Abstract
BACKGROUND:
American Indians have excess risk of depression, which can contribute to cerebrovascular and cognitive disability, with effects on memory, processing speed, executive function, and visuospatial ability. However, studies examining depression and cognition in American Indians are limited; this study aims to report associations of depression with general cognition, verbal fluency and memory, and processing speed.
DESIGN:
Cohort study.
SETTING:
The Cerebrovascular Disease and its Consequences in American Indians study was an ancillary examination of Strong Heart Study participants from 3 U.S. regions.
PARTICIPANTS:
All eligible were included in this analysis (N=818).
MEASUREMENTS:
Participants completed evaluations for depressive symptomology, cognition, and physical function—including Center for Epidemiologic Studies Depression (CESD), Modified Mini-Mental State Examination (3MSE), Wechsler Adult Intelligence Scale-Fourth Edition coding (WAIS), Controlled Oral Word Association (COWA), California Verbal and Learning Test, Halstead finger tapping, grip strength, and Short Physical Performance Battery (SPPB) tests. Linear mixed models were adjusted for site, age, sex, education, income, marital status, alcohol, smoking, diabetes, hypertension, obesity, cholesterol, stroke, infarct, and hemorrhage.
RESULTS:
Symptoms of depression were common, with 20% (N=138) endorsing CES-D scores of 16+. More depressive symptoms were associated with older age, female sex, lower education, lower income, non-married status, not using alcohol, not smoking, hypertension, diabetes, and stroke. In adjusted analyses, processing speed (WAIS: β −0.13, 95%CI −0.25, −0.03), general cognition (3MSE: β −0.10, 95%CI −0.17, −0.03), verbal fluency (COWA: β −0.10, 95%CI −0.19, −0.01), and motor function (SPPB: β −0.05, 95%CI −0.07, −0.03) were significantly associated with more symptoms of depression.
CONCLUSION:
These findings maybe informative for health disparities populations, especially those with depressive risk. Clinicians may require particular training in cultural humility. Future studies should validate use of the CES-D scale in this population; longitudinal studies may focus on causal mechanisms and potential secondary prevention, such as social support.
Keywords: cognitive assessment, depression, American Indians/Alaska Natives, education, culture
Major depressive disorder and clinical depression can confer profound disability. Studies of depression in older adults have reported associations with cognitive impairment, poorer quality of life, diminished global functioning, and decreased overall physical health, independent of age and education.1,2 Specific domains of depression-related cognitive impairment include episodic memory, information-processing speed, executive function, and visuospatial ability.2–6 These effects can persist, even after the primary depression resolves.7 Further, depressive symptoms seem to be dose dependently related to disability, non-response to antidepressant treatment, depression relapse, and clinical dementia including both vascular dementia and Alzheimer’s disease.8,9
Older American Indians, one of the fastest growing segments of the US population,10 endure excess exposure to several cerebrovascular risk factors11 including smoking, obesity, diabetes, hypertension, and high cholesterol, each of which can contribute to excess morbidity from depression, cognitive decline, and loss of independent functionality. Conversely, increases in depression and cognitive decline lead to a decreased ability to adopt preventive lifestyle strategies and to adhere to care guidelines, once chronic diseases are diagnosed. Despite this, studies on depressive symptoms and cognitive function in older American Indians have been limited. One study of 140 American Indians in the Northern Plains reported that participants with depressive symptoms scored more poorly in conceptualization than those without such symptoms.12 Another study in 2017 of approximately 500 residents of one tribal community in the US Southeast assessed Center for Epidemiologic Studies Depression (CES-D) scale validity13 and associations with all-cause mortality.14
Biological mechanisms relating depressive symptoms and cognitive function may not differ for older American Indians compared with other ethnic or racial groups. However, culturally unique forms of oppression, historical trauma, and adverse experiences, such as forced removal from childhood homes and placement in Indian boarding schools15 that often emphasized assimilation over education, with harsh, military-style disciplinary techniques, were linked to mental health issues that are acutely experienced by older American Indians, warranting these examinations.16–18 Additionally, older American Indians face numerous challenges simply obtaining care19 including underfunding of the Indian Health Service with a shortage of providers, services, and public health awareness,20 along with cultural beliefs regarding care seeking in mental and cognitive health.21–23
In addition, cultural-specific forms of resilience, such as participation in regular cultural events like powwows, speaking Native language, and having strong community identity and social support, may ameliorate the relationship between psychological symptoms and health consequences in American Indians.24–28 The association between depressive symptomology and cognitive function, and how it may differ for older American Indians compared with members of the majority population, is poorly understood. Very few studies are available on the clinical course and treatment of depressive symptoms in this vulnerable and understudied population. Improving cultural competencies in healthcare providers to serve this population is also critical to address this gap.29
The Cerebrovascular Disease and Its Consequences in American Indians study conducted cross-sectional examinations on American Indian participants of the Strong Heart Study in 2010–2013 including depression symptomology, cognition and physical function, and cerebrovascular risk. In this article, we provide a description of measured cognition in association with symptoms of depression in this multi-region study of community-dwelling older American Indian adults.
METHODS
Study Setting
The Strong Heart Study cohort (1989–1991) recruited 4,549 American Indians aged 45 to 74 years living in 13 tribal communities in the US Northern Plains, Southern Plains, and Southwest.30 The Cerebrovascular Disease and Its Consequences in American Indians study (2010–2013) comprised an ancillary neurologic examination of all available participants from the original cohort (N = 1,033).31,32 All waves of the Strong Heart Study including the neurologic examination successfully examined more than 85% of eligible surviving participants, as previously described. After data collections were completed, one community withdrew consent and was excluded from subsequent analyses. The resulting analytic data set includes 818 participants.
Depression Symptoms
The CES-D assesses depressive symptoms in the general population.33 It is among the most widely used instruments in clinical medicine and psychiatric epidemiology.34 The original scale consists of 20 symptom assessment questions, with 5-point responses ranging from “Not at all or less than one day” (scored as 0) to “Nearly every day for 2 weeks” (scored as 4), with a possible score range of 0 to 80; scores of 16 or higher are considered evidence of clinically significant depressive symptoms.35 Four items (eg, “During the past week I enjoyed life”) were reverse coded, which was accounted for in total score accounting. Our study protocol included a minor deviation from conventional scale procedures. The original wording for one item was “I could not get going”; this item was amended in our study to “I felt like I couldn’t do what I needed to do,” to accommodate potential cultural and linguistic ambiguity with the idiomatic phrase “get going.”
Cognitive Function
The Modified Mini-Mental State Examination (3MSE) is a global screening tool for cognitive functioning adapted from the Mini-Mental State Examination to reduce ceiling effects and improve sensitivity to change over time.36 It consists of 40 questions and was scored on a 100-point scale. The Wechsler Adult Intelligence Scale-Fourth Edition Coding Test (WAIS Coding) measures visuospatial processing speed and working memory.37 Along with the 3MSE, the Coding subtest (termed the Digit Symbol Test in previous WAIS versions) was a primary screening tool for cognitive function used by the Cardiovascular Health Study.38 Respondents were asked to pair a set of symbols with specific numbers (1–9) within a span of 120 seconds. Scoring was based on the total number of symbols coded correctly with a range from 0 to 135.
The Controlled Oral Word Association (COWA) test is a widely used measure of phonemic verbal fluency that also provides an index of executive functioning.39 In three successive 1-minute trials, participants are asked to say as many words as possible that begin with the letter F, then A, and finally S. A total score was derived from the summation of the three trials, ranging from 0 to more than 53. The California Verbal and Learning Test-II Short Form (CVLT-II SF) provides several indices of verbal learning and memory.40 A list of nine words from three semantic categories was presented over four learning trials, and participants are asked to repeat as many words as possible after each trial. Memory indices include long-term (10-minute delay) free recall (eg, without cues).
Physical Function
The Finger Tapping Test is a test of motor dexterity41 used as an index of laterality and hemispheric dysfunction. Respondents tap the index finger as quickly as possible using the Lafayette Finger Tapping instrument over five 10-second trials that are averaged for both the dominant and nondominant hands. The Grip Strength Test is a measure of gross physical strength that has been used as an index of laterality and hemispheric dysfunction.42 Grip strength is measured in kilograms using the 200-pound Hydraulic Hand Dynamometer,43 with the average of three consecutive trials recorded for both dominant and nondominant hands. The Short Physical Performance Battery (SPPB) is used to measure lower body functioning; components of the battery include timed standing balance, gait speed, and chair stands.44 Scores on each of the three tasks range from 0 to 4 and are summed to generate a total score ranging from 0 to 12, with a threshold of 9 or lower denoting poor physical function.45
Other Participant Characteristics
Participants self-reported age, sex, marital status, household income, educational attainment, and tobacco and alcohol use behaviors. Annual household income was coded in intervals of less than $10,000, $10,000 to $20,000, $20,000 to $35,000, or more than $35,000. Formal education was coded as less than high school, high school or equivalent, some college, or a college degree; marital status was coded as single, married or living with partner, or separated, widowed, or divorced; and alcohol use was coded as never, former, or current. Prevalent diabetes was established based on high fasting plasma glucose or use of glucose-lowering medications or insulin; prevalent hypertension was based on high systolic blood pressure (≥140 mm Hg), high diastolic blood pressure (≥90 mm Hg), or use of anti-hypertensive medications. Obesity was defined as a body mass index (BMI) of 30 kg/m2 or above. Prior stroke was defined via semiannual electronic medical record surveillance with physician panel adjudication for detected events. Cranial magnetic resonance imaging (MRI), read by trained neuroradiologists, provided evidence of vascular lesions including cerebral infarct or hemorrhage.
Statistical Analyses
Graphical analysis of depression symptoms (CES-D), overall and separated by findings of vascular brain injury (VBI) (presence of infarcts, hemorrhages, or white matter disease) or cerebral atrophy (sulcal dilatation or ventricle enlargement), used histogram, scatter, and box plots. One-way analysis of variance (ANOVA) tested for differences in variation in CES-D scores between categories of brain imaging findings. Partial correlation coefficients and Student t tests examined associations between depression symptoms (CES-D) and cognitive (3MSE, COWA, WAIS coding, CVLT-II SF) or physical (grip, finger tap, SPPB) tests.
Adjustment models included site, age, sex, education, income, marital status, alcohol, and tobacco use (model 1); additionally diabetes, hypertension, obesity, and low-density lipoprotein (LDL) (model 2); and additionally prior stroke, infarct, or hemorrhage (model 3). Model 3 analyses were the ones interpreted for causal mediation. Scatterplots with loess fit and 95% confidence intervals (CIs) showed visual associations of depression symptoms with cognitive or physical function tests.
Secondary analyses examined generalized linear models as well as modeling assumptions including splines for linearity; variance inflation factor for multicollinearity; residual-fitted value, residual-leverage, and quantile-quantile plots for normality, heteroscedasticity, and outliers; Cook’s distance for influential outliers; and Box-Cox for need for power transformation. Generalized linear models β coefficients and 95% confidence intervals (CIs) were interpreted as the independent change in outcome (eg, cognitive or physical function test score), per additional endorsed symptom of depression. All analyses were conducted using Stata v.14 (StataCorp, College Station, TX) and R v.3.3 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
The overall distribution of CES-D scores (Figure 1) somewhat followed a normal distribution with excess endorsements at the lowest value (eg, 0 reported symptoms), a skew toward lower scores (eg, below the mean), and a long upper “tail” (eg, many reported symptoms). Mean was 10.7 and standard deviation was 8.0. Participants who endorsed more depressive symptoms were, on average, older, female, had lower educational attainment, lower income, not currently married, noncurrent alcohol users, nonsmokers, hypertensive, had diabetes, and with prevalent stroke (Table 1). Participants with high BMI, high LDL, or vascular brain lesions did not appear to differ in terms of CES-D scores. Approximately 20% (N = 138) had evidence of clinical depressive symptomology (CES-D ≥16; Supplementary Table S1). These participants were not substantively different from those endorsing fewer depressive symptoms with respect to age or smoking. However, a larger proportion of those with depressive symptoms were female, had lower education and income; single or previously married (eg, widowed, separated, or divorced); obese; not alcohol users; and had hypertension, diabetes, or low LDL.
Figure 1.
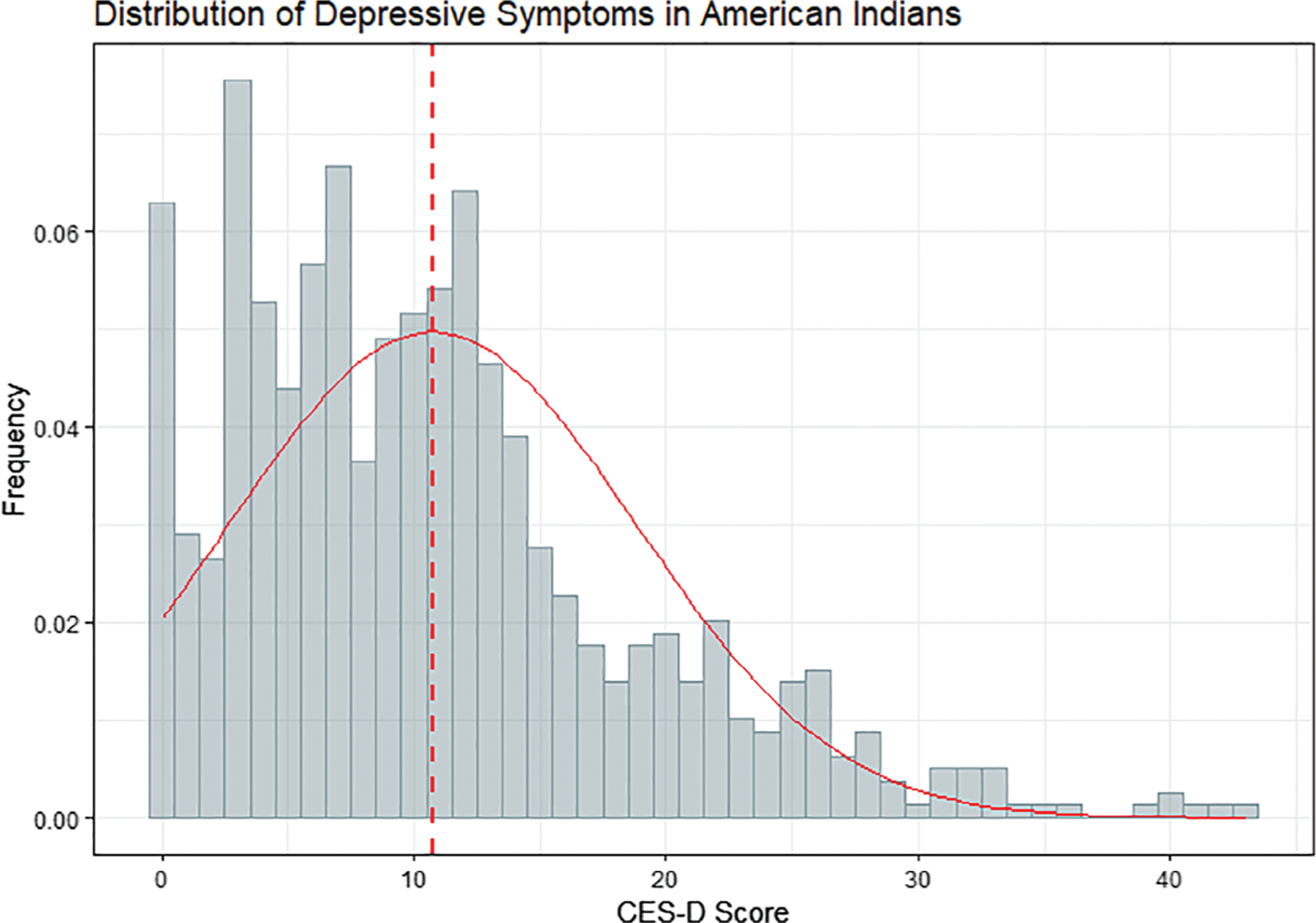
Distribution of depressive symptoms, as measured by Centers for Epidemiologic Studies Depression scale (CES-D), in older American Indians from the Cerebrovascular Disease and Its Consequences in American Indians study (2010–2013). Score distribution shown by blue bars and red normal (Gaussian) density curve. Mean score (red dashed line) is 10.7 (standard deviation = 8.0). Score of 16 is often considered the threshold for clinical depression, although standards vary by population and setting.
Table 1.
Centers for Epidemiologic Studies Depression Scale Scores by Categories of Selected Participant Characteristics from the Cerebrovascular Disease and Its Consequences in American Indians Study (2010–2013)
| Distribution | CES-D Score | ||||
|---|---|---|---|---|---|
| Characteristic | Category | N | % | Mean | SD |
| Age, y | 65–69 | 337 | 41.2 | 9.8 | 7.9 |
| 70–79 | 380 | 46.5 | 11.1 | 8.0 | |
| ≥80 | 101 | 12.3 | 11.7 | 8.4 | |
| Sex | Female | 555 | 67.8 | 11.3 | 8.4 |
| Male | 263 | 32.2 | 9.5 | 6.9 | |
| Education | Up to/Any high school | 162 | 19.8 | 12.0 | 8.8 |
| Graduated high school | 210 | 25.7 | 11.8 | 8.1 | |
| Any college | 322 | 39.4 | 10.3 | 7.5 | |
| Graduated college | 124 | 15.2 | 8.3 | 7.5 | |
| Annual household income | <$10,000 | 251 | 30.7 | 12.3 | 8.3 |
| $10,000-$20,000 | 234 | 28.6 | 11.3 | 8.1 | |
| $20,000-$35,000 | 175 | 21.4 | 9.8 | 7.2 | |
| >$35,000 | 158 | 19.3 | 8.3 | 7.8 | |
| Study field site | Northern Plains | 374 | 45.7 | 11.3 | 8.4 |
| Southern Plains | 346 | 42.3 | 10.3 | 7.7 | |
| Southwest | 98 | 12.0 | 10.1 | 7.3 | |
| Marital status | Single | 43 | 5.3 | 13.8 | 8.5 |
| Married or partnered | 310 | 37.9 | 9.5 | 7.1 | |
| Previously married | 465 | 56.8 | 11.3 | 8.5 | |
| BMI categories | Normal | 124 | 15.3 | 9.8 | 7.9 |
| Overweight | 244 | 30.0 | 10.9 | 7.9 | |
| Obese | 445 | 54.7 | 10.9 | 8.2 | |
| Alcohol use | Never | 200 | 24.4 | 11.5 | 8.7 |
| Former (>1 y ago) | 475 | 58.1 | 11.2 | 8.0 | |
| Current (within 1 y) | 143 | 17.5 | 8.2 | 6.5 | |
| Current smoking | Nonsmoker | 278 | 34.0 | 10.7 | 8.0 |
| Smoker | 540 | 66.0 | 9.8 | 7.5 | |
| Hypertension | No | 157 | 19.2 | 9.4 | 7.6 |
| Yes | 661 | 80.8 | 11.0 | 8.1 | |
| Diabetes | No | 414 | 50.6 | 10.0 | 7.8 |
| Yes | 404 | 49.4 | 11.5 | 8.2 | |
| High LDL | No | 418 | 53.3 | 11.3 | 8.3 |
| Yes | 366 | 46.7 | 10.0 | 7.7 | |
| Self-reported prior stroke | No | 737 | 90.1 | 10.3 | 7.8 |
| Yes | 81 | 9.9 | 15.3 | 9.2 | |
| Infarct or hemorrhage on MRI | No | 497 | 63.4 | 10,.6 | 8.3 |
| Yes | 287 | 36.6 | 11.1 | 7.7 | |
Abbreviations: BMI, body mass index; LDL, low-density lipoprotein; MRI, magnetic resonance imaging; SD, standard deviation.
Box plots and one-way ANOVA comparing CES-D scores for participants with or without history of stroke, MRI findings of VBI (infarct, hemorrhage, or white matter disease), and MRI findings of atrophy (sulcal dilatation or ventricle enlargement) suggested that stroke, but not MRI findings, were significantly associated with symptoms of depression (P < .001, P = .136, and P = .631, respectively; Figure 2).
Figure 2.
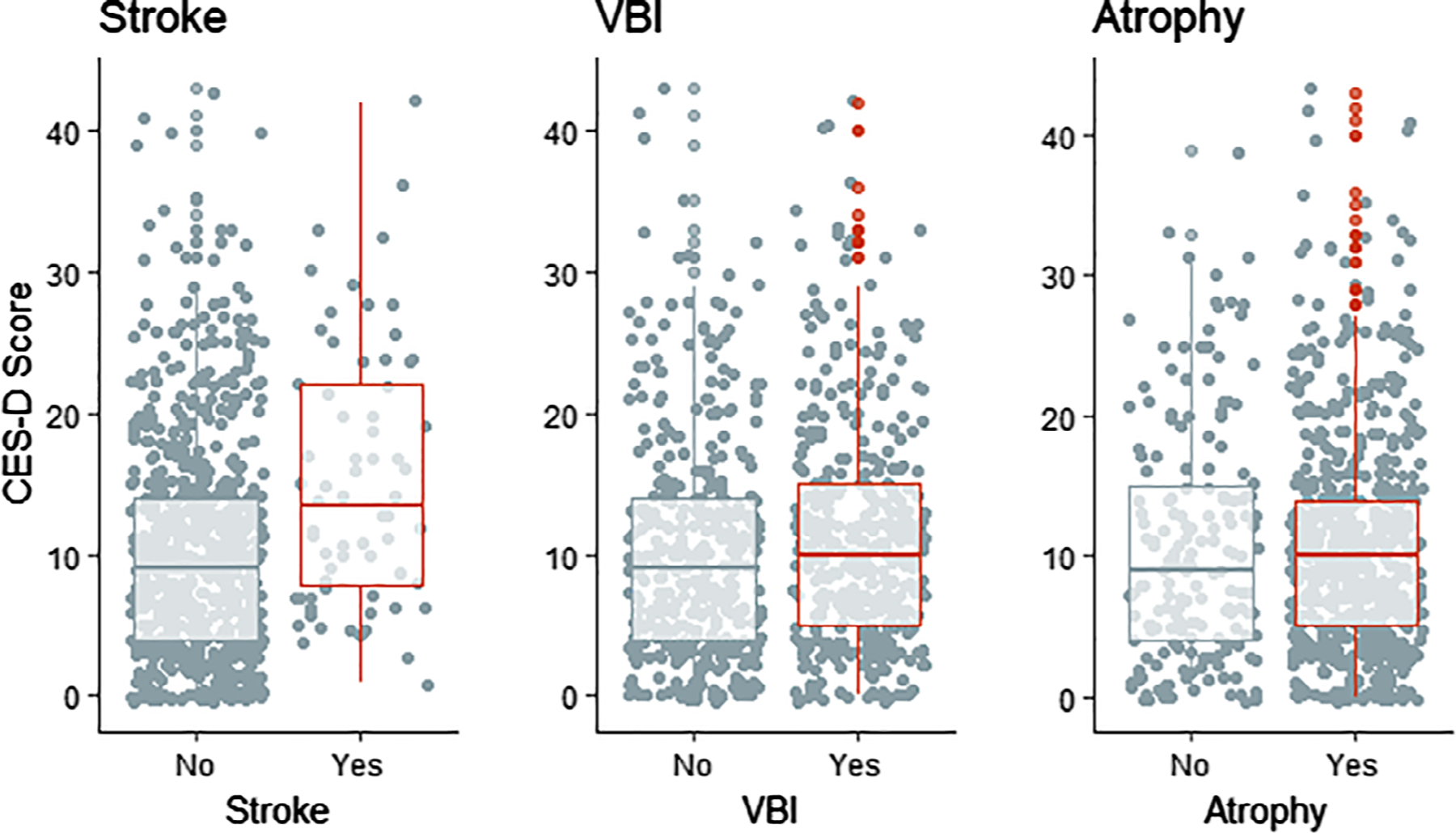
Scatter and box plots comparing Centers for Epidemiologic Studies Depression (CES-D) scores (Y-axes) for participants with (red) and without stroke (left), vascular brain injury (VBI; center), or cerebral atrophy (right) in older American Indians (2010–2013). VBI is defined as the presence of infarcts, hemorrhages, or white matter disease. Atrophy is defined as sulcal dilatation or ventricle enlargement. One-way analysis of variance testing for differences in variation in CES-D scores between groups for stroke, VBI, and atrophy detected P values <.001, .136, and .631, respectively.
Associations between cognitive examination scores with CES-D scores were graphically evident in scatterplots (Figure 3), with loess fit for 3MSE, COWA, and WAIS coding tests declining linearly with an increase in CES-D scores; no statistical association was detected for CVLT long delay free recall. Similar findings were noted for grip strength, Halstead finger tap, and SPPB (Supplementary Figure S1).
Figure 3.
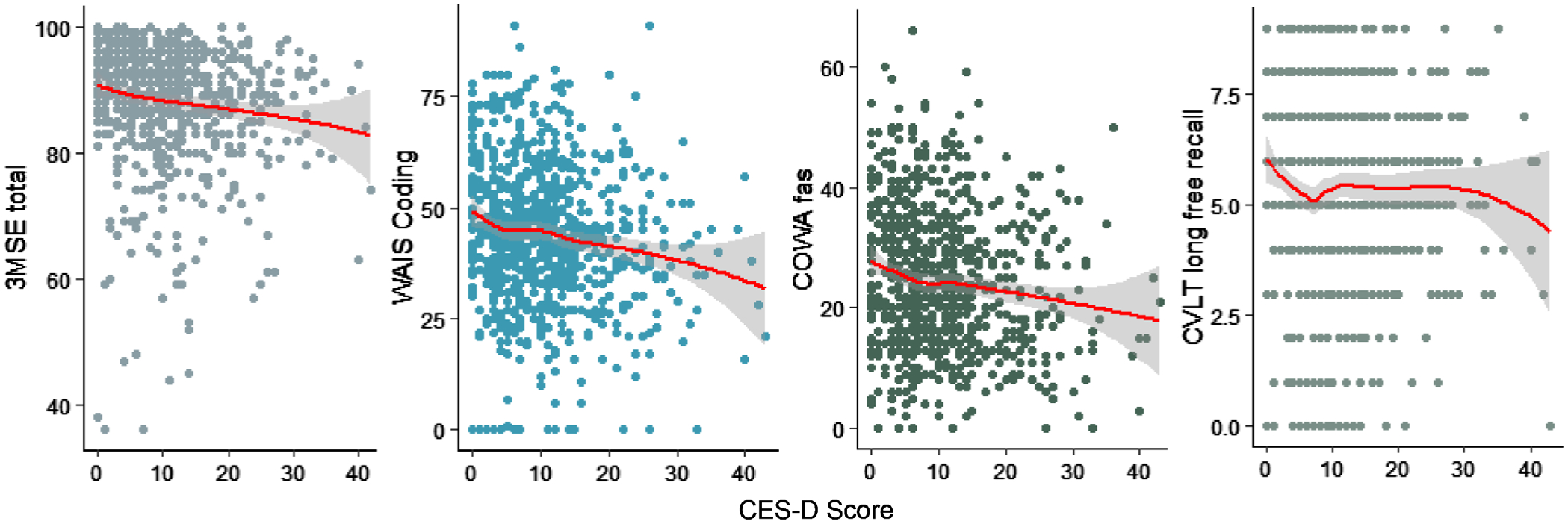
Scatterplots with loess fit (red curve) and 95% confidence interval (gray shading) showing association of cognitive examination scores (Y-axes) with Centers for Epidemiologic Studies Depression (CES-D) scores (X-axes) in older American Indians (2010–2013). Cognitive examinations include Modified Mini-Mental Status Examination (3MSE), Weschler Adult Intelligence Scale (WAIS) coding subtest, Controlled Oral Word Association (COWA) Test-FAS, and California Verbal Learning Test (CVLT), 2nd edition short form long delay free recall. Unadjusted Pearson’s coefficient tests for correlation between CES-D scores and 3MSE, WAIS, COWA, or CVLT LF examinations detected P values .0002, <.001, <.001, and .387, respectively.
Adjusted correlation coefficients between cognitive and physical function examination scores with CES-D suggested that 3MSE (ρ = −.11; P = .009), COWA (ρ = −.10; P = .017), WAIS coding (ρ −.14; P = .001), and SPPB (ρ −.17, P < .001) were all significantly negatively correlated with increased endorsement of depressive symptoms (Table 2), adjusting for site, sex, age, education, income, marital status, alcohol, smoking, diabetes, hypertension, obesity, and LDL (model 2). Additional adjustment for possible mediators of prior stroke and cerebrovascular lesions from MRI (model 3) was slightly attenuated but did not substantively change these findings.
Table 2.
Partial Correlation Coefficients for Association between Centers for Epidemiologic Studies Depression Scores with Cognitive and Physical Function Tests in Older American Indians (2010–2013)
| Examination Score | Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Minimum | Maximum | PCCa | P value | PCCa | P value | PCCa | P value | |
| 3MSE | 88.4 | 9.3 | 36 | 100 | −.10 | .018 | −.11 | .009 | −.08 | .026 |
| COWA | 24.4 | 11.4 | 0 | 66 | −.10 | .011 | −.10 | .017 | −.09 | .038 |
| WAIS coding | 44.0 | 15.9 | 0 | 91 | −.14 | <.001 | −.14 | .001 | −.13 | .003 |
| CVLT-II long delay free recall | 5.5 | 2.2 | 0 | 9 | .01 | .785 | −.01 | .842 | −.01 | .830 |
| Grip strength | 24.8 | 9.5 | 0 | 68 | −.08 | .069 | −.06 | .126 | −.04 | .341 |
| Halstead finger tap | 35.0 | 9.9 | 0 | 61 | −.06 | .155 | −.07 | .119 | −.06 | .159 |
| SPPB | 7.3 | 3.0 | 0 | 12 | −.20 | <.001 | −.171 | <.001 | −.16 | <.001 |
Abbreviations: 3MSE, Modified Mini-Mental State Examination Total Score; COWA-FAS, Controlled Oral Word Association Test-FAS; CVLT-II, California Verbal Learning Test 2nd edition short form long delay free recall; PCC, partial correlation coefficient; SPPB, Short Physical Performance Battery (SPPB) with chair stand, tandem stand, and timed walk; WAIS coding, Weschler Adult Intelligence Scale Digit Symbol Substitution/Coding subtest.
With adjustment for Student t tests.
Secondary evaluations of association using generalized linear models (fit data not shown) suggest that there is little evidence for multicollinearity in these data, with variable inflation factors all less than 1.1. Polynomial splines suggested that continuous covariate associations, including those for CES-D, were mostly linear in form. Cook’s distance estimates suggested one to four outliers for each model, although none unduly influenced model fit. Examination of residual, quantile, and leverage distributions suggested normality, and homoscedasticity assumptions are reasonably fulfilled, although 3MSE had heavy tails in the lower range of scores, and errors for CVLT-II SF may be better captured with a Poisson function (3MSE, WAIS coding, and COWA were modeled with Gaussian error distribution). Box-Cox analysis suggested a power transformation for 3MSE would be appropriate (λ = 5).
Generalized linear models with full model 3 covariate adjustment (Supplementary Table S2) estimated similar associations as for the partial correlations for CES-D including 3MSE (β = −.10; 95% CI = −.17 to −.03); COWA (β = −.10; 95% CI = −.19 to −.01); WAIS coding (β = −.13; 95% CI = −.25 to −.03); and SPPB (β = −.05; 95% CI = −.07 to −.03). Limited association was detected for dominant hand grip strength (β = −.04; 95% CI = −.10 to +.01), but the finding was not significant (P = .06). No association was detected for CVLT-II SF long delay free recall (β = −.01; 95% CI = −.06 to +.03) or for dominant hand finger tap (β = −.01; 95% CI = −.08 to +.07).
DISCUSSION
This report is a comprehensive evaluation of the association of depression symptoms that were somewhat commonly reported with cognitive and motor function status in a large cohort study of older American Indians. General cognition (3MSE), verbal fluency and executive function (COWA), processing speed (WAIS coding), and physical motor function (SPPB) were all consistently negatively associated with a greater number of reported symptoms of depression. The two different analytic methods, partial correlations and generalized linear models, yielded similar results, and models were not different based on the covariables included in the adjustment scheme, suggesting robustness. Additionally, adjustment for sociodemographics, vascular risk factors, vascular imaging findings, and prior stroke were not adequate to attenuate the findings, suggesting these imaging features were not solely responsible for mediating the observed associations between depressive symptoms and cognition or physical function.
Findings in Other Populations
The relatively high prevalence of clinically significant depressive symptoms (20.2% with score ≥16; mean score = 10.7) in this older population is somewhat lower than previous findings among middle-aged and older adults including both African Americans with and without diabetes from the Jackson Heart Study (range = 33%−54%).46 They are on the low end compared with multiethnic Asian studies including both Chinese47 and Singaporean48 populations (range = 19.9%−33.2% and 27.4%−43.2%, respectively) and consistent with findings among indigenous Mexicans (range = 8.5%−21.3%).49 All of these population groups are much higher than non-Hispanic whites of similar age ranges, who reportedly number 5.4% with clinically significant depressive symptomology,50 and approximately .8% increased prevalence per additional year of age, 8% additional prevalence for women compared with men, and 10.8% additional prevalence for those who are “sick,” based on their self-rated health.51
One report found excellent factor structure, reliability, and validity for both short- and long-form CES-D in a separate sample of 491 American Indians,13 similar to findings from other community-dwelling older populations, after amendments were made to accommodate linguistic and other cultural differences.48,52–54 The authors found, somewhat contrary to our findings, that depressive symptoms in their study sample were associated with younger age and not with sex or marital status.13,14 That sample included individuals from a single tribe in the US Southeast, were somewhat younger overall, with prevalence of those with a CES-D score of 16 or above (13.2%). It is possible that social support is more meaningful as individuals age, with the association with age and marital status or other measure of psychosocial connection carrying more weight in an older or more heterogeneous group.
We detected cross-sectional associations between depression symptoms and older age, female sex, lower education, lower income, single marital status, less alcohol use, less tobacco smoking, prevalent hypertension, prevalent diabetes, and previous stroke. The directionality or causality of these findings is not yet clear. A previous investigation in American Indians also detected associations with genetic biomarkers of accelerated biological aging.32 One large study of more than 15,000 older Chinese also discovered inverse associations of smoking and drinking with depressive symptoms,55 although, as in our study, due to the cross-sectional nature of the data, it was unclear whether drinkers and smokers were less likely to develop depression or whether depressed participants were less likely to smoke and drink. It should be noted that drinking was particularly uncommon in this study, with 82.5% of participants not having had any alcohol in the past year.
Studies of other populations that identified similar associations with economic adversity include lower socioeconomic status in Chinese Americans52 and with fewer years of education, material hardship, and other sociodemographics in African Americans.56 Studies that found similar clinical associations include functional status and diabetes in African Americans46 and high blood pressure with a younger onset of hypertension, especially among women, in non-Hispanic whites and African Americans.57 Depression was associated with lower BMI in Chinese Asians,47 a finding we did not replicate. Psychosocial associations that other populations have detected, which may guide additional future research, include chronic stress in African Americans, Latinos, and non-Hispanic whites, with unhealthy coping behaviors in Latinos,58 poorer self-rated health in African Americans,56 and poorer “evaluation of self” in non-Hispanic whites but not in African Americans.59
Strengths and Limitations
This study included a very large sample of a previously underrepresented, understudied population. However, these cross-sectional data make it difficult to interpret causality or temporality. Carefully designed longitudinal studies may be needed to delineate whether intervention to alleviate depressive symptoms can ameliorate cognitive symptoms or dementia risk.
These analyses included data collected from comprehensive standardized examinations with extensive clinical and neuropsychological evaluations. Most associations in this analysis were detected in continuous evaluations not in dichotomizations. This may be due to limitations to statistical power, from small strata, or the relevant threshold for assessing risk for clinically significant depression in this population may not be the conventional limit of a CES-D score or 16 or above as often used to define depression in clinical populations. Additionally, standard deviations were large compared with mean estimates for most categorical strata, suggesting a high degree of residual variability.
Overall, these findings were identified at the population level and are not interpretable for individual-level inference. Also, these scales, although well characterized in many other populations, have not yet been validated in American Indians. Furthermore, it is important to note that the presence of depressive symptoms may be a causal factor, a result, or a confounder for cognitive or functional decline, and it is therefore difficult to infer directionality from these cross-sectional associations. Future analyses should make use of more finely tuned a priori analytic structuring and hypothesis testing to strengthen statistical power and further examine these questions of causality.
Social support, here represented as marital status, and socioeconomic status may be important factors in evaluating depressive symptoms in older American Indians. Annual household income and marital status had the strongest associations with CES-D scores of the factors that were evaluated, with the exception of prior stroke. Future research should include these or possibly more comprehensive factors such as social engagement60 when evaluating depressive symptomology in this population.
As a voluntary research study, and because active and willing participants may be less likely to have clinically significant depression or increased depressive symptomology, some potentially affected individuals may not have been recruited or included in examinations. However, previous evaluation of inclusion criteria for this study identified 86% participation of survivors from the baseline cohort31 and little evidence of differential selection or survival on the basis of cardiovascular or cerebrovascular risk factors. Although our retention rate, compared with previous cohort examinations, was excellent, it is unknown whether our sample findings would hold for the entire population including those who did not volunteer.
In conclusion, this study included a high-quality extensive standardized study protocol that systematically evaluated neuropsychological factors including depressive symptomology and cognition in the largest population-based cohort study of older American Indians assessed to date. These data report the associations between depression symptoms and cognition in this population. These findings may be informative for both this as well as other understudied minority and health disparities populations experiencing depression symptoms or depression. Some American Indian communities and tribes have struggled to maintain their cultural identity as major relocations and other federal policies disrupted and displaced entire communities of American Indian peoples throughout the United States. Intergenerational trauma resulting from these policies may have contributed to a higher burden of depression in American Indians and warrants particular attention by clinicians to improve the clinical course and treatment of depressive symptoms in members of this population.29
Supplementary Material
Supplementary Figure S1: Scatterplots with loess fit (red curve) and 95% confidence interval (gray shading) showing association of physical function examination scores (Y-axes) with Centers for Epidemiologic Studies Depression Scale (CES-D) scores (X-axes) in older American Indians (2010–2013). Physical examinations include grip strength (kg) for dominant hand, Halstead finger tap for dominant hand, and Short Physical Performance Battery (SPPB).
Supplementary Table S1: Center for Epidemiologic Studies Depression Scale (CES-D) scores, by categories of selected participant characteristics from the Cerebrovascular Disease and Its Consequences in American Indians study (2010–2013)
Supplementary Table S2: Generalized linear model findings for associations between Centers for Epidemiologic Studies Depression Scale (CES-D) scores with cognitive and physical function tests in older American Indians (2010–2013)
ACKNOWLEDGMENTS
We wish to thank all Strong Heart Study participants and communities. The opinions expressed in this article are those of the author(s) and do not necessarily reflect the views of the Indian Health Service.
Financial Disclosure: This work was supported by funding from the National Institute on Aging and the National Heart Lung and Blood Institute including cooperative agreement grants U01-HL41642, U01-HL41652, U01-HL41654, U01-HL65520, and U01-HL65521; and research grants R01-HL109315, R01HL109301, R01HL109284, R01HL109282, R01HL109319, R01HL093086, and K01AG057821.
Sponsor’s Role: Sponsor had no role other than funding for the data collection and investigator time in analysis.
Footnotes
Conflict of Interest: The authors have declared no conflicts of interest for this article.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.Butters MA, Whyte EM, Nebes RD, et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004;61:587–595. [DOI] [PubMed] [Google Scholar]
- 2.Sheline YI, Barch DM, Garcia K, et al. Cognitive function in late life depression: relationships to depression severity, cerebrovascular risk factors and processing speed. Biol Psychiatry. 2006;60(1):58–65. [DOI] [PubMed] [Google Scholar]
- 3.Lockwood KA, Alexopoulos GS, van Gorp WG. Executive dysfunction in geriatric depression. Am J Psychiatry. 2002;159:1119–1126. [DOI] [PubMed] [Google Scholar]
- 4.Elderkin-Thompson V, Kumar A, Bilker WB, et al. Neuropsychological deficits among patients with late-onset minor and major depression. Arch Clin Neuropsychol. 2003;18:529–549. [DOI] [PubMed] [Google Scholar]
- 5.Rapp MA, Dahlman K, Sano M, Grossman HT, Haroutunian V, Gorman JM. Neuropsychological differences between late-onset and recurrent geriatric major depression. Am J Psychiatry. 2005;162:691–698. [DOI] [PubMed] [Google Scholar]
- 6.Koenig AM, DeLozier IJ, Zmuda MD, et al. Neuropsychological functioning in the acute and remitted states of late-life depression. J Alzheimers Dis. 2015;45:175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nebes RD, Pollock BG, Houck PR, et al. Persistence of cognitive impairment in geriatric patients following antidepressant treatment: a randomized, double-blind clinical trial with nortriptyline and paroxetine. J Psychiatr Res. 2003;37:99–108. [DOI] [PubMed] [Google Scholar]
- 8.Koenig AM, Bhalla R, Butters MA. Cognitive functioning and late-life depression. J Int Neuropsychol Soc. 2014;20:461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF. Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013; 202:329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US Census Bureau. 2012 American Community Survey. Washington, DC: US Census Bureau; 2012. [Google Scholar]
- 11.Hutchinson RN, Shin S. Systematic review of health disparities for cardiovascular diseases and associated factors among American Indian and Alaska Native populations. PLOS One. 2014;9:e80973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verney SP, Jervis LL, Fickenscher A, Roubideaux Y, Bogart A, Goldberg J. Symptoms of depression and cognitive functioning in older American Indians. Aging Ment Health. 2008;12:108–115. [DOI] [PubMed] [Google Scholar]
- 13.Schure M, Goins RT. Psychometric examination of the Center for Epidemiologic Studies Depression scale with older American Indians: the Native Elder Care Study. Am Indian Alsk Native Ment Health Res. 2017;24:1–17. [DOI] [PubMed] [Google Scholar]
- 14.Goins RT, Noonan C, Winchester B, Brock D. Depressive symptoms and all-cause mortality in older American Indians with type 2 diabetes mellitus. J Am Geriatr Soc. 2019;67:1940–1945. [DOI] [PubMed] [Google Scholar]
- 15.Lomawaima T, McCarty TL. To Remain Indian: Lessons in Democracy from a Century of Native American Education. New York, NY: Teachers College Press; 2006. [Google Scholar]
- 16.Roh S, Burnette CE, Lee KH, Lee YS, Easton SD, Lawler MJ. Risk and protective factors for depressive symptoms among American Indian older adults: adverse childhood experiences and social support. Aging Ment Health. 2015;19:371–380. [DOI] [PubMed] [Google Scholar]
- 17.Clouston SA, Rubin MS, Phelan JC, Link BG. A social history of disease: contextualizing the rise and fall of social inequalities in cause-specific mortality. Demography. 2016;53:1631–1656. [DOI] [PubMed] [Google Scholar]
- 18.Link BG, Phelan J. Social conditions as fundamental causes of disease. J Health Soc Behav. 1995;80–94. https://pubmed.ncbi.nlm.nih.gov/7560851 [PubMed] [Google Scholar]
- 19.Noe TD, Kaufman CE, Kaufmann LJ, Brooks E, Shore JH. Providing culturally competent services for American Indian and Alaska Native veterans to reduce health care disparities. Am J Public Health. 2014;104(suppl 4):S548–S554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim C. Recruitment and retention in the Navajo Area Indian Health Service. West J Med. 2000;173:240–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jervis LL, Manson SM. American Indians/Alaska natives and dementia. Alzheimer Dis Assoc Disord. 2002;16(suppl 2):S89–S95. [DOI] [PubMed] [Google Scholar]
- 22.Griffin-Pierce T, Silverberg N, Connor D, et al. Challenges to the recognition and assessment of Alzheimer’s disease in American Indians of the southwestern United States. Alzheimers Dement. 2008;4:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henderson JN, Traphagan JW. Cultural factors in dementia: perspectives from the anthropology of aging. Alzheimer Dis Assoc Disord. 2005;19: 272–274. [DOI] [PubMed] [Google Scholar]
- 24.Reinschmidt KM, Attakai A, Kahn CB, Whitewater S, Teufel-Shone N. Shaping a Stories of Resilience Model from urban American Indian elders’ narratives of historical trauma and resilience. Am Indian Alsk Native Ment Health Res. 2016;23:63–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahn-John Dine M, Koithan M. Living in health, harmony, and beauty: the Diné (Navajo) Hózhó wellness philosophy. Glob Adv Health Med. 2015;4: 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ward CJ, Cope MR, Elmont L. Native American Vietnam-era veterans’ access to VA healthcare: vulnerability and resilience in two Montana reservation communities. J Community Health. 2017;42:887–893. [DOI] [PubMed] [Google Scholar]
- 27.Whitewater S, Reinschmidt KM, Kahn C, Attakai A, Teufel-Shone NI. Flexible roles for American Indian elders in community-based participatory research. Prev Chronic Dis. 2016;13:E72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duncan GE, McDougall CL, Dansie E, Garroutte E, Buchwald D, Henderson JA. Association of American Indian cultural identity with physical activity. Ethn Dis. 2014;24:1–7. [PMC free article] [PubMed] [Google Scholar]
- 29.Bassett D, Tsosie U, Nannauck S. “Our culture is medicine”: perspectives of native healers on posttrauma recovery among American Indian and Alaska Native patients. Perm J. 2012;16:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee ET, Welty TK, Fabsitz R, et al. The strong heart study. A study of cardiovascular disease in American Indians: design and methods. Am J Epidemiol. 1990;132:1141–1155. [DOI] [PubMed] [Google Scholar]
- 31.Suchy-Dicey AM, Shibata D, Best LG, et al. Cranial magnetic resonance imaging in elderly American Indians: design, methods, and implementation of the cerebrovascular disease and its consequences in American Indians study. Neuroepidemiology. 2016;47:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Q, Zhu Y, Yeh F, et al. Depressive symptoms are associated with leukocyte telomere length in American Indians: findings from the Strong Heart Family Study. Aging (Albany NY). 2016;8:2961–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radloff R The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 34.Murphy JM. Symptom scales and diagnostic schedules in adult psychiatry In: Tsuan MT, Tohen M, eds. Textbook in Psychiatric Epidemiology. New York, NY: Wiley-Liss; 2002:273–332. [Google Scholar]
- 35.Eaton WW, Muntaner C, Smith C, Tien A, Ybarra M. Center for Epidemiologic Studies Depression Scale: review and revision (CESD and CESD-R) In: Maruish ME, ed. The Use of Psychological Testing for Treatment Planning and Outcome Assessment. 3rd ed. Mahwah, NJ: Lawrence Erlbaum; 2004: 363–377. [Google Scholar]
- 36.Teng EL, Chang CH. The modified Mini-Mental (3MS) Examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 37.Wechsler D Wechsler Adult Intelligence Scale. 4th ed. Pearson: San Antonio, TX; 2008. [Google Scholar]
- 38.Lopez OL, Kuller LH, Fitzpatrick A, Ives D, Becker JT, Beauchamp N. Evaluation of dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22:1–12. [DOI] [PubMed] [Google Scholar]
- 39.Benton AL, Hansher K. Multilingual Aphasia Examination. 2nd ed. Iowa City, IA: AJA Associates; 1976. [Google Scholar]
- 40.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test (CVLT-II). 2nd ed. San Antonio, TX: Manual Psychological Corporation; 2000. [Google Scholar]
- 41.Halstead WC. Brain and Intelligence. Chicago, IL: University of Chicago Press; 1947. [Google Scholar]
- 42.Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Applications. Tucson, AZ: Neuropsychology Press; 1993. [Google Scholar]
- 43.Fabrication Enterprises I. Baseline 200 lb STD Hydraulic Hand Dynamometer Manual. White Plains, NY: Fabrication Enterprises; https://www.fab-ent.com/evaluation/strength/baseline-hydraulic-hand-dynamometers/ [Google Scholar]
- 44.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 45.Bean JF, Leveille SG, Kiely DK, Bandinelli S, Guralnik JM, Ferrucci L. A comparison of leg power and leg strength within the InCHIANTI study: which influences mobility more? J Gerontol A Biol Sci Med Sci. 2003;58:M728–M732. [DOI] [PubMed] [Google Scholar]
- 46.Kalyani RR, Ji N, Carnethon M, et al. Diabetes, depressive symptoms, and functional disability in African Americans: the Jackson Heart Study. J Diabetes Complications. 2017;31:1259–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qian J, Li N, Ren X. Obesity and depressive symptoms among Chinese people aged 45 and over. Sci Rep. 2017;7:45637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stahl D, Sum CF, Lum SS, et al. Screening for depressive symptoms: validation of the Center for Epidemiologic Studies Depression scale (CES-D) in a multiethnic group of patients with diabetes in Singapore. Diabetes Care. 2008;31:1118–1119. [DOI] [PubMed] [Google Scholar]
- 49.Franco-Diaz KL, Fernandez-Nino JA, Astudillo-Garcia CI. Prevalence of depressive symptoms and factorial invariance of the Center for Epidemiologic Studies (CES-D) Depression Scale in a group of Mexican indigenous population. Biomedica. 2018;38:127–140. [DOI] [PubMed] [Google Scholar]
- 50.Rawlings AM, Sharrett AR, Golden SH, Windham BG, Selvin E. Prevalence and correlates of depressive symptoms in older adults across the glycaemic spectrum: the Atherosclerosis Risk in Communities (ARIC) study. Diabet Med. 2018;35:583–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thielke SM, Diehr P, Unutzer J. Prevalence, incidence, and persistence of major depressive symptoms in the Cardiovascular Health Study. Aging Ment Health. 2010;14:168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ying YW. Depressive symptomatology among Chinese-Americans as measured by the CES-D. J Clin Psychol. 1988;44:739–746. [DOI] [PubMed] [Google Scholar]
- 53.Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging. 1997;12:277–287. [DOI] [PubMed] [Google Scholar]
- 54.Kumar S, Nakulan A, Thoppil SP, Parassery RP, Kunnukattil SS. Screening for depression among community-dwelling elders: usefulness of the Center for Epidemiologic Studies Depression Scale. Indian J Psychol Med. 2016;38: 483–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng HG, Chen S, McBride O, Phillips MR. Prospective relationship of depressive symptoms, drinking, and tobacco smoking among middle-aged and elderly community-dwelling adults: results from the China Health and Retirement Longitudinal Study (CHARLS). J Affect Disord. 2016;195:136–143. [DOI] [PubMed] [Google Scholar]
- 56.Weaver A, Taylor RJ, Chatters LM, Himle JA. Depressive symptoms and psychological distress among rural African Americans: the role of material hardship and self-rated health. J Affect Disord. 2018;236:207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shah MT, Zonderman AB, Waldstein SR. Sex and age differences in the relation of depressive symptoms with blood pressure. Am J Hypertens. 2013;26: 1413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodriquez EJ, Gregorich SE, Livaudais-Toman J, Perez-Stable EJ. Coping with chronic stress by unhealthy behaviors: a re-evaluation among older adults by race/ethnicity. J Aging Health. 2017;29:805–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Assari S, Lankarani MM. Reciprocal associations between depressive symptoms and mastery among older adults; black-white differences. Front Aging Neurosci. 2016;8:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nelson LA, Noonan CJ, Goldberg J, Buchwald DS. Social engagement and physical and cognitive health among American Indian participants in the Health and Retirement Study. J Cross Cult Gerontol. 2013;28:453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1: Scatterplots with loess fit (red curve) and 95% confidence interval (gray shading) showing association of physical function examination scores (Y-axes) with Centers for Epidemiologic Studies Depression Scale (CES-D) scores (X-axes) in older American Indians (2010–2013). Physical examinations include grip strength (kg) for dominant hand, Halstead finger tap for dominant hand, and Short Physical Performance Battery (SPPB).
Supplementary Table S1: Center for Epidemiologic Studies Depression Scale (CES-D) scores, by categories of selected participant characteristics from the Cerebrovascular Disease and Its Consequences in American Indians study (2010–2013)
Supplementary Table S2: Generalized linear model findings for associations between Centers for Epidemiologic Studies Depression Scale (CES-D) scores with cognitive and physical function tests in older American Indians (2010–2013)


