Abstract
Objective
Laser technology in urology is currently used for both stone lithotripsy and prostate enucleation. Thulium fiber laser (TFL) is a novel laser, with initial studies showing potential benefits over other lasers both in terms of its effectiveness and safety profile.
Material and methods
In the first part of this review, a descriptive analysis of the theoretical concepts behind TFL was performed. This part focused on the physics and laser parameters as applied to the clinical practice. These were interpreted in the context of other lasers, namely, the Holmium:YAG laser to highlight the theoretical advantages as well as potential pitfalls offered by the TFL. In the second part of the review, a narrative synthesis of in-vitro studies regarding TFL and its modifications is performed assessing stone-related parameters, namely, ablation rate, operative time retropulsion, and safety.
Results
TFL achieved high ablation rates in most studies and performed better than Holmium:YAG laser across a range of different settings and ablation modes when the two lasers were compared. Moreover, its ability to use low pulse energy ensures minimal stone retropulsion with the retropulsion threshold estimated to be 2–4 times higher than that of Holmium:YAG laser. From a safety viewpoint, TFL poses no additional risks than other lasers, although it does potentially lead to slightly higher temperatures in the surrounding tissues during lithotripsy.
Conclusion
The unique properties of TFL have made it an attractive alternative to conventional laser techniques currently used in urology. Clinical studies are required before its application can become more widespread.
Keywords: BPH, fiber laser, fragmentation, laser, stones, thulium, urolithiasis
Introduction
The first use of lasers in urology was reported in 1976 by Staehler et al.[1] whereby an argon laser beam was experimentally tested on the bladder wall. Advancements of fiber-optic technology, newer digital ureteroscopes, and novel laser techniques have enabled modern lasers to be used for various indications, most notably for lithotripsy and prostate enucleation.
Holmium:YAG laser remains the most commonly used laser technique in urology, which was first investigated 30 years ago and subsequently introduced in clinical practice in 1993.[2] Its ability to cut and coagulate tissue, multipurpose usability, and suitability to be used with modern endoscopes meant that Holmium:YAG laser technology quickly gained popularity.[2]
As technology improves, the quest for the better laser technology with safety and efficacy in mind has led to the evolution of thulium fiber laser (TFL), which has started to achieve due recognition with the first experimental lithotripsy studies conducted in 2005.[3] Initial results from ex vivo studies have been quite promising with improved and quicker stone ablation and reduced retropulsion[3] among its numerous advantages over more conventional lasers, although these findings are yet to be replicated in clinical studies.
The aim of this review was to examine the theoretical and technological aspects behind the TFL and how these correlate with its use in clinical practice, thereby assessing available evidence from in-vitro and clinical studies. A systematic review of data regarding TFL lithotripsy will be conducted at the end of this article.
Material and methods
We look at the theoretical aspects of the TFL as well as the laser parameters and their application in the clinical setting. The review article was based on a search of various bibliographic databases including MEDLINE, EMBASE, Cochrane Controlled Registered of Trials, and Google Scholar. They were searched for relevant English language studies published anytime. The keywords “thulium fiber laser,” “TFL,” “lasertripsy,” and “lithotripsy” were used. Boolean operators (AND, OR) were used to refine the search. Chain searching of references of all included articles was performed to identify further relevant articles. Because of heterogeneity of published data, a meta-analysis of the various clinical and technological parameters was not possible; thus, a narrative synthesis has been carried out.
Theoretical concepts
Thulium is a rare-earth element, which exists in the trivalent state (Tm3+).[4] It is silvery grey in color and rather soft and malleable.[4] It undergoes slow oxidation in air and melts at a temperature of 1550°C to form Thulium oxide (Tm2O3).[4]
The infrared light emitted by the TFL has a typical wavelength of 1940 nm, although it can vary between 1810 nm and 2100 nm, depending on the design of the TFL.[5] This is in contrast with other lasers used in urology, namely, the FREDDY laser, which has a wavelength of 532–1064 nm and the more popular Holmium:YAG laser with a wavelength of 2100 nm.[5] The water absorption coefficient (WAC) determines how well the infrared radiation emitted by a fiber laser is absorbed by water and consequently the efficiency of stone ablation.[6] TFL has a WAC μa=129.2 cm−1, whereas its wavelength closely matches the water absorption peak.[7] This is potentially clinically advantageous for two reasons, namely, that it theoretically leads to more efficient lithotripsy while keeping photothermal damage to surrounding tissue to a minimum. To put this into context, Holmium:YAG laser’s WAC at a wavelength of 2100 nm is μa=28 cm−1[8], which means that water absorbs TFL energy approximately four times higher than it does with Holmium:YAG laser energy (Figure 1). Like any other laser, TFL’s WAC is temperature dependent, with an experimental study showing a linear decrease in WAC as the temperature is increased from 20°C to 80°C.[9] The opposite effect was observed at a wavelength of 1920 nm,[9] which raises the prospect of whether manipulating laser wavelength can further optimize TFL’s ablation efficiency as tissue is heated toward vaporization.
Figure 1.
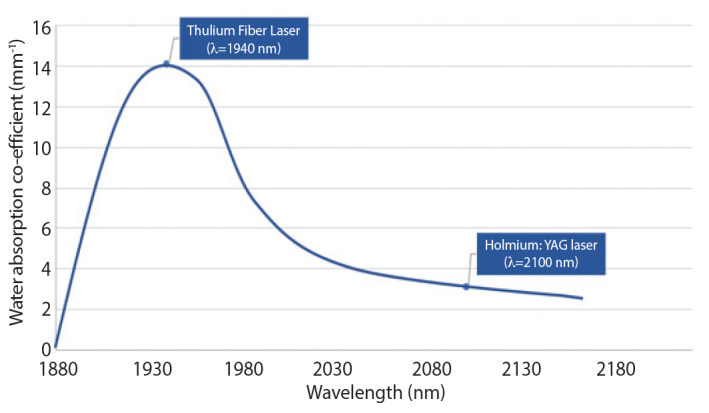
Graph showing relationship between wavelength (nm) and water absorption coefficient (mm−1) for TFL and Holmium:YAG laser[10]
Energy source
In a traditional TFL, the pump energy is provided by the laser diodes as compared with a flash lamp pump used in a Holmium:YAG laser. These semiconductor devices work by converting electrical energy to optical energy, which is then used for the excitation of Thulium ions.[10] Laser diodes have several advantages over flash lamps, namely, that it can operate at a lower power and is smaller in size. Moreover, the presence of an air cooling system, as will be explained below, overcomes any thermal issues associated with laser diodes.[10] The main pitfall with such energy source is that it has a critical heating problem, which can result in thermal stress to the diode, although this is more likely to become an issue with high-powered industrial devices.[11]
Fiber laser structure
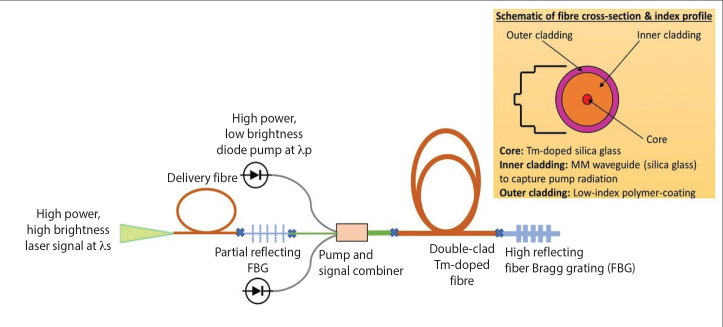
Most of the high power fiber laser consists of a rare-earth-doped optical fiber coated with a low-index polymer as shown in Figure 2. Multimode laser diodes (pump) are launched at one end of the active fiber. The pump is guided by the cladding and subsequently absorbed by the rare-earth ions doped in the core. The emission of rare-earth ions, spatial distribution of light in core, and fiber Bragg gratings ensure a stable laser output beam. Fiber laser can be considered as a device that converts low brightness of laser diodes into a high brightness source, defined by the waveguiding properties of the core.[12]
Figure 2.
Schematic representation of a cladding pumped fiber laser
In the case of TFL, the core of the optical fiber is doped with Tm2O3 and additional dopants such as Al2O3 in a silica matrix to avoid quenching of rare-earth ions. Both Al2O3 and Tm2O3 are refractive index increasing components; hence, they constitute a high refractive index core of fiber. In contrast, cladding is normally pure silica and the outermost cladding is a polymer coating. The fiber is cladding pumped at 793 nm, which results in the excitation of thulium ions within the core region, which is typically 10–20 μm in diameter fiber. Consequently, a laser beam is generated with a wavelength of ~1940 nm. Furthermore, a small cross-sectional area of the silica fiber is also very important as it allows extreme deflection of flexible ureteroscope to perform difficult lithotripsy procedures such as in the case of lower pole stones and also allows optimal irrigation through the miniature working channel.[13]
Beam profile
The thermal distortion of the fiber laser output beam is negligible as compared with the solid-state lasers, thanks to the large surface-area-to-volume ratio offered by fiber.[14] Furthermore, a small core size in thulium-doped silica fiber ensures a near-diffraction limited output beam profile of the TFL unlike the Holmium:YAG laser (Figure 3). In practical terms, this means that a laser beam can be focused onto a small spot, resulting in more efficient tissue ablation or lithotripsy. Moreover, the beam profile offered by the TFL would allow delivery of high power laser beams through a very small core fiber, 50–200 μm in diameter.[15]
Figure 3.
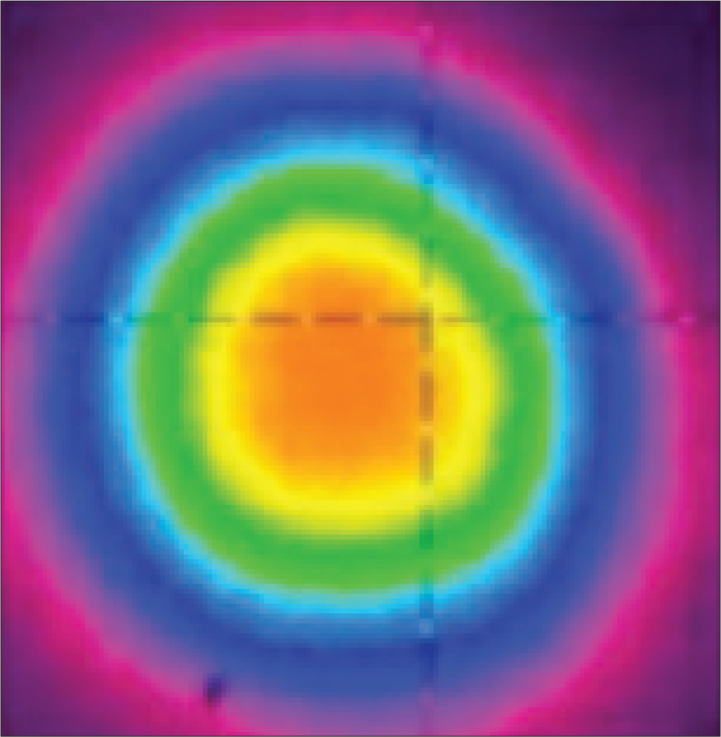
Spatial collimated beam profile of 30-W TFL in continuous wave operation. Adapted with permission from Kneis et al.[14] © The Optical Society.
The initial spike in the temporal beam profile of TFL is less steep and shorter than that for Holmium:YAG laser.[15] This initial energy spike produces a laser beam, which is directly related to the size of the vapor bubble produced, which, in turn, translates to the pressure exerted on the stone and subsequently its retropulsion. The lower energy spike produced by TFL ensures a more uniform temporal beam profile and, consequently, a more even energy distribution.[15] In a study of bubble dynamics, Hardy et al.[16] and Gonzalez et al.[17] showed that the vapor bubble produced by TFL was four times smaller than that of Holmium:YAG laser owing to lower peak power and smaller core fibers.
TFL in practice
Pulse energy
The pulse energy for current TFL ranges between 0.025 and 6 J. The provision for low energy setting allows the production of finer particles during dusting and reduced stone retropulsion as the stone vibrates rather than recoils during ablation.[17]
Pulse frequency
TFL can reach pulse frequencies of 2100 Hz, which is about 21 times more than the maximum frequency achieved by a Holmium:YAG laser.[7,18] This high pulse frequency is probably unnecessary, and the highest repetition rate reported in medical literature currently is 500 Hz.[19] The advantage of such high frequencies is that it can be used in combination with low pulse energies in a highly efficient stone dusting mode.[19]
Pulse duration (width)
TFL pulse duration settings range from 200 μs to 1100 μs, thereby allowing the operator to use the TFL in both short and long pulse modes. Studies have shown that pulse width does not affect ablation volume, although a difference in crater size is observed between the short and long pulse mode.[20] The short pulse mode will, however, lead to more stone retropulsion and more fiber-tip degradation, which is one advantage of TFL over Holmium:YAG laser as the latter is restricted to the short pulse mode.[21] Furthermore, this reduction in retropulsion decreases the non-contact lithotripsy phase by 3.5 times, improving the fragmentation efficiency in the popcorn mode and decreases the overall operative time.[22]
Power
TFL can operate at a high peak power, with newer models reaching up to 500 W (superpulse mode), far exceeding those that can be achieved with the Holmium:YAG laser. This is possible because of less heat dissipation with TFL as compared with Holmium:YAG laser.[22] Nevertheless, the clinical utility of such high power is debatable as lithotripsy settings rarely exceed powers of 30W and the risk of collateral damage to surrounding tissues, at high powers, must also be acknowledged.
Dimensions
A typical TFL 50-W generator console measures 44.8×50.4×17.7 cm and weighs <30 kg,[23] making it compact, portable, and space-efficient especially in cumbersome operating theaters. Its portability is further highlighted by its use of a standard power outlet (220–240 V). This also reduces costs related to installation of specialized high amperage systems, as in the case of the Holmium:YAG laser system.[23] These features raise the prospect of the TFL being used in the outpatient setting to carry out local anesthetic ureteroscopy and lithotripsy in the foreseeable future.[24]
Cooling system
TFL uses an air cooling system as compared with Holmium:YAG laser, which uses a water cooling system.[25] This is possible because the TFL is more energy-efficient and, therefore, an air cooling system is sufficient to dissipate any residual heat energy.[20,26] Moreover, additional benefits of using a fan ventilation cooling system are that it allows the TFL to operate at a higher power and also reduces the size of the generator required when compared with the Holmium:YAG refrigeration system. It is worth noting that for power generators >50 W, an external water cooling system is still required.[26]
Power consumption
A standard 50-W TFL consumes <900 W of power, with even lower values for the 10 W and 30 W models. Moreover, TFL has a wall-plug efficiency, i.e., its ability to convert electrical energy to optical energy, of approximately 10%.[26] This contrasts with that of Holmium:YAG laser, which stands at around 1%–2%.[27] The reasons for TFL’s high wall-plug efficiency are multifactorial, namely, increased laser diode efficiency, ability for direct coupling of laser beams, and low quantum defect.[27]
Cost savings
In an era of cost-cutting and sensible spending within health care, the TFL appears to fit the bill for a variety of reasons. Unlike Holmium:YAG laser, TFL can operate using the main power supply and does not require a high amperage power outlet.[28] The lifetime of a laser diode is estimated to be around 70,000 h,[29] and it is less susceptible to wear and tear unlike flash lamps in Holmium:YAG lasers. The uniform laser beam profile and certain modifications to the silica core fiber such as a hollow steel tip reduce fiber-tip degradation secondary to burn-back, which makes TFL fibers reusable, thereby also highlighting its environmental credentials.[30] Although theoretical expenditure for both production and maintenance of TFL appears to be lower than that of other lasers, detailed cost-effectiveness analysis within clinical studies will be required before its use becomes more mainstream.
Hazards
Ocular injury remains the primary concern with the use of lasers. This potential hazard has been extensively investigated with regard to the use of Holmium:YAG lasers, with studies showing that eye injury is rare and three conditions need to be satisfied for eye injury to occur, namely, high energy settings, short distance (<5 cm), and the absence of protective eyewear. With a wavelength of >1400 nm, most of the absorbed radiation is restricted to the cornea and not the more sensitive retina, with cataracts and corneal abrasions, therefore, being the more likely sequelae.[31]
Another potential hazard is the risk of the thermal injury such as skin burns. It is unclear whether TFL poses any additional risks to those already specific to laser fibers. It is likely that any potential hazard is due to factors unrelated to TFL’s intrinsic characteristics, such as the absorption and scattering coefficients of the skin, irradiance of the laser beam, duration of the exposure, and size of the area irradiated.[31]
Clinical applications
Stone lithotripsy
Multiple in-vitro analytic studies, assessing the characteristics of TFL for lithotripsy have been published (Table 1). It was not possible to combine the results of the different studies because of variable laser settings, experimental setup, and different core fibers, and therefore, a narrative synthesis and analysis regarding the different outcomes can be found below.
Table 1.
List of studies on thulium fiber laser lithotripsy including laser settings and outcomes (Continue)
| Study | Study Objectives | Laser Fiber | Laser Settings | Results | ||||
|---|---|---|---|---|---|---|---|---|
| Power | Pulse Frequency | Pulse Energy | Pulse duration | Primary Outcome | Power | |||
| Andreeva et al.[32] | Preclinical comparison of superpulse TFL and a holmium:YAG laser for lithotripsy | 200-μm core thulium-doped silica fiber (λ=1940 nm) | 100–500 W | 6–50 Hz | 0.2–1 J | 400–10,000 μs |
|
|
| Hardy et al.[39] | Compare stone ablation rates in dusting mode for Holmium:YAG and TFL | 100-μm core thulium-doped silica fiber (λ=1940 nm) | 10–32 W | 50–80 Hz | 2–4 mJ | 500–1000 μs |
|
|
| Hardy et al.[34] | Investigate operative time and saline temperatures around stone samples during lithotripsy | 100-μm core thulium-doped silica fiber (λ=1908 nm) | 500 W | 150–500 Hz | 35 mJ | 500 μs |
|
|
| Blackmon et al.[33] | Compare Holmium:YAG and TFL in terms of ablation thresholds, ablation rates, and retropulsion for COMb stones | 200-μm core thulium-doped silica fiber (λ=1908 nm) | - | 10–400 Hz | 5–35 mJ | 500 μs |
|
|
| Blackmon et al.[36] | Compare stone vaporization rates for holmium:YAG and TFL | 100-μm core thulium-doped silica fiber (λ=1908 nm) | 100 W | 10 Hz | 70 mJ | 1000 μs |
|
|
| Fried et al.[3] | In-vitro analysis of stone fragmentation | 300-μm core thulium-doped silica fiber (λ=1940 nm) | 110 W | 10 Hz | 1 J | 20 ms |
|
|
| Studies which tested modifications to the TFL | ||||||||
| Hall et al.[41] | Use of an automated, vibrating fiber tip in dusting of kidney stones using TFL | 50–150 μm core thulium-doped silica fiber (λ=1908 nm) | 100 W | 300 Hz | 33 mJ | 500 μs |
|
|
| Hutchens et al.[42] | Investigate use of fiber-optic muzzle brake tip for reducing fiber burn-back and stone retropulsion during TFL lithotripsy | 100-μm core thulium-doped silica fiber (λ=1908 nm) | 100 W | 300 Hz | 35 mJ | 500 μs |
|
|
| Wilson et al.[43] | Investigate use of miniature ball-tip optical fibers during TFL lithotripsy | 100-μm core thulium-doped silica fiber (λ=1908 nm) | 100 W | 300 Hz | 35 mJ | 500 μs |
|
|
| Wilson et al.[44] | Compare ablation rate and burn-back for novel steel tip fiber and conventional bare tip fiber for TFL lithotripsy of UA stones | 100-μm core thulium-doped silica fiber (λ=1940 nm) | - | 500 Hz | 30 mJ | 500 μs |
|
|
| Hutchens et al.[30] | Investigate use of hallow steel tips to reduce distal fiber burn-back during TFL lithotripsy | 150-μm core thulium-doped silica fiber (λ=1908 nm) | 110 W | 150 Hz | 34 mJ | 500 μs |
|
|
| Blackmon et al.[45] | To investigate the efficiency of lithotripsy of TFL when used in the MPTd mode | 100-μm core thulium-doped silica fiber (λ=1908 nm) | - | 10–50 Hz and MPT | 35 mJ | 500 μs |
|
|
TFL: thulium fiber laser.
COM: calcium oxalate monohydrate.
UA: uric acid. dMPT: micropulse train.
Ablation rates
TFL achieved high ablation rates in most studies and performed better than Holmium:YAG laser across a range of different settings and ablation modes when the two lasers were compared. TFL’s superiority in the dusting mode is highlighted in the study by Andreeva et al.[32] whereby its stone ablation rates for calcium oxalate monohydrate (COM) and uric acid (UA) stones were threefold and twofold higher, respectively, than those of Holmium:YAG laser at equivalent laser settings.[32] This difference in ablation rate is due to a lower ablation threshold for TFL, which has been estimated to be fourfold less than that of Holmium:YAG laser. This lower ablation threshold is likely because of the higher WAC of TFL, both for water bound to the stone and the hydrophilic environment surrounding it.[33]
Given that an increase in pulse frequency in the Holmium laser is limited to 80 Hz, one way to increase ablation rates is by increasing the pulse energy. One study,[33] in fact, showed a linear relationship between an increase in the pulse energy and stone ablation rates, though the trade-off to this would be more stone retropulsion. Such problems are not encountered with TFL as stone ablation rates are a function of pulse frequency, especially with its ability to reach high frequencies of up to a 1000 Hz while keeping the pulse energy low, a combination which works optimally in the dusting mode. The same aforementioned study did depict this relationship, although this was a non-linear one. Nevertheless, at exceedingly high frequencies, retropulsion will become an issue.[33]
Retropulsion
Studies show that for the same pulse energy, stone retropulsion during TFL lithotripsy is weaker than both short and long pulse Holmium:YAG lasers, whereas the retropulsion threshold is 2–4 times higher. The main factor is likely to be a reduced water pressure from the vapor bubble generated during TFL lithotripsy.[32,33] Blackmon et al.[33] defined retropulsion as a stone fragment movement over a distance of >2 mm. Their results were consistent with those of other studies,[33] namely, that with a combination of long pulse duration, moderately high pulse frequency and low pulse energy, there is minimal retropulsion and the diode-pumped TFL was an ideal laser to operate within such parameters. Although multiple studies suggest that TFL causes lower retropulsion when compared with Holmium:YAG laser, it is worth noting that every study uses a different type of stone phantom, making comparisons of retropulsion distances difficult.
Laser and operative time
In-vitro studies have allowed differentiation between laser time and operative time. Laser time can be defined as the duration of time during which the stone is being lasered and is inherently dependent on the intrinsic laser settings and stone characteristics, whereas operative time is the more clinically important measure as it also takes into account other factors such as re-adjusting the stone due to retropulsion effects as well as other confounding human, operative, and non-operative factors. Multiple studies have shown that laser time and operative time for TFL were significantly shorter than those for Holmium:YAG laser, by approximately 2.5–4 times.[34] Shorter ablation time for UA stones than COM stones was also seen with TFL, and these findings are similar to those of other lasers with stone composition rather than type of laser being the main determining factor.[34]
The difference in laser time was even larger when TFL was operated at higher frequencies of 300 Hz and 500 Hz, highlighting the important role pulse frequency plays in regulating laser time. Interestingly, for TFL, there were no statistically significant differences in laser time at 300 Hz and 500 Hz, suggesting that higher pulse frequencies may be unnecessary to achieve adequate stone dusting. In addition, the ability to use lower pulse energy and reduced stone retropulsion both contribute to shorter laser time.[34] Whether these benefits of TFL translate into shorter operative times in real-life situations is a question that can only be answered through clinical studies.
Safety
The safety of TFL during lithotripsy was assessed by Hardy et al.[34] by measuring peak saline irrigation temperature using thermocouples, at 3 mm from the point of lithotripsy. This showed that temperatures for TFL were on average 9–12°C higher than those for the Holmium:YAG laser, with the temperature difference increasing as the pulse frequency went up, thereby suggesting that pulse frequency should ideally be kept below 500 Hz to maintain safe operation of the TFL. Nonetheless, the highest temperatures were only observed for a period of <4 s, which correlated with a reduced flow of irrigation due to obstructing stone debris, which further highlights the importance of a constant flow of irrigation, for an efficacious lithotripsy procedure and to reduce thermal damage to surrounding tissue. Optimal irrigation flow was also identified as a determining factor in reducing thermal stress in a more recent study whereby TFL was operated on its own in the absence of stones.[35] This showed that power >15 W and low irrigation are both predisposing factors to a temperature rise and potentially thermal stress to surrounding tissue. Although low irrigation rates are usually preferred during lithotripsy to reduce the retropulsion effect, TFL’s intrinsic characteristics help to counterbalance this and allow for a steadier irrigation flow than other lasers would.[35,36]
Fragmentation, dusting, or popcorning
Recent studies using the Holmium:YAG laser show the effect of dusting and pop-dusting on stone fragmentation.[37–39] The cost of treatment for these procedures also depends on the consumables used.[40] Although these techniques of breaking the stones are well established with regard to the Holmium:YAG laser, future clinical work will need to establish optimal stone fragmentation techniques for TFL whereby the pulse modulation is likely to play a big part in shaping these techniques. Moreover a number of studies, detailed in the second part of Table 1 have explored the use of certain modifications such as a muzzle brake or ball-tip in an attempt to reduce fiber tip burn-back and retropulsion.[41–45]
Prostate enucleation
Prostate surgery to assess the clinical effectiveness of TFL enucleation of the prostate (ThuFLEP) was performed by Enikeev et al.[46] where they prospectively randomized patients with prostates <80 g to have either ThuFLEP or the gold standard transurethral resection of the prostate (TURP). This should not be confused with the more conventional Thulium:Yttrium Aluminium Garnet (Tm:YAG) laser also used for laser enucleation of the prostate and which has been in clinical use for a longer period.[46] ThuFLEP resulted in statistically significant improvement in clinically important outcomes, namely, quality of life, international prostate symptom score (IPSS), maximum flow rate (Qmax), and post-void residual volume. IPSS decreased by 15.3 points, whereas Qmax improved by 10.8 mls/s. There was no statistically significant difference in outcomes apart from Qmax whereby the improvement was greater in the ThuFLEP group. Although these results are certainly encouraging, they have to be interpreted with caution given that long-term outcomes are not known with the follow-up period of only 12 months.[46]
One of the mainstream criticisms of laser enucleation of smaller prostates is the time factor, with studies consistently showing that it takes longer than other techniques. The findings of this study support this trend with ThuFLEP taking on average 7 min longer than TURP. This is, however, still an improvement on the mean 10- to 15-min difference when TURP was compared with other laser techniques. From a safety viewpoint, none of the patients in the ThuFLEP suffered complications higher than Clavie-Dindo grade 2, with no statistically significant differences between the two groups suggesting that ThuFLEP is at least as safe as the more conventional techniques. The obvious limiting factor of this study was the small number of patients recruited. Larger randomized studies are required to further assess the role and applicability of TFL for prostates <80 g. Moreover, its role in larger prostates (>80 g) and how it compares with Holmium laser enucleation of the prostate is yet to be studied.[46]
Limitations
The obvious limitation with this review is that TFL is a novel technology, which is in the process of being introduced into clinical practice, and as a result, most of the data available are from studies carried out in vitro. The limited clinical data available are from conference abstracts with small patient numbers and no method of assessing the study’s quality and rigor.
Conclusion
The unique properties of TFL have made it an attractive alternative to conventional laser techniques currently used in urology. Results from in-vitro studies show that it matches or outperforms its nearest competitors, namely, Holmium:YAG laser. Multi-center randomized trials are required to further ascertain both its clinical applicability and cost-effectiveness.
Main Points.
TFL is a novel laser technique with ideal physical properties for use in stone lithotripsy and prostate enucleation.
In-vitro studies show that TFL outperforms Holmium:YAG laser both with regard to stone ablation rate and reduced stone retropulsion during lithotripsy.
Randomized clinical studies are required before the use of TFL can become more widespread.
Footnotes
Peer-review: This manuscript was prepared by the invitation of the Editorial Board and its scientific evaluation was carried out by the Editorial Board.
Author Contributions: Concept - B.K.S.; Design - M.S.; Supervision - B.K.S.; Materials - J.S.; Data Collection and/or Processing - M.S.; Analysis and/or Interpretation - M.S., A.P.; Literature Search - M.S.; Writing Manuscript - M.S.; Critical Review - O.A., B.K.S.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Staehler G, Hofstetter A, Gorisch W, Keiditsch E, Müssiggang M. Endoscopy in Experimental Urology Using an Argon-Laser Beam. Endoscopy. 1976;8:1–4. doi: 10.1055/s-0028-1098365. [DOI] [PubMed] [Google Scholar]
- 2.Kronenberg P, Somani B. Advances in Lasers for the Treatment of Stones-a Systematic Review. Curr Urol Rep. 2018;19:45. doi: 10.1007/s11934-018-0807-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fried N. Thulium fiber laser lithotripsy: An in vitro analysis of stone fragmentation using a modulated 110-watt Thulium fiber laser at 1.94 μm. Lasers Surg Med. 2005;37:53–8. doi: 10.1002/lsm.20196. [DOI] [PubMed] [Google Scholar]
- 4.National Center for Biotechnology Information. PubChem Database. Thulium, AtomicNumber=69. [accessed on Feb. 3, 2020]. Available from: https://pubchem.ncbi.nlm.nih.gov/element/Thulium.
- 5.Fried N, Hardy L. Comparison of 1908 and 1940 nm wavelengths for thulium fiber laser lithotripsy. Therapeutics Diag Urol. 2019 doi: 10.1117/12.2506779.. doi: 10.1117/12.2506779.. [DOI] [Google Scholar]
- 6.Fried N, Irby P. Advances in laser technology and fiber-optic delivery systems in lithotripsy. Nat Rev Urol. 2018;15:563–73. doi: 10.1038/s41585-018-0035-8. [DOI] [PubMed] [Google Scholar]
- 7.Traxer O, Keller E. Thulium fiber laser: the new player for kidney stone treatment? A comparison with Holmium:YAG laser. World J Urol. 2019 doi: 10.1007/s00345-019-02654-5. doi: 10.1007/s00345-019-02654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blomley M, Nicholson D, Bartal G, Bradley A, Myers M, Allison D. Penetration of the Holmium:YAG Laser Through Fluid. J Vasc Interv Radiol. 1995;6:903–10. doi: 10.1016/S1051-0443(95)71210-6. [DOI] [PubMed] [Google Scholar]
- 9.Theisen-Kunde D, Danicke V, Wendt M, Brinkmann R. Temperature dependence of water absorption for wavelengths at 1920 nm and 1940 nm. IFMBE Proceedings. 2009:2228–9. doi: 10.1007/978-3-540-89208-3_533. [DOI] [Google Scholar]
- 10.Kronenberg P, Traxer O. The laser of the future: reality and expectations about the new thulium fiber laser-a systematic review. Transl Androl Urol. 2019;8(Suppl 4):S398–S417. doi: 10.21037/tau.2019.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Souto J, Pura JL, Jiménez J. Thermomechanical issues of high power laser diode catastrophic optical damage. J Phys D: Applied Physics. 2019;52:343002. doi: 10.1088/1361-6463/ab243f. [DOI] [Google Scholar]
- 12.Duarte F. Tunable laser applications. 2nd ed. New York: CRC Press; 2009. [DOI] [Google Scholar]
- 13.Scott N, Cilip C, Fried N. Thulium Fiber Laser Ablation of Urinary Stones Through Small-Core Optical Fibers. IEEE Journal of Selected Topics in Quantum Electronics. 2009;15:435–40. doi: 10.1109/JSTQE.2008.2012133. [DOI] [Google Scholar]
- 14.Kneis C, Donelan B, Berrou A, Manek-Hönninger I, Robin T, Cadier B, et al. Actively mode-locked Tm^3+-doped silica fiber laser with wavelength-tunable, high average output power. Opt Lett. 2015;40:1464–7. doi: 10.1364/OL.40.001464. [DOI] [PubMed] [Google Scholar]
- 15.Fried N. Recent advances in infrared laser lithotripsy [Invited] Biomed Opt Express. 2018;9:4552–68. doi: 10.1364/BOE.9.004552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hardy L, Kennedy J, Wilson C, Irby P, Fried N. Analysis of thulium fiber laser induced bubble dynamics for ablation of kidney stones. J Biophotonics. 2016;10:1240–9. doi: 10.1002/jbio.201600010. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez DA, Hardy LA, Hutchens TC, Irby PB, Fried NM. Thulium fiber laser-induced vapor bubble dynamics using bare, tapered, ball, hollow steel, and muzzle brake fiber optic tips. Optical Engineering. 2018;57:036106. doi: 10.1117/1.OE.57.3.036106. [DOI] [Google Scholar]
- 18.Black K, Aldoukhi AH, Ghani K. A Users Guide to Holmium Laser Lithotripsy Settings in the Modern Era. Front Surg. 2019;6:48. doi: 10.3389/fsurg.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardy LA, Wilson CR, Irby PB, Fried NM. Rapid Thulium fiber laser lithotripsy at pulse rates up to 500 Hz using a stone basket. IEEE Journal of Selected Topics in Quantum Electronics. 2014;20:138–41. doi: 10.1109/JSTQE.2014.2305715. [DOI] [Google Scholar]
- 20.Hardy LA, Irby PB, Fried NM. Scanning electron microscopy of real and artificial kidney stones before and after Thulium fiber laser ablation in air and water. Therapeutics and Diagnostics in Urology. 2018 doi: 10.1117/12.2285069. doi: 10.1117/12.2285069. [DOI] [Google Scholar]
- 21.Sroka R, Pongratz T, Scheib G, Khoder W, Stief CG, Herrmann T, et al. Impact of pulse duration on Ho: YAG laser lithotripsy: treatment aspects on the single-pulse level. World J Urol. 2015;33:479–85. doi: 10.1007/s00345-015-1504-9. [DOI] [PubMed] [Google Scholar]
- 22.Hardy LA, Kennedy JD, Wilson CR, Irby PB, Fried NM. Cavitation bubble dynamics during thulium fiber laser lithotripsy. Photonic Therapeutics and Diagnostics XII. 2016 doi: 10.1117/12.2208168.. doi: 10.1117/12.2208168.. [DOI] [Google Scholar]
- 23.Fiber Laser Sources & Solutions | IPG Photonics [Internet] Ipgphotonics.com. 2020. [cited 19 February 2020]. Available from: https://www.ipgphotonics.com/en.
- 24.Pai A, Kadhim H, Mackie S, Watson G. Local Anesthetic Flexible Ureterorenoscopy in the Management of Urolithiasis. J Endourol. 2019;33:696–8. doi: 10.1089/end.2019.0107. [DOI] [PubMed] [Google Scholar]
- 25.Jackson SD, Lauto A. Diode-pumped fiber lasers: A new clinical tool? Lasers Surg Med. 2002;30:184–90. doi: 10.1002/lsm.10023. [DOI] [PubMed] [Google Scholar]
- 26.nu2μTM Thulium Fiber Laser [Internet] Nufern.com. 2020. [cited 20 February 2020]. Available from: http://www.nufern.com/filestorage/fiber_lasers/N2U_1940_NA_0025_10_spec.pdf?4605.
- 27.Hecht J. Photonic Frontiers: High-efficiency Optical Pumping: ‘Going green’ cranks up the laser power [Internet] Laser Focus World. 2020. [cited 20 February 2020]. Available from: https://www.laserfocusworld.com/lasers-sources/article/16547048/photonic-frontiers-highefficiency-optical-pumping-going-green-cranks-up-the-laser-power.
- 28.Thulium-Doped Fiber Lasers: 1800 nm to 2050 nm [Internet] Thorlabs.com. 2020. [cited 20 February 2020]. Available from: https://www.thorlabs.com/newgrouppage9.cfm?objectgroup_id=11137.
- 29.Gale P. Estimating laser diode lifetimes and activation energy. ILX Lightwave application note. 2008:1–2. [Google Scholar]
- 30.Hutchens TC, Blackmon RL, Irby PB, Fried NM. Hollow steel tips for reducing distal fiber burn-back during thulium fiber laser lithotripsy. J Biomed Opt. 2013;18:078001. doi: 10.1117/1.JBO.18.7.078001. [DOI] [PubMed] [Google Scholar]
- 31.Althunayan AM, Elkoushy MA, Elhilali MM, Andonian S. Adverse events resulting from lasers used in urology. J Endourol. 2014;28:256–60. doi: 10.1089/end.2013.0451. [DOI] [PubMed] [Google Scholar]
- 32.Andreeva V, Vinarov A, Yaroslavsky I, Kovalenko A, Vybornov A, Rapoport L, et al. Preclinical comparison of superpulse thulium fiber laser and a holmium: YAG laser for lithotripsy. World J Urol. 2020;38:497–503. doi: 10.1007/s00345-019-02785-9. [DOI] [PubMed] [Google Scholar]
- 33.Blackmon RL, Fried NM, Irby PB. Comparison of holmium: YAG and thulium fiber laser lithotripsy: ablation thresholds, ablation rates, and retropulsion effects. J Biomed Opt. 2011;16:071403. doi: 10.1117/1.3564884. [DOI] [PubMed] [Google Scholar]
- 34.Hardy LA, Wilson CR, Irby PB, Fried NM. Thulium fiber laser lithotripsy in an in vitro ureter model. J Biomed Opt. 2014;19:128001. doi: 10.1117/1.JBO.19.12.128001. [DOI] [PubMed] [Google Scholar]
- 35.Peng Y, Liu M, Ming S, Yu W, Li L, Lu C, et al. Safety of a novel Thulium fiber laser for lithotripsy: an in vitro study on the thermal effect and its impact factor. J Endourol. 2020;34:88–92. doi: 10.1089/end.2019.0426. [DOI] [PubMed] [Google Scholar]
- 36.Blackmon RL, Irby PB, Fried NM. Holmium: YAG (λ= 2,120 nm) versus thulium fiber (λ= 1,908 nm) laser lithotripsy. Lasers Surg Med. 2010;42:232–6. doi: 10.1002/lsm.20893. [DOI] [PubMed] [Google Scholar]
- 37.Pietropaolo A, Jones P, Whitehurst L, Somani BK. Role of ‘dusting and pop-dusting’ using a high-powered (100W) laser machine in the treatment of large stones (≥ 15 mm): Prospective outcomes over 16 months. Urolithiasis. 2019;47:391–4. doi: 10.1007/s00240-018-1076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reeves T, Griffin S, Pietropaolo A, Somani BK. Feasibility of dusting and pop-dusting using high-power (100W) Holmium YAG (Ho:YAG) laser in treatment of paediatric stones: results of first worldwide clinical study. Cent European J Urol. 2019;72:398–401. doi: 10.5173/ceju.2019.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hardy LA, Vinnichenko V, Fried NM. High power holmium: YAG versus thulium fiber laser treatment of kidney stones in dusting mode: ablation rate and fragment size studies. Lasers Surg Med. 2019;51:522–30. doi: 10.1002/lsm.23057. [DOI] [PubMed] [Google Scholar]
- 40.Somani B, Robertson A, Kata SG. Decreasing the cost of flexible ureterorenoscopic procedures. Urology. 2011;78:528–30. doi: 10.1016/j.urology.2010.12.073. [DOI] [PubMed] [Google Scholar]
- 41.Hall L, Gonzalez D, Fried N. Thulium fiber laser ablation of kidney stones using an automated, vibrating fiber. J Biomed Opt. 2019;24:1–10. doi: 10.1117/1.JBO.24.3.038001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hutchens TC, Gonzalez DA, Irby PB, Fried NM. Fiber optic muzzle brake tip for reducing fiber burnback and stone retropulsion during thulium fiber laser lithotripsy. J Biomed Opt. 2017;22:018001. doi: 10.1117/1.JBO.22.1.018001. [DOI] [PubMed] [Google Scholar]
- 43.Wilson CR, Hardy LA, Kennedy JD, Irby PB, Fried NM. Miniature ball-tip optical fibers for use in thulium fiber laser ablation of kidney stones. J Biomed Opt. 2016;21:018003. doi: 10.1117/1.JBO.21.1.018003. [DOI] [PubMed] [Google Scholar]
- 44.Wilson CR, Hutchens TC, Hardy LA, Irby PB, Fried NM. A miniaturized, 1.9 F integrated optical fiber and stone basket for use in thulium fiber laser lithotripsy. J Endourol. 2015;29:1110–4. doi: 10.1089/end.2015.0124. [DOI] [PubMed] [Google Scholar]
- 45.Blackmon RL, Case JR, Trammell SR, Irby PB, Fried NM. Fiber-optic manipulation of urinary stone phantoms using holmium: YAG and thulium fiber lasers. J Biomed Opt. 2013;18:028001. doi: 10.1117/1.JBO.18.2.028001. [DOI] [PubMed] [Google Scholar]
- 46.Enikeev D, Netsch C, Rapoport L, Gazimiev M, Laukhtina E, Snurnitsyna O, et al. Novel thulium fiber laser for endoscopic enucleation of the prostate: A prospective comparison with conventional transurethral resection of the prostate. Int J Urol. 2019;26:1138–43. doi: 10.1111/iju.14115. [DOI] [PubMed] [Google Scholar]