Abstract
A ureteral stent is a commonly implanted urological device in patients with urinary tract obstruction. The main role of these stents is to allow adequate drainage of urine from the kidney into the bladder. Individuals with strictures, tumors, or obstructions from urinary stones do not have adequate urine flow and require ureteral stents as a part of their treatment to avoid potential hydronephrosis and renal failure. Although ureteral stents are highly effective in treating urinary tract obstructions, they have associated morbidities, such as biofilm formation and encrustation. Researchers have studied about how to diminish these negative outcomes by developing novel stent materials. Different coatings and biomaterials have been developed to reduce bacterial adhesion and crystal deposition onto the stent surfaces. Moreover, new investigation technologies, such as microfluidic platforms and encrustation sensors, have been utilized to better study the stents. Biofilms and encrustations can stem from bacterial origins; therefore, understanding the urinary microbiome will also provide insight into the solutions for treating them. There are still some gaps in our knowledge regarding the exact underlying mechanisms of stent-associated biofilms and encrustation. Future studies should include continuous testing of novel stent biomaterials for safety and efficacy, developing new technologies for identifying and extracting biofilms, enriching the assessment of stent encrustation, and diving deeper into understanding the urinary microbiome.
Keywords: Antibiotic prophylaxis, biofilms, stent encrustation, ureteral stents, urinary microbiome
Introduction
Ureteral stents are urological devices that are commonly utilized to treat obstructions in the upper urinary tract by allowing the drainage of urine from the kidney into the bladder.[1] Numerous morbidities can lead to the retention of urine and require the use of ureteral stents for adequate urine flow, including kidney stones and obstructing tumors.[2] Indwelling stents are often associated with complications that can cause pain and discomfort to the patient, biofilm formation, and stent encrustation.[3] These morbidities can lead to serious problems in individuals, including renal failure, pyelonephritis, hydronephrosis, bacteremia, and death. Researchers are working toward improving or redesigning the ureteral stents in the hopes of targeting and solving stent-associated problems for improved patient care and quality of life.
Biofilms
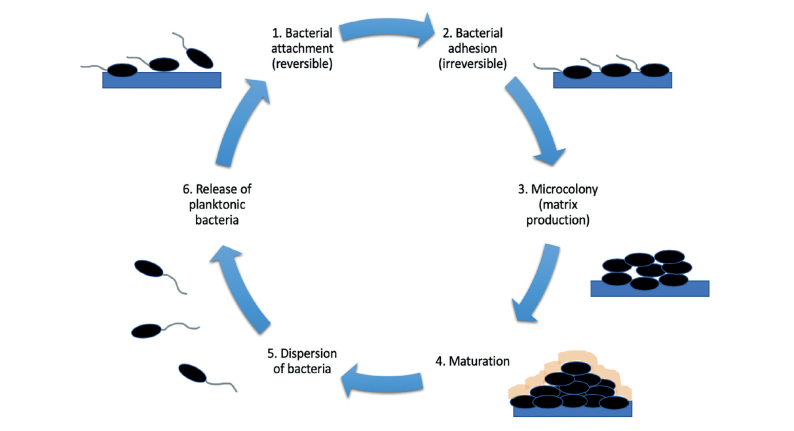
Biofilms are large communities of bacteria that adhere onto surfaces and form highly resistant matrices through the release of different polysaccharides, lipids, nucleic acids, and proteins that form a highly protective layer around the bacteria (Figure 1).[4] These microbial communities can pose a major problem for urological devices that are implanted inside the human body, such as stents. If left untreated, the bacterial aggregates can lead to detrimental outcomes, such as bacteremia and urosepsis.[5] Ureteral stents provide a scaffolding surface for bacterial attachment[6] and lead to strengthening of their survival even in unfavorable conditions, such as with the use of antibiotics. Researchers in the field of urology have employed many different methods to assess these stent-associated morbidities and tackle biofilm formation in novel and promising ways.
Figure 1.
The process of biofilm formation
To better understand the underlying mechanisms of encrustation and biofilm formation, multiple patients with ureteral stents were observed and their symptoms analyzed to understand the contributing factors that lead to these morbidities, such as duration of stenting, symptom correlations, and bacterial colonization rates.
In a study by Betschart et al.,[7] patients’ symptoms post-stenting as well as the effect of biofilms associated with these symptoms were observed. In this prospective observational study, patients who underwent ureteral stenting and consecutive stent exchanges on a regular basis were included. They were asked to complete a German version of the ureteral stent symptoms questionnaire (USSQ) before their scheduled stent exchange and had urine samples collected before each exchange. The biofilm mass on the stent surface as well as the USSQ scores were observed for correlations as the primary outcome of the study. There were no significant correlations between the biofilms and stent-associated symptoms. However, the total biofilm mass was 36% higher in patients with long-term indwelling catheters than those with median indwelling catheter durations. The data suggested that biofilms in the patients with long-term stents were not the main driver of stent-associated symptoms.
In a separate study, Betschart et al.[8] also studied the influence of biofilms on morbidity associated with short-term stenting. They concluded that there was no significant correlation between the total biofilm mass extracted from the patients’ stent and the USSQ score. However, significant correlations were found between the biofilm mass and hematuria as well as between the number of bacteria quantified using quantitative polymerase chain reaction (qPCR) and the USSQ pain score. Their findings indicated that ureteral stent biofilms can aggravate the symptoms in the lower urinary tract; however, they are not the main driver for stent-related symptoms in short-term stenting. This conclusion was drawn from the fact that stent-associated symptoms in patients decreased when the indwelling time increased. However, biofilm formation is known to increase over time, which showcases the fact that the increase in biofilm formation was not the main determining factor of the symptoms.
Toprak et al.[9] assessed if the duration of stenting had any effect on the increased risk of a clinical infection. They conducted their study with a cohort of patients who received ureteral stents after urinary stone treatment. Half of the patients had their implants removed at 15 days and the remaining at 30 days; following removal all of the patient’s stents were sent for microbiological examination. Preoperative and postoperative urine cultures were also examined. Their goal was to provide an insight about when a urological device should be withdrawn in case of an infection. Although they did not find a direct relationship between bacterial colonization and symptomatic infection, it was evident that patients stented for a longer period had increased bacterial growth on their stents. It was also concluded that the bacterial urine cultures were not representative of overall stent colonization; therefore, analysis should be aimed toward not only preoperative urine cultures but also appropriate stent use and prophylaxis. Similar results were seen when Shabeena et al.[10] assessed bacterial colonization on ureteral stents during different time periods, ranging 7–120 days. They found that bacterial colonization of the stent significantly increased as the indwelling time of the stent increased, implying that longer period of stent implantation allowed the colonized bacteria to strengthen their communities and form biofilms.
Techniques to quantify stent biofilm
Detection of bacterial colonization in the urinary tract is an important step for targeting biofilm formation and encrustation on ureteral stents. A recent study looked at different types of assessments to determine the presence of bacteria in stented patients to find the relationships between these diverse methods.[11] Their results showed that there was no strong correlation between routine preoperative urine cultures and stent biofilm cultures. There was also a poor correlation between the preoperative and intraoperative urine cultures as well as between intraoperative urine and stent bacterial cultures. This showcases the discrepancy between how microbial colonization in stenting is assessed and treated and the actual bacterial cultures that are present at the time of stent removal. The clinical relevance of preoperative urine cultures for biofilm identification in stent removal may need to be reconsidered because they poorly correlate with the actual intraoperative bacteria that are detected in urine and on indwelling stents. They concluded that detecting bacteria in patients with ureteral stents depends on a multitude of factors, including timing and detection method. These data may lead to reconsideration of the given preoperative antibiotic prophylaxis.
Targeting the ureteral stent biofilm formation requires enhanced methods for biofilm extraction and quantification. A major problem in the field has been standardizing the extraction protocol for indwelling stents. Buhmann et al.[12] have developed a contamination-free method for extracting the biofilms from ureteral stents. The pinhole method is an abrasion-based method that includes passing the stent with its associated biofilm through a pinhole in a steel plate with a diameter slightly smaller than that of the stent. Biofilm extraction was effective for both cultivation-dependent and cultivation-independent analyses. Compared with the standard sonication and vortexing biofilm extraction protocols, the pinhole method allowed for significantly more effective biofilm removal from the stents.
Furthermore, a platform that has recently gained momentum in studying the ureteral stents has been the use of microfluidic chips. De Grazia et al.[13] employed microfluidic-based methods for modeling the fluid dynamics and investigating the bacterial attachment to ureteral stents. Using computational fluid dynamic simulations, they were able to analyze the flow dynamics of an obstructed and stented ureter to better understand the role it can have on bacterial attachment.
Encrustation
Alongside biofilm formation, ureteral stent encrustation is a closely associated and a major problem that researchers are working on. Encrustation and biofilms on stents are interdependent processes that can both stem from bacterial colonization. The process of encrustation is thought to start with the host proteins found in the urine attaching onto the indwelling ureteral stent surface. This creates a scaffold for the bacteria in the urine to bind to, and the bacteria start producing a biofilm on the stent surface. In the case of urease-negative bacteria, the exopolysaccharide of the biofilm is believed to retain crystals from the urine, allowing them to form a nidus for encrustation. In the case of urease-positive bacteria, such as Proteus mirabilis, indwelling device encrustation and incrustation become a significant problem owing to the rapid crystal growth induced by these bacterial species. This is because of the urease splitting the urea into carbon dioxide and ammonia,[14] with the excess ammonia increasing the urinary pH, significantly resulting in magnesium ammonium phosphate precipitation and crystal formation. Device encrustation and incrustation in this environment tends to be significant, often resulting in device failure because of obstruction, and its removal requires invasive procedures. Furthermore, hematuria and extreme pain are also the known complications of stent encrustation.[15]
As with the studies observing biofilm formation over varying indwelling stent times discussed earlier, similar experiments were performed to assess the relationship between stent indwelling time and encrustation. Kartal et al.[16] found that increased indwelling time leads to a greater stent encrustation, with more complicated treatments becoming necessary for removal. Another study further investigated the potential risk factors for stent encrustation in patients with urolithiasis and found that the indwelling time of the stent is the main risk factor for encrustation.[17] To better understand the potential physical factors of the stents that lead to stent encrustation, such as stent discoloration proposed by studies from Japan, Chew et al.[18] studied the potential mechanisms that lead to stent discoloration and whether it promotes encrustation, biofilm formation, and other changes that would compromise the function as was suggested by the previous studies. Overall, a potential mechanism for discoloration that was identified was the reaction between sulfur in urine and bismuth subcarbonate in the stent materials used for radiopacity. However, discoloration on the stents removed from the patients was not found to increase bacterial adhesion or encrustation and was not associated with significant material changes of the stent. These results suggested that stent discoloration has only cosmetic implications as opposed to increasing the complications that compromise stent function.
Solutions
To deal with stent-associated morbidities, ureteral stents are undergoing innovative improvements in design that can help them evade biofilm formation and encrustation. These innovations include coating the stent with antimicrobials, changing the materials that they are made from, or altering the ureteral stent architecture (Table 1).
Table 1.
List of novel ureteral stents, materials, and technologies
| Product | Author | Publication Year |
|---|---|---|
| Microfluidic-based urodynamic device | De Grazia et al.[13] | 2020 |
| Pellethane® thermoplastic polyurethane (TPU) stent with 2-hydroxyethyl methacrylate (HEMA) and tetraethylene glycol dimethyl ether (TETRA) coatings | Cottone et al.[19] | 2020 |
| Silver sulfadiazine (SSD)-coated stent | El-Nahas et al.[20] | 2018 |
| Poly(N,N-dimethylacrylamide) (PDMAA) coating | Szell et al.[21] | 2018 |
| Magnesium-zinc-strontium alloy stents | Tian et al.[24] | 2019 |
| Zinc alloy stents | Paramitha et al.[26] | 2019 |
| Copper (Cu)-bearing stainless steel stents | Zhao et al.[27] | 2019 |
| Natural-based polymer biodegradable stent | Barros et al.[28] | 2017 |
| Stent with novel side-hole configuration | Mosayyebi et al.[31] | 2019 |
| Quartz crystal microbalance (QCM) sensor | Abadian et al.[33] | 2018 |
Novel coatings
Cottone et al.[19] evaluated PellethaneÒ thermoplastic polyurethane, a polyether-based compound, which is known for its porosity, overall resistance to solvents, and strength. It was assessed in conjunction with 2 surface coatings: 2-hydroxyethyl methacrylate (HEMA) and tetraethylene glycol dimethyl ether as a solution to ureteral stent encrustation. Their results indicated that PellethaneÒ with a HEMA-based coating yielded lower encrustation when placed in an artificial urinary environment. This could be a potential alternative for controlling ureteral stent encrustation.
El-Nahas et al.[20] conducted a study comparing silver sulfadiazine (SSD)-coated ureteral stents with their uncoated counterparts. Their results could not justify the widespread application of this coating because the SSD-coated stents did not significantly reduce the stent-related bacteriuria; however, the SSD-coated stents did have a trend toward diminished bacterial colonization and incidence of bacteriuria compared with that of the control. Although this reduction was not statistically significant, further studies are warranted to assess whether there is a clinical benefit. Another group implemented a nonfouling coating including a cross-linked poly-(N,N-dimethylacrylamide) (PDMAA) hydrogel network.[21] The mechanism of action aims to reduce bacterial adhesion on the stents to prevent biofilm formation rather than using antibiotics to kill the bacteria.[21] PDMAA does this by evading protein adsorption and cellular adhesion via entropic shielding, which is the thermodynamic principle that entropy loss of an already stretched gel is higher than the entropy gain when mixed with a penetrating protein; therefore, no penetration occurs.[22] PDMAA resists the adsorption of the conditioning film components of biofilm formation, inhibiting bacterial colonization. Their results showed a significant decrease in the adhesion of Escherichia coli, a prominent uropathogen, to the surface of the coated ureteral stents. Although clinical applications of this coating are yet to be observed, the results of this study provide a strong foundation for moving the coating down the testing pathway and observing the in vivo efficacy of PDMAA as a method for combating biofilm formation on ureteral stents.
Novel materials
Although some groups looked into novel coatings as a solution to biofilm formation and encrustation, many others tackled these problems by altering the stent material. Rebl et al.[23] assessed and identified which stent surface parameters prevent encrustation in the hopes of developing novel ureteral stents. They observed the contact angle, surface charge, and encrustation of different materials to provide guidance on the beneficial surface parameters. Their findings suggested that materials with a slight hydrophilicity and a strong zeta potential had the best outcome in reducing encrustation.
Conventional ureteral stents are often made from polyurethane; however, a group studied magnesium-zinc-strontium alloys and the effect they would have on human urothelial cells (HUC) if they were used to produce biodegradable ureteral stents.[24] They found that the cytocompatibility of HUCs differs depending on the varying concentrations of these alloys, their degradation rates, and degradation products. Magnesium alloy degradation products can affect HUC viability; therefore, reducing these degradation rates will be an important step. Another group observed the degradation of zinc alloys in an artificial urine system in a study of zinc alloys as a potential biomaterial for ureteral stents and found that zinc alloys degraded at a slower rate than magnesium alloys and could be the ideal biomaterial to prevent bacterial adhesion and encrustation on stents.[25]
Furthermore, Paramitha et al.[26] studied the biological effects of utilizing zinc-based absorbable metals for ureteral stents. Their results indicated that zinc alloys had higher cytocompatibility with HUC than magnesium alloys and that damaged cells could repair and colonize these areas to maintain proper functioning. Their studies provided an insight into expanding and optimizing the absorbable metals for ureteral stents. Zhao et al.[27] extended their development of a copper-bearing stainless steel stent into an in vivo model with the aim of decreasing bacterial growth and encrustation. This stent resulted in significantly reducing the bacterial attachment on the stent surface compared with that in the control. By targeting the cell membranes of the microbes and penetrating them, instead of being coated with antibiotics, the copper-bearing stent may prevent bacterial adherence and encrustation while also decreasing the risk of antibiotic resistance.
Although different metallic stents have been identified and examined for efficacy, biodegradable ureteral stents (BUS) produced from natural-based polymers have also been studied. Barros et al.[28] conducted a study on an in vivo animal model observing the capabilities of their novel BUS. The stent showed homogenous degradation; however, the mechanical properties were diminished compared with the commercial control stents over time. However, the level of hydronephrosis was lower in the BUS, and it also demonstrated effective urine drainage and high biocompatibility, making it a viable option to pursue in a clinical setting. In the pursuit of developing and implementing biodegradable stents, a study by Chew et al.[29] observed the efficacy of the next generation UripreneÒ ureteral stent. This novel biodegradable stent was implanted in a pig model and studied for biodegradation, effects on renal function, and hydronephrosis. Their results indicated that the UripreneÒ stent was biocompatible, biodegradable, and able to provide good renal drainage, all while inducing considerably less hydronephrosis and inflammation. The control stents also had higher levels of uropathy and nephropathy than those of the UripreneÒ stents. This study lays the groundwork for testing the biodegradable UripreneÒ stent in a clinical model as a means for reducing the stent-associated morbidities, such as infection and hydronephrosis.
With regard to the commercial stents that are clinically being used, polyurethane is currently the most common material. A study by Gadzhiev et al.[30] examined the symptoms that the patients experience with polyurethane stents compared with silicone stents. Among the stent-related symptoms that were assessed, encrustation was a secondary outcome that was measured. Although no significant differences were found regarding encrustation between polyurethane and silicone stents, a trend toward decreased encrustation was observed with silicone stents. Moreover, the patients with silicone stents also expressed fewer pain symptoms than those who had polyurethane stents.
Novel technologies
Mosayyebi et al.[31] focused their efforts on altering the architecture of ureteral stents to manipulate flow in a way that reduced crystal deposition. By employing a microfluidic platform for their hydrodynamic analyses, they introduced a stent with a different side-hole configuration to tackle the issue of stent encrustation by decreasing particle deposition and improving overall stent function. By altering the stent thickness and side-hole design, they were able to observe a significant increase in the wall-shear stress of the inactive side-holes where encrustation was seen to occur. This increase in wall-shear stress from the novel stent architecture significantly decreased encrustation and particle build-up compared with the control stents, which provided future clinical applications to target stent encrustation in patients.
In addition to reimaging the ureteral stent, a group developed a noninvasive technique for the ultrasonic sanitation of stents to evade bacterial colonization and encrustation.[32] They found their sanitation treatment to be safe and effective for reducing the risk of infectious complications caused by stenting.
As urological research moves toward targeting ureteral stent encrustation as a major key to improving patient care, detection and monitoring of the encrustation process will be necessary. A recent study utilized a quartz crystal microbalance (QCM) to monitor urinary crystallization.[33] The QCM sensor was able to detect encrustation in less than an hour and surpassed other encrustation assays in efficiency and sensitivity. Through early detection of surface encrustation, complications, including blockage and pain arising from crystalline deposits on urological devices, can be avoided or tackled to improve patient care.
Pharmacological treatment and prophylaxis
Prophylactic antibiotics given before endourologic procedures, such as urinary stone extraction with subsequent ureteral stenting, have been in common practice for many decades. Antibiotic prophylaxis has been proven to significantly reduce early postoperative bacteriuria without any significant side effects.[34] Decreasing the bacterial growth can lead to less bacteria adhering onto the stent surface, thereby preventing the formation of biofilm or encrustation. Lee et al.[35] studied the use of antibiotic prophylaxis in immunosuppressed patients with kidney transplantation undergoing ureteral stenting compared with that in patients with general urinary stones. Kidney transplant recipients are regularly given sulfamethoxazole/trimethoprim (SMZ/TMP) as a prophylaxis against Pneumocystis jirovecii, and this study investigated the potential beneficial effects of providing additional antibiotic prophylaxis. Their results indicated that additional antibiotics did not provide any significant protection from bacterial infection to the patients. In another study evaluating prophylaxis against infections postrenal transplant, Rosado-Canto et al.[36] studied fosfomycin disodium administration in transplant recipients. Owing to the high rates of microbial resistance observed with SMZ/TMP, they studied the use of this novel treatment utilizing fosfomycin in conjuction. The results showed that fosfomycin administration in addition to SMZ/TMP prophylaxis reduced the hospitalizations with symptomatic urinary tract infection in patients undergoing urological procedure, such as a kidney transplant.
Uric acid stones are obstructions in the urinary tract that can arise from low urine pH and low urine output.[37] They are often treated with potassium citrate as an alkalinizing agent. A recent study evaluated the safety of ureteral stenting in patients undergoing potassium citrate treatment with regard to stent encrustation and infection.[38] They concluded that potassium citrate leads to stone dissolution, thereby decreasing the rates of stent encrustation. Furthermore, they concluded that the combined use of ureteral stents and potassium citrate treatment was safe and did not lead to higher rates of encrustation or infection in patients. This can be seen as a potential alternative to surgery for uric acid stone removal in patients.
Urinary microbiome
To better understand the underlying mechanisms of biofilm formation and encrustation on ureteral stents, many researchers are studying the urinary microbiome. Investigating the urinary microbiome can provide insights into solving stent-associated problems, including biofilm formation and encrustation. Identifying the commensal and resident bacteria of the urinary tract may assist in correlating the individual differences of patient microbiome profiles and their prognoses. Buhmann et al.[39] studied individual urinary microbiota by sequencing, qPCR, and imaging of ureteral stent encrustations in patients without urinary tract infections or bacteriuria. They were able to classify the patients into different urotypes to provide an understanding of the commensal bacteria and their effect on the health of the urinary tract. There are still gaps in our knowledge of the role of the urinary microbiome in stent colonization, in part owing to the difficulty in extracting bacteria from indwelling ureteral stents. Furthermore, a preliminary study found that Bacteroides fragilis could play an important role in stent encrustation,[40] without causing a positive urinary tract infection in culture. Further studies are required to better understand the bacterial flora of urine and its implications on stent encrustation.
In conclusion, the field of urological research has progressed immensely in translational and clinically relevant research that can improve patient quality of care; however, some gaps still exist regarding how to effectively solve the 2 interdependent problems of biofilm formation and encrustation on ureteral stents. Regarding stent composition, there is still not a perfect design or material that evades bacterial adhesion or crystal deposition. Numerous stent materials that are being tested in vitro or in vivo will need to continue on to human clinical trials for further safety and efficacy testing because the human urinary environment is more complex than that of the currently available animal models. Although we are beginning to realize that the urinary tract is not a perfectly sterile environment and that commensal bacteria may play a role in patient susceptibility to infection, further work must be performed to identify and understand the role of urinary microbiome composition in stent complications. The exact underlying mechanisms of encrustation and biofilm formation in patients with ureteral stent are not yet fully understood. Researchers in the field of urological sciences must continue their exceptional work to fill the gaps on mechanisms of action, stent development, investigation methods, prophylaxis, and the urinary microbiome for improved patient care and diminished morbidities.
Main Points.
Encrustation and biofilm formation continue to contribute to stent-associated morbidities, including pain, bacteremia, and urosepsis.
The improvement of ureteral stent design and function is a main goal of researchers in the field in order to reduce the associated morbidities from encrustation and biofilm formation.
Innovations, such as altering stent architecture or utilizing coatings, have shown to aid in the reduction of bacterial adherence and encrustation propagation on stent surfaces.
Footnotes
Peer-review: This manuscript was prepared by the invitation of the Editorial Board and its scientific evaluation was carried out by the Editorial Board.
Author Contributions: Concept – S.K., B.H.C., D.L.; Design – S.K., B.H.C., D.L.; Supervision – S.K., B.H.C., D.L.; Literature Search – S.K., D.L.; Writing Manuscript – S.K., D.L.; Critical Review – S.K., B.H.C., D.L.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Zimskind PD, Fetter TR, Wilkerson JL. Clinical use of long-term indwelling silicone rubber ureteral splints inserted cystoscopically. J Urol. 1967;97:840–4. doi: 10.1016/S0022-5347(17)63130-6. [DOI] [PubMed] [Google Scholar]
- 2.Pariente JL, Conort P. Biomaterials used in contact with the urinary tract for urine drainage: catheters and ureteric stents. Prog Urol. 2005;15:897–906. [PubMed] [Google Scholar]
- 3.Riedl CR, Plas E, Hübner WA, Zimmerl H, Ulrich W, Pflüger H. Bacterial colonization of ureteral stents. Eur Urol. 1999;36:53–9. doi: 10.1159/000019927. [DOI] [PubMed] [Google Scholar]
- 4.Lange D, Scotland K. The role of bacteria in urology. Cham: Springer; 2019. pp. 27–43. [DOI] [Google Scholar]
- 5.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113:5–13. doi: 10.1016/S0002-9343(02)01054-9. [DOI] [PubMed] [Google Scholar]
- 6.Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13:269–84. doi: 10.1038/nrmicro3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Betschart P, Zumstein V, Buhmann MT, Altenried S, Babst C, Müllhaupt G, et al. Symptoms associated with long-term double-J ureteral stenting and influence of biofilms. J Urol. 2019;134:72–8. doi: 10.1016/j.urology.2019.08.028. [DOI] [PubMed] [Google Scholar]
- 8.Betschart P, Zumstein V, Buhmann MT, Albrich WC, Nolte O, Güsewell S, et al. Influence of biofilms on morbidity associated with short-term indwelling ureteral stents: a prospective observational study. World J Urol. 2019;37:1703–11. doi: 10.1007/s00345-018-2569-z. [DOI] [PubMed] [Google Scholar]
- 9.Toprak T, Aytaç S. Does duration of stenting increase the risk of clinical infection? Arch Ital Urol Androl. 2019;91:237–40. doi: 10.4081/aiua.2019.4.237. [DOI] [PubMed] [Google Scholar]
- 10.Shabeena KS, Bhargava R, Manzoor MAP, Mujeeburahiman M. Characteristics of bacterial colonization after indwelling double-J ureteral stents for different time duration. Urol Ann. 2018;10:71–5. doi: 10.4103/UA.UA_158_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zumstein V, Betschart P, Buhmann MT, Albrich WC, Nolte O, Güsewell S, et al. Detection of microbial colonization of the urinary tract of patients prior to secondary ureterorenoscopy is highly variable between different types of assessment: results of a prospective observational study. Biofouling. 2019;35:1083–92. doi: 10.1080/08927014.2019.1692000. [DOI] [PubMed] [Google Scholar]
- 12.Buhmann MT, Abt D, Altenried S, Rupper P, Betschart P, Zumstein V, et al. Extraction of biofilms from ureteral stents for quantification and cultivation-dependent and -independent analyses. Front Microbiol. 2018;9:1470. doi: 10.3389/fmicb.2018.01470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Grazia A, LuTheryn G, Meghdadi A, Mosayyebi A, Espinosa-Ortiz EJ, Gerlach R, et al. A Microfluidic-based investigation of bacterial attachment in ureteral stents. Micromachines. 2020;11:408. doi: 10.3390/mi11040408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broomfield RJ, Morgan SD, Khan A, Stickler DJ. Crystal-line bacterial biofilm formation on urinary catheters by urease-producing urinary tract pathogens: a simple method of control. J Med Microbiol. 2009;58:1367–75. doi: 10.1099/jmm.0.012419-0. [DOI] [PubMed] [Google Scholar]
- 15.Lange D, Bidnur S, Hoag N, Chew BH. Ureteral stent-associated complications-where we are and where we are going. Nat Rev Urol. 2015;12:17–25. doi: 10.1038/nrurol.2014.340. [DOI] [PubMed] [Google Scholar]
- 16.Kartal IG, Baylan B, Gok A, Sagnak AL, Karakoyunlu N, Cakici MC, et al. The association of encrustation and ureteral stent indwelling time in urolithiasis and KUB grading system. Endoruol Stones Disease. 2018;15:323–8. doi: 10.22037/uj.v0i0.4592. [DOI] [PubMed] [Google Scholar]
- 17.Scarneciu I, Bratu OG, Cobelschi CP, Neculoiu CD, Scarneciu CC, Lupu S, et al. The risk factors and chemical composition of encrustation of ureteral double J stents in patients with urolithiasis. Rev Chim. 2018;69:3406–9. doi: 10.37358/RC.18.12.6759. [DOI] [Google Scholar]
- 18.Chew BH, Chan J, Choy D, Hirayama T, Iwamura M, Branda N, et al. The interaction of urinary components with biomaterials in the urinary tract: ureteral stent discoloration. J Endourol. 2020;34:608–16. doi: 10.1089/end.2019.0551. [DOI] [PubMed] [Google Scholar]
- 19.Cottone CM, Lu S, Wu YX, Guan K, Yoon R, Limfueco L, et al. Surface-treated pellethanes: comparative quantification of encrustation in artificial urine solution. J Endourol. 2020;34:868–73. doi: 10.1089/end.2020.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Nahas AR, Lachine M, Elsawy E, Mosbah A, El-Kappany H. A randomized controlled trial comparing antimicrobial (silver sulfadiazine)-coated ureteral stents with non-coated stents. Scand J Urol. 2018;52:76–80. doi: 10.1080/21681805.2017.1376353. [DOI] [PubMed] [Google Scholar]
- 21.Szell T, Dressler FF, Goelz H, Bluemel B, Miernik A, Brandstetter T, et al. In vitro effects of a novel coating agent on bacterial biofilm development on ureteral stents. J Endourol. 2018;33:225–31. doi: 10.1089/end.2018.0616. [DOI] [PubMed] [Google Scholar]
- 22.Pandiyarajan C, Prucker O, Zieger B, Rühe J. Influence of the molecular structure of surface attached poly (N-alkyl Acrylamide) zoatings on the interaction of surfaces with Proteins, cells and blood platelets. Macromol Biosci. 2013;13:873–84. doi: 10.1002/mabi.201200445. [DOI] [PubMed] [Google Scholar]
- 23.Rebl H, Renner J, Kram W, Springer A, Fritsch N, Hansmann H, et al. Prevention of encrustation on ureteral stents: which surface parameters provide guidance for the development of novel stent materials? Polymers. 2020;12:558. doi: 10.3390/polym12030558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian Q, Zhang C, Deo M, Rivera-Castaneda L, Masoudipour N, Guan R, et al. Responses of human urothelial cells to magnesium-zinc-strontium alloys and associated insoluble degradation products for urological stent applications. Mat Sci Eng. 2018;96:248–62. doi: 10.1016/j.msec.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 25.Champagne S, Mostaed E, Safizadeh F, Ghali E, Vedani M, Hermawan H. In vitro degradation of absorbable zinc alloys in artificial urine. Materials. 2019;12:295. doi: 10.3390/ma12020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paramitha D, Chabaud S, Bolduc S, Hermawan H. Biological assessment of Zn-based absorbable metals for ureteral stent applications. Materials. 2019;12:3325. doi: 10.3390/ma12203325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao J, Cao Z, Lin H, Yang H, Li J, Li X, et al. In vivo research on Cu-bearing ureteral stent. Jour Mat Sci Mater Med. 2019;30:83. doi: 10.1007/s10856-019-6285-z. [DOI] [PubMed] [Google Scholar]
- 28.Barros AA, Oliveira C, Ribeiro AJ, Autorino R, Reis RL, Duarte ARC, et al. In vivo assessment of novel biodegradable ureteral stent. World J Urol. 2018;36:277–83. doi: 10.1007/s00345-017-2124-3. [DOI] [PubMed] [Google Scholar]
- 29.Chew BH, Lange D, Paterson RF, Hendlin K, Monga M, Clinkscales KW, et al. Next generation biodegradable ureteral stent in a Yucatan pig model. J Urol. 2010;183:765–71. doi: 10.1016/j.juro.2009.09.073. [DOI] [PubMed] [Google Scholar]
- 30.Gadzhiev N, Gorelov D, Malkhasyan V, Akopyan G, Harchelava R, Mazurenko D, et al. Comparison of silicone versus polyurethane ureteral stents: a prospective controlled study. BMC Urol. 2020;20:10. doi: 10.1186/s12894-020-0577-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosayyebi A, Lange D, Yann Yue Q, Somani BK, Zhang X, Manes C, et al. Reducing deposition of encrustation in ureteric stents by changing the stent architecture: a microfluidic investigation. Biomicrofluidics. 2019;13:014101. doi: 10.1063/1.5059370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Novikov AA, Tsukanov AY, Akhmetov DS, Mustafaev RF, Mulyukova AR. Noninvasive ultrasonic sanitation of stents for drainage of upper urinary tract. Biomed Eng. 2018;52:173–6. doi: 10.1007/s10527-018-9807-y. [DOI] [Google Scholar]
- 33.Abadian PN, Buch PJ, Goluch ED, Li J, Zhang Z. Real-time monitoring of urinary encrustation using a quartz crystal microbalance. Anal Chem. 2018;90:1531–5. doi: 10.1021/acs.analchem.7b04047. [DOI] [PubMed] [Google Scholar]
- 34.Fourcade RO. Antibiotics prophylaxis with cefotaxime in endoscopic extraction of upper urinary tract stones: a randomized study. J Antimicrob Chemother. 1990;26:77–83. doi: 10.1093/jac/26.suppl_A.77. [DOI] [PubMed] [Google Scholar]
- 35.Lee JH, Muthukumar T, Kim J, Aull MJ, Watkins A, Kapur S, et al. Antibiotic prophylaxis for ureteral stent removal after kidney transplantation. Clin Transplant. 2019;33:e13491. doi: 10.1111/ctr.13491. [DOI] [PubMed] [Google Scholar]
- 36.Rosado-Canto R, Parra-Avila I, Tejeda-Maldonado J, Kauffman-Ortega C, Rodriguez-Covarrubias FT, Trujeque-Matos M, et al. Perioperative foscomycin disodium prophylaxis against urinary tract infection in renal transplant recipients: a randomized clinical trial. Nephrol Dial Transplant. 2019:1–8. doi: 10.1093/ndt/gfz261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson DM. Clinical and laboratory approaches for evaluation of nephrolithiasis. J Urol. 1989;141:770–4. doi: 10.1016/S0022-5347(17)41006-8. [DOI] [PubMed] [Google Scholar]
- 38.Alenezi NA, Zanaty F, Hodhod A, El-Gharabawy M, El-Sherif E, Badawy A, et al. The safety of ureteral stenting with the use of potassium citrate for management or renal urine acid stones. Urol Anna. 2020;12:37–41. doi: 10.4103/UA.UA_60_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buhmann MT, Abt D, Nolte O, Neu TR, Strempel S, Albrich WC, et al. Encrustations on ureteral stents from patients without urinary tract infection revel distinct urotypes and low bacterial load. Microbiome. 2019;7:60. doi: 10.1186/s40168-019-0674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X, Gao H, Sun X, Huang Z, Wang B, Li Y, et al. A preliminary study on the role of Bacteroides fragilis in stent encrustation. World J Urol. 2020 doi: 10.1007/s00345-020-03185-0. doi: 10.1007/s00345-020-03185-0. [DOI] [PubMed] [Google Scholar]