Abstract
Vitamin D is essential for bone health and is known to be involved in immunomodulation and cell proliferation. Vitamin D status remains a significant health issue worldwide. However, there has been no clear consensus on vitamin D deficiency and its measurement in serum, and clinical practice of vitamin D deficiency treatment remains inconsistent. The major circulating metabolite of vitamin D, 25-hydroxyvitamin D (25(OH)D), is widely used as a biomarker of vitamin D status. Other metabolic pathways are recognised as important to vitamin D function and measurement of other metabolites may become important in the future. The utility of free 25(OH)D rather than total 25(OH)D needs further assessment. Data used to estimate the vitamin D intake required to achieve a serum 25(OH)D concentration were drawn from individual studies which reported dose-response data. The studies differ in their choice of subjects, dose of vitamin D, frequency of dosing regimen and methods used for the measurement of 25(OH)D concentration. Baseline 25(OH)D, body mass index, ethnicity, type of vitamin D (D2 or D3) and genetics affect the response of serum 25(OH)D to vitamin D supplementation. The diversity of opinions that exist on this topic are reflected in the guidelines. Government and scientific societies have published their recommendations for vitamin D intake which vary from 400–1000 IU/d (10–25 μg/d) for an average adult. It was not possible to establish a range of serum 25(OH)D concentrations associated with selected non-musculoskeletal health outcomes. To recommend treatment targets, future studies need to be on infants, children, pregnant and lactating women.
Background
Vitamin D deficiency is a major public health problem worldwide in all age groups, even in those residing in countries with sun exposure all year round.1 The serum 25-hydroxyvitamin D (25(OH)D) concentration has long been used as a parameter of choice for the assessment of vitamin D status. There has, however, been a long debate on the cut-off points of 25(OH)D used to diagnose vitamin D deficiency. While 25(OH)D metabolism forms the backbone of vitamin D physiology, there are other metabolic and catabolic pathways that are now recognised as important for vitamin D function.2 Several government bodies were requested to derive updated guidelines for the acceptable range of vitamin D intakes for adults, children and infants. The European Commission requested the European Food Safety Authority (EFSA) to provide advice on population dietary intakes,3 the Scientific Advisory Committee on Nutrition (SACN) Working Group on Vitamin D remit was to make dietary recommendations for the UK,4 and the Institute of Medicine (IOM) was requested by the US and Canadian governments to conduct a review of data to identify daily vitamin D requirements.5 The Endocrine Society provides information on treatment and prevention of vitamin D deficiency in their Clinical Practice Guideline.6 The Working Group of the Australian and New Zealand Bone and Mineral Society, Endocrine Society of Australia and Osteoporosis Australia prepared a position statement on vitamin D status in Australia and New Zealand.7,8 There is disagreement in the guidelines with regard to dosage and optimal concentration of 25(OH)D. The evidence on the relationship between serum 25(OH)D concentration and musculoskeletal health outcomes in adults, infants and children, and adverse pregnancy-related health outcomes, is widely variable. In the first instance this review will summarise recent advances in the understanding of vitamin D metabolism, tissue distribution and pharmacokinetics used to analyse data which determine the response curves of 25(OH)D to vitamin D supplementation. Genetic determinants, obesity, exposure to sunlight, co-existing pathology (e.g. malabsorption) and drug interaction are other factors that affect the response of 25(OH)D to vitamin D. The data provide the baseline for comparison of the different guidelines on optimal vitamin D status and their application to health outcomes.
Metabolism of Vitamin D
Vitamin D is made up of two forms. Vitamin D2 or ergocalciferol is made by plants and fungi and vitamin D3 or cholecalciferol is made by animals. Vitamin D3 is formed in the skin upon exposure to ultraviolet light (wavelengths 290–320 nm) on its precursor 7-dehydrocholesterol or found in the diet in oil-rich fish. Vitamin D (D2 and D3) is ingested incorporated in chylomicrons. Once in the circulation, it is converted to 25(OH)D. This is achieved primarily in the liver by several enzymes which include CYP2R1 and CYP27A1 (25-OHase), but can also occur in a variety of tissues in an autocrine/paracrine fashion. The conversion rate to 25(OH)D may be slower in subjects receiving larger doses of vitamin D,9 and may vary with nutritional state.10 The major circulating form of vitamin D, 25(OH)D, is present in human serum with a reported half-life of 2–3 w. The stability of this metabolite is mainly attributed to its strong affinity for the vitamin D binding protein (VDBP) in blood, with a dissociation constant of ~10−8 mol.11,12 In the kidney, filtered 25(OH)D bound to VDBP is endocytosed by megalin/cubilin receptors in the proximal tubules. The final hydroxylation of the reabsorbed, intracellular 25(OH)D occurs mainly in the kidneys and is carried out by the 25(OH)-1α-OHase (CYP27B1) to form the biologically active form of vitamin D, 1,25(OH)2D. For vitamin D and 1,25(OH)2D the dissociation constant with VDBP is ~10−7 mol.11 These dissociation constants may contribute to the half-life of these proteins; for vitamin D approximately 1 d,9 and for 1,25(OH)2D a few hours.13 Loss into tissues may further explain the short half-life of vitamin D. The dissociation constants may further dictate the ‘free’ concentration of compound that is available to enter cells.14
1,25(OH)2D initiates or suppresses gene transcription by binding to the vitamin D receptor (VDR). Binding to VDR triggers hetero-dimerisation of VDR with retinoid X receptor. The heterodimer then translocates to the nucleus where the complex binds to vitamin D response elements and alters gene transcription. In addition, 1,25(OH)2D stimulates its own destruction by enhancing the expression of 25 hydroxyvitamin-D-24OHase (CYP24A1) to metabolise 25(OH)D and 1,25(OH)2D into water-soluble inactive forms (Figure 1). The control of circulating concentrations of vitamin D is affected by many hormones (e.g. parathyroid hormone (PTH)) and metabolites (e.g. phosphate). Important though these topics are, they are not the subject of this review and the reader is referred to several previous publications including those by the current author.15,16
Figure 1.
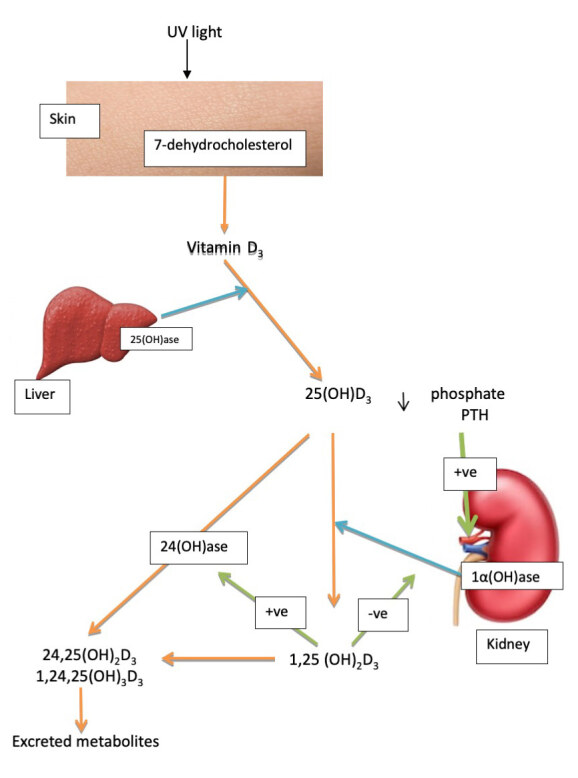
Simplified metabolism of vitamin D. Vitamin D3 is synthesised in the skin from 7-dehydrocholesterol, hydroxylated to 25(OH)D3 in the liver, and converted to 1,25(OH)2D3 in the kidney. 25(OH)D3 and 1,25(OH)2D are converted by 24(OH)ase to 24,25(OH)2D and 1,24,25(OH)3D.
blue arrows, enzyme pathways; green arrows, pathway activation/inhibition.
A number of other vitamin D (D2 and D3) metabolites have been reported to be produced by alternative pathways other than the pathway for 1,25(OH)2D. These pathways include C3-epimerisation, CYP24A1 hydroxylase, CYP11A1 and phase II metabolism by conjugation. There are several reviews on the metabolism of vitamin D and only a brief overview of the subject will be given.2 The initial C24 hydroxylation of 1,25(OH)2D3 forms 1,24,25(OH)3D3 which is less active than 1,25(OH)2D3. CYP24A1 is a bifunctional enzyme capable of 24 and 23 hydroxylation of 1,25(OH)2D3. The dominant metabolite of vitamin D in serum, 25(OH)D3, is a further substrate for CYP24A1 with C24, C23, C26 hydroxylation forming 24(R),25(OH)2D3, 23(S)25(OH)2D3 and 25(R)26(OH)2D3 respectively.17,18 There have been claims that intermediates of the C24 oxidation pathway retain some biological activity. There may be a physiologic role for 24,25(OH)2D3 in growth plate formation19 or for the terminal product of C23 hydroxylation as a potent VDR antagonist.20 CYP3A4 can catalyse the hydroxylation of 1,25(OH)2D3 at C23R and C24S positions. The catabolic pathway for 25(OH)D3 by CYP3A4 results in the formation of 4α,25(OH)2D3 and 4β,25(OH)2D3.21 CYP3A4 mutations that oxidise 1,25(OH)2D at a greater activity have been linked to rickets.22 Induction of CYP3A4 by anti-epileptic drugs and increased metabolism of 1,25(OH)2D3 may contribute to osteomalacia.23 CYP11A1 catalyses the first step of steroidogenesis where cholesterol is converted to pregnenolone. Products of CYP11A1 metabolism of vitamin D3, 20(OH)D3, 22(OH)D3, 20,22(OH)2D3, 20,23(OH)2D3 and 17,20,23(OH)3D3 were found in the human epidermis and serum. These compounds can be further acted on by CYP27B1 (producing 1,20(OH)2D3 and 1,20,23(OH)3D3) or CYP27A1 and/or CYP24A1 (producing 20,24(OH)2D3, 20,25(OH)2D3 and 20,26(OH)2D3). Major metabolites of this pathway were shown to display proliferative, pro-differentiation and anti-inflammatory activities in a cell-dependent fashion.24 This raises the possibility that the measurement of the major CYP11A-derived metabolites of vitamin D3 in human serum may be necessary to fully assess vitamin D deficiency or sufficiency.
Epimerisation of vitamin D and its metabolites is the nonreversible conversion of the hydroxyl group on C3 from the 3β orientation to the 3α position in a reaction catalysed by a non-P450 enzyme, 3-epimerase. All major vitamin D metabolites can be epimerised. Following epimerisation the epimers can be metabolised by the same enzymes involved in the hydroxylation pathways for 25(OH)D3. In adults, 3-epi-25(OH)D3 makes up 0–13.3% of the total 25(OH)D,25 though higher percentages have been reported.26 There was a correlation between the percentage of 3-epi-25(OH)D3 and total 25(OH)D.25 Genetic polymorphisms in the vitamin D-related pathways can contribute to variation in the C3-epimer levels.27 In infants the proportion of 3-epi-25(OH)D3 was increased in the first year.28 The epimer 3-epi-25(OH)D3 is present in all blood samples of pregnant women29 and reported as approximately 25% of total vitamin D levels in a further study.30 In cell culture studies the C3-epimers of 1,25(OH)2D3, 25(OH)D3, 24,25(OH)2D3 had a lower binding affinity for VDR and lower transcriptional activity towards certain genes, and lower anti-proliferative/differentiation inducing activity.31 Higher metabolic stability of 3-epi-1,25(OH)2D3 is stated as a possible explanation for the PTH suppression in cultured bovine parathyroid cells.32 Though a limited number of studies support a physiological role for the epimers, the physiological function of the epimer forms still needs further clarification. LC-MS/MS techniques that can chromatographically separate epimeric interference and detect vitamin D metabolites more accurately are recommended especially in young infants.33
Phase II metabolism of vitamin D 25(OH)D3 by sulfo-transferases or uridine 5′-diphosphoglucuronyltransferase forms the sulfate and glucuronide conjugate. While these enzymes can contribute to a catabolic inactivating process, it has been suggested that the conjugated forms of 25(OH)D3 may be metabolite storage forms and may need to be taken into account in determining vitamin D status. Variations in gene polymorphism of the phase II enzymes may contribute to interindividual variation in vitamin D homeostasis.34,35
Biological Functions of Vitamin D
1,25(OH)2D has a wide range of biological functions which are mediated through the VDR. Traditionally 1,25(OH)2D has been associated with calcium and phosphorus homeostasis and maintenance of bone content. However there are several tissues and cells that possess 1-OHase activity and VDR is present in cells other than bone, intestine, kidney and parathyroid gland. This observation led to recognition of non-calcaemic actions of vitamin D. Vitamin D is also known to be involved in cell proliferation, differentiation and immunomodulation.36
Treatment with vitamin D increases levels of 25(OH)D without a measurable effect on serum 1,25(OH)2D.37,38 Other studies suggest no relationship between serum 25(OH)D and 1,25(OH)2D.39,40 It is possible that 25(OH)D which is a precursor of 1,25(OH)2D has a role in calcium absorption.
Pharmacokinetics of Vitamin D Metabolism
Literature characterising the dose-response curve to vitamin D shows varied results. Clinical studies investigating these relationships vary in dosing regimen, administrative routes, assay methods for 25(OH)D and demographics as well as control of endogenous vitamin D production. There is lack of consensus as to the optimal level and no unanimity as to the dose that will bring individual patients to that level. Aloia et al. suggest that determination of intake required to attain optimal serum 25(OH)D concentration must take into account the wide variability in dose-response curve and basal 25(OH)D concentration.41 Pharmacokinetics of the distribution of vitamin D must consider absorption, distribution, metabolism and excretion as well as different routes of administration (Figure 2).
Figure 2.
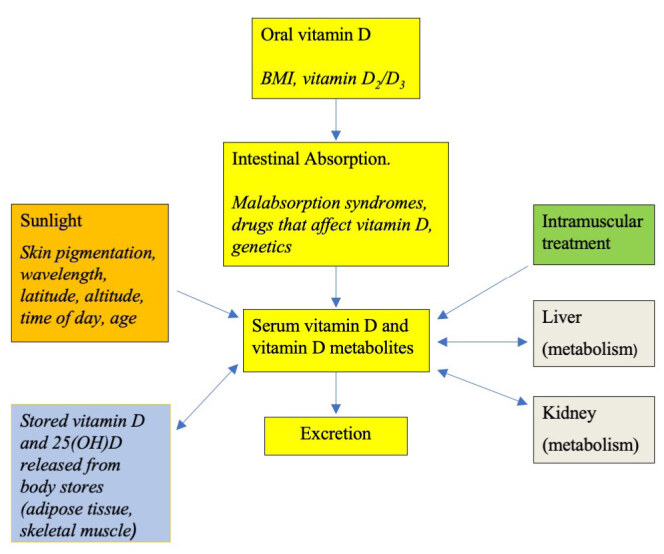
Compartmental structure of factors that contribute to tissue pools of vitamin D and its metabolites.
Analytical Methods for the Measurement of 25(OH)D
Comparisons of methodology for different assays for 25(OH)D suggest a 10–15% variation between methods, suggesting caution is required when comparing different methods. When assays were first introduced, laboratories were using in-house competitive binding assays. In the 1990s, development of an iodinated tracer led to the development of a commercial radioimmunoassay. Fully automated immunoassay procedures introduced in 2007 were prone to matrix effects. Immunoassays do not always distinguish between 25(OH)D2 and 25(OH)D3. Recognising the limitations of the automated methods led to the increased adoption of LC-MS/MS assays. The US National Institute of Standards and Technology (NIST) had their LC-MS/MS assay accepted as the reference measurement procedure and the vitamin D standardisation program was established in 2010. The NIST introduced the standard reference material for vitamin D in 2009.42 Assay analytical factors may explain the difference in response to vitamin D intake in some of the studies.43
Serum 25(OH)D Response to Dosing with Vitamin D
It is not well established how much vitamin D or its metabolites may be needed to bring serum levels in individuals to desired levels. Generally, serum 25(OH)D is used as a functional marker of vitamin D nutritional status. Ease of analysis, stability, half-life and concentration in serum have contributed to 25(OH)D as the main assay form of vitamin D. Several studies have investigated the relationship between oral dosing with vitamin D and achieved serum levels. Studies used pharmacology,44 low doses of vitamin D,45 focused on patient groups (elderly individuals),45 those on drugs known to affect vitamin D metabolism,46 or were limited in that they were conducted for another purpose.47 A further limitation of the earlier studies was the different assay methodologies used in the studies and the inability to evaluate or control sunlight exposure or dietary intake of vitamin D.
Studies have shown that dosing with vitamin D3 required in the order of 150–180 d to reach something approaching a steady state. During the study period, concentrations of 25(OH)D increased with a slope (change/dose) of 0.70 nmol/L (0.28 ng/mL) for each 40 IU (1 μg) of vitamin D3 input, in subjects with mean baseline 25(OH)D value of 70.3 nmol/L (28.1 ng/mL).48 In an earlier study by the same authors, the change in 25(OH)D over the study period was 1.23 nmol/L (0.49 ng/mL) for each 100 IU (2.5 μg) of vitamin D3 input. In a summary of 13 studies carried out during the period 1977–1996, the increase in 25(OH)D varied from 1.47–8.75 nmol/L (0.59–3.5 ng/mL) per 100 IU (2.5 μg) of oral vitamin D intake.38 In subjects with an average 25(OH)D concentration of 47 nmol/L (18.8 ng/mL), treatment with vitamin D at concentrations of 1000 IU/d (25 μg/d) or 4000 IU/d (100 μg/d) increased serum 25(OH)D over 3 m to 69 nmol/L (27.6 ng/mL) and 96 nmol/L (38.4 ng/mL) respectively.49 In a review of 49 studies, authors conclude that the ‘rule of thumb’ is 5.0 nmol/L (2 ng/mL) increase of 25(OH)D for each 100 IU/d (2.5 μg/d) of ingested vitamin D.50 In a review of 76 trials published from 1984 to 2011 the average increase was 1.95 nmol/L (0.78 ng/mL) per 40 IU (1 μg) of vitamin D3 supplement per day.51 A summary suggests that trials that used similar doses could obtain changes in 25(OH)D that can vary 3–4-fold. A mean slope of 0.66 nmol/L per 40 IU/d (0.26 ng/mL per μg/d) was reported by Aloia et al.41 The individual slopes ranged from 0.15–1.49 nmol/L (0.06–0.60 ng/mL), virtually a 10-fold increase. Investigators either report the same slope for different dosage concentrations48 or a variable response dependent on the IU of vitamin D intake.41 A further review of the effect of vitamin D supplementation on 25(OH)D levels in children and adolescents is in progress.52
In summary, the literature characterising the dose response of 25(OH)D contains varied results. Baseline 25(OH)D concentration has been shown to contribute to the variation in 25(OH)D response to vitamin D supplementation.53 Because hepatic hydroxylation of vitamin D may be a saturable process, response to vitamin D could be affected by baseline 25(OH)D concentrations. Studies suggest that baseline 25(OH)D influenced vitamin D response.38
Factors Affecting Response to Vitamin D Supplementation
Patient-specific factors may further affect the amount of vitamin D required to attain a sufficient concentration49 and it is important to substantiate what factors influence subjects’ responses to vitamin D supplements. Zittermann et al.demonstrated an association between vitamin D dose per kg body weight and increment in circulating 25(OH)D.54 Body weight has an influence on the blood volume and amount of muscle and adipose tissue. Higher body fat percentage or higher body mass index (BMI) have been associated with smaller increases in 25(OH)D concentrations in response to vitamin D supplementation. The effect of BMI and 25(OH)D response to supplementation has been reported in younger55 and older adults.56 In a regression model to predict 25(OH)D, BMI was the main predictor explaining 21.6% of the inverse variation in serum 25(OH)D.57 Wortsman et al. demonstrated a significant relationship between BMI and peak vitamin D2 after vitamin D2 load as well as serum vitamin D3 concentration after UV-B irradiation.58 The authors suggest that the inverse correlation observed between BMI and peak vitamin D levels was due to decreased bioavailability of vitamin D due to its deposition in body fat compartments.
There are few trials examining the effects of calcium supplementation on serum 25(OH)D response to vitamin D dose and the results are variable. Studies suggest that addition of calcium (a) had a comparable effect to placebo in its effect on 25(OH)D response to vitamin D;59 (b) decreased the response of 25(OH)D to vitamin D;60 and (c) increased the response of 25(OH) to vitamin D compared to vitamin D alone.61
Trang et al.,53 Armas et al.,62 Heaney et al.63 and Tripokovic et al.64 demonstrated that vitamin D3 is more potent at raising serum 25(OH)D than equimolar vitamin D2. In a further meta-analysis, vitamin D3 was more proficient than vitamin D2 in raising serum 25(OH)D concentration.65 There are reports that the rate of 25 hydroxylation of vitamin D3 is higher than that of vitamin D2,66 and that vitamin D3 metabolites have a higher affinity for VDBP67 and altered binding with vitamin D receptor68 compared to vitamin D2 metabolites. The more efficient hydroxylation of vitamin D3 can contribute to the increased production of 25(OH)D3 compared to 25(OH)D2. Another probable reason for the difference between vitamin D2 and vitamin D3 is that the weaker affinity of vitamin D2 metabolites to VDBP would lead to shorter half-life and an increased rate of clearance from circulation.69 Previous investigators have shown that 24 hydroxylation of 1,25(OH)2D2 is a significant in vivo deactivation step whereas 1,25,24(OH)3D3 still requires additional steps for deactivation, suggesting another reason for cholecalciferol to remain biologically active and maintain vitamin D status.70 Studies report a fall in 25(OH)D3 and 1,25(OH)2D3 in vitamin D2-treated subjects and a similar decrease in 25(OH)D2 and 1,25(OH)2D2 in vitamin D3-treated subjects.71,72 Total 1,25(OH)2D did not change.72 Taken together with reports of a non-linear response in 25(OH)D with increasing doses of vitamin D, it is possible that total 25(OH)D level is tightly regulated.
In summary, BMI, calcium intake and type of vitamin D (D2 or D3) can affect the dose response of 25(OH)D to vitamin D.
Intestinal Absorption
Following oral intake, vitamin D is rapidly absorbed to reach a maximum level at around 24 h. Levels of 25(OH)D increase gradually to peak at 7–14 d depending on dose.73 The response rate to the same oral dose can be very broad.74 The response rate is the result of input as well as metabolic degradation and tissue distribution. Clinical trials and studies using radioactive forms of vitamin D showed that different malabsorptive conditions modified vitamin D absorption.75,76 Aging did not interfere with vitamin D absorption in elderly subjects with normal kidney function.77
25(OH)D is better absorbed than the non-hydroxylated forms of vitamin D.44 It has been suggested that the absorption of 25(OH)D is less dependent on bile acids and chylomicrons than vitamin D and occurs through the portal route.78 The available data indicate that the amount of fat has no effect on vitamin D bioavailability, though the type of fatty acid can affect vitamin D bioavailability.79 Data on the effect of fibre on vitamin D absorption are contradictory.80,81 Several anti-obesity drugs have been shown to reduce the absorption of vitamin D,82 and certain absorption enhancers (e.g. water-soluble form of vitamin E) have been shown to increase vitamin D absorption. 83
Studies on mice suggest that vitamin D is absorbed in the median intestine.84 It has long been assumed that vitamin D was absorbed by an unsaturable passive diffusion process. Recent studies using human Caco-2 and human embryonic kidney (HEK) transfected cell lines suggest that membrane transporters SR-B1, CD36 and Nieman-Pick C1-Like 1 inhibitor (NPC1L1), the three main cholesterol transporters, may be involved in vitamin D transport. It was further suggested that protein-mediated transport occurred at dietary, low concentrations of vitamin D and passive diffusion occurred at pharmacological, high concentrations of vitamin D.85 The possibilities of other membrane protein transporters cannot be ruled out. It can be hypothesised that genetic variations in SR-B1, CD36 and NPC1L1 may affect vitamin D intestinal absorption and status in humans.
Storage Sites for Vitamin D in the Body
Little is known about the quantity and location of vitamin D or its metabolites in the adult human body. In a study using pigs and HPLC for analysis of vitamin D and metabolites, substantial amounts of 25(OH)D were found distributed in the body, principally in fat, muscle and serum.86 Using LC/MS, vitamin D was detectable in fat tissue of obese subjects and was positively correlated with serum vitamin D.87 New high resolution tandem mass spectrometry methods for the simultaneous measurement of vitamin D and its metabolites in mouse tissue have been developed.88 In a further study using dual-energy X-ray absorptiometry for body composition, obese women were shown to have greater adipose tissue storage and the increased amount of vitamin D required to saturate the depot may predispose individuals to inadequate serum 25(OH)D.89 The authors report vitamin D total storage of 84.8 ng/g in adipose tissue and total body stores as 2.29 mg in obese patients compared with 55.5 ng/g in adipose tissue and total body stores of 0.44 mg in control subjects. Similar levels in adipose tissue have been reported by Lawson et al.,90 in adipose tissue of obese subjects about to undergo bariatric surgery,87 and in baseline samples of adipose tissue prior to vitamin D treatment.61
Early studies showed that voluntary muscle and adipose tissue are the principal sites of storage of vitamin D in humans.91 Whether this storage is of clinical importance is uncertain and there are arguments that the amounts of vitamin D stored in adipose tissue are too small and have only negligible effects on serum 25(OH)D levels.86 The relation between stored vitamin D3 and 25(OH)D was investigated in a further study. At typical vitamin D inputs, 25(OH)D constitutes the bulk of vitamin D reserves. However, at supraphysiologic inputs, large quantities of vitamin D3 are stored and slowly released to be converted to 25(OH)D.92 In a further study, subjects were treated with a weekly intake of 20,000 IU (500 μg) of vitamin D for a period of 3–5 y. During the follow up period, 25(OH)D gradually declined over 12 m, with a pre-terminal phase (0–3 m) half-life of 83.4 d and a further fall thereafter with a half-life of 255 d. The authors suggest that the longer half-life was the result of gradual release of vitamin D stored in adipose and other tissues.93
The half-life of VDBP is ~1–2 d,94 surprisingly shorter than the half-life of 25(OH)D. VDBP has an additional binding site for actin. As the plasma membrane of skeletal muscle contains megalin, this will transfer VDBP into the cytoplasm of skeletal muscle cells where it binds to actin filaments. It can be postulated that the process of 25(OH)D passaging into and out of muscle cells can further explain the long residence time for this metabolite in circulation. Studies with cells in culture support the proposal that mature muscle cells, but not adipocytes, take up 25(OH)D for intracellular retention by VDBP.95,96 The authors suggest that PTH is a candidate for regulator of 25(OH)D storage in muscle cells.97 However, cell culture models may not reflect vitamin D homeostatic mechanisms in humans. The authors suggest that the storage of 25(OH)D is enhanced in winter which would enable adequate vitamin D status to be maintained during the seasonal variation in vitamin D supply from solar irradiation.98
Ultraviolet Radiation and Vitamin D Production
During exposure to sunlight, ultraviolet (UV) B radiation converts 7-dehydrocholesterol to previtamin D3 which in turn is isomerised to vitamin D3. In the past, the primary source of vitamin D for the human body was synthesis in the skin in response to UV exposure. Skin pigment and skin type, sunscreen use, aging, time of day (very little vitamin D3 is produced in the skin in the early morning and late afternoon), season, latitude and altitude can affect previtamin D3 synthesis.99 Continued exposure of 7-dehydrocholesterol to UV radiation resulted in the production of other photoproducts lumisterol and tachysterol, which may prevent excessive vitamin D from being produced in the skin.100 The amount of vitamin D that is produced by exposure to sunlight is controversial. To quantify vitamin D production following UV radiation, exposure values are compared to serum levels following oral vitamin D intake.
In a study carried out in 1977, daily whole-body artificial UVB light radiation resulted in changes in plasma 25(OH)D equivalent to 10,000 IU (250 μg) oral dose vitamin D daily, taken over 3–4 w, and resulted in an increase in 25(OH)D in the range 50–100 nmol/L (20–40 ng/mL).44 In a further experiment a single full-body exposure to 1 minimal erythemal dose (MED; causes slight pinkness of skin 24 h after exposure) of simulated sunlight resulted in serum levels of vitamin D3 that compared to an oral dose of 10,000–25,000 IU (250–625 μg) of vitamin D3.101 Similar results were found in a further study where subjects received a single whole-body UV radiation exposure of 1–4 MED. Changes in serum concentration over time showed an increase in vitamin D from approximately 50 nmol/L (20 ng/mL) which peaked at 1–2 d, in contrast to increased 25(OH)D over 7–14 d to approximately 100 nmol/L (40 ng/mL).102 Over a 12-week period, UV radiation treatment of one-sixth of the body area increased serum 25(OH)D levels to the same levels as 400 IU (10 μg) oral dose.103 Sophisticated computational models permit theoretical prediction of the amount of natural sunlight required to match specific levels of oral vitamin D supplementation. However there are several limitations to these models.104 Over-exposure to solar radiation is responsible for skin cancer and it is important to estimate a balance between avoiding skin cancer and maintaining sufficient vitamin D production. The choice of sunlight exposure is an option to those who consider this an alternative to daily supplement. Optimal sun exposure advice can be tailored to each location.105
An observational study from the north of England found that average sun exposure in the white-skinned population in summer was sufficient to prevent vitamin D deficiency in winter, however it was not adequate in a South Asian population.106 Studies in the Greater Manchester Area in the UK suggest that a late summer level of 76 nmol/L (30.4 ng/mL) in women and 87.3 nmol/L (34.9 ng/mL) in men was required for a winter level of 50 nmol/L (20 ng/mL) 25(OH)D. The authors observed a longer half-life for 25(OH)D suggesting that stored vitamin D can be utilised, thus lengthening the apparent half-life. The majority of the population fail to reach this level and become vitamin D insufficient.107 In a review of 15 studies, the estimated rise in 25(OH)D per standard erythema dose (SED) was 0.19 nmol/L (0.08 ng/mL). The authors suggest that partial body surface area (10%) exposure with moderate UV doses (1 SED) was effective for generating and maintaining vitamin D status.108 Holick suggests a sensible approximation is that for a Caucasian living at 42°N, in June, exposure of arms and legs to sunlight on a clear day between 10 am and 3 pm for approximately 5–15 min (approximately 25% of MED) 2–3 times per week is adequate to satisfy the body’s vitamin D requirements.101 Webb et al. calculate that white Caucasians (at UK latitudes) should receive 9 min of daily sunlight from March to September, with forearms and lower legs exposed for adequate vitamin D status during winter.109 They suggest a longer exposure time of 25 min for individuals with darker skin.110 There are inconsistencies in the literature in the recommendations for sunlight exposure. Further, there are practical difficulties and detrimental effects of UV exposure, and oral supplementation may be a safer way of increasing vitamin D status. In previous studies dietary supply of vitamin D was considered important in maintaining serum 25(OH)D during winter, and it was likely that the supplement requirement was dependent on sun exposure.111
Pharmacokinetic Modelling of Vitamin D Metabolism
Mathematical models that describe the absorption, distribution, metabolism and excretion of vitamin D are limited. Sawyer et al. developed a model that predicted the response of the body to low concentrations of vitamin D in a sunlight-restricted environment.112 A further pharmacokinetic model was developed to predict mean vitamin D3 and 25(OH)D3 exposure from varied doses and administrative routes.113 The model compared 25(OH)D2 which showed a first-order clearance and 25(OH)D3 which showed a saturable non-linear clearance.114 Models were optimised for the expression and enzymatic parameters of enzymes involved in the vitamin D cascade (CYP2R1, CYP27A1, CYP27B1 and CYP24A1) as well as vitamin D binding to lipids and VDBP.115
Free or Total 25(OH)D
The majority of vitamin D and its metabolites are tightly bound to VDBP, with smaller amounts bound to albumin (10–15%); less than 1% of circulating vitamin D metabolites exist in a free, unbound form. Gene sequencing identified >120 variants of the VDBP gene and 3 main phenotypes (Gc1F, Gc2, Gc1S) have been described. When measured with a variety of polyclonal antibodies using an ELISA method, VDBP concentrations in Gc2 genotype are slightly lower than the Gc1 carriers. Whether different forms of VDBP have different affinities for vitamin D metabolites is a matter of debate. Individuals with the Gc2 variant have been shown to respond to vitamin D treatment with a stronger increase in 25(OH)D. Differences in alleles have not been shown to contribute to differences in fracture rate though a number of chronic diseases have been associated with VDBP variants. Further studies are needed to show if these observations can be repeated in all populations.116 Decreased VDBP concentrations are found in liver cirrhosis, malnutrition and nephrotic syndrome. Exposure to oestrogens increases serum concentrations of VDBP. Exposure to androgens, vitamin D deficiency or excess, osteoporosis, hyperthyroidism, sarcoidosis, cancer and Addison’s disease have no effect on VDBP concentrations. VDBP is an acute phase reactant, but severe trauma or illness can decrease VDBP by >10%.94 Serum VDBP levels were shown to be lower in primary hyperparathyroidism but no significant differences in free 25(OH)D were observed between primary hyperparathyroidism patients and controls.117 Congenital absence of the VDBP in a patient resulted in normocalcaemia and a relatively mild disruption of bone metabolism. Despite very low 25(OH)D in his hemizygous brother, free 25(OH)D levels were within reference limits and he had no signs of abnormal calcium homeostasis, supporting the free hormone hypothesis.118
In the kidney, epithelial renal cells in the proximal tubule express both megalin and cubulin, which facilitate the entry of 25(OH)D-VDBP complex into the cell. Once internalised, 25(OH)D dissociates from VDBP and is metabolised to 1,25(OH)2D. VDBP-bound 25(OH)D may play a role in the renal synthesis of 1,25(OH)2D. Megalin knockout mice are characterised by major developmental brain abnormalities and the changes are usually lethal.119
A reference standard for VDBP based on protein purified from Gc1F, Gc2, Gc1S individuals and a reference method for an MS/MS-based assay of VDBP have been developed.120 The concentration of VDBP in human serum is in the micromolar range as measured by MS/MS.94 As VDBP has a high affinity for vitamin D and its metabolites and is present in high molar concentrations compared to its ligands, the free concentration of vitamin D and its metabolites is quite low. Formulae used to calculate the free hormone are based on the measurement of total concentrations of metabolite and VDBP, the best estimate of its affinity and the law of mass action. As the VDBP assay has been standardised only recently and the affinity constant is not perfectly known, the predictive value of the calculated free hormone is variable. Free 25(OH)D can also be measured by ultrafiltration121 or ELISA.122 Levels of free 25(OH)D measured by ELISA and calculated levels were correlated.123
Pathological conditions where total 25(OH)D and free 25(OH)D diverge as a marker of vitamin D status have been reviewed previously. Bikle et al. suggest that concentrations differ in pregnant women, people with cirrhosis, and elderly people with multiple comorbidities.116 Further clinical studies need to be conducted to assess the utility of free 25(OH)D measurement in different physiological/pathological situations.
Vitamin D Metabolism During Pregnancy and Lactation
Circulating concentrations of 1,25(OH)2D, 25(OH)D and VDBP have been shown to be increased during pregnancy and the trend was higher as subjects progressed through pregnancy.124 Other studies suggest a decrease in the molar ratio of 1,25(OH)2D:VDBP and 25(OH)D:VDBP at delivery compared to values taken during early pregnancy.125,126 Early studies suggest that the increase in free 1,25(OH)2D3 during pregnancy cannot be solely accounted for by increased VDBP.127 In other studies neither total nor free 25(OH)D were significantly different in pregnant and non-pregnant women.128 Further studies are required to evaluate the role of free 25(OH)D and total 25(OH)D in pregnancy as studies are contradictory on their role as predictors of circulating markers of calcium metabolism.125,126
During pregnancy, circulating 25(OH)D had a direct influence on 1,25(OH)2D concentrations.124 There are still some knowledge gaps regarding the regulation of 1,25(OH)2D production during pregnancy, though it has been suggested that maternal kidneys upregulate the synthesis of 1,25(OH)2D. Earlier it was thought that the increase in 1,25(OH)2D was to ensure adequate delivery of calcium for maternal skeleton preservation and foetal skeletal development. The increase in intestinal calcium absorption is probably mediated in part by calcitriol, but other factors may contribute to pregnancy-induced increase in calcium absorption.129 During pregnancy, the efficiency of intestinal calcium absorption increases to meet the foetal requirement for calcium, whereas during lactation, skeletal resorption increases to provide calcium to milk. In lactating women, mean levels of 25(OH)D and 1,25(OH)2D were similar to non-pregnant control groups. There appears to be a trend towards lower PTH concentrations in lactating women. Increased bone loss observed during lactation is attributed to mammary gland-derived PTH-related protein (PTHrP). Women undergo a substantial increase in bone mass and mineralisation after weaning, reversing the losses that transiently occur during lactation.129,130
The placenta meets the foetal need for minerals by actively transporting calcium, phosphorus and magnesium from the maternal circulation. The placenta appears capable of providing minerals even when faced with reduced concentrations of these minerals in the maternal circulation. Foetal bone and mineral metabolism are dependent on PTH and PTHrP, whereas vitamin D/1,25(OH)2D, fibroblast growth factor 23 (FGF23), calcitonin and the sex steroids may not be required. The placenta and foetal parathyroids are possible sources of foetal PTHrP. Intestinal absorption becomes the main source of minerals after birth. Intestinal calcium absorption is initially a passive process facilitated by lactose, but later becomes active and 1,25(OH)2D-dependent. 1,25(OH)2D is low in cord blood and in the neonate increases to adult values during the first 2 d after birth.131
The placenta expresses all components of the vitamin D pathway: megalin/cubilin, CYP2R1, CYP27B1 and CYP24A1. This raises the possibility that the placenta can contribute to maternal circulating vitamin D metabolites.132 1,25(OH)2D circulates at low levels in foetal blood, typically 50% of the maternal value; it is suggested that the foetal kidneys and not the placenta contribute to 1,25(OH)2D in the foetal circulation. Maternal 25(OH)D crosses the placenta and is the main pool of 25(OH)D in the foetus and achieves cord blood levels that are typically 75–100% of the maternal value at term with a foetal-maternal correlation coefficient of 0.8–0.9.131
Fragility fractures of the spine and other sites rarely occur during lactation and pregnancy. They may result from pre-existing disorders of skeletal fragility, a greater-than-expected skeletal resorption that may have occurred during pregnancy or low calcium intake during lactation. Since breast milk normally contains little vitamin D, breast-fed babies may require vitamin D supplements.129,131,133 The immunomodulatory effects of 1,25(OH)2D lead to the hypothesis that 1,25(OH)2D may act as an autocrine/paracrine regulator of immunity at the foetal/maternal interface.134
Anti-Epileptic Drug Therapy and Vitamin D Metabolism
Long-term use of anti-epileptic drug (AED) treatment was associated with an increased risk of fractures.135 Studies have shown an association between anticonvulsant medications and decreased bone mineral density (BMD) and increased fracture risk, especially with older agents such as phenobarbital, carbamazepine, phenytoin and valproate.136,137 Vitamin D was metabolised at a more rapid rate when exposed to AEDs, due to increased metabolism of 25(OH)D by CYP24 hydroxylation to 24,25(OH)2D, an inactive metabolite. The induction was mediated through the pregnane X receptor (PXR). PXR is a nuclear receptor activated by phenobarbital, valproic acid and phenytoin. PXR shares homology with VDR and when activated promotes expression of CYP24, a VDR target gene,138 and increases catabolism of 25(OH)D, which leads to vitamin D deficiency.139 Anti-epileptic drugs can induce CYP3A4 expression in liver and small intestine and accelerate vitamin D metabolism and contribute to vitamin D deficiency.140 In other studies, vitamin D deficiency was not associated with low BMD after correcting for age and time on AEDs.141 Long-term treatment with AEDs has a negative effect on growth in children.142 The mechanisms of AED-induced bone disease appear to be multiple.143 In their review, Fernandez et al. state that heterogeneity in study methods, differences in dosing and duration of study made the effect of vitamin D on bone mineralisation (in patients treated with AEDs) difficult to generalise, but observed that studies showed that at least one indicator of bone health improved.144 Drezner et al. recommend a prophylactic dose of up to 2000 IU/d (50 μg/d) for patients on the initiation of anticonvulsant treatment; 2000–4000 IU/d (50–100 μg/d) if osteoporotic/osteopenic disorders exist; 5000–15,000 IU/d (125–375 μg/d) for 3–4 w for osteomalacia.145 The authors recommend careful monitoring as the selection of dose is arbitrary and titration to an effective dose may be necessary. They recommend further research to define optimal therapy for affected patients.
Renal Disease and Vitamin D Metabolism
Chronic kidney disease (CKD) is associated with low 25(OH)D levels. Low 25(OH)D has been associated with muscle weakness and possible risk of falls. Patients with CKD have elevated serum phosphate levels, elevated FGF23 and elevated CYP24A1.146 The pathogenesis of secondary hyperparathyroidism in CKD is a complex process. In the classic hypothesis, the retained phosphate levels led to a triad of hyperphosphataemia, low 1,25(OH)2D and hypocalcaemia which can lead to increased PTH. Newer discoveries have led to different explanations for the pathogenesis for secondary hyperparathyroidism. FGF23 is derived from osteocytes and requires Klotho (a transmembrane protein) to enable it to bind to the FGF receptor and enhance phosphate excretion in the kidneys and decrease intestinal phosphate absorption. FGF23 also reduces the formation of 1,25(OH)2D leading to hypocalcaemia and secondary hyperparathyroidism. The excess PTH leads to mobilisation of calcium from the bone leading to renal bone disease.147 Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines suggest that treatment with vitamin D analogues or 1,25(OH)2D be reserved for severe and progressive secondary hyperparathyroidism. Treatment choice may be guided by the patient’s concomitant treatment and current calcium and phosphate levels.148
Bariatric Surgery and Vitamin D Deficiency
Vitamin D deficiency and secondary hyperparathyroidism can be found in obese patients. Vitamin D deficiency and elevated PTH are common findings following gastric bypass surgery.149 It is important to recognise and treat vitamin D deficiency before bariatric surgery to avoid postoperative complications and metabolic bone disease with increased fracture risk. The negative skeletal effects of bariatric surgery on bone are probably multifactorial and include nutritional factors such as vitamin D deficiency, inadequate calcium intake and calcium malabsorption.150 Depending on the procedure, it may be difficult to normalise 25(OH)D and PTH levels on an individual basis; it cannot always be assumed that providing a single dose of vitamin D may normalise vitamin D status in all patients.151 A recent review on pre- and post-bariatric vitamin D supplementation suggested a lack of consensus on the dosage and frequency of optimal supplementation regimen.152
Guidelines for Prevention and Treatment of Vitamin D Deficiency
Definition of Vitamin D Deficiency
A central controversy in vitamin D is how to define hypovitaminosis. The blood level of 25(OH)D that is defined as vitamin D deficiency remains controversial. The controversy is reflected in the diversity of recommendations from the European authorities, IOM and Endocrine Society. Vitamin D deficiency as defined by SACN, IOM, EFSA, the Endocrine Practice Guidelines and the Australian Working Group are given in Table 1. Details of the methodology and literature search carried out by the IOM and EFSA are given in the published monographs.3–6
Table 1.
Recommended serum levels for 25(OH)D.
| ESPG | SACN | IOM | EFSA | Australian Working Group | |
|---|---|---|---|---|---|
| Vitamin D deficiency | <50 nmol/L (<20 ng/mL) | <25 nmol/L (<10 ng/mL) | Persons are at risk of deficiency relative to bone health at serum 25OHD levels <30 nmol/L (<12 ng/mL) | Severe <12.5 nmol/L (<5 ng/mL); Moderate 12.5–29 nmol/L (5–11.6 ng/mL) | |
| Vitamin D insufficiency | 52.5–72.5 nmol/L (21–29 ng/mL) | Some, but not all, persons are potentially at risk of inadequacy at serum 25OHD levels 30–50 nmol/L (12–20 ng/mL) | 30–49 nmol/L (12–19.6 ng/mL) Mild deficiency |
||
| Sufficient | 75–250 nmol/L (30–100 ng/mL) | 50 nmol/L (20 ng/mL) (covers the requirements of 97.5% of population)* | ≥50 nmol/L (≥20 ng/mL) | ≥50 nmol/L (≥20 ng/mL) (at the end of winter)*† |
Levels are higher 10–20 nmol/L (4–8 ng/mL) at the end of summer to allow for the seasonal decrease in winter.
Includes pregnant women.
ESPG, Endocrine Society Practice Guideline; SACN, Scientific Advisory Committee on Nutrition; IOM, Institute of Medicine; EFSA, European Food Safety Authority.
Scientific Advisory Committee on Nutrition
The SACN Working Group on Vitamin D reviewed the relationship between vitamin D and various health outcomes.4 The committee took into account a detailed report published by the IOM in the US on Dietary Reference Intakes for Calcium and Vitamin D.5 SACN suggested that (a) the risk of rickets increased in children with 25(OH)D levels <25 nmol/L (10 ng/mL); and (b) serum concentrations of 25(OH)D ranged from 4–20 nmol/L (1.6–8 ng/mL) in case reports of osteomalacia. Although there are many uncertainties in the data, the evidence was suggestive overall of an increased risk of poor musculoskeletal health at serum 25(OH)D concentrations <~20–30 nmol/L (<8–12 ng/mL). The review suggests that a threshold serum 25(OH)D concentration of 25 nmol/L (10 ng/mL) is not diagnostic of disease but denotes the concentration below which risk of poor musculoskeletal health is increased at a population level. It could, therefore, be considered a ‘population protective’ concentration. Since the data were insufficient or inadequate to ascertain whether the threshold serum 25(OH)D concentration associated with increased risk of poor musculoskeletal health differs during pregnancy and lactation, the population protective concentration of 25 nmol/L (10 ng/mL) was extended to these groups. The review further suggests that data on vitamin D benefit on any non-musculoskeletal health outcome (e.g. hypertension, cancer) were insufficient to set a requirement for vitamin D. Musculoskeletal health outcomes were suggested as the basis for setting vitamin D requirements (Table 2).4
Table 2.
Recommendations on non-musculoskeletal outcomes.
| IOM | SACN | EFSA | |
|---|---|---|---|
| Non-musculoskeletal outcomes | Outcomes related to cancer/neoplasms, cardiovascular disease and hypertension, diabetes and metabolic syndrome, falls and physical performance, immune functioning and autoimmune disorders, infections, neuropsychological functioning, and pre-eclampsia could not be linked reliably with calcium or vitamin D intake and were often conflicting. | Non-musculoskeletal health outcomes considered were: reproductive health (on maternal & newborn outcomes), cancer, cardiovascular disease, hypertension, all-cause mortality, immune modulation, infectious diseases, neuropsychological functioning, oral health and age-related macular degeneration. Results from vitamin D supplementation are inconsistent. Data insufficient to set nutritional requirements. | The available evidence on non-musculoskeletal health outcomes is insufficient to be used as criterion for setting nutritional requirements for vitamin D. |
IOM, Institute of Medicine; SACN, Scientific Advisory Committee on Nutrition; EFSA, European Food Safety Authority.
Endocrine Society Practice Guidelines
In their review, Bischoff-Ferrari et al. estimated thresholds for serum 25(OH)D in relation to BMD, lower extremity function, dental health, and risk of falls, fractures and colorectal cancer.153 The authors suggest that for all these endpoints the most advantageous serum concentrations of 25(OH)D begin at 75 nmol/L (30 ng/mL) and are best at 90–100 nmol/L (36–40 ng/mL). The authors also suggest that health risks defined by hypercalcaemia and nephrolithiasis were not increased at this level, but suggest that the recommended calcium intake may require a downward adjustment if vitamin D intake is adequate. The study suggests an optimal 25(OH)D status at 75 nmol/L (30 ng/mL).154
The Endocrine Society Practice Guidelines use intact PTH suppression by serum 25(OH)D level to define hypovitaminosis. Several studies have reported that PTH levels are inversely associated with 25(OH)D and plateau in adults who have blood 25(OH)D levels of 75–100 nmol/L (30–40 ng/mL).155,156 Based on this and other studies, they define vitamin D deficiency as 25(OH)D <50 nmol/L (<20 ng/mL). The plasma PTH concentration varies widely within and among individuals at any given concentration of 25(OH)D.5 Other studies did not find a threshold of 25(OH)D at which PTH plateaus, or found threshold levels for PTH varied at serum 25(OH)D levels of 25–62.4 nmol/L (10–24.9 ng/mL). Calcium intake has been shown to affect serum PTH levels and this may be dependent on serum 25(OH)D levels.157 Establishment of a threshold has been further hampered by standardisation of PTH assays and specimen stability.158
Other arguments for a lower level of 25(OH)D to be used in the definition of vitamin D deficiency are that the regulation of calcium homeostasis is based on several hormones (1,25(OH)2D, PTH and FGF23) and that the measurement of these hormones can be used to evaluate optimal vitamin D status as reflected by serum 25(OH)D. Plateau levels of 1,25(OH)2D are reached once 25(OH)D levels exceed 10 nmol/L (4 ng/mL). Similarly, calcium absorption was maintained until the mean level of 25(OH)D fell below 10 nmol/L (4 ng/mL).159 Using different methodology, calcium absorption was higher in patients with 25(OH)D levels ranging up to 86 nmol/L (34.4 ng/mL).160 One possible explanation is that high levels of 25(OH)D act as agonists for VDR.
EFSA Recommendations
The EFSA Panel suggests that at 25(OH)D concentrations >50 nmol/L (>20 ng/mL), the risk of increased BMD/bone mineral content (BMC) loss in free-living adults, osteomalacia, rickets and fractures is decreased. They did not assign a target range for muscle strength function or risk of falling. Overall, the EFSA Panel considered that there is some evidence that, in infants and children, increasing mean serum 25(OH)D from about 40–60 nmol/L (16–24 ng/mL) to higher values is not associated with further benefit on BMC/BMD.3
Factors that Affect Treatment with Vitamin D
Pregnancy
Maternal vitamin D status has been investigated as a determinant of offspring bone development. Evidence either supports a role for vitamin D status that can affect bone mineral accrual during the intrauterine period or does not as it did not lead to increased offspring whole-body BMC compared with placebo. The Maternal Vitamin D Osteoporosis Study (MAVIDOS) is a randomised double-blind placebo-controlled trial of antenatal vitamin D supplementation of 1000 IU/d from 14 w gestation. First reports suggest that whole-body BMC and BMD were higher in children born to mothers in winter randomised to cholecalciferol.4,161,162 In a further review, Curtis et al. suggest that although there is evidence to support a positive relationship between vitamin D status and offspring bone mass/birthweight, conflicting evidence still exists.163 They suggest that maternal vitamin D supplementation decreases the risk of neonatal hypocalcaemia.
Musculoskeletal Function
Other described effects of vitamin D supplementation on musculoskeletal function are: (a) increase in muscle function and strength in participants <40 y;164 (b) increase in BMD in older men and women;165,166 and (c) with combined calcium supplementation, reduced risk of any type of fracture.167,168 Evidence was mixed on falls,4 but overall was suggestive of a beneficial effect of vitamin D supplementation in reducing fall risk in adults ≥50 y with mean baseline serum 25(OH)D concentrations ranging between <25 nmol/L (<10 ng/mL) and ~80 nmol/L (~32 ng/mL). However, a high dose of vitamin D (500,000 IU/y; 12,500 μg/y) resulted in higher risk of falls and fractures.169 Two recent studies suggest no changes in BMD and/or a decrease in BMD at high-dose vitamin D supplementation.170,171 A further study showed that high-dose vitamin D treatment had neither beneficial nor adverse effects on falls and muscle function.172 The US Preventive Services Task Force guidelines found inadequate evidence to estimate the benefits of vitamin D, calcium or combined supplementation to prevent fractures in community-dwelling men and premenopausal women.173
Extraskeletal Effects
Vitamin D as a hormone is likely to have many extraskeletal effects. VDR is present in a number of tissues and cells in the body and this resulted in a much greater appreciation of the physiological role of vitamin D. Experimental studies suggest a biological plausibility for a role for vitamin D in extraskeletal biology. This has led to a widespread investigation on the use of vitamin D in the prevention and treatment of chronic diseases. Table 2 summarises recent reviews on the use of vitamin D in non-musculoskeletal disorders.
Personalised Vitamin D Index
Stimulation of cell culture models with high-dose 1,25(OH)2D promotes genomic VDR binding and chromatin opening. Two trials investigated long-term vitamin D intervention and short term vitamin D bolus and measured a wide range of vitamin D-triggered parameters such as changes in gene expression, chromatin accessibility and serum proteins and metabolites suitable for determining vitamin D response. This allowed the study participants to be classified into high, mid and low responders. The study suggests that the dose of daily vitamin D supplementation can be adapted to the vitamin D response index.174 A further study confirmed the difference in genomic response between ‘weak’ and ‘strong’ responders. Though treatment with vitamin D changed gene expression in all the subjects, there was a considerable difference in inter-individual response. The authors suggest that there was a dissociation between calcaemic and non-calcaemic function of vitamin D3 especially in functions involved in immunoreactivity.175
Individualised Assessment of Vitamin D Status
Circulating concentrations of 25(OH)D and 24,25(OH)2D are closely related. It has been suggested that both 25(OH)D and 24,25(OH)2D concentrations can be compared to those of healthy subjects. Theoretically, vitamin D-deficient patients cannot afford to waste 25(OH)D and CYP24A1 is down regulated. It is likely that lower 25(OH)D concentrations and low or undetectable 24,25(OH)2D are indicative of functional vitamin D deficiency. When 25(OH)D is high, 24,25(OH)2D is formed to protect against hypercalcaemia. Undetectable 24,25(OH)2D in this context may suggest CYP24A1 deficiency or granulomatous disease. However, the interpretation of 25(OH)D and 24,25(OH)2D is still a matter of debate. The authors suggest that the results show that vitamin D deficiency, as defined biochemically, could be around concentrations of 25(OH)D of 50 nmol/L (20 ng/mL) in infants, children, adolescents and young adults, and that vitamin D deficiency could be evaluated on a more individual basis.176
Upper Limit of Vitamin D Intake
Clinical symptoms of vitamin D toxicity are the result of hypercalcaemia and hypercalciuria. Symptoms include neuropsychiatric manifestations such as lethargy and confusion, stupor, coma, GIT symptoms such as anorexia, vomiting and constipation, cardiovascular manifestations, polyuria and renal colic from the passage of renal stones.177
Intervention studies with vitamin D varied in design, administered dose regimen and duration of the study. Case reports of vitamin D intoxication have been reported in the literature. A summary of a few of the intervention studies and case reports is given in Table 3. Serum 25(OH)D levels as a result of exposure to sunshine can be as high as 225 nmol/L (90 ng/mL). In a review of multiple studies, Vieth et al. suggest that serum 25(OH)D rises steeply at intakes of vitamin D >10,000 IU/d (250 μg/d).178 In an early study, Adams et al. suggest that vitamin D and calcium intake with values of serum 25(OH)D in the range 177–222 nmol/L (70.8–88.8 ng/mL) were associated with hypercalciuria.179 In a further study, vitamin D and calcium supplementation increased the risk of renal stones.180 The suggested maximum intakes that can be consumed every day over a lifetime without appreciable risk to health, from several health authorities, are summarised in Table 4.
Table 3.
Studies which contributed to recommended upper intake levels for vitamin D.
| Dose | Interval | Duration | Basal 25(OH)D concentration nmol/L (ng/mL) | Final 25(OH)D concentration nmol/L (ng/mL) | |
|---|---|---|---|---|---|
| 1000 IU (25 μg) | Daily | 3 m | 43.3±16.8 (17.3±6.7) | 68.7±16.9 (27.5±6.8) | No significant change in serum calcium49 |
| 4000 IU (100 μg) | Daily | 3 m | 37.9 ±13.4 (15.2±5.4) | 96.4±14.6 (38.6±5.8) | No significant change in serum calcium49 |
| 12,000/24,000/48,000 IU (300/600/1200 μg) | Monthly | 1 y | 41.6, 39.5, 38.9 (16.6, 15.9, 15.6) | 55.9, 64.6, 79.0 (22.4, 25.8, 31.6) | No symptomatic case of hypercalcaemia when measured every 3 m170 |
| 400/4000/10,000 IU (10/100/250 μg) | Daily | 3 y | 76.3, 81.3, 78.4 (30.5, 32.5, 31.4) | 77.4, 132.2, 144.4 (30.9, 52.9, 57.8) | Serum calcium measured at 6-monthly intervals. Report mild dose-dependent hypercalcaemia171 |
| 50,000IU (1250 μg ) | Daily | 2 m | 735 (294) | Clinical signs of vitamin D toxicity: failure to thrive, dehydration, nausea, vomiting181 | |
| Milk over-fortified with vitamin D | 731±434 (292±174) | Hypercalcaemia182 |
Table 4.
Acceptable upper limit of vitamin D intake stated by various health bodies.*
| Age | ESPG | SACN | IOM | EFSA |
|---|---|---|---|---|
| Infant | 0–6 m: 1000 IU (25 μg)/d |
1000 IU (25 μg)/d | 0–6 m: 1000 IU (25 μg)/d |
1000 IU (25 μg)/d |
| 6–12 m: 1500 IU (37.5 μg)/d |
6–12 m: 1500 IU (37.5 μg)/d |
|||
| 1–10 y | 1–3 y: 2500 IU (62.5 μg)/d |
2000 IU (50 μg)/d | 1–3 y: 2500 IU (62.5 μg)/d |
2000 IU (50 μg)/d (children and adolescents) |
| 4–8 y: 3000 IU (75 μg)/d |
4–8 y: 3000 IU (75 μg)/d |
|||
| 11–17 y | >8 y: 4000 IU (100 μg)/d |
4000 IU (100 μg)/d | 9–18 y: 4000 IU (100 μg)/d |
|
| Adult | 4000 IU (100 μg)/d | 4000 IU (100 μg)/d | 19->70 y: 4000 IU (100 μg)/d |
4000 IU (100 μg)/d |
The upper limit did not apply to individuals with certain medical conditions such as normocalcaemic hyperparathyroidism and granulomatous conditions (sarcoidosis and tuberculosis) which predispose to hypercalcaemia, or to those with genetic conditions such as familial hypocalciuric hypercalcaemia.
ESPG, Endocrine Society Practice Guideline; SACN, Scientific Advisory Committee on Nutrition; IOM, Institute of Medicine; EFSA, European Food Safety Authority.
Nutritional Intake of Vitamin D
Experts recommend an age-dependent minimum intake of calcium to avoid calcium deficiency-associated rickets. Adequate levels of both vitamin D and dietary calcium are needed to ensure optimum serum calcium levels, and to prevent long-term adverse effects on the bone. Several online calcium calculators for adequate calcium intake are available, such as the Institute of Genetics and Molecular Medicine calcium calculator.183 There are disagreements concerning the nature of the available data regarding the dietary intake of vitamin D and the serum level of 25(OH)D that can constitutes vitamin D deficiency.
Institute of Medicine
IOM defines the following components of dietary reference intake (DRI): (i) Estimated Average Requirement (EAR), reflects the estimated median requirement; (ii) Recommended Dietary Allowance (RDA), derived from the EAR and meets or exceeds the requirement for 97.5% of the population (i.e. 2 standard deviations above the EAR); and (iii) Adequate Intake (AI), used when an EAR/RDA cannot be developed.5 Recommendations are based on the assumptions of minimal sun exposure and that all vitamin D comes from the diet. The potential contribution from body stores is unknown and this introduces further uncertainty. Serum levels of 25(OH)D were used as a measure of adequacy for vitamin D intake. Recent studies suggest that more than one metabolite of vitamin D may be required to assess adequate intake. The IOM selected bone health as the indicator to serve as the basis of DRI for calcium and vitamin D. The dose-response between bone health and vitamin D intake is lacking and instead serum 25(OH)D is used as a biomarker of vitamin D intake. The committee further concluded that a dose-response relationship can be simulated based on measured serum 25(OH)D concentrations. If the estimated requirement range for 25(OH)D was 30–50 nmol/L (12–20 ng/mL), what remained was to ascertain the level of vitamin D intake that would achieve these levels of 25(OH)D in serum. IOM carried out a regression analysis of the relationship between serum 25(OH)D level and total intake of vitamin D during the winter season. The IOM acknowledges the wide variability in the observed dose-response curves in the published literature (large inter-study variance) as well as a nonlinear dose-response relationship with increasing vitamin D intake. They found that age does not influence the change in serum 25(OH)D level in response to vitamin D intake. The following equation was predicted: achieved 25(OH)D in nmol/L = 9.9 loge (total vitamin D intake). They suggest that an intake of 400 IU (10 μg) is associated with a predicted mean circulating 25(OH)D level of 59 nmol/L (23.6 ng/mL) in children and adolescents, young and middle-aged adults and older adults (Table 5). There is a large confidence interval for the predicted values and IOM advises caution in the presence of several uncertainties in the calculation. The IOM recommends an EAR of 400 IU/d (10 μg/d) with RDA of 600 IU/d (15 μg/d) for ages 1–70 y and during pregnancy and lactation. Data were not sufficient to establish EAR for infants <1 y and an AI of 400 IU/d was suggested. For adults >70 y they suggest a RDA of 800 IU/d (20 μg/d) to decrease the risk of fracture and to allow the physiological aging of individuals, but IOM acknowledges the lack of dose-response data for this recommendation.
Table 5.
Vitamin D intake recommended by the various government bodies and working groups.
| IOM* | SACN* | EFSA* | Endocrine Society | Australian Working Group† | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | AI | EAR | RDA | Age | RDA | Age | AI | Age | Daily requirement | Age | Daily requirement |
| 0–1 y | 400 IU (10 μg)/d | 0–1 y | 340–400 IU (8.5–10 μg)/d | 7–11 m | 400 IU (10 μg)/d | 0–1 y | 400–1000 IU (10–25 μg)/d | ≤70 y | 600 IU (15 μg)/d | ||
| 1–70 y | 400 IU (10 μg)/d | 600 IU (15 μg) /d | >1 y | 400 IU (10 μg)/d | 1-≥18 y | 600 IU (15 μg)/d | 1–8 y | 600–1000 IU (15–25 μg)/d | >70 y | 800 IU (20 μg)/d | |
| Pregnancy and lactation | 400 IU (10 μg)/d | 600 IU (15 μg) /d | Pregnancy and lactation | 400 IU (10 μg)/d | Pregnancy and lactation | 600 IU (15 μg)/d | 9–18 y‡ | 600–1000 IU (15–25 μg)/d | |||
| 19–>70 y | 1500–2000 IU (37.5–50 μg)/d | ||||||||||
Under conditions of minimal cutaneous vitamin D synthesis.
In older people, 1000 IU (25 μg)/d with adequate calcium intake to prevent fracture.
Pregnancy and lactation daily intake was adjusted according to age as given.
AI, adequate intake; RDA, recommended dietary allowance; EAR, estimated average requirement.
Scientific Advisory Committee on Nutrition
SACN used a serum 25(OH)D concentration of 25 nmol/L (10 ng/mL) as the basis for establishing the RDA (also called reference nutrient intake, the amount of a nutrient that is sufficient to meet the needs of 97.5% of the population) for vitamin D.4 They suggest that, as the individuals have <25 nmol/L (<10 ng/mL) 25(OH)D during summer and winter despite exposure to sunlight, the RDA should apply throughout the year. SACN chose the threshold serum 25(OH)D concentration of 25 nmol/L (10 ng/mL) as indicative of the serum 25(OH)D concentration below which risk of poor musculoskeletal health is increased. Their approach differed from the IOM. The approach used to determine the RDA for vitamin D used data on the individual response of serum 25(OH)D concentration in winter to increased vitamin D intake from three randomised control trials (RCT) in adults aged 20–40 y,184 adults aged ≥64 y,185 and adolescent girls aged 11 y.186 These three RCTs were used because they were conducted in winter which minimised sunlight-dependent synthesis of vitamin D. There is no evidence to suggest that the RDA for children aged 4–<11 y should be a different value to the RDA proposed for the UK population aged ≥11 y. Based on these studies, they recommend an RDA of 400 IU/d (10 μg/d) for a population (in the UK) aged ≥4 y, and this included pregnant and breast feeding women. Data are not available to suggest the requirement for an additional increment during pregnancy and the RDA recommended for the general population is extended to pregnant and lactating women. A Safe Intake (SI) range of 340–400 IU/d (8.5–10 μg/d) is proposed for infants aged 0–11 m and includes those who are exclusively breast fed. A SI of 400 IU/d (10 μg/d) is proposed for children aged 1–<4 y. Since data are not available to relate serum 25(OH)D concentration in the infant, SIs rather than RDAs are proposed for ages 0–4 y. This reflects the insecurity of the data and SIs are stated as a level or range of intake at which there is no risk of deficiency, and below a level where there is a risk of undesirable effects. On the basis that there are currently insufficient data to set a higher RDA for people from different ethnic groups, the proposed RDA of 400 IU/d (10 μg/d) is considered appropriate to cover the needs of ethnic groups within the population.
European Food Safety Authority
The meta-regression dose-response model used by EFSA was based solely on randomised trials data.3 A non-linear model was used to describe the dose-response shape and the model was able to include results from trials using higher supplemental doses (i.e. up to 2000 IU/d, 50 μg/d). Due to the uncertainties in the calculation, EFSA considered that the average requirements for a population could not be derived and defined AIs instead:
y = 23.2 ln (total vitamin D intake in μg/d) (equation 1, unadjusted model)
y = 16.3 ln (total vitamin D intake) + 0.5 mean baseline 25(OH)D − 0.5 latitude + 0.9 study start year − 2.0 HPLC − 4.7 LC−MS + 0.6 CPBA − 6.4 ELISA/not reported + 1.3 Other assay +7.8 compliance not assessed (equation 2, adjusted model).
(y, achieved 25(OH)D concentration; CPBA, competitive protein binding assay)
The adjusted model predictions were interpreted as referring to an average ideal population in which the major factors influencing the heterogeneity across different populations have been ruled out. EFSA set AIs for vitamin D for adults, pregnant and lactating women and children (1–17 y) at 600 IU/d (15 μg/d) to achieve a serum 25(OH)D concentration near or above the target of 50 nmol/L (20 ng/mL). For infants aged 7–11 m, the EFSA sets an AI for vitamin D at 400 IU/d (10 μg/d). EFSA states that there was no evidence of difference in absorption capacity between younger and older adults and decided not to set specific AIs for the two groups. EFSA acknowledges the paucity of data for setting the AIs for children, pregnant and lactating women.
Endocrine Society Guidelines
The Endocrine Society clinical practice guideline recommends the following: children aged 0–1 y, 400 IU/d (10 μg/d), and for 1–18 y at least 400–600 IU/d (10–15 μg/d); but may require at least 1000 IU/d to achieve a serum level of 25(OH)D >75 nmol/L (>30 ng/mL).6 For adults aged 19–50 y and pregnant and lactating women, the guideline recommends at least 600 IU/d (15 μg/d), and at least 1500–2000 IU/d (37.5–50 μg/d) to keep the serum level of 25(OH)D >75 nmol/L (>30 ng/mL). For adults aged ≥50 y the guidelines suggest at least 600–800 IU/d (15–20 μg/d), and at least 1500–2000 IU/d (37.5–50 μg/d) to raise the blood level of 25(OH)D above 75 nmol/L (30 ng/mL). The authors include meta-analysis as well as individual studies to support their recommendations. The disagreements between the Endocrine Society guideline and IOM resulted in a commentary by the IOM suggesting that some aspects of the Endocrine Society guidelines are not supported.187
Treatment of Vitamin D Deficiency
Most guidelines recommend oral vitamin D3 as a treatment of choice for vitamin D deficiency. However, vitamin D2 may be preferred by vegetarians and those who prefer to avoid vitamin D from animal origin. The National Osteoporosis Society guideline recommends treatment of symptomatic vitamin D deficiency, or when treatment is about to be started with a potent anti-resorptive agent such as zoledronate or densosumab, with a fixed loading dose vitamin D. Treatment is given either as separate weekly or daily doses over 6–10 w: 50,000 IU (1250 μg) given weekly for 6 w, a total of 300,000 IU (7500 μg); 40,000 IU (1000 μg) given weekly for 7 w, a total of 280,000 IU (7000 μg); 4000 IU (100 μg) given daily over 10 w, a total of 280,000 IU (7000 μg). This is followed by maintenance doses of 800–2000 IU (20–50 μg) daily or on occasion 4000 IU (100 μg) daily given either daily or intermittently at high doses. When correction of vitamin D is less urgent or when co-prescribing antiresorptive agents, the guideline recommends starting the maintenance therapy without the loading dose. The authors state that ‘there is no available evidence with clinical outcomes that giving loading doses in excess of 15,000 μg or 600,000 IU of vitamin D3/D2 has any beneficial effect, in the absence of clinical osteomalacia’.188,189 The principal aim of the therapy is to replenish vitamin D stores following which patients are continued on a maintenance dose.
In subjects given a single oral dose of 100,000 IU (2500 μg) of vitamin D3 the 25(OH)D peaked at 103 nmol/L (41.2 ng/mL) and fell slowly to baseline by day 112.92 Ilahi et al. report the highest concentration achieved at a single oral dose of 100,000 IU (2500 μg) was 160 nmol/L (64 ng/mL).74 A single annual oral dose of 500,000 IU (12,500 μg) of vitamin D resulted in 25(OH)D levels at 1 m of approximately 120 nmol/L (48 ng/mL) and at 3 m, 90 nmol/L (36 ng/mL).169 Monthly oral doses of 12,000 IU (300 μg), 24,000 IU (600 μg) and 48,000 IU (1200 μg) increased serum 25(OH)D to 55.9 nmol/L (22.4 ng/mL), 64.6 nmol/L (25.8 ng/mL) and 79 nmol/L (31.6 ng/mL) respectively at 12 m.170 Aspray et al.170 report no changes in BMD between the three doses of vitamin D over 12 m, but Sanders et al.169 report an increased risk of falls and fractures. In a further study, a total of approximately 600,000 IU was given to vitamin D-deficient subjects in different doses of (i) 10,000 IU/d (250 μg/d) for 8 w followed by 1000 IU/d (25 μg/d) for 4 w; (ii) 50,000 IU/d (1250 μg/d) for 12 w; and (iii) 100,000 IU (2500 μg)/every other week for 12 w. Mean 25(OH)D levels increased to ≥50 nmol/L (≥20 ng/mL) at 4 w and remained at ≥50 nmol/L (≥20 ng/mL) at 3 m.190 An oral regimen of 60,000 IU (1500 μg) weekly normalised serum 25(OH)D levels compared to daily dosing of 1000 IU/d (25 μg/d) vitamin D over a period of 10 w.191 Authors report a similar efficacy of monthly, weekly or daily doses192 while others report a slightly lower efficacy.193 In studies in children with nutritional rickets, hypercalcaemia was observed in approximately 10% of children treated with a single oral dose of 300,000 IU (7500 μg) or 600,000 IU (15,000 μg).194 In a further study, the authors report healing of rickets in children aged <5 y given 90,000 IU (2250 μg) of vitamin D given as a single oral dose, though they reported non-symptomatic transient hypercalcaemia in <10% of the children.195 It is likely that at high loading doses transient hypercalcaemia and hypercalciuria is observed, and possibly a transient increase in risks of falls and fractures. Longer dosing periods at lower treatment doses may, however, result in poor compliance.196
For infants aged 0–1 y and children aged 1–18 y who are vitamin D deficient, the Endocrine Society guidelines suggest a treatment with 2000 IU/d (50 μg/d) for 6 w or 50,000 IU (1250 μg) of vitamin D once weekly for 6 w to achieve a blood level of 75 nmol/L (30 ng/mL), followed by the recommended daily requirement as maintenance treatment. For adults they suggest 50,000 IU (1250 μg) of vitamin D once a week for 6 w or its equivalent of 6000 IU/d (150 μg/d) followed by the daily requirement as recommended by the Endocrine Society guidelines. Gordon et al. treated infants aged 8–24 m with either 2000 IU (50 μg) oral vitamin D daily or 50,000 IU (1250 μg) vitamin D weekly.197 In all participants, 25(OH)D increased from an average of 43 nmol/L to 90 nmol/L (17.2 ng/mL to 36 ng/mL). Very few studies have been conducted on vitamin D replacement in obese children and adults and children and adults on anticonvulsant medications. The Endocrine Society guidelines suggest that they should be given 2–3 times more vitamin D than suggested for their age group.6
The Australian Working Group recommends 3000–5000 IU/d (75–125 μg/d) for at least 6–12 w followed by 1000–2000 IU/d (25–50 μg/d) for 6–12 w followed by 1000 IU/d (25 μg/d). An alternative suggestion is 50,000 IU (1250 μg) vitamin D3 once per month for 3–6 m. They suggest most patients will require 1000 IU/day (25 μg/day) as ongoing treatment.7,8
Amid such differing evidence, recommendation on treating vitamin D deficiency is based on expert opinion. In theory, a titrated treatment approach is more likely to be more effective than a fixed loading dose regime at restoring vitamin D levels and reducing the risk of vitamin D toxicity. The disadvantages are the increased costs of titration testing and the effect of increasing complexity of the treatment on the community medical practitioner and the patient. It is suggested that patients under treatment are monitored if vitamin D deficiency is suspected after loading or during maintenance. Serum calcium is monitored to detect primary hyperparathyroidism uncovered by vitamin D treatment.198
Conclusion
Although the health benefits of vitamin D in musculoskeletal systems are established, vitamin D insufficiency is common across the globe. International guidelines differ in their recommendations for adequate vitamin D status. Dose-response studies of 25(OH)D to vitamin D intake vary in their study design, choice of patients, dose of vitamin D, and length of follow up of study patients. Another confounding factor is the analytical methods used to quantify 25(OH)D. Inconsistencies between the methods have to be taken into consideration when setting desired levels of 25(OH)D. Guidelines use systemic reviews of studies to recommend mean doses of vitamin D at various stages in life. Though consensus exists among the guidelines that serum levels of 25(OH)D <25 nmol/L (10 ng/mL) are to be avoided, there is dispute on the recommended vitamin D intake, in line with different study designs and expert opinion. Genetic variation in the metabolism of vitamin D can give rise to the concept of personalised vitamin D response and new research emphasises the importance of personalised medicine. Biomarkers of vitamin D status other than 25(OH)D have been identified in new metabolic pathways of vitamin D. Further studies are needed to identify the role of vitamin D in non-musculoskeletal outcomes. There is a lack of data on acceptable 25(OH)D concentrations in infants, children, pregnant and lactating women and certain ethnic groups. Further prospective randomised controlled studies are required to examine the determinants of vitamin D intake and adequacy in specialised patient groups that include patients on co-prescription drugs that affect vitamin D metabolism, patients with malabsorption or patients following gastric bypass surgery.
References
- 1.Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol. 2014;144:138–45. doi: 10.1016/j.jsbmb.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jenkinson C. The vitamin D metabolome: An update on analysis and function. Cell Biochem Funct. 2019;37:408–23. doi: 10.1002/cbf.3421. [DOI] [PubMed] [Google Scholar]
- 3.EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) Dietary reference values for vitamin D. EFSA Journal. 2016;14:e04547. [Google Scholar]
- 4.Scientific Advisory Committee on Nutrition. SACN vitamin D and health report. 2016. [Accessed 23 September 2020]. https://www.gov.uk/government/publications/sacn-vitamin-d-and-health-report.
- 5.Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Dietary Reference Intakes for Calcium and Vitamin. Washington DC: National Academic Press; 2011. [PubMed] [Google Scholar]
- 6.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 7.Nowson CA, McGrath JJ, Ebeling PR, Haikerwal A, Daly RM, Sanders KM, et al. Working Group of Australian and New Zealand Bone and Mineral Society, Endocrine Society of Australia and Osteoporosis Australia. Vitamin D and health in adults in Australia and New Zealand: a position statement. Med J Aust. 2012;196:686–7. doi: 10.5694/mja11.10301. [DOI] [PubMed] [Google Scholar]
- 8.Homer CS, Oats J, Middleton P, Ramson J, Diplock S. Updated clinical practice guidelines on pregnancy care. Med J Aust. 2018;209:409–12. doi: 10.5694/mja18.00286. [DOI] [PubMed] [Google Scholar]
- 9.Smith JE, Goodman DS. The turnover and transport of vitamin D and of a polar metabolite with the properties of 25-hydroxycholecalciferol in human plasma. J Clin Invest. 1971;50:2159–67. doi: 10.1172/JCI106710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mawer EB, Schaefer K, Lumb GA, Stanbury SW. The metabolism of isotopically labelled vitamin D3 in man: the influence of the state of vitamin D nutrition. Clin Sci. 1971;40:39–53. doi: 10.1042/cs0400039. [DOI] [PubMed] [Google Scholar]
- 11.Haddad JG, Jr, Walgate J. 25-Hydroxyvitamin D transport in human plasma. Isolation and partial characterization of calcifidiol-binding protein. J Biol Chem. 1976;251:4803–9. [PubMed] [Google Scholar]
- 12.Kawakami M, Imawari M, Goodman DS. Quantitative studies of the interaction of cholecalciferol ((vitamin D3) and its metabolites with different genetic variants of the serum binding protein for these sterols. Biochem J. 1979;179:413–23. doi: 10.1042/bj1790413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levine BS, Singer FR, Bryce GF, Mallon JP, Miller ON, Coburn JW. Pharmacokinetics and biologic effects of calcitriol in normal humans. J Lab Clin Med. 1985;105:239–46. [PubMed] [Google Scholar]
- 14.Hollis BW, Wagner CL. New insights into the vitamin D requirements during pregnancy. Bone Res. 2017;5:17030. doi: 10.1038/boneres.2017.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramasamy I. Inherited disorders of calcium homeostasis. Clin Chim Acta. 2008;394:22–41. doi: 10.1016/j.cca.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Ramasamy I. Recent advances in physiological calcium homeostasis. Clin Chem Lab Med. 2006;44:237–73. doi: 10.1515/CCLM.2006.046. [DOI] [PubMed] [Google Scholar]
- 17.Reddy GS, Omdahl JL, Robinson M, Wang G, Palmore GTR, Vicchio D, et al. 23-carboxy-24,25,26,27-tetranorvitamin D3 (calcioic acid) and 24-carboxy-25,26,27-trinorvitamin D3 (cholacalcioic acid): end products of 25-hydroxyvitamin D3 metabolism in rat kidney through C-24 oxidation pathway. Arch Biochem Biophys. 2006;455:18–30. doi: 10.1016/j.abb.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 18.Prosser DE, Kaufmann M, O’Leary B, Byford V, Jones G. Single A326G mutation converts human CYP24A1 from 25-OH-D3-24-hydroxylase into -23-hydroxylase, generating 1α,25-(OH)2D3-26,23-lactone. Proc Natl Acad Sci USA. 2007;104:12673–8. doi: 10.1073/pnas.0702093104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plachot JJ, Du Bois MB, Halpern S, Cournot-Witmer G, Garabedian M, Balsan S. In vitro action of 1,25-dihydroxycholecalciferol and 24,25-dihydroxycholecalciferol on matrix organization and mineral distribution in rabbit growth plate. Metab Bone Dis Relat Res. 1982;4:135–42. doi: 10.1016/0221-8747(82)90027-3. [DOI] [PubMed] [Google Scholar]
- 20.Ishizuka S, Kurihara N, Reddy SV, Cornish J, Cundy T, Roodman GD. (23S)-25-Dehydro-1{alpha}-hydroxyvitamin D3-26,23-lactone, a vitamin D receptor antagonist that inhibits osteoclast formation and bone resorption in bone marrow cultures from patients with Paget’s disease. Endocrinology. 2005;146:2023–30. doi: 10.1210/en.2004-1140. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Lin YS, Zheng XE, Senn T, Hashizume T, Scian M, et al. An inducible cytochrome P450 3A4-dependent vitamin D catabolic pathway. Mol Pharmacol. 2012;81:498–509. doi: 10.1124/mol.111.076356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roizen JD, Li D, O’Lear L, Javaid MK, Shaw NJ, Ebeling PR, et al. CYP3A4 mutation causes vitamin D-dependent rickets type 3. J Clin Invest. 2018;128:1913–8. doi: 10.1172/JCI98680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Y, Hashizume T, Shuhart MC, Davis CL, Nelson WL, Sakaki T, et al. Intestinal and hepatic CYP3A4 catalyze hydroxylation of 1alpha,25-dihydroxyvitamin D(3): implications for drug-induced osteomalacia. Mol Pharmacol. 2006;69:56–65. doi: 10.1124/mol.105.017392. [DOI] [PubMed] [Google Scholar]
- 24.Slominski AT, Li W, Kim TK, Semak I, Wang J, Zjawiony JK, et al. Novel activities of CYP11A1 and their potential physiological significance. J Steroid Biochem Mol Biol. 2015;151:25–37. doi: 10.1016/j.jsbmb.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engelman CD, Bo R, Zuelsdorff M, Steltenpohl H, Kirby T, Nieto FJ. Epidemiologic study of the C-3 epimer of 25-hydroxyvitamin D3 in a population-based sample. Clin Nutr. 2014;33:421–5. doi: 10.1016/j.clnu.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berger SE, Van Rompay MI, Gordon CM, Goodman E, Eliasziw M, Holick MF, et al. Investigation of the C-3-epi-25(OH)D3 of 25-hydroxyvitamin D3 in urban schoolchildren. Appl Physiol Nutr Metab. 2018;43:259–65. doi: 10.1139/apnm-2017-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torugsa S, Nimitphong H, Warodomwichit D, Chailurkit LO, Srijaruskul K, Chanprasertyothin S, et al. The genetic determinants of circulating C3-epimers of 25-hydroxyvitamin D. J Clin Transl Endocrinol. 2018;12:36–41. doi: 10.1016/j.jcte.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yazdanpanah M, Bailey D, Walsh W, Wan B, Adeli K. Analytical measurement of serum 25-OH-vitamin D3, 25-OH-vitamin D2 and their C3-epimers by LC-MS/MS in infant and pediatric specimens. Clin Biochem. 2013;46:1264–71. doi: 10.1016/j.clinbiochem.2012.11.030. [DOI] [PubMed] [Google Scholar]
- 29.Aghajafari F, Field CJ, Rabi D, Kaplan BJ, Maggiore JA, O’Beirne M, et al. APrON Study Team. Plasma 3-epi-25-hydroxycholecalciferol can alter the assessment of vitamin D status using the current reference ranges for pregnant women and their newborns. J Nutr. 2016;146:70–5. doi: 10.3945/jn.115.220095. [DOI] [PubMed] [Google Scholar]
- 30.Karras SN, Shah I, Petroczi A, Goulis DG, Bili H, Papadopoulou F, et al. An observational study reveals that neonatal vitamin D is primarily determined by maternal contributions: implications of a new assay on the roles of vitamin D forms. Nutr J. 2013;12:77. doi: 10.1186/1475-2891-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamao M, Tatematsu S, Hatakeyama S, Sakaki T, Sawada N, Inouye K, et al. C-3 epimerization of vitamin D3 metabolites and further metabolism of C-3 epimers: 25-hydroxyvitamin D3 is metabolized to 3-epi-25-hydroxyvitamin D3 and subsequently metabolized through C-1alpha or C-24 hydroxylation. J Biol Chem. 2004;279:15897–907. doi: 10.1074/jbc.M311473200. [DOI] [PubMed] [Google Scholar]
- 32.Brown AJ, Ritter C, Slatopolsky E, Muralidharan KR, Okamura WH, Reddy GS. 1Alpha,25-dihydroxy-3-epi-vitamin D3, a natural metabolite of 1alpha,25-dihydroxyvitamin D3, is a potent suppressor of parathyroid hormone secretion. J Cell Biochem. 1999;73:106–13. [PubMed] [Google Scholar]
- 33.Al-Zohily B, Al-Menhali A, Gariballa S, Haq A, Shah I. Epimers of vitamin D: a review. Int J Mol Sci. 2020;21:e470. doi: 10.3390/ijms21020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong T, Wang Z, Chapron BD, Suzuki M, Claw KG, Gao C, et al. Polymorphic human sulfotransferase 2A1 mediates the formation of 25-hydroxyvitamin D3-3-O-sulfate, a major circulating vitamin D metabolite in humans. Drug Metab Dispos. 2018;46:367–79. doi: 10.1124/dmd.117.078428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z, Wong T, Hashizume T, Dickmann LZ, Scian M, Koszewski NJ, et al. Human UGT1A4 and UGT1A3 conjugate 25-hydroxyvitamin D3: metabolite structure, kinetics, inducibility, and interindividual variability. Endocrinology. 2014;155:2052–63. doi: 10.1210/en.2013-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26:662–87. doi: 10.1210/er.2004-0002. [DOI] [PubMed] [Google Scholar]
- 37.Chapuy MC, Chapuy P, Meunier PJ. Calcium and vitamin D supplements: effects on calcium metabolism in elderly people. Am J Clin Nutr. 1987;46:324–8. doi: 10.1093/ajcn/46.2.324. [DOI] [PubMed] [Google Scholar]
- 38.Barger-Lux MJ, Heaney RP, Dowell S, Chen TC, Holick MF. Vitamin D and its major metabolites: serum levels after graded oral dosing in healthy men. Osteoporos Int. 1998;8:222–30. doi: 10.1007/s001980050058. [DOI] [PubMed] [Google Scholar]
- 39.Vieth R, Ladak Y, Walfish PG. Age-related changes in the 25-hydroxyvitamin D versus parathyroid hormone relationship suggest a different reason why older adults require more vitamin D. J Clin Endocrinol Metab. 2003;88:185–91. doi: 10.1210/jc.2002-021064. [DOI] [PubMed] [Google Scholar]
- 40.Vaes AMM, Tieland M, de Regt MF, Wittwer J, van Loon LJC, de Groot LCPGM. Dose-response effects of supplementation with calcifediol on serum 25-hydroxyvitamin D status and its metabolites: A randomized controlled trial in older adults. Clin Nutr. 2018;37:808–14. doi: 10.1016/j.clnu.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 41.Aloia JF, Patel M, Dimaano R, Li-Ng M, Talwar SA, Mikhail M, et al. Vitamin D intake to attain a desired serum 25-hydroxyvitamin D concentration. Am J Clin Nutr. 2008;87:1952–8. doi: 10.1093/ajcn/87.6.1952. [DOI] [PubMed] [Google Scholar]
- 42.Phinney KW, Bedner M, Tai SS, Vamathevan VV, Sander LC, Sharpless KE, et al. Development and certification of a standard reference material for vitamin D metabolites in human serum. Anal Chem. 2012;84:956–62. doi: 10.1021/ac202047n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carter GD, Berry J, Durazo-Arvizu R, Gunter E, Jones G, Jones J, et al. Hydroxyvitamin D assays: An historical perspective from DEQAS. J Steroid Biochem Mol Biol. 2018;177:30–5. doi: 10.1016/j.jsbmb.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 44.Stamp TC, Haddad JG, Twigg CA. Comparison of oral 25-hydroxycholecalciferol, vitamin D, and ultraviolet light as determinants of circulating 25-hydroxyvitamin D. Lancet. 1977;1:1341–3. doi: 10.1016/s0140-6736(77)92553-3. [DOI] [PubMed] [Google Scholar]
- 45.Lips P, Wiersinga A, van Ginkel FC, Jongen MJ, Netelenbos JC, Hackeng WH, et al. The effect of vitamin D supplementation on vitamin D status and parathyroid function in elderly subjects. J Clin Endocrinol Metab. 1988;67:644–50. doi: 10.1210/jcem-67-4-644. [DOI] [PubMed] [Google Scholar]
- 46.Davie MW, Lawson DE, Emberson C, Barnes JL, Roberts GE, Barnes ND. Vitamin D from skin: contribution to vitamin D status compared with oral vitamin D in normal and anticonvulsant-treated subjects. Clin Sci (Lond) 1982;63:461–72. doi: 10.1042/cs0630461. [DOI] [PubMed] [Google Scholar]
- 47.Byrne PM, Freaney R, McKenna MJ. Vitamin D supplementation in the elderly: review of safety and effectiveness of different regimes. Calcif Tissue Int. 1995;56:518–20. doi: 10.1007/BF00298580. [DOI] [PubMed] [Google Scholar]
- 48.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–10. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- 49.Vieth R, Chan PC, MacFarlane GD. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am J Clin Nutr. 2001;73:288–94. doi: 10.1093/ajcn/73.2.288. [DOI] [PubMed] [Google Scholar]
- 50.McKenna MJ, Murray BF. Vitamin D dose response is underestimated by Endocrine Society’s Clinical Practice Guideline. Endocr Connect. 2013;2:87–95. doi: 10.1530/EC-13-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Autier P, Gandini S, Mullie P. A systematic review: influence of vitamin D supplementation on serum 25-hydroxyvitamin D concentration. J Clin Endocrinol Metab. 2012;97:2606–13. doi: 10.1210/jc.2012-1238. [DOI] [PubMed] [Google Scholar]
- 52.Asghari G, Farhadnejad H, Hosseinpanah F, Moslehi N, Mirmiran P, Azizi F. Effect of vitamin D supplementation on serum 25-hydroxyvitamin D concentration in children and adolescents: a systematic review and meta-analysis protocol. BMJ Open. 2018;8:e021636. doi: 10.1136/bmjopen-2018-021636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trang HM, Cole DE, Rubin LA, Pierratos A, Siu S, Vieth R. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr. 1998;68:854–8. doi: 10.1093/ajcn/68.4.854. [DOI] [PubMed] [Google Scholar]
- 54.Zittermann A, Ernst JB, Gummert JF, Börgermann J. Vitamin D supplementation, body weight and human serum 25-hydroxyvitamin D response: a systematic review. Eur J Nutr. 2014;53:367–74. doi: 10.1007/s00394-013-0634-3. [DOI] [PubMed] [Google Scholar]
- 55.Zwart SR, Mehta SK, Ploutz-Snyder R, Bourbeau Y, Locke JP, Pierson DL, et al. Response to vitamin D supplementation during Antarctic winter is related to BMI, and supplementation can mitigate Epstein-Barr virus reactivation. J Nutr. 2011;141:692–7. doi: 10.3945/jn.110.134742. [DOI] [PubMed] [Google Scholar]
- 56.Blum M, Dallal GE, Dawson-Hughes B. Body size and serum 25 hydroxy vitamin D response to oral supplements in healthy older adults. J Am Coll Nutr. 2008;27:274–9. doi: 10.1080/07315724.2008.10719700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tepper S, Shahar DR, Geva D, Ish-Shalom S. Predictors of serum 25(Oh)D increase following bimonthly supplementation with 100,000IU vitamin D in healthy men aged 25–65 years. J Steroid Biochem Mol Biol. 2014;144:163–6. doi: 10.1016/j.jsbmb.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 58.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–3. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 59.Goussous R, Song L, Dallal GE, Dawson-Hughes B. Lack of effect of calcium intake on the 25-hydroxyvitamin d response to oral vitamin D3. J Clin Endocrinol Metab. 2005;90:707–11. doi: 10.1210/jc.2004-1380. [DOI] [PubMed] [Google Scholar]
- 60.Bell NH, Shaw S, Turner RT. Evidence that calcium modulates circulating 25-hydroxyvitamin D in man. J Bone Miner Res. 1987;2:211–4. doi: 10.1002/jbmr.5650020307. [DOI] [PubMed] [Google Scholar]
- 61.Thomas SDC, Need AG, Nordin BEC. Suppression of C-terminal telopeptide in hypovitaminosis D requires calcium as well as vitamin D. Calcif Tissue Int. 2010;86:367–74. doi: 10.1007/s00223-010-9354-3. [DOI] [PubMed] [Google Scholar]
- 62.Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89:5387–91. doi: 10.1210/jc.2004-0360. [DOI] [PubMed] [Google Scholar]
- 63.Heaney RP, Recker RR, Grote J, Horst RL, Armas LA. Vitamin D3 is more potent than vitamin D2 in humans. J Clin Endocrinol Metab. 2011;96:E447–52. doi: 10.1210/jc.2010-2230. [DOI] [PubMed] [Google Scholar]
- 64.Tripkovic L, Wilson LR, Hart K, Johnsen S, de Lusignan S, Smith CP, et al. Daily supplementation with 15 μg vitamin D2 compared with vitamin D3 to increase wintertime 25-hydroxyvitamin D status in healthy South Asian and white European women: a 12-wk randomized, placebo-controlled food-fortification trial. Am J Clin Nutr. 2017;106:481–90. doi: 10.3945/ajcn.116.138693. [DOI] [PubMed] [Google Scholar]
- 65.Tripkovic L, Lambert H, Hart K, Smith CP, Bucca G, Penson S, et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95:1357–64. doi: 10.3945/ajcn.111.031070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Holmberg I, Berlin T, Ewerth S, Björkhem I. 25-Hydroxylase activity in subcellular fractions from human liver. Evidence for different rates of mitochondrial hydroxylation of vitamin D2 and D3. Scand J Clin Lab Invest. 1986;46:785–90. doi: 10.3109/00365518609084051. [DOI] [PubMed] [Google Scholar]
- 67.Hollis BW. Comparison of equilibrium and disequilibrium assay conditions for ergocalciferol, cholecalciferol and their major metabolites. J Steroid Biochem. 1984;21:81–6. doi: 10.1016/0022-4731(84)90063-3. [DOI] [PubMed] [Google Scholar]
- 68.Horst R, Prapong S, Reinhardt T, Koszewski N, Knutson J, Bishop C. Comparison of the relative effects of 1,24-dihydroxyvitamin D(2) [1,24-(OH)(2)D(2)], 1,24-dihydroxyvitamin D(3) [1,24-(OH)(2)D(3)], and 1,25-dihydroxyvitamin D(3) [1,25-(OH)(2)D(3)] on selected vitamin D-regulated events in the rat. Biochem Pharmacol. 2000;60:701–8. doi: 10.1016/s0006-2952(00)00378-6. [DOI] [PubMed] [Google Scholar]
- 69.Houghton LA, Vieth R. The case against ergocalciferol (vitamin D2) as a vitamin supplement. Am J Clin Nutr. 2006;84:694–7. doi: 10.1093/ajcn/84.4.694. [DOI] [PubMed] [Google Scholar]
- 70.Horst RL, Reinhardt TA, Ramberg CF, Koszewski NJ, Napoli JL. 24-Hydroxylation of 1,25-dihydroxyergocalciferol. An unambiguous deactivation process. J Biol Chem. 1986;261:9250–6. [PubMed] [Google Scholar]
- 71.Hammami MM, Abuhdeeb K, Hammami S, Yusuf A. Vitamin-D2 treatment-associated decrease in 25(OH)D3 level is a reciprocal phenomenon: a randomized controlled trial. BMC Endocr Disord. 2019;19:8. doi: 10.1186/s12902-019-0337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Biancuzzo RM, Clarke N, Reitz RE, Travison TG, Holick MF. Serum concentrations of 1,25-dihydroxyvitamin D2 and 1,25-dihydroxyvitamin D3 in response to vitamin D2 and vitamin D3 supplementation. J Clin Endocrinol Metab. 2013;98:973–9. doi: 10.1210/jc.2012-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Silva MC, Furlanetto TW. Intestinal absorption of vitamin D: a systematic review. Nutr Rev. 2018;76:60–76. doi: 10.1093/nutrit/nux034. [DOI] [PubMed] [Google Scholar]
- 74.Ilahi M, Armas LA, Heaney RP. Pharmacokinetics of a single, large dose of cholecalciferol. Am J Clin Nutr. 2008;87:688–91. doi: 10.1093/ajcn/87.3.688. [DOI] [PubMed] [Google Scholar]
- 75.Davies M, Mawer EB, Krawitt EL. Comparative absorption of vitamin D3 and 25-hydroxyvitamin D3 in intestinal disease. Gut. 1980;21:287–92. doi: 10.1136/gut.21.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Davies M, Mawer EB, Klass HJ, Lumb GA, Berry JL, Warnes TW. Vitamin D deficiency, osteomalacia, and primary biliary cirrhosis. Response to orally administered vitamin D3. Dig Dis Sci. 1983;28:145–53. doi: 10.1007/BF01315144. [DOI] [PubMed] [Google Scholar]
- 77.Clemens TL, Zhou XY, Myles M, Endres D, Lindsay R. Serum vitamin D2 and vitamin D3 metabolite concentrations and absorption of vitamin D2 in elderly subjects. J Clin Endocrinol Metab. 1986;63:656–60. doi: 10.1210/jcem-63-3-656. [DOI] [PubMed] [Google Scholar]
- 78.Compston JE, Merrett AL, Hammett FG, Magill P. Comparison of the appearance of radiolabelled vitamin D3 and 25-hydroxy-vitamin D3 in the chylomicron fraction of plasma after oral administration in man. Clin Sci (Lond) 1981;60:241–3. doi: 10.1042/cs0600241. [DOI] [PubMed] [Google Scholar]
- 79.Borel P, Caillaud D, Cano NJ. Vitamin D bioavailability: state of the art. Crit Rev Food Sci Nutr. 2015;55:1193–205. doi: 10.1080/10408398.2012.688897. [DOI] [PubMed] [Google Scholar]
- 80.Batchelor AJ, Compston JE. Reduced plasma half-life of radio-labelled 25-hydroxyvitamin D3 in subjects receiving a high-fibre diet. Br J Nutr. 1983;49:213–6. doi: 10.1079/bjn19830027. [DOI] [PubMed] [Google Scholar]
- 81.Natri AM, Salo P, Vikstedt T, Palssa A, Huttunen M, Kärkkäinen MU, et al. Bread fortified with cholecalciferol increases the serum 25-hydroxyvitamin D concentration in women as effectively as a cholecalciferol supplement. J Nutr. 2006;136:123–7. doi: 10.1093/jn/136.1.123. [DOI] [PubMed] [Google Scholar]
- 82.McDuffie JR, Calis KA, Booth SL, Uwaifo GI, Yanovski JA. Effects of orlistat on fat-soluble vitamins in obese adolescents. Pharmacotherapy. 2002;22:814–22. doi: 10.1592/phco.22.11.814.33627. [DOI] [PubMed] [Google Scholar]
- 83.Argao EA, Heubi JE, Hollis BW, Tsang RC. d-α-Tocopheryl polyethylene glycol-1000 succinate enhances the absorption of vitamin D in chronic cholestatic liver disease of infancy and childhood. Pediatr Res. 1992;31:146–50. doi: 10.1203/00006450-199202000-00011. [DOI] [PubMed] [Google Scholar]
- 84.Goncalves A, Roi S, Nowicki M, Dhaussy A, Huertas A, Amiot MJ, et al. Fat-soluble vitamin intestinal absorption: absorption sites in the intestine and interactions for absorption. Food Chem. 2015;172:155–60. doi: 10.1016/j.foodchem.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 85.Reboul E, Goncalves A, Comera C, Bott R, Nowicki M, Landrier JF, et al. Vitamin D intestinal absorption is not a simple passive diffusion: evidences for involvement of cholesterol transporters. Mol Nutr Food Res. 2011;55:691–702. doi: 10.1002/mnfr.201000553. [DOI] [PubMed] [Google Scholar]
- 86.Heaney RP, Horst RL, Cullen DM, Armas LA. Vitamin D3 distribution and status in the body. J Am Coll Nutr. 2009;28:252–6. doi: 10.1080/07315724.2009.10719779. [DOI] [PubMed] [Google Scholar]
- 87.Blum M, Dolnikowski G, Seyoum E, Harris SS, Booth SL, Peterson J, et al. Vitamin D3 in fat tissue. Endocrine. 2008;33:90–4. doi: 10.1007/s12020-008-9051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bonnet L, Margier M, Svilar L, Couturier C, Reboul E, Martin JC, et al. Simple fast quantification of cholecalciferol, 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D in adipose tissue using LC-HRMS/MS. Nutrients. 2019;11:e1977. doi: 10.3390/nu11091977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carrelli A, Bucovsky M, Horst R, Cremers S, Zhang C, Bessler M, et al. Vitamin D storage in adipose tissue of obese and normal weight women. J Bone Miner Res. 2017;32:237–42. doi: 10.1002/jbmr.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lawson DE, Douglas J, Lean M, Sedrani S. Estimation of vitamin D3 and 25-hydroxyvitamin D3 in muscle and adipose tissue of rats and man. Clin Chim Acta. 1986;157:175–81. doi: 10.1016/0009-8981(86)90223-8. [DOI] [PubMed] [Google Scholar]
- 91.Mawer EB, Backhouse J, Holman CA, Lumb GA, Stanbury SW. The distribution and storage of vitamin D and its metabolites in human tissues. Clin Sci. 1972;43:413–31. doi: 10.1042/cs0430413. [DOI] [PubMed] [Google Scholar]
- 92.Heaney RP, Armas LA, Shary JR, Bell NH, Binkley N, Hollis BW. 25-Hydroxylation of vitamin D3: relation to circulating vitamin D3 under various input conditions. Am J Clin Nutr. 2008;87:1738–42. doi: 10.1093/ajcn/87.6.1738. [DOI] [PubMed] [Google Scholar]
- 93.Martinaityte I, Kamycheva E, Didriksen A, Jakobsen J, Jorde R. Vitamin D stored in fat tissue during a 5-year intervention affects serum 25-hydroxyvitamin D levels the following year. J Clin Endocrinol Metab. 2017;102:3731–8. doi: 10.1210/jc.2017-01187. [DOI] [PubMed] [Google Scholar]
- 94.Bouillon R, Schuit F, Antonio L, Rastinejad F. Vitamin D binding protein: a historic overview. Front Endocrinol (Lausanne) 2020;10:910. doi: 10.3389/fendo.2019.00910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Abboud M, Gordon-Thomson C, Hoy AJ, Balaban S, Rybchyn MS, Cole L, et al. Uptake of 25-hydroxyvitamin D by muscle and fat cells. J Steroid Biochem Mol Biol. 2014;144(Pt A):232–6. doi: 10.1016/j.jsbmb.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 96.Abboud M, Puglisi DA, Davies BN, Rybchyn M, Whitehead NP, Brock KE, et al. Evidence for a specific uptake and retention mechanism for 25-hydroxyvitamin D (25OHD) in skeletal muscle cells. Endocrinology. 2013;154:3022–30. doi: 10.1210/en.2012-2245. [DOI] [PubMed] [Google Scholar]
- 97.Abboud M, Rybchyn MS, Liu J, Ning Y, Gordon-Thomson C, Brennan-Speranza TC, et al. The effect of parathyroid hormone on the uptake and retention of 25-hydroxyvitamin D in skeletal muscle cells. J Steroid Biochem Mol Biol. 2017;173:173–9. doi: 10.1016/j.jsbmb.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 98.Mason RS, Rybchyn MS, Abboud M, Brennan-Speranza TC, Fraser DR. The Role of Skeletal Muscle in Maintaining Vitamin D Status in Winter. Curr Dev Nutr. 2019;3:nzz087. doi: 10.1093/cdn/nzz087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Holick MF, Chen TC, Lu Z, Sauter E. Vitamin D and skin physiology: a D-lightful story. J Bone Miner Res. 2007;22(Suppl 2):V28–33. doi: 10.1359/jbmr.07s211. [DOI] [PubMed] [Google Scholar]
- 100.Obi-Tabot ET, Tian XQ, Chen TC, Holick MF. A human skin equivalent model that mimics the photoproduction of vitamin D3 in human skin. In Vitro Cell Dev Biol Anim. 2000;36:201–4. doi: 10.1290/1071-2690(2000)036<0201:AHSEMT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 101.Holick MF. Sunlight, UV-radiation, vitamin D and skin cancer: how much sunlight do we need? Adv Exp Med Biol. 2008;624:1–15. doi: 10.1007/978-0-387-77574-6_1. [DOI] [PubMed] [Google Scholar]
- 102.Adams JS, Clemens TL, Parrish JA, Holick MF. Vitamin-D synthesis and metabolism after ultraviolet irradiation of normal and vitamin-D-deficient subjects. N Engl J Med. 1982;306:722–5. doi: 10.1056/NEJM198203253061206. [DOI] [PubMed] [Google Scholar]
- 103.Chel VG, Ooms ME, Popp-Snijders C, Pavel S, Schothorst AA, Meulemans CC, et al. Ultraviolet irradiation corrects vitamin D deficiency and suppresses secondary hyperparathyroidism in the elderly. J Bone Miner Res. 1998;13:1238–42. doi: 10.1359/jbmr.1998.13.8.1238. [DOI] [PubMed] [Google Scholar]
- 104.Terushkin V, Bender A, Psaty EL, Engelsen O, Wang SQ, Halpern AC. Estimated equivalency of vitamin D production from natural sun exposure versus oral vitamin D supplementation across seasons at two US latitudes. J Am Acad Dermatol. 2010;62:929.e1–9. doi: 10.1016/j.jaad.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 105.Samanek AJ, Croager EJ, Gies P, Milne E, Prince R, McMichael AJ, et al. Estimates of beneficial and harmful sun exposure times during the year for major Australian population centres. Med J Aust. 2006;184:338–41. doi: 10.5694/j.1326-5377.2006.tb00267.x. [DOI] [PubMed] [Google Scholar]
- 106.Kift R, Rhodes LE, Farrar MD, Webb AR. Is sunlight exposure enough to avoid wintertime vitamin D deficiency in United Kingdom population groups? Int J Environ Res Public Health. 2018;15:e1624. doi: 10.3390/ijerph15081624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Webb AR, Kift R, Durkin MT, O’Brien SJ, Vail A, Berry JL, et al. The role of sunlight exposure in determining the vitamin D status of the U.K. white adult population. Br J Dermatol. 2010;163:1050–5. doi: 10.1111/j.1365-2133.2010.09975.x. [DOI] [PubMed] [Google Scholar]
- 108.Jager N, Schöpe J, Wagenpfeil S, Bocionek P, Saternus R, Vogt T, et al. The impact of UV-dose, body surface area exposed and other factors on cutaneous vitamin D synthesis measured as serum 25(OH)D concentration: systematic review and meta-analysis. Anticancer Res. 2018;38:1165–71. doi: 10.21873/anticanres.12336. [DOI] [PubMed] [Google Scholar]
- 109.Webb AR, Kazantzidis A, Kift RC, Farrar MD, Wilkinson J, Rhodes LE. Meeting vitamin D requirements in white caucasians at UK latitudes: providing a choice. Nutrients. 2018;10:e497. doi: 10.3390/nu10040497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Webb AR, Kazantzidis A, Kift RC, Farrar MD, Wilkinson J, Rhodes LE. Colour counts: sunlight and skin type as drivers of vitamin D deficiency at UK latitudes. Nutrients. 2018;10:e457. doi: 10.3390/nu10040457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ashwell M, Stone EM, Stolte H, Cashman KD, Macdonald H, Lanham-New S, et al. UK Food Standards Agency Workshop Report: an investigation of the relative contributions of diet and sunlight to vitamin D status. Br J Nutr. 2010;104:603–11. doi: 10.1017/S0007114510002138. [DOI] [PubMed] [Google Scholar]
- 112.Sawyer ME, Tran HT, Evans MV. A physiologically based pharmacokinetic model of vitamin D. J Appl Toxicol. 2017;37:1448–54. doi: 10.1002/jat.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ocampo-Pelland AS, Gastonguay MR, French JF, Riggs MM. Model-based meta-analysis for development of a population-pharmacokinetic (PPK) model for Vitamin D3 and its 25OHD3 metabolite using both individual and arm-level data. J Pharmacokinet Pharmacodyn. 2016;43:191–206. doi: 10.1007/s10928-016-9465-1. [DOI] [PubMed] [Google Scholar]
- 114.Ocampo-Pelland AS, Gastonguay MR, Riggs MM. Model-based meta-analysis for comparing Vitamin D2 and D3 parent-metabolite pharmacokinetics. J Pharmacokinet Pharmacodyn. 2017;44:375–88. doi: 10.1007/s10928-017-9525-1. [DOI] [PubMed] [Google Scholar]
- 115.Ramakrishnan V, Yang QJ, Quach HP, Cao Y, Chow EC, Mager DE, et al. Physiologically-based pharmacokinetic-pharmacodynamic modeling of 1α,25-dihydroxyvitamin D3 in mice. Drug Metab Dispos. 2016;44:189–208. doi: 10.1124/dmd.115.067033. [DOI] [PubMed] [Google Scholar]
- 116.Bikle DD, Schwartz J. Vitamin D binding protein, total and free vitamin D levels in different physiological and pathophysiological conditions. Front Endocrinol (Lausanne) 2019;10:317. doi: 10.3389/fendo.2019.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang X, Sheng Z, Meng L, Su C, Trooskin S, Shapses SA. 25-Hydroxyvitamin D and vitamin D binding protein levels in patients with primary hyperparathyroidism before and after parathyroidectomy. Front Endocrinol (Lausanne) 2019;10:171. doi: 10.3389/fendo.2019.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Henderson CM, Fink SL, Bassyouni H, Argiropoulos B, Brown L, Laha TJ, et al. Vitamin D-binding protein deficiency and homozygous deletion of the GC gene. N Engl J Med. 2019;380:1150–7. doi: 10.1056/NEJMoa1807841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, et al. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96:507–15. doi: 10.1016/s0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]
- 120.Kilpatrick L, Bouillon R, Davis C, Henderson C, Hoofnagle A, Pauwels S, et al. Evaluating LC-IDMS for bias during quantification of VDBP proteoforms. Poster at MSACL Annual congress in clinical mass spectrometry; Palm Springs CA. 2019. [Google Scholar]
- 121.Bikle DD, Siiteri PK, Ryzen E, Haddad JG. Serum protein binding of 1,25-dihydroxyvitamin D: a reevaluation by direct measurement of free metabolite levels. J Clin Endocrinol Metab. 1985;61:969–75. doi: 10.1210/jcem-61-5-969. [DOI] [PubMed] [Google Scholar]
- 122.Heureux N, Lindhout E, Swinkels L. A direct assay for measuring free 25-hydroxyvitamin D. J AOAC Int. 2017;100:1318–22. doi: 10.5740/jaoacint.17-0084. [DOI] [PubMed] [Google Scholar]
- 123.Nielson CM, Jones KS, Bouillon R Osteoporotic Fractures in Men (MrOS) Research Group. Role of Assay Type in Determining Free 25-Hydroxyvitamin D Levels in Diverse Populations. N Engl J Med. 2016;374:1695–6. doi: 10.1056/NEJMc1513502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res. 2011;26:2341–57. doi: 10.1002/jbmr.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Best CM, Pressman EK, Queenan RA, Cooper E, O’Brien KO. Longitudinal changes in serum vitamin D binding protein and free 25-hydroxyvitamin D in a multiracial cohort of pregnant adolescents. J Steroid Biochem Mol Biol. 2019;186:79–88. doi: 10.1016/j.jsbmb.2018.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tsuprykov O, Buse C, Skoblo R, Haq A, Hocher B. Reference intervals for measured and calculated free 25-hydroxyvitamin D in normal pregnancy. J Steroid Biochem Mol Biol. 2018;181:80–7. doi: 10.1016/j.jsbmb.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 127.Bikle DD, Gee E, Halloran B, Haddad JG. Free 1,25-dihydroxyvitamin D levels in serum from normal subjects, pregnant subjects, and subjects with liver disease. J Clin Invest. 1984;74:1966–71. doi: 10.1172/JCI111617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schwartz JB, Lai J, Lizaola B, Kane L, Weyland P, Terrault NA, et al. Variability in free 25(OH) vitamin D levels in clinical populations. J Steroid Biochem Mol Biol. 2014;144(Part A):156–8. doi: 10.1016/j.jsbmb.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kovacs CS. Maternal mineral and bone metabolism during pregnancy, lactation, and post-weaning recovery. Physiol Rev. 2016;96:449–547. doi: 10.1152/physrev.00027.2015. [DOI] [PubMed] [Google Scholar]
- 130.Carneiro RM, Prebehalla L, Tedesco MB, Sereika SM, Hugo M, Hollis BW, et al. Lactation and bone turnover: a conundrum of marked bone loss in the setting of coupled bone turnover. J Clin Endocrinol Metab. 2010;95:1767–76. doi: 10.1210/jc.2009-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kovacs CS. Bone development and mineral homeostasis in the fetus and neonate: roles of the calciotropic and phosphotropic hormones. Physiol Rev. 2014;94:1143–218. doi: 10.1152/physrev.00014.2014. [DOI] [PubMed] [Google Scholar]
- 132.Park H, Wood MR, Malysheva OV, Jones S, Mehta S, Brannon PM, et al. Placental vitamin D metabolism and its associations with circulating vitamin D metabolites in pregnant women. Am J Clin Nutr. 2017;106:1439–48. doi: 10.3945/ajcn.117.153429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.við Streym S, Højskov CS, Møller UK, Heickendorff L, Vestergaard P, Mosekilde L, et al. Vitamin D content in human breast milk: a 9-mo follow-up study. Am J Clin Nutr. 2016;103:107–14. doi: 10.3945/ajcn.115.115105. [DOI] [PubMed] [Google Scholar]
- 134.Karras SN, Wagner CL, Castracane VD. Understanding vitamin D metabolism in pregnancy: from physiology to pathophysiology and clinical outcomes. Metabolism. 2018;86:112–23. doi: 10.1016/j.metabol.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 135.Souverein PC, Webb DJ, Weil JG, Van Staa TP, Egberts AC. Use of antiepileptic drugs and risk of fractures: case-control study among patients with epilepsy. Neurology. 2006;66:1318–24. doi: 10.1212/01.wnl.0000210503.89488.88. [DOI] [PubMed] [Google Scholar]
- 136.Lee RH, Lyles KW, Colón-Emeric C. A review of the effect of anticonvulsant medications on bone mineral density and fracture risk. Am J Geriatr Pharmacother. 2010;8:34–46. doi: 10.1016/j.amjopharm.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pack AM, Morrell MJ, Marcus R, Holloway L, Flaster E, Doñe S, et al. Bone mass and turnover in women with epilepsy on antiepileptic drug monotherapy. Ann Neurol. 2005;57:252–7. doi: 10.1002/ana.20378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pascussi JM, Robert A, Nguyen M, Walrant-Debray O, Garabedian M, Martin P, et al. Possible involvement of pregnane X receptor-enhanced CYP24 expression in drug-induced osteomalacia. J Clin Invest. 2005;115:177–86. doi: 10.1172/JCI21867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gröber U, Holick MF, Kisters K. Vitamin D and drugs. Med Monatsschr Pharm. 2011;34:377–87. [PubMed] [Google Scholar]
- 140.Wang Z, Schuetz EG, Xu Y, Thummel KE. Interplay between vitamin D and the drug metabolizing enzyme CYP3A4. J Steroid Biochem Mol Biol. 2013;136:54–8. doi: 10.1016/j.jsbmb.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Andress DL, Ozuna J, Tirschwell D, Grande L, Johnson M, Jacobson AF, et al. Antiepileptic drug-induced bone loss in young male patients who have seizures. Arch Neurol. 2002;59:781–6. doi: 10.1001/archneur.59.5.781. [DOI] [PubMed] [Google Scholar]
- 142.Lee HS, Wang SY, Salter DM, Wang CC, Chen SJ, Fan HC. The impact of the use of antiepileptic drugs on the growth of children. BMC Pediatr. 2013;13:211. doi: 10.1186/1471-2431-13-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fitzpatrick LA. Pathophysiology of bone loss in patients receiving anticonvulsant therapy. Epilepsy Behav. 2004;5(Suppl 2):S3–15. doi: 10.1016/j.yebeh.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 144.Fernandez H, Mohammed HT, Patel T. Vitamin D supplementation for bone health in adults with epilepsy: a systematic review. Epilepsia. 2018;59:885–96. doi: 10.1111/epi.14015. [DOI] [PubMed] [Google Scholar]
- 145.Drezner MK. Treatment of anticonvulsant drug-induced bone disease. Epilepsy Behav. 2004;5(Suppl 2):S41–7. doi: 10.1016/j.yebeh.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 146.Petkovich M, Jones G. CYP24A1 and kidney disease. Curr Opin Nephrol Hypertens. 2011;20:337–44. doi: 10.1097/MNH.0b013e3283477a7b. [DOI] [PubMed] [Google Scholar]
- 147.Waziri B, Duarte R, Naicker S. Chronic kidney disease-mineral and bone disorder (CKD-MBD): current perspectives. Int J Nephrol Renovasc Dis. 2019;12:263–76. doi: 10.2147/IJNRD.S191156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ketteler M, Block GA, Evenepoel P, Fukagawa M, Herzog CA, McCann L, et al. Diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder: synopsis of the Kidney Disease: Improving Global Outcomes 2017 Clinical Practice Guideline Update. Ann Intern Med. 2018;168:422–30. doi: 10.7326/M17-2640. [DOI] [PubMed] [Google Scholar]
- 149.Johnson JM, Maher JW, DeMaria EJ, Downs RW, Wolfe LG, Kellum JM. The long-term effects of gastric bypass on vitamin D metabolism. Ann Surg. 2006;243:701–4. doi: 10.1097/01.sla.0000216773.47825.c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schafer AL. Vitamin D and intestinal calcium transport after bariatric surgery. J Steroid Biochem Mol Biol. 2017;173:202–10. doi: 10.1016/j.jsbmb.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Borges JLC, Miranda ISM, Sarquis MMS, Borba V, Maeda SS, Lazaretti-Castro M, et al. Obesity, bariatric surgery, and vitamin D. J Clin Densitom. 2018;21:157–62. doi: 10.1016/j.jocd.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 152.Peterson LA, Zeng X, Caufield-Noll CP, Schweitzer MA, Magnuson TH, Steele KE. Vitamin D status and supplementation before and after bariatric surgery: a comprehensive literature review. Surg Obes Relat Dis. 2016;12:693–702. doi: 10.1016/j.soard.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 153.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 154.Bischoff-Ferrari HA, Shao A, Dawson-Hughes B, Hathcock J, Giovannucci E, Willett WC. Benefit-risk assessment of vitamin D supplementation. Osteoporos Int. 2010;21:1121–32. doi: 10.1007/s00198-009-1119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lips P, Hosking D, Lippuner K, Norquist JM, Wehren L, Maalouf G, et al. The prevalence of vitamin D inadequacy amongst women with osteoporosis: an international epidemiological investigation. J Intern Med. 2006;260:245–54. doi: 10.1111/j.1365-2796.2006.01685.x. [DOI] [PubMed] [Google Scholar]
- 156.Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, et al. Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90:3215–24. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 157.Sai AJ, Walters RW, Fang X, Gallagher JC. Relationship between vitamin D, parathyroid hormone, and bone health. J Clin Endocrinol Metab. 2011;96:E436–46. doi: 10.1210/jc.2010-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bensalah M, Bouayadi O, Rahmani N, Lyagoubi A, Lamrabat S, Choukri M. Comparative study of the serum measurement of PTH on Roche Cobas e411® versus the Abbott Architect ci8200®. Ann Biol Clin (Paris) 2018;76:61–7. doi: 10.1684/abc.2017.1309. [DOI] [PubMed] [Google Scholar]
- 159.Need AG, O’Loughlin PD, Morris HA, Coates PS, Horowitz M, Nordin BE. Vitamin D metabolites and calcium absorption in severe vitamin D deficiency. J Bone Miner Res. 2008;23:1859–63. doi: 10.1359/jbmr.080607. [DOI] [PubMed] [Google Scholar]
- 160.Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr. 2003;22:142–6. doi: 10.1080/07315724.2003.10719287. [DOI] [PubMed] [Google Scholar]
- 161.Viljakainen HT, Saarnio E, Hytinantti T, Miettinen M, Surcel H, Mäkitie O, et al. Maternal vitamin D status determines bone variables in the newborn. J Clin Endocrinol Metab. 2010;95:1749–57. doi: 10.1210/jc.2009-1391. [DOI] [PubMed] [Google Scholar]
- 162.Cooper C, Harvey NC, Bishop NJ, Kennedy S, Papageorghiou AT, Schoenmakers I, et al. Maternal gestational vitamin D supplementation and offspring bone health (MAVIDOS): a multicentre, double-blind, randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 2016;4:393–402. doi: 10.1016/S2213-8587(16)00044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Curtis EM, Moon RJ, Harvey NC, Cooper C. Maternal vitamin D supplementation during pregnancy. Br Med Bull. 2018;126:57–77. doi: 10.1093/bmb/ldy010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tomlinson PB, Joseph C, Angioi M. Effects of vitamin D supplementation on upper and lower body muscle strength levels in healthy individuals. A systematic review with meta-analysis. J Sci Med Sport. 2015;18:575–80. doi: 10.1016/j.jsams.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 165.Kärkkäinen M, Tuppurainen M, Salovaara K, Sandini L, Rikkonen T, Sirola J, et al. Effect of calcium and vitamin D supplementation on bone mineral density in women aged 65–71 years: a 3-year randomized population-based trial (OSTPRE-FPS) Osteoporos Int. 2010;21:2047–55. doi: 10.1007/s00198-009-1167-8. [DOI] [PubMed] [Google Scholar]
- 166.Ensrud KE, Taylor BC, Paudel ML, Cauley JA, Cawthon PM, Cummings SR, et al. Serum 25-hydroxyvitamin D levels and rate of hip bone loss in older men. J Clin Endocrinol Metab. 2009;94:2773–80. doi: 10.1210/jc.2008-2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Avenell A, Mak JC, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014:CD000227. doi: 10.1002/14651858.CD000227.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bischoff-Ferrari HA, Willett WC, Orav EJ, Lips P, Meunier PJ, Lyons RA, et al. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med. 2012;367:40–9. doi: 10.1056/NEJMoa1109617. [DOI] [PubMed] [Google Scholar]
- 169.Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303:1815–22. doi: 10.1001/jama.2010.594. [DOI] [PubMed] [Google Scholar]
- 170.Aspray TJ, Chadwick T, Francis RM, McColl E, Stamp E, Prentice A, et al. Randomized controlled trial of vitamin D supplementation in older people to optimize bone health. Am J Clin Nutr. 2019;109:207–17. doi: 10.1093/ajcn/nqy280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Burt LA, Billington EO, Rose MS, Raymond DA, Hanley DA, Boyd SK. Effect of high-dose vitamin D supplementation on volumetric bone density and bone strength: a randomized clinical trial. JAMA. 2019;322:736–45. doi: 10.1001/jama.2019.11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Glendenning P, Zhu K, Inderjeeth C, Howat P, Lewis JR, Prince RL. Effects of three-monthly oral 150,000 IU cholecalciferol supplementation on falls, mobility, and muscle strength in older postmenopausal women: a randomized controlled trial. J Bone Miner Res. 2012;27:170–6. doi: 10.1002/jbmr.524. [DOI] [PubMed] [Google Scholar]
- 173.Grossman DC, Curry SJ, Owens DK, Barry MJ, Caughey AB, Davidson KW, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1592–9. doi: 10.1001/jama.2018.3185. [DOI] [PubMed] [Google Scholar]
- 174.Carlberg C, Haq A. The concept of the personal vitamin D response index. J Steroid Biochem Mol Biol. 2018;175:12–7. doi: 10.1016/j.jsbmb.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 175.Shirvani A, Kalajian TA, Song A, Holick MF. Disassociation of vitamin D’s calcemic activity and non-calcemic genomic activity and individual responsiveness: a randomized controlled double-blind clinical trial. Sci Rep. 2019;9:17685. doi: 10.1038/s41598-019-53864-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cavalier E, Huyghebaert L, Rousselle O, Bekaert AC, Kovacs S, Vranken L, et al. Simultaneous measurement of 25(OH)-vitamin D and 24,25(OH)2-vitamin D to define cut-offs for CYP24A1 mutation and vitamin D deficiency in a population of 1200 young subjects. Clin Chem Lab Med. 2020;58:197–201. doi: 10.1515/cclm-2019-0996. [DOI] [PubMed] [Google Scholar]
- 177.Tebben PJ, Singh RJ, Kumar R. Vitamin D-mediated hypercalcemia: mechanisms, diagnosis, and treatment. Endocr Rev. 2016;37:521–47. doi: 10.1210/er.2016-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999;69:842–56. doi: 10.1093/ajcn/69.5.842. [DOI] [PubMed] [Google Scholar]
- 179.Adams JS, Lee G. Gains in bone mineral density with resolution of vitamin D intoxication. Ann Intern Med. 1997;127:203–6. doi: 10.7326/0003-4819-127-3-199708010-00004. [DOI] [PubMed] [Google Scholar]
- 180.Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, et al. Women’s Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669–83. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 181.Ketha H, Wadams H, Lteif A, Singh RJ. Iatrogenic vitamin D toxicity in an infant – a case report and review of literature. J Steroid Biochem Mol Biol. 2015;148:14–8. doi: 10.1016/j.jsbmb.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 182.Jacobus CH, Holick MF, Shao Q, Chen TC, Holm IA, Kolodny JM, et al. Hypervitaminosis D associated with drinking milk. N Engl J Med. 1992;326:1173–7. doi: 10.1056/NEJM199204303261801. [DOI] [PubMed] [Google Scholar]
- 183.Calcium Calculator. [Accessed 22 October 2020]. https://www.cgem.ed.ac.uk/research/rheumatological/calcium-calculator.
- 184.Cashman KD, Hill TR, Lucey AJ, Taylor N, Seamans KM, Muldowney S, et al. Estimation of the dietary requirement for vitamin D in healthy adults. Am J Clin Nutr. 2008;88:1535–42. doi: 10.3945/ajcn.2008.26594. [DOI] [PubMed] [Google Scholar]
- 185.Cashman KD, Wallace JM, Horigan G, Hill TR, Barnes MS, Lucey AJ, et al. Estimation of the dietary requirement for vitamin D in free-living adults >=64 y of age. Am J Clin Nutr. 2009;89:1366–74. doi: 10.3945/ajcn.2008.27334. [DOI] [PubMed] [Google Scholar]
- 186.Cashman KD, FitzGerald AP, Viljakainen HT, Jakobsen J, Michaelsen KF, Lamberg-Allardt C, et al. Estimation of the dietary requirement for vitamin D in healthy adolescent white girls. Am J Clin Nutr. 2011;93:549–55. doi: 10.3945/ajcn.110.006577. [DOI] [PubMed] [Google Scholar]
- 187.Rosen CJ, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, et al. IOM committee members respond to Endocrine Society vitamin D guideline. J Clin Endocrinol Metab. 2012;97:1146–52. doi: 10.1210/jc.2011-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Francis RM, Aspray TJ, Bowring CE, Fraser WD, Gittoes NJ, Javaid MK, et al. National Osteoporosis Society practical clinical guideline on vitamin D and bone health. Maturitas. 2015;80:119–21. doi: 10.1016/j.maturitas.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 189.Aspray TJ, Bowring C, Fraser W, Gittoes N, Javaid MK, Macdonald H, et al. National Osteoporosis Society vitamin D guideline summary. Age Ageing. 2014;43:592–5. doi: 10.1093/ageing/afu093. [DOI] [PubMed] [Google Scholar]
- 190.Fassio A, Adami G, Rossini M, Giollo A, Caimmi C, Bixio R, et al. Pharmacokinetics of oral cholecalciferol in healthy subjects with vitamin D deficiency: a randomized open-label study. Nutrients. 2020;12:E1553. doi: 10.3390/nu12061553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Singh V, Misra AK, Singh M, Midha NK, Kumar B, Ambwani S, et al. An open-label, randomized, 10 weeks prospective study on the efficacy of vitamin D (daily low dose and weekly high dose) in vitamin D deficient patients. J Family Med Prim Care. 2019;8:1958–63. doi: 10.4103/jfmpc.jfmpc_272_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ish-Shalom S, Segal E, Salganik T, Raz B, Bromberg IL, Vieth R. Comparison of daily, weekly, and monthly vitamin D3 in ethanol dosing protocols for two months in elderly hip fracture patients. J Clin Endocrinol Metab. 2008;93:3430–5. doi: 10.1210/jc.2008-0241. [DOI] [PubMed] [Google Scholar]
- 193.Chel V, Wijnhoven HA, Smit JH, Ooms M, Lips P. Efficacy of different doses and time intervals of oral vitamin D supplementation with or without calcium in elderly nursing home residents. Osteoporos Int. 2008;19:663–71. doi: 10.1007/s00198-007-0465-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Mittal H, Rai S, Shah D, Madhu SV, Mehrotra G, Malhotra RK, et al. 300,000 IU or 600,000 IU of oral vitamin D3 for treatment of nutritional rickets: a randomized controlled trial. Indian Pediatr. 2014;51:265–72. doi: 10.1007/s13312-014-0399-7. [DOI] [PubMed] [Google Scholar]
- 195.Mittal M, Yadav V, Khadgawat R, Kumar M, Sherwani P. Efficacy and safety of 90,000 IU versus 300,000 IU single dose oral vitamin D in nutritional rickets: a randomized controlled trial. Indian J Endocrinol Metab. 2018;22:760–5. doi: 10.4103/ijem.IJEM_84_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lubani MM, al-Shab TS, al-Saleh QA, Sharda DC, Quattawi SA, Ahmed SA, et al. Vitamin-D-deficiency rickets in Kuwait: the prevalence of a preventable disease. Ann Trop Paediatr. 1989;9:134–9. doi: 10.1080/02724936.1989.11748616. [DOI] [PubMed] [Google Scholar]
- 197.Gordon CM, Williams AL, Feldman HA, May J, Sinclair L, Vasquez A, et al. Treatment of hypovitaminosis D in infants and toddlers. J Clin Endocrinol Metab. 2008;93:2716–21. doi: 10.1210/jc.2007-2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.National Institute for Health and Care Excellence Clinical Knowledge Summaries. [Accessed 22 October 2020]. https://cks.nice.org.uk/vitamin-d-deficiency-in-adults-treatment-and-prevention.


