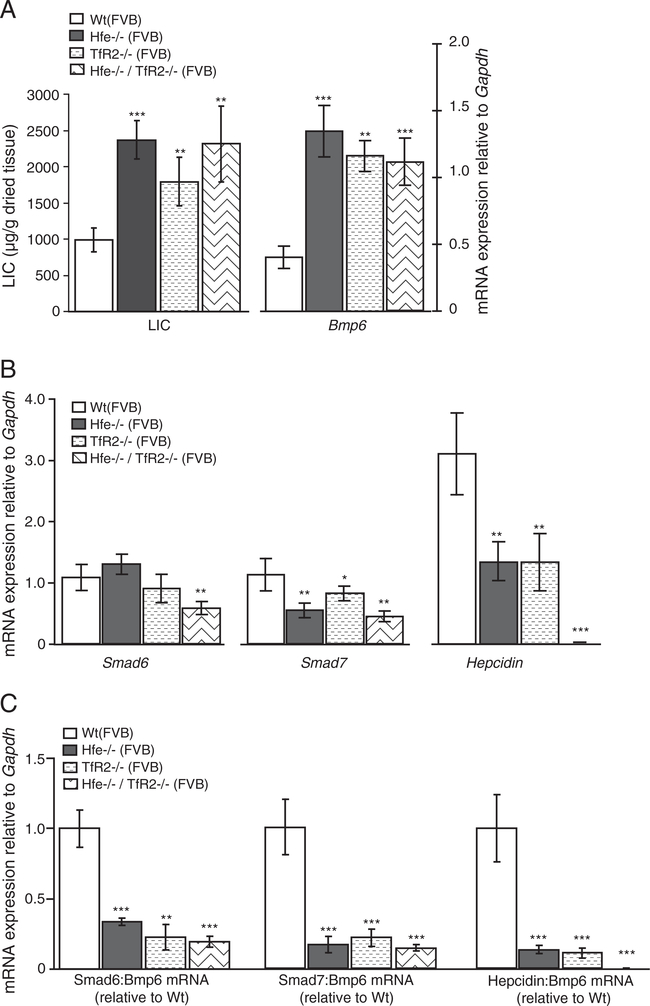
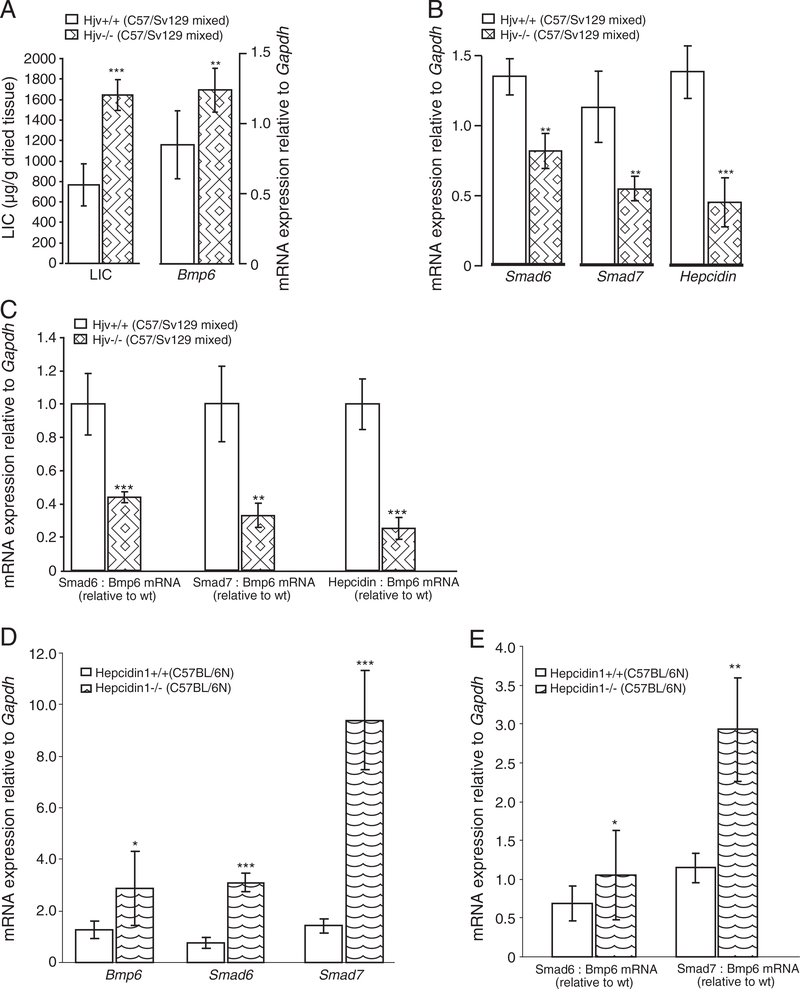
Mice with a functional loss of the HH proteins Hfe, TfR2, Hfe and TfR2, or Hjv are hallmarked by severe iron overload that associates with increased hepatic Bmp6 and inappropriately low hepcidin mRNA levels (Figs. 2A,B, 3A,B, 4A,B; Suppl. Figs. 2C, 3A,C), being consistent with previous reports [8,10,11,13,14]. The observation that Smad6 and Smad7 are co-regulated with hepcidin and Bmp6 in response to iron overload in Wt mice prompted us to examine their relationship in each of murine HH models. While Bmp6 mRNA levels positively associate with high hepatic iron burden in HH mice, the mRNA expression of Smad6, Smad7 and hepcidin is inappropriately low in regard to liver iron levels. Thus, Smad6 and Smad7 mRNA expression are co-regulated with hepcidin mRNA levels in the mutant mice (Figs. 2B, 3B, 4B; Suppl. Figs. 2C, 3A,C). To substantiate this finding, we expressed Smad6, Smad7 and hepcidin mRNA expression relative to Bmp6 mRNA as a ratio in each of HH mouse model, since iron overload in these animals associates with increased hepatic Bmp6 mRNA levels. Whereas the ratios between Smad6:Bmp6, Smad7:Bmp6 and hepcidin:Bmp6 mRNA levels were similar between Wt mice challenged by systemic iron injections and dextran-injected control mice (Fig. 2C), we measured a significant decrease of Smad6, Smad7 and hepcidin mRNA expression in Hfe −/−, TfR2 −/−, Hfe/TfR2 double knockout mice and in Hjv −/− mice (Figs. 2C, 3C, 4C, respectively). Thus, the lack of Hfe, TfR2, Hfe/TfR2 or Hjv affects the relationship between Smad6, Smad7, hepcidin and Bmp6 expression. Jointly, our data suggest that Hfe, TfR2, Hfe/TfR2 and Hjv are not only required for the appropriate hepcidin mRNA expression but likewise control the mRNA levels of Smad6 and Smad7.
Fig. 2.
Deregulated Smad6, Smad7 and hepcidin mRNA expression in Hfe −/− mice. The liver iron content (LIC; μg of iron per gram of dried liver tissue) was measured in Hfe −/− (n=19) and Wt (n=12) mice maintained on C57BL/6J genetic background and fed standard chow until the age of 8–12 weeks (A). Steady-state mRNA expression of Bmp6, Smad6, Smad7 and hepcidin in Hfe −/− was quantified by real-time PCR and normalized to the expression of Gapdh. Results are shown as the mean ± S.D. (A, B). To provide the relationship between iron mediatedregulation of Smad6, Smad7, hepcidin and Bmp6, we used iron-dextran injected Wt mice (n=5) in comparison to dextran-injected Hfe −/− (n=4) and Wt (n=4) mice (C). The ratios between Smad6, Smad7,or hepcidin to Bmp6 mRNA expression were determined individually for each sample and expressed as the mean ± S.D. relative to Wt, dextran-injected mice.
Fig. 3.
Deregulated Smad6, Smad7 and hepcidin mRNA expression in Hfe −/−, TfR2 −/− and Hfe/TfR2 mutant mice. The liver iron content (LIC; μg of iron per gram of dried liver tissue) was analyzed in Wt (n=6) and each of the HH mouse models: Hfe −/− (n=5), TfR2 −/− (n=6), and Hfe/TfR2 (n=5) mice (A), all raised on FVB genetic background. Steady-state mRNA expression of Bmp6, Smad6, Smad7 and hepcidin was quantified by real-time PCR and normalized to the expression of Gapdh. Results are shown as the mean ± S.D. (A, B). The ratios between Smad6, Smad7 or hepcidin to Bmp6 mRNA expression were determined individually for each sample and expressed as the mean ± S.D. relative to Wt mice (C).
Fig. 4.
Deregulated Smad6, Smad7 and hepcidin mRNA expression in Hjv −/− but not in hepcidin1 −/− mutant mice. The liver iron content (LIC; μg of iron per gram of dried liver tissue) was analyzed in Hjv +/+ (n=8) and Hjv −/− (n=6) mice (A). Steady-state mRNA expression of Bmp6, Smad6, Smad7 and hepcidin in Hjv −/− (A, B), hepcidin1 −/− (n=6) and hepcidin1 +/+ control (n=6) mice (D) was quantified by real-time PCR and normalized to the expression of Gapdh. Results are shown as the mean ± S.D. (A, B, D). The ratios between Smad6, Smad7 or hepcidin to Bmp6 mRNA expression were determined individually for each sample and expressed as the mean ± S.D. relative to control mice (C, E).
Importantly, hepcidin1-deficient mice show high mRNA levels of Bmp6 [24], Smad6 and Smad7 (Fig. 4D; Suppl. Fig. 3D) that is consistent with the response to the severe hepatic iron overload [2] and increased Bmp/Smad signaling in these mice (Suppl. Fig. 4C). In line with the adequate Bmp6, Smad6 and Smad7 response to iron, the ratios of Smad6:Bmp6 and Smad7:Bmp6 ratio were significantly higher in hepcidin1-deficient mice in regard to control mice (Fig. 4E). Jointly, our data suggest that signaling pathway(s) affected by the lack of Hfe, TfR2, Hfe/TfR2 or Hjv proteins rather than hepatic iron levels per se are critical for the regulation of Smad6, Smad7 and hepcidin mRNA expression.
3.3. Attenuated Bmp/Smad signaling correlates with low Smad6, Smad7 and hepcidin mRNA expression in HH mouse models
Previous studies suggested thatiron and Bmp6 induced hepcidin expression via phosphorylation of Smad1/5/8 proteins [24,16]. Recent findings highlighted the critical importance of the Bmp/Smad signaling pathway for maintaining physiological hepcidin mRNA expression in HH mouse models and in HFE-HH patients [10–12]. We measured the levels of pSmad1/5/8 proteins in Wt mice upon systemic iron injections and observed the anticipated increase in response to iron loading (Suppl. Fig. 4A,B). By contrast, Hfe −/− mice with high hepatic iron overload did not show increased pSmad1/5/8 protein levels upon iron injections when compared to levels measured in control Hfe −/− mice (Suppl. Fig. 4A,B). This suggests that the attenuated hepatic pSmad1/5/8 levels in mice with deficiencies in Hfe [11,10,13], TfR2 and Hfe/TfR2 [5,13] or Hjv [15], are responsible for the inappropriate low hepcidin, Smad6 and Smad7 mRNA expression. Thus, hepcidin, Smad6 and Smad7 mRNA expression is directly coupled to the activity of the Bmp/Smad signaling pathway in conditions of genetic iron overload rather than to the hepatic iron levels per se. By contrast, the HH-associated proteins and the activity of the Bmp/Smad signaling pathway are dispensable for elevated Bmp6 mRNA expression proposing that the signaling pathways that regulate hepcidin, Smad6 and Smad7 mRNA expression differ from those regulating Bmp6.
3.4. Systemic iron injections activate Smad6, Smad7 and hepcidin expression despite the loss of Hfe
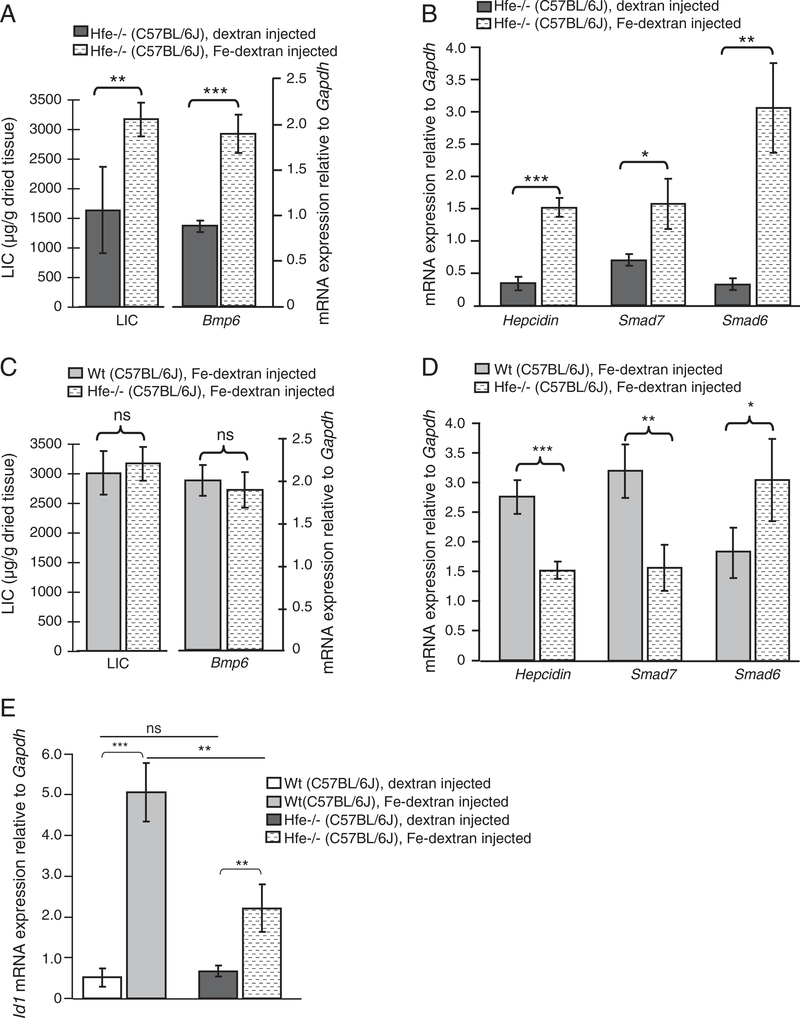
We next investigated whether systemic iron injections correlate with an increase in Smad6, Smad7 and hepcidin mRNA expression and the activity of Bmp/Smad signaling in the context of Hfe deficiency. Repeated iron injections in Wt and Hfe −/− mice increased the LIC and Bmp6 mRNA expression to similar levels (Fig. 5C; Suppl. Fig. 2A,B). However, a concomitant increase of pSmad1/5/8 was not observed in Hfe −/− mice (Suppl. Fig. 4A,B). In iron-injected Hfe −/− mice, hepcidin and Smad7 mRNA expression increased by 4.5-fold (p<0.0005) and 2.25-fold (p<0.0003), respectively, as compared to dextran-injected Hfe −/− mice (Fig. 5B; Suppl. Fig. 2B). Yet the mRNA levels of hepcidin and Smad7 are lower in iron-loaded Hfe −/− mice compared to the levels in iron-loaded Wt mice (Fig. 5D), suggesting that Hfe is partially required for the full hepcidin and Smad7 response during systemic iron injections. Smad6 mRNA levels increase by 6-fold (p<0.0012) in iron-loaded Hfe −/− mice relative to the levels in dextran-injected Hfe −/− controls (Fig. 5B; Suppl. Fig. 2B), and by 1.7-fold (p<0.002) relative to the levels in iron-treated Wt mice (Fig. 5D). We also measured a marked increase of Id1, another Bmp/Smad target gene, in response to iron injections in Wt and in Hfe −/− mice (Fig. 5E; Suppl. Fig. 2D) being in line with previously published data [13]. The increase in Smad6, Smad7, hepcidin mRNA levels is unlikely to be explained by increased hepatic Stat3 phosphorylation, which remained unaltered in iron-loaded Hfe −/− and Wt mice compared to the dextran-injected controls (Suppl. Fig. 4A). Thus, systemic iron injections in Hfe −/− mice correlate with the activation of Smad6, Smad7 and hepcidin despite the lack of concomitant Smad1/5/8 phosphorylation.
Fig. 5.
Systemic iron injections in Hfe −/− mice cause increased hepatic iron levels and induction of Bmp6, Smad6, Smad7 and hepcidin mRNA expression. Wt (n=5) and Hfe −/− (n=5) mice were subjected to repeated iron-dextran injections over a period of 3 weeks. In parallel 4 Wt and 4 Hfe −/− mice were injected with dextran solution only. Mice were sacrificed at the age of 8 weeks and analyzed for hepatic iron content (LIC; indicated as μg of iron per g of dried liver tissue) (A, C). The mRNA expression of Bmp6 (A, C), hepcidin, Smad7 and Smad6 mRNA (B, D) and Id1 (E) was quantified by real-time PCR and expressed relative to Gapdh mRNA expression as the mean ± S.D.
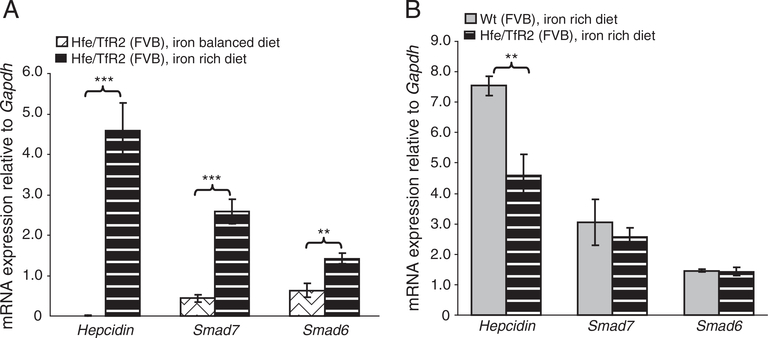
Similar results were obtained in Hfe −/− mice raised on FVB genetic background and maintained on iron-rich diet (data not shown). Whereas hepcidin, Smad6 and Smad7 mRNA expression was the lowest in Hfe/TfR2 double knockout mice in comparison to single mutant mice (see Fig. 3B) their mRNA expression was raised by several folds upon iron treatment (Fig. 6A; Suppl. Fig. 3B). Notably, the expression levels of Smad6 and Smad7 reached the levels present in Wt mice fed an iron-rich diet (Fig. 6B). This activation occurred even in the absence of activated Bmp/Smad and Jak/Stat3 signaling cascades [13].
Fig. 6.
Smad6, Smad7 and hepcidin mRNA expression in Hfe/TfR2 mutant mice upon dietary iron loading. Mice with combined loss of Hfe and TfR2 (Hfe/TfR2 mice) were maintained on iron balanced (n=5) or on a high-iron diet (n=3) containing 25,000 ppm iron as previously described [13]. Four Wt mice were maintained on the same iron-rich diet. All mice were raised on FVB genetic background. Liver Smad6, Smad7 and hepcidin mRNA expression were quantified by real time PCR and normalized to Gapdh mRNA. Data are expressed as the mean ± S.D. in Hfe/TfR2 mice fed an iron-rich diet in regard to (A) Hfe/TfR2 mice fed an iron-balanced diet or (B) Wt mice fed an iron-rich diet.
3.5. Overexpression of Smad6 suppresses hepcidin mRNA expression in primary hepatocytes
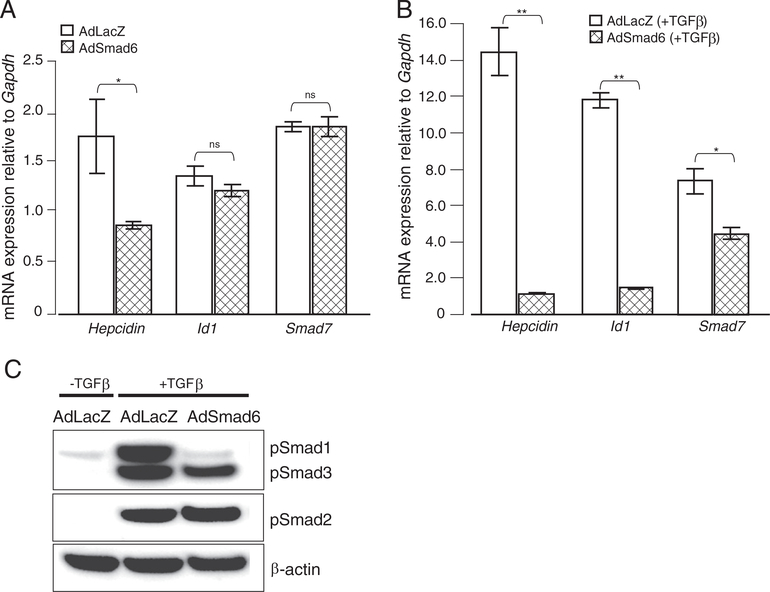
Both iSmads quickly respond to signals triggered by Tgf-β family members and play important roles in a negative feedback regulatory loop to attenuate Tgf-β/Bmp/Smad signaling. Additionally, iSmads are critically regulated by iron levels and the activity of the Bmp/Smad signaling pathway (Figs. 1–6). We therefore questioned whether Smad6, like previously shown for the Smad7 [32], can suppress hepcidin transcription in a dominant way. We overexpressed Smad6 in primary hepatocytes by adenovirus-mediated gene delivery (AdSmad6 hepatocytes). Compared to hepatocytes infected by a control virus (AdLacZ hepatocytes), hepcidin mRNA expression was suppressed upon Smad6 overexpression under steady-state conditions (Fig. 7A). Even more strikingly, Smad6 gene delivery completely eliminated hepcidin activation by Tgf-β treatment (Fig. 7B). Interestingly, mRNA responses of Smad7 and Id1 were not significantly altered in primary hepatocytes overexpressing Smad6 (AdSmad6 hepatocytes) compared to AdLacZ Wt primary hepatocytes (Fig. 7A). However, mRNA expression of both genes was suppressed in Tgf-β treated hepatocytes overexpressing Smad6 (Fig. 7B). The mRNA responses are consistent with attenuated Bmp/Smad signaling (i.e. decreased pSmad1 protein levels) as a consequence of Smad6 overexpression (Fig. 7C).
Fig. 7.
Smad6 overexpression suppresses hepcidin mRNA levels in primary hepatocytes. Primary hepatocytes from C57/BL6 mice were transduced with adenoviral vectors expressing the full length murine Smad6 gene under the control of the CMV promoter (AdSmad6) or with adenoviral vectors encoding β-galactosidase as a control (AdLacZ). Cells were left untreated or treated with TGFβ and harvested for RNA and protein isolation. (A, B) mRNA expression of hepcidin, Id1 and Smad7 was analyzed by quantitative real-time PCR and normalized to the expression of Gapdh. Results are shown as the mean ± S.D. (C) The levels of pSmad1, pSmad2 and pSmad3 and β-actin proteins were analyzed by Western blot analysis.
4. Discussion
Inappropriately low hepcidin levels and severe iron accumulation in the liver are two major hallmarks of HH [32]. Recent data revealed that physiological hepcidin mRNA expression is maintained by the activity of the Bmp/Smad signaling pathway, which is impaired in mice with mutations in HH-proteins such as Hfe, Hjv, Tfr2, Bmp6, Smad4 and in HFE-HH patients [8–13,16–18]. Using mouse models for HH and iron-loaded Wt mice, we now show that two feed-back regulators of Tgf-β/Bmp/Smad-signaling are responsive to iron-levels and are co-regulated with hepcidin via the Bmp/Smad signaling pathway.
Our data demonstrate that Smad6, Smad7 and hepcidin mRNA expression is increased together with liver iron levels when the Bmp/Smad signaling pathway is intact. Deficiency of the HH-associated membrane proteins that are involved in the surveillance of systemic iron levels attenuates Bmp/Smad signaling as well as Smad6, Smad7 and hepcidin mRNA expression. This suggests that each of the HH proteins (Hfe, TfR2, and Hjv) is critical to maintain the responses of Smad6, Smad7 and hepcidin to iron levels. Of note is that mice with a combined loss of Hfe and TfR2 proteins show the lowest hepatic Smad6, Smad7 and hepcidin levels compared to mice with the loss of either gene individually (Fig. 3). We suspect that Hfe and TfR2 either act as a complex and/or employ multiple signaling pathways that synergistically influence the expression of the responsive genes. Our observations in mice are partially reflected upon by a study of non-venesected Hfe-HH patients, in which a significantly higher Smad6 mRNA expression compared to systemic iron levels and the activity of the Bmp/Smad signaling pathway was observed [12].Other studies reported weaker or absent induction of Smad7 in Hfe-HH patients [33,12] suggesting that the difference in the magnitude of Smad6 and Smad7 response may be due to differences in a patients’ diet, demographic status, gender, medical status or genetically determined set point for iron stores.
Equally interesting is our finding that stimuli known to affect hepcidin expression also control Smad6 and Smad7 levels, yet in a manner not correlating with the activity of the Bmp/Smad signaling pathway. For example, systemic iron injections of Hfe −/− mice increase hepatic iron stores without a concomitant increase of pSmad1/5/8 (Suppl. Fig. 4). Under this condition, Smad6, Smad7 and hepcidin expression is significantly upregulated. Likewise, dietary iron loading of mice with a combined loss of Hfe and TfR2 resulted in a significant increase of Smad6, Smad7 and hepcidin expression (Fig. 6), bypassing the activation of a Bmp/Smad signaling cascade [13]. While currently we cannot fully exclude that unaltered pSmad1/5/8 levels under the conditions of systemic iron loading in Hfe or Hfe/TfR2 mice may be explained by subtle increases of pSmad1/5/8 that cannot be detected by Western blot analysis or by transient kinetics that differ from target gene expression, our data support the view shared by other investigators that other, yet to be discovered alternative signaling pathway(s) exist to communicate chronically high iron levels to hepcidin [13,34] and to Smad6, Smad7 and Id1. This action is independent of Hfe and Hfe/TfR2.
The striking co-regulatory expression pattern of Smad6 and Smad7 in mouse models of iron overload and the fi ndings that Smad7 overexpression suppresses hepcidin in vitro [26], led us to question whether a relationship between Smad6 and the regulation of hepcidin may exist. We show that overexpression of Smad6 in murine primary hepatocytes inhibits hepcidin expression (Fig. 7A). Even more strikingly, Smad6 gene delivery completely eliminates hepcidin activation by TGFβ, a potent inducer of liver fibrosis (Fig. 7B). The mechanism by which the two inhibitory Smads, Smad6 and Smad7 control Hepcidin mRNA expression differs. Smad7 overexpression in primary hepatocytes strongly decreases Smad1 and Smad2/3 phosphorylation, that links to Bmp and TGFβ ligands, respectively [26]. On the other hand, Smad6 affects hepcidin suppression by inhibiting the phosphorylation of Smad1 (and not Smad2/3) (Fig. 7C). Possibly, additional regulatory modes may be operational such as binding to cognate SMAD regulatory motif GTCAAGAC within the hepcidin promoter [26], or through SMAD4 degradation [35]. We propose that Smad6 and Smad7 may therefore constitute novel layers of hepcidin regulation.
5. Conclusions
In this study we define the mRNA expression of Smad6 and Smad7 within pathways known to regulate hepcidin levels. Dietary iron or systemic iron injections trigger co-ordinated increase in hepcidin, Smad6 and Smad7 mRNA expression, the response that is however abolished in mice with genetic iron overload due to mutation in Hfe, TfR2, Hfe/ TfR2, or Hjv. We thus conclude that Smad6, Smad7 and hepcidin mRNA expression correlates with the activity of the Bmp/Smad signaling pathway rather than with liver iron levels. Interestingly, this regulatory circuitry isuncoupledbyiron treatmentof Hfe −/− and Hfe/TfR2 mice, that results in a significantincrease of hepcidin, Smad6 and Smad7 mRNA expression being suggestive that additional regulatory mechanisms contribute to their regulation which do not exclusively require Hfe. When overexpressed in cells, the inhibitory Smad6 and Smad7 suppress hepcidin mRNA levels suggesting that conditions hallmarked by high levels of Smad6 and Smad7 may be linked to altered iron homeostasis via Smad6 or Smad7 mediated hepcidin suppression.
Supplementary Material
Acknowledgements
The authors would like to thank Matthias W. Hentze, the staff of the EMBL laboratory animal facility, and Jean-Christophe Deschemin for their expert support. This work was supported by grant support from the BMBF (HepatoSys and Virtual Liver—0315761; to MUM and SD), eRARE-01GM1005 (to MUM) and by the University of Heidelberg Award (to MVS).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.bbadis.2012.08.013.
References
- [1].Andrews NC, Iron homeostasis: insights from genetics and animal models, Nat. Rev. Genet. 1 (2000) 208–217. [DOI] [PubMed] [Google Scholar]
- [2].Lesbordes-Brion JC, Viatte L, Bennoun M, Lou DQ, Ramey G, Houbron C, Hamard G, Kahn A, Vaulont S, Targeted disruption of the hepcidin 1 gene results in severe hemochromatosis, Blood 108 (2006) 1402–1405. [DOI] [PubMed] [Google Scholar]
- [3].Feder JN, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy DA, Basava A, Dormishian F, Domingo R Jr., Ellis MC, Fullan A, Hinton LM, Jones NL, Kimmel BE, Kronmal GS, Lauer P, Lee VK, Loeb DB, Mapa FA, McClelland E, Meyer NC, Mintier GA, Moeller N, Moore T, Morikang E, Prass CE, Quintana L, Starnes SM, Schatzman RC, Brunke KJ, Drayna DT, Risch NJ, Bacon BR, Wolff RK, A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis, Nat. Genet. 13 (1996) 399–408. [DOI] [PubMed] [Google Scholar]
- [4].Bridle KR, Frazer DM, Wilkins SJ, Dixon JL, Purdie DM, Crawford DH, Subramaniam VN, Powell LW, Anderson GJ, Ramm GA, Disrupted hepcidin regulation in HFE-associated haemochromatosis and the liver as a regulator of body iron homoeostasis, Lancet 361 (2003) 669–673. [DOI] [PubMed] [Google Scholar]
- [5].Wallace DF, Summerville L, Crampton EM, Frazer DM, Anderson GJ, Subramaniam VN, Combined deletion of Hfe and transferrin receptor 2 in mice leads to marked dysregulation of hepcidin and iron overload, Hepatology 50 (2009) 1992–2000. [DOI] [PubMed] [Google Scholar]
- [6].Wallace DF, Summerville L, Subramaniam VN, Targeted disruption of the hepatic transferrin receptor 2 gene in mice leads to iron overload, Gastroenterology 132 (2007) 301–310. [DOI] [PubMed] [Google Scholar]
- [7].Kawabata H, Fleming RE, Gui D, Moon SY, Saitoh T, O’Kelly J, Umehara Y, Wano Y, Said JW, Koeffler HP, Expression of hepcidin is down-regulated in TfR2 mutant mice manifesting a phenotype of hereditary hemochromatosis, Blood 105 (2005) 376–381. [DOI] [PubMed] [Google Scholar]
- [8].Niederkofler V, Salie R, Arber S, Hemojuvelin is essential for dietary iron sensing, and its mutation leads to severe iron overload, J. Clin. Invest. 115 (2005) 2180–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Huang FW, Pinkus JL, Pinkus GS, Fleming MD, Andrews NC, A mouse model of juvenile hemochromatosis, J. Clin. Invest. 115 (2005) 2187–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kautz L, Meynard D, Besson-Fournier C, Darnaud V, Al Saati T, Coppin H, Roth MP, BMP/Smad signaling is not enhanced in Hfe-deficient mice despite increased Bmp6 expression, Blood 114 (2009) 2515–2520. [DOI] [PubMed] [Google Scholar]
- [11].Corradini E, Garuti C, Montosi G, Ventura P, Andriopoulos B Jr., Lin HY, Pietrangelo A, Babitt JL, Bone morphogenetic protein signaling is impaired in an HFE knockout mouse model of hemochromatosis, Gastroenterology 137 (2009) 1489–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ryan JD, Ryan E, Fabre A, Lawless MW, Crowe J, Defective bone morphogenic protein signaling underlies hepcidin deficiency in HFE hereditary hemochromatosis, Hepatology 52 (2010) 1266–1273. [DOI] [PubMed] [Google Scholar]
- [13].Corradini E, Rozier M, Meynard D, Odhiambo A, Lin HY, Feng Q, Migas MC, Britton RS, Babitt JL, Fleming RE, Iron regulation of hepcidin despite attenuated Smad1,5,8 signaling in mice without transferrin receptor 2 or Hfe, Gastroenterology 141 (5) (2011) 1907–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Babitt JL, Huang FW, Wrighting DM, Xia Y, Sidis Y, Samad TA, Campagna JA, Chung RT, Schneyer AL, Woolf CJ, Andrews NC, Lin HY, Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression, Nat. Genet. 38 (2006) 531–539. [DOI] [PubMed] [Google Scholar]
- [15].Zhang AS, Gao J, Koeberl DD, Enns CA, The role of hepatocyte hemojuvelin in the regulation of bone morphogenic protein-6 and hepcidin expression in vivo, J. Biol. Chem. 285 (2010) 16416–16423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Andriopoulos B Jr., Corradini E, Xia Y, Faasse SA, Chen S, Grgurevic L, Knutson MD, Pietrangelo A, Vukicevic S, Lin HY, Babitt JL, BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism, Nat. Genet. 41 (2009) 482–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Meynard D, Kautz L, Darnaud V, Canonne-Hergaux F, Coppin H, Roth MP, Lack of the bone morphogenetic protein BMP6 induces massive iron overload, Nat. Genet. 41 (2009) 478–481. [DOI] [PubMed] [Google Scholar]
- [18].Wang RH, Li C, Xu X, Zheng Y, Xiao C, Zerfas P, Cooperman S, Eckhaus M, Rouault T, Mishra L, Deng CX, A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression, Cell Metab 2 (2005) 399–409. [DOI] [PubMed] [Google Scholar]
- [19].Miyazono K, Maeda S, Imamura T, BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk, Cytokine Growth Factor Rev. 16 (2005) 251–263. [DOI] [PubMed] [Google Scholar]
- [20].Afrakhte M, Moren A, Jossan S, Itoh S, Sampath K, Westermark B, Heldin CH, Heldin NE, ten Dijke P, Induction of inhibitory Smad6 and Smad7 mRNA by TGF-beta family members, Biochem. Biophys. Res. Commun. 249 (1998) 505–511. [DOI] [PubMed] [Google Scholar]
- [21].Itoh S, ten Dijke P, Negative regulation of TGF-beta receptor/Smad signal transduction, Curr. Opin. Cell Biol. 19 (2007) 176–184. [DOI] [PubMed] [Google Scholar]
- [22].Ulloa L, Doody J, Massague J, Inhibition of transforming growth factor-beta/SMAD signalling by the interferon-gamma/STAT pathway, Nature 397 (1999) 710–713. [DOI] [PubMed] [Google Scholar]
- [23].Bitzer M, von Gersdorff G, Liang D, Dominguez-Rosales A, Beg AA, Rojkind M, Bottinger EP, A mechanism of suppression of TGF-beta/SMAD signaling by NF-kappa B/RelA, Genes Dev. 14 (2000) 187–197. [PMC free article] [PubMed] [Google Scholar]
- [24].Kautz L, Meynard D, Monnier A, Darnaud V, Bouvet R, Wang RH, Deng C, Vaulont S, Mosser J, Coppin H, Roth MP, Iron regulates phosphorylation of Smad1/5/8 and gene expression of Bmp6, Smad7, Id1, and Atoh8 in the mouse liver, Blood 112 (2008) 1503–1509. [DOI] [PubMed] [Google Scholar]
- [25].Corradini E, Meynard D, Wu Q, Chen S, Ventura P, Pietrangelo A, Babitt JL, Serum and liver iron differently regulate the bone morphogenetic protein 6 (BMP6)—SMAD signaling pathway in mice, Hepatology 54 (2011) 273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mleczko-Sanecka K, Casanovas G, Ragab A, Breitkopf K, Muller A, Boutros M, Dooley S, Hentze MW, Muckenthaler MU, SMAD7 controls iron metabolism as a potent inhibitor of hepcidin expression, Blood 115 (2010) 2657–2665. [DOI] [PubMed] [Google Scholar]
- [27].Vujic Spasic M, Kiss J, Herrmann T, Kessler R, Stolte J, Galy B, Rathkolb B, Wolf E, Stremmel W, Hentze MW, Muckenthaler MU, Physiological systemic iron metabolism in mice deficient for duodenal Hfe, Blood 109 (2007) 4511–4517. [DOI] [PubMed] [Google Scholar]
- [28].Muckenthaler M, Roy CN, Custodio AO, Minana B, deGraaf J, Montross LK, Andrews NC, Hentze MW, Regulatory defects in liver and intestine implicate abnormal hepcidin and Cybrd1 expression in mouse hemochromatosis, Nat. Genet. 34 (2003) 102–107. [DOI] [PubMed] [Google Scholar]
- [29].Pfaffl MW, Horgan GW, Dempfle L, Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR, Nucleic Acids Res. 30 (2002) e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Klingmuller U, Bauer A, Bohl S, Nickel PJ, Breitkopf K, Dooley S, Zellmer S, Kern C, Merfort I, Sparna T, Donauer J, Walz G, Geyer M, Kreutz C, Hermes M, Gotschel F, Hecht A, Walter D, Egger L, Neubert K, Borner C, Brulport M, Schormann W, Sauer C, Baumann F, Preiss R, MacNelly S, Godoy P, Wiercinska E, Ciuclan L, Edelmann J, Zeilinger K, Heinrich M, Zanger UM, Gebhardt R, Maiwald T, Heinrich R, Timmer J, von Weizsacker F, Hengstler JG, Primary mouse hepatocytes for systems biology approaches: a standardized in vitro system for modelling of signal transduction pathways, Syst. Biol. (Stevenage) 153 (2006) 433–447. [DOI] [PubMed] [Google Scholar]
- [31].Ludwiczek S, Theurl I, Artner-Dworzak E, Chorney M, Weiss G, Duodenal HFE expression and hepcidin levels determine body iron homeostasis: modulation by genetic diversity and dietary iron availability, J. Mol. Med. 82 (2004) 373–382. [DOI] [PubMed] [Google Scholar]
- [32].Camaschella C, Understanding iron homeostasis through genetic analysis of hemochromatosis and related disorders, Blood 106 (2005) 3710–3717. [DOI] [PubMed] [Google Scholar]
- [33].Bolondi G, Garuti C, Corradini E, Zoller H, Vogel W, Finkenstedt A, Babitt JL, Lin HY, Pietrangelo A, Altered hepatic BMP signaling pathway in human HFE hemochromatosis, Blood Cells Mol. Dis. 45 (2010) 308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ramos E, Kautz L, Rodriguez R, Hansen M, Gabayan V, Ginzburg Y, Roth MP, Nemeth E, Ganz T, Evidence for distinct pathways of hepcidin regulation by acute and chronic iron loading in mice, Hepatology. 53 (2011) 1333–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Moren A, Imamura T, Miyazono K, Heldin CH, Moustakas A, Degradation of the tumor suppressor Smad4 by WW and HECT domain ubiquitin ligases, J. Biol. Chem. 280 (2005) 22115–22123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.