Abstract
9-(2′,3′-Dideoxy-2′,3′-difluoro-β-d-arabinofuranosyl)adenine (20), 2-chloro-9-(2′,3′-dideoxy-2,3-difluoro-β-d-arabinofuranosyl)adenine (22), as well as their respective α-anomers 21 and 23, were synthesized by the nucleobase anion glycosylation of intermediate 5-O-benzoyl-2,3-dideoxy-2,3-difluoro-α-d-arabinofuranosyl bromide (13) starting from methyl 5-O-benzyl-3-deoxy-3-fluoro-α-d-ribofuranoside (3) and methyl 5-O-benzoyl-α-d-xylofuranoside (10). These compounds were evaluated as potential inhibitors of HIV-1 and hepatitis C virus in human PBM and Huh-7 Replicon cells, respectively. The adenosine analog 20 demonstrated potent activity against HIV-1 in primary human lymphocytes with no apparent cytotoxicity. Conformation of pentofuranose ring of nucleoside 20 in solution was studied by PSEUROT calculations.
Keywords: Fluoro-nucleosides, purines, antiviral activity, conformational analysis
INTRODUCTION
Fluorine substitution of a hydrogen atom and/or a hydroxyl group in a nucleoside analog can impart a significant influence on its biological properties. Addition of a fluorine atom to the sugar moiety of nucleosides can improve their antiviral properties by enhancing potency and selectivity of modified nucleosides against HIV or other viruses.[1–4] The structure-activity relationships for pyrimidine and purine monofluoro 2′,3′-dideoxy nucleosides as potential anti-HIV agents has been previously reported.[1,5,6] Of the different fluoro-substituted nucleosides, purine 2′-β-fluoro nucleosides attract particular attention in view of their interesting biological activity, primarily due to the stabilization of the glycosidic bond. For example, lodenosine 1 is anti-HIV agent which possesses increased chemical and metabolic stability (Figure 1).[7]
FIGURE 1.
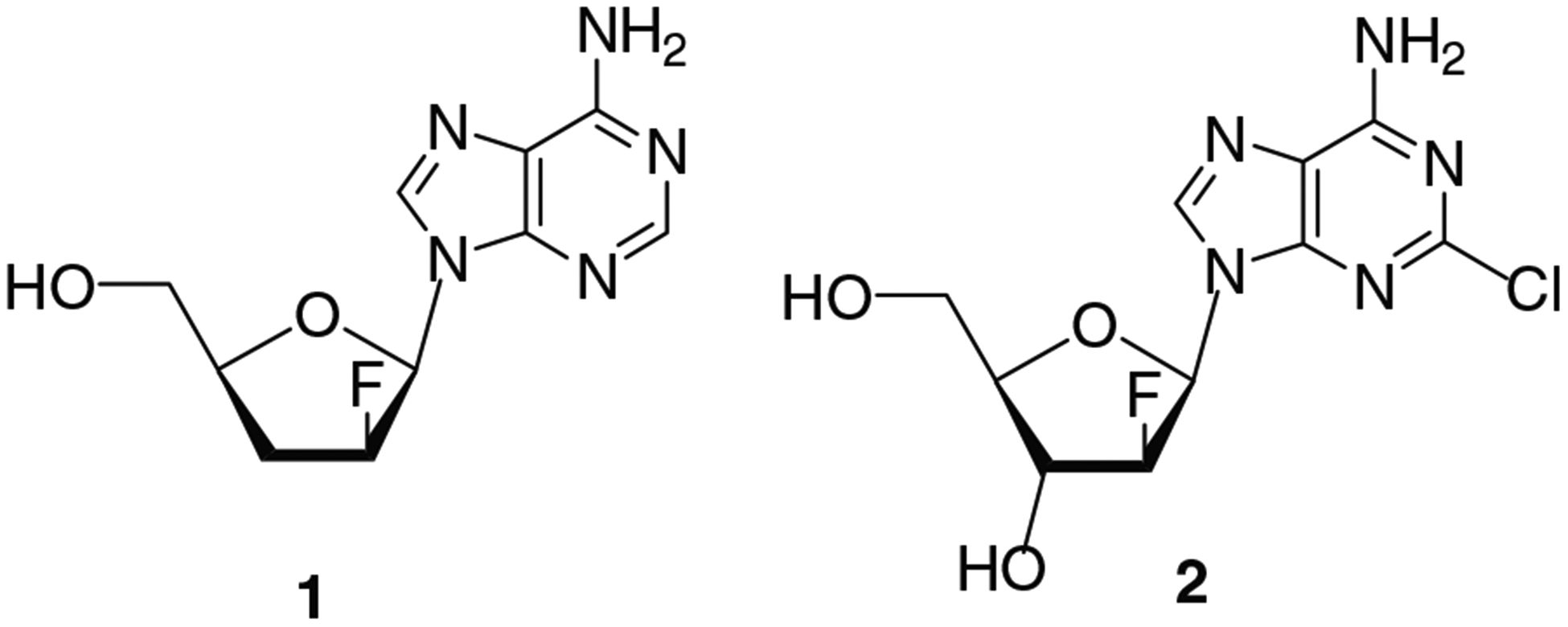
Biologically active purine 2′-β-fluoro nucleosides.
Among dideoxydifluoro nucleosides with β-d-arabino-configuration, a set of pyrimidine nucleosides have been synthesized and evaluated against HIV-1.[5] However, synthesis and study of the antiviral properties of purine nucleosides is of interest for a full biological evaluation of the antiviral potential for this family of nucleosides. Thus, we have synthesized 9-(2′,3′-dideoxy-2′,3′-difluoro-β-d-arabinofuranosyl)adenine (20) and its 2-chloro derivative 22 which can also be considered as new, closely related nucleoside analogues of purine 2′-β-fluoro nucleosides, lodenosine 1 and clofarabine 2,[8] respectively, the latter being used as anticancer agent for treatment of pediatric acute leukemia (Figure 1).
We describe herein the synthesis of purine 2′,3′-dideoxy-2′,3′-difluoro nucleosides with β-d-arabino-configuration 20 and 22, their α-anomers 21 and 23, along with the in vitro anti-HIV and anti-HCV evaluation.
RESULTS AND DISCUSSION
The synthetic route to adenine nucleoside 20 and its α-anomer 21 was briefly reported earlier from methyl 5-O-benzyl-2-deoxy-2-fluoro-α-d-arabinofuranoside.[9] Herein, we studied alternative pathways for the preparation of difluoride 9 from the benzyl derivative of methyl 3-fluoro-3-deoxy-d-ribofuranoside (3) and methyl 5-O-benzoyl-α-d-xylofuranoside (10) (Scheme 1). Riboside 3, prepared from methyl 5-O-benzyl-3-deoxy-3-fluoro-α-d-arabinofuranoside,[10] gave the 2-O-Ts derivative 4 under standard tosylation conditions. The 2-O-SO2Imd derivative 5 was synthesized from 3 using the method proposed by Hanessian for the preparation of imidazolylsulfonate derivatives of sugars.[11] Debenzylation of 4 and 5 by 20% Pd(OH)2/C in ethanol in the presence of cyclohexene followed by benzoylation resulted in benzoates 6 and 7. Further, two approaches involving nucleophilic displacement of the 2-OTs group of the riboside 6 by CsF in DMSO/HMPA[12,13] or 2-O-SO2Imd group of the riboside 7 under treatment with KHF2/HF/2,3-butanediol[14] were tested for the introduction of a fluorine atom at C-2 position. Difluoride 9 was synthesized under these conditions from the tosylate 6 and the imidazolylsulfonate 7 in 10 and 49% yields (Scheme 1), respectively, after chromatographic isolation.
SCHEME 1.
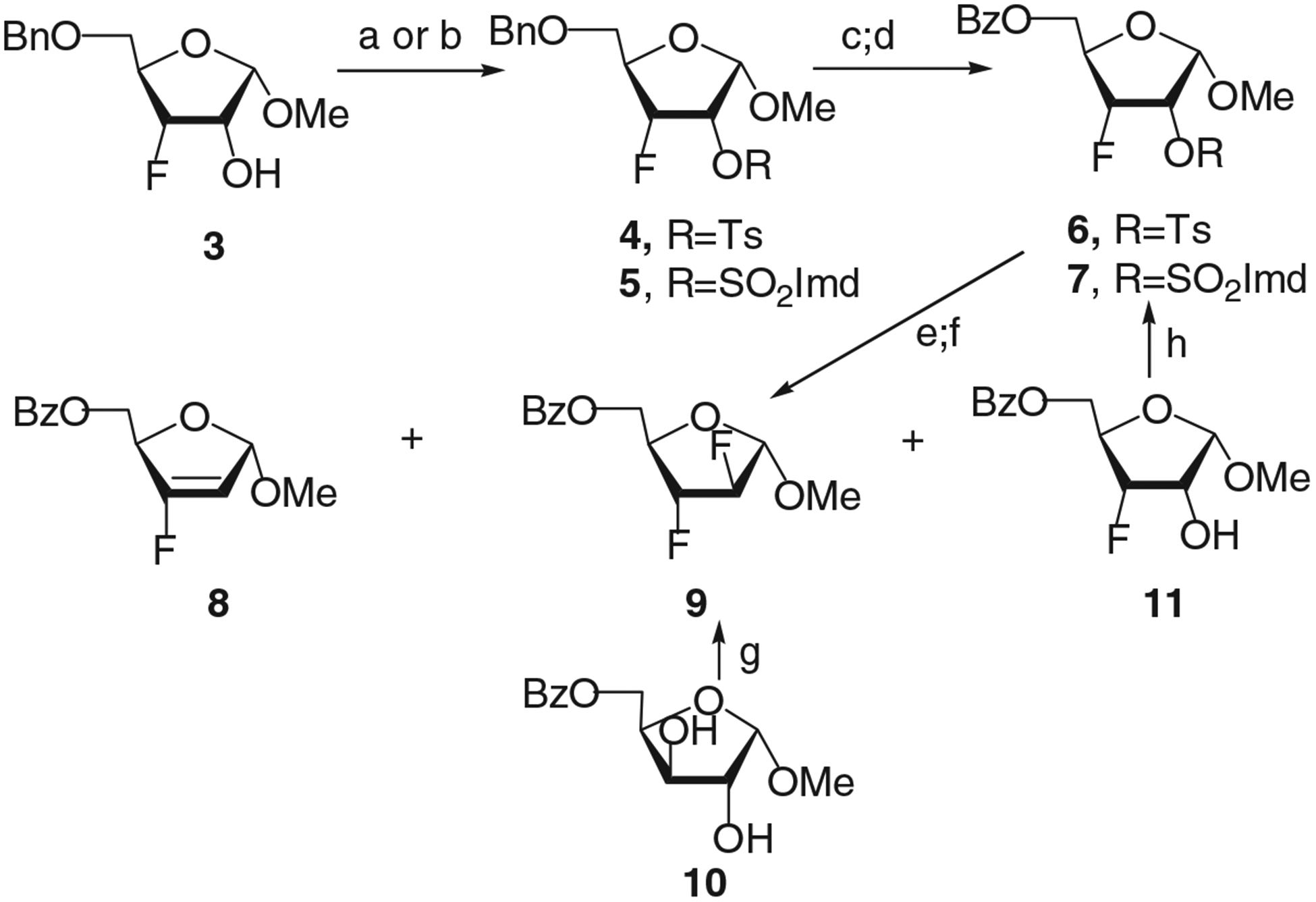
Reagents and conditions: a) TsCl/Py, room temperature 18 hours, (4, 84%); b) SO2Cl2/CH2Cl2, −40°C to room temperature, Imd, 0°C to room temperature (5, 64%); c) 20% Pd(OH)2/C/cycloxehene, EtOH, reflux, 2–3 hours; d) BzCl/Py, room temperature (c + d, 6 Σ70%; 7 Σ64%); e) KHF2/45%HF/2,3-butanediol, 160°C, 45 minutes (9 from 7, 49%); f) CsF/anh.DMSO/anh.HMPA (11:1,v/v), 190°C, 1 hour (9, 10%; 8, 26%); g) DAST, CH2Cl2, room temperature and 27–29°C, 9, 34%; 11, 19%; h) SO2Cl2/CH2Cl2/DMF, −40°C to room temperature, Imd, 0°C to room temperature (7, 92%).
Concomitant with the nucleophilic displacement of the 2-O-tosyl group in 6 by the fluoride anion, elimination of the tosyl group generated the the 3-fluorovinyl compound 8 as a by-product isolated in 26% yield after chromatography. Thus, the investigated four-step route to 9 from riboside 3 via imidazolylsulfonate 7 resulted in the target difluoride in a moderate 20% combined yield.[9]
The most efficient synthetic route to difluoride 9 was from derivative of methyl α-d-xylofuranoside 10, which is readily available from D-xylose.[15] The fluorination of 10 was accomplished with excess of DAST in methylene chloride under 27–29°C for 20 hours resulted in the target sugar 9 and methyl 5-O-benzoyl-3-deoxy-3-fluoro-α-d-ribofuranoside (11) with 34% and 19% yields, respectively. The synthesis of difluoride 9 from 10 involves fluorination of 10 at C-3 resulting in the formation of intermediate 3-fluorodeoxy sugar 11 followed by introduction of a fluorine atom with DAST at C-2 of 11 under mild heating. It should be noted that the preparation of 9 under these conditions is the first example of relatively effective synthesis of vicinal substituted 2,3-dideoxy-2,3-difluoro d-pentofuranose via a double fluorination of a 5-O-acyl derivative of d-pentofuranose with double inversion at the C-2 and C-3 atoms resulting from of a single treatment of the starting sugar by a fluorinating agent. The riboside 11 prepared from xyloside 10 can also be converted into difluoride 9 via imidazolylsulfonate 7. Synthesis of sulfonate 7 was performed by treatment of 11 with SO2Cl2 and imidazole in CH2Cl2-DMF in high yield (92%) after column chromatography on silica gel.
The condensation of difluoride 9 with persilylated N6-benzoyladenine in the presence of SnCl4 in acetonitrile afforded a mixture of β- and α-nucleosides 14 and 15 which was separated into individual anomers by column chromatography on silica gel.[9] The formation of desired β-nucleoside 14 was observed in low yield as a result of the glycosylation of N6-benzoyladenine by α-methyl glycoside 9. Therefore, another and more effective approach to β-nucleoside 14 was studied via bromide 13 and experimental details of it are reported in this communication (Scheme 2). Conventional acetolysis of 9 gave a mixture of acetates 12 after chromatography on silica gel, bromination (TMSiBr/CDCl3)[16] of the latter generated intermediate glycosyl bromide 13 that was reacted, without isolation, with the sodium salt of N6-benzoyladenine under reflux in tetrahydrofuran to give a mixture of β- and α-nucleosides 14 and 15. The target β-nucleoside 14 and its α-anomer 15 were isolated by column chromatography on silica gel in 23% and 9% yield, respectively. An analogous route via coupling of 1-α-bromo sugar with the nucleobase was used for the preparation of 2-chloro derivative of 18.
SCHEME 2.
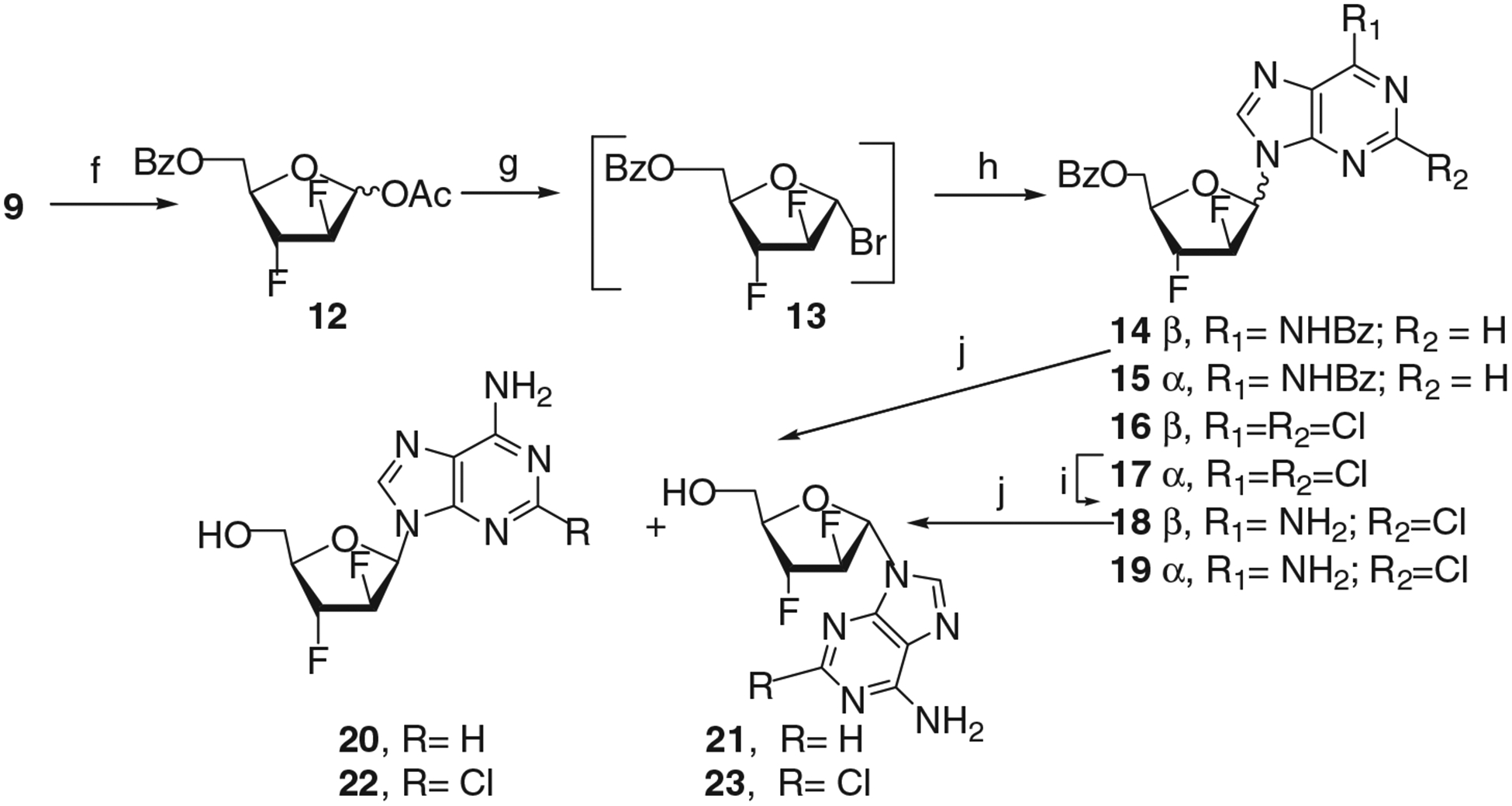
Reagents and conditions: f) AcOH/Ac2O/concn. H2SO4 (77%); g) TMS-Br/CDCl3, 2 weeks, room temperature; (13, ≈50%); h) 13/Na-salt of N6BzAde/THF, reflux, 5 hours (14, 23%; 15, 9%); h) 11/Na-salt of 2,6-di-Cl purine/CH3CN, room temperature (1:1.04), 18 hours (16+17, 48%); i) saturated NH3/1,2-DME, room temperature (18, 67%; 19, 25%); j) saturated NH3/MeOH, room temperature (20, 88%, 21, 81%, 22, 75%, 23, 80%).
The condensation of bromide 13 with the sodium salt of 2,6-dichloropurine in acetonitrile[17] when compared to the one of N6-benzoyladenine gave more complex mixture of products from which a mixture of protected N9-β/α-nucleosides 16 and 17 (β/α-ratio ≈ 2.9:1 according to 1H NMR data) was isolated in 48% overall yield after column chromatography on silica gel (Scheme 2). Treatment of the 16/17 mixture with a saturated solution of ammonia in 1,2-dimethoxyethane[18] at room temperature for 24 hours afforded protected β- and α-nucleosides of 2-chloroadenine 18 and 19 which were successively isolated by chromatography on silica gel in 67 and 25% yield, respectively.
Standard deprotection of individual blocked nucleosides 14, 15, 18, and 19 with methanolic ammonia and subsequent chromatographic purification gave pure 9-(2′,3′-dideoxy-2′,3′-difluoro-β-d-arabinofuranosyl)adenine (20) and its α-anomer 21, 2-chloro-9-(2′,3′-dideoxy-2′,3′-difluoro-β-darabinofuranosyl)adenine (22) and its α-anomer 23, respectively. The structure of nucleosides 20 and 21, 22, and 23 was verified by 1H, 19F, 13C NMR and by mass spectroscopy, UV, CD.
The assignments of the configurations of nucleosides 20–23 at the anomeric centers were based upon NMR data. The diagnostic for the β-anomeric configurations of 20 and 22 is the 5J H,F long-range coupling of H-C(8) to a 2′-β-fluorine atom of 2.3 and 2.27 Hz, respectively, exhibited in their 1H NMR spectra (Tables 1 and 2). Similarly, the presence of the similar five-bond coupling of 2.9 and 3.0 Hz (see Experimental) for intermediate protected nucleosides 14 and 18 supported their β-structural assignment. This coupling is generally indicative of a spatial proximity of the nuclei involved and is not observed in the α-anomers 15 and 19, 21, and 23. The most informative feature of the 1H NMR spectra for the ones in comparison with the corresponding β-nucleosides 14 and 19, 20, and 22 is the shift of H-2′ and H-4′ resonances in a lower field for the α-anomers.[19,20]
TABLE 1.
1H NMR chemical shifts of 2,3-dideoxy-2,3-difluoro sugars 9, 12 and nucleosides 20–23 with d-arabino-configurations. δ in ppm; J in Hz
| Cmpd. | H-1 or H-1′ | H-2 or H-2′ | H-3 or H-3′ | H-4 or H-4′ | H-5a or H-5′a | H-5b or H-5′b | Others |
|---|---|---|---|---|---|---|---|
| 9 | 5.13 br.d | 5.08 ddd | 5.10 dddt | 4.49–4.61 m | 3.46 (s, OMe), 8.08 (m, 1H), 7.60 (m, 2H), 7.48 (m, 2H, Bz) | ||
| 12 | 6.42 br.d | 5.21 dd | 5.20 ddd | 4.70 ddt | 4.57 dd | 4.55 dd | 2.12 (s, 3H, CH3CO), 8.04 (d, 1H), 7.57 (t, 2H), 7.44 (t, 2H, Bz) |
| 20 | 6.53 ddd | 5.50 dddd | 5.46 ddd | 4.30 dm | 3.89 ddd | 3.85 dd | 8.30 (d, 1H, J = 2.3, H-8), 8.21 (s, 1H, H-2) |
| 22 | 6.42 ddd | 5.74 dddd | 5.57 dddd | 4.20 dm | 3.72 br.m | 3.69 br.m | 8.27 (d, 1H, J = 2.27, H-8), 7.94 (br.s, 2H, NH2),5.27 (br.s, 1H, 5′-OH),gemJ 5′a,5′b ~ 13 |
| 21 | 6.53 dd | 6.04 ddt | 5.47 dddd | 4.81 ddt | 3.83 dd | 3.79 ddd | 8.40 (s, 1H, H-8) 8.39 (s, 1H, H-2) 4J 5′b,F3′ = 1.15 |
| 23 | 6.35 dd | 6.06 ddt | 5.45 ddt | 4.63 dm | 3.61 dd | 3.58 dd | 8.31 (s, 1H, H-8), 5.26 (t, 1H, J = 5.66, 5′-OH), 7.90 (br.s, 2H, NH2) |
Spectra were obtained in CDCl3 for sugars 9 and 12, in CD3OD and DMSO-d6 for nucleosides 20, 21 and 22, 23, respectively. Spectral data for pure α-anomer of 12 are presented.
TABLE 2.
Coupling constants (in Hz) for 1H NMR data of 2,3-dideoxy-2,3-difluoro sugars 9, 12 and nucleosides 20–23 with d-arabino-configurations
| 3J (H,H) | 3J (H,F) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1,2 or 1′,2′ | 2,3 or 2′,3′ | 3,4 or 3′,4′ | 4,5a/4,5b or 4′,5′a/4′,5′b | H1,F2 or H1′,F2 | H3,F2 or H3′,F2 | H2,F3 or H2′,F3 | H4,F3 or H4′,F3 | Others | |
| 9 | <0.8 | 0.91 | 4.11 | n.d. | 10.03 | 19.76 | 13.44 | 22.60 | 4J 3,1 = 0.91 |
| 12 | <1.0 | <1.0 | 3.24 | 4.5/4.69 | 10.57 | 17.49 | 12.21 | 24.14 |
gemJ
2,F2 = 48.17 gemJ 3,F3 = 51.0 gemJ H5a,H5b = 12.71 |
| 20 | 3.97 | 2.17 | 3.76 | 4.27/4.85 | 17.67 | 15.29 | 12.82 | 24.80 |
5J
F2′,H8 = 2.3 4J 1′,F3′ = 1.86 gemJ 2′,F2′ = 50.52 gemJ 3′,F3′ = 51.24 J H5′a,H5′b = 12.8 |
| 22 | 4.76 | 3.35 | 4.73 | 4.78 | 14.54 | 16.58 | 14.77 | 22.31 |
5J
F2′,H8 = 2.27 4J 1′,F3′ = 0.9 gemJ 2′,F2′ = 50.86 gemJ 3′,F3′ = 52.16 |
| 21 | 2.42 | 2.74 | 3.90 | 5.04/4.98 | 15.04 | 15.90 | 14.0 | 21.16 |
gemJ
2′,F2′ = 49.70 gemJ 3′F3′ = 51.70 gemJ H5′a,H5′b = 12.8 |
| 23 | 3.24 | 3.37 | 4.32 | 6.30/5.26 | 15.11 | 15.39 | 17.06 | 20.44 |
gemJ
H5′a,H5′b = 2.29 gemJ 3′,F3′ = 52.35 gemJ 2′,F2′ = 50.28 |
Of interest are some other observations from the NMR data (Table 2). Long-range coupling of H-1′ to 3′-fluorine atom of 1.86 and 0.9 Hz in 1H NMR spectra of nucleosides 20 and 22 argues for W-shape configuration of H-1′ and F-3′, and the β-anomeric configuration of adenine and 2-chloroadenine nucleosides. A four-bond coupling (0.91 Hz) between H-1 and H-3 atoms of difluoride 9 is exhibited in its 1H NMR spectrum due to W-arrangement[21] between these protons in the case of α-methyl glycoside 9, but it is not found for α-1-O-acetate 12. Interesting stereochemical peculiarities of compounds 20, 22, and 9 is well represented by 1H NMR data described above.
13C NMR data presented in Tables 3 and 4 provide further support for the assignments of the configurations of nucleosides 20–23 at the anomeric centers which were made in terms of 1H NMR data, long-range coupling constants between C-8 of heterocyclic base and 2′-β-fluorine atom (4.6 and 3.75 Hz) that were observed only for β-nucleosides 20 and 22, respectively. The carbon resonances of the bases in 13C NMR spectra of nucleosides 20–23 are in good accord with 13C NMR spectral data obtained earlier for closely related purine 2′-β-fluoro nucleosides.[20] 19F NMR data (see Experimental) of 2,3-dideoxy-2,3-difluoro sugars 9, 12 and nucleosides 20–23 with D-arabino-configurations are evidence in favour of the assigned structures of synthesized sugars and nucleosides. F-2′ and F-3′ resonance signals of sugars and nucleosides with two fluorine atoms in trans-arrangement are revealed as complex multiplets in their 19F NMR spectra, but the magnitude of 3J F-2,F-3 (8.08–8.48 Hz) did not profoundly differ for sugars 9, 12, and target nucleosides.
TABLE 3.
The 13C NMR data (chemical shifts in ppm)
| Chemical shifts, δTMS, ppm [J (C,F)in Hz] | ||||||
|---|---|---|---|---|---|---|
| Compd. | C-1 or C-1′ | C-2 or C-2′ | C-3 or C-3′ | C-4 or C-4′ | C-5 or C-5′ | Others |
| 9 | 106.03 dd | 96.81 dd | 95.04 dd | 80.19 d | 62.95 d | 166.14 (s, Ph-C=O, arom), 133.30, 129.78, 129.55, 128.47 (Ph-C=O), 54.98 (s, OCH3) |
| 12 | 98.84 dd | 96.86 dd | 94.38 dd | 82.76 d | 62.81 d | 169.34 (s, CH3-C=O), 166.21 (s, Ph-C=O), 133.54, 129.91, 129.8, 128.59 (Ph-C=O), 21.05 (s, CH3-C=O) |
| 20 | 83.07 dd | 93.57 dd | 92.69 dd | 82.15 dd | 60.26 d | 156.09 (C-6) 152.83 (C-2) 149.13 (C-4) 140.26 (d, 5J C8,F2′ = 4.6, C-8) 118.49 (C-5) |
| 22 | 81.75 dd | 93.93 dd | 92.99 dd | 81.11 dd | 60.40 d | 157.38(C-6) 153.95(C-2) 150.70(C-4) 140.69 (d, 5J C8,F2′ = 3.75, C-8) 117.89 (C-5) |
| 21 | 87.95 dd | 96.90 dd | 93.93 dd | 85.13 dd | 60.30 d | 152.93(C-6) 148.68(C-2) 147.94(C-4) 141.02 (s, 5J C8,F2′ <1.0, C-8) 119.37 (C-5) |
| 23 | 86.58 dd | 97.03 dd | 94.46 dd | 84.04 dd | 60.66 d | 157.42(C-6) 153.87(C-2) 150.56(C-4) 140.06 (s, C-8) 118.69 (d, J = 5.51, C-5) |
TABLE 4.
The 13C NMR data (coupling constants in Hz)
| F-2 | F-3 | |||||||
|---|---|---|---|---|---|---|---|---|
| 2J (C,F) | 3J (C,F) | 4J (C,F) | 3J (C,F) | 2J (C,F) | 3J (C,F) | |||
| Compd. | C1,F2 or C1′,F2 | C3,F2 or C3′,F2 | C4,F2 or C4′,F2 | C5,F2 or C5′,F2 | C1,F3 or C1′,F3 | C2,F3 or C2′,F3 | C4,F3 or C4′,F3 | C5,F3 or C5′,F3 |
| 9 | 35.30 | 30.45 | <1.0 | <1.0 | 4.20 | 28.10 | 27.20 | 5.40 |
| 12 | 36.87 | 30.85 | <1.0 | <1.0 | 1.52 | 29.43 | 28.23 | 6.49 |
| 20 | 17.35 | 28.9 | 2.84 | <1.0 | 3.55 | 28.70 | 25.51 | 6.39 |
| 22 | 17.42 | 27.30 | 3.35 | <1.0 | 5.89 | 27.63 | 24.36 | 4.63 |
| 21 | 36.40 | 28.02 | 2.08 | <1.0 | 4.98 | 28.71 | 24.55 | 5.66 |
| 23 | 35.50 | 27.36 | 3.40 | <1.0 | 6.42 | 27.11 | 24.30 | 4.68 |
Compounds 20–23 were evaluated as potential inhibitors of HIV-1 and HCV in primary human peripheral blood mononuclear (PBM) and Huh-7 Replicon cells, respectively. The adenine nucleoside 20 demonstrated potent activity against HIV-1 in primary human lymphocytes with a median effective concentration (EC50) of 0.72 μM with no apparent cytotoxicity in three different cell systems up to 100 μM. The 2-chloro derivative 22 was moderately active with an EC50 of 14.1 μM, but antiviral activity could be secondary to its cytotoxicity in human PBM cells (Table 5).
TABLE 5.
Anti-HIV and anti-HCV activities of nucleosides 20–23
| Anti-HIV-1 activity in human PBM cells and cytotoxicity in | Anti-HCV activity and toxicity in replicon assay Huh7 cells | |||||||
|---|---|---|---|---|---|---|---|---|
| CC50(μM) | ||||||||
| Compound | EC50 (μM) | EC90 (μM) | PBM | CEM | Vero | EC50 (μM) | EC90 (μM) | CC50b (μM) |
| 20 | 0.72 | 10.3 | >100 | >100 | >100 | >10 | >10 | >10 |
| 21 | 5.6 | >100 | >100 | >100 | >100 | >10 | >10 | >10 |
| 22 | 14.1 | 46.3 | 13.6 | 70.2 | >100 | >10 | >10 | >10 |
| 23 | 60.1 | >100 | 9.4 | >100 | >100 | >10 | >10 | >10 |
| 2′-β-FddAa | 4.4 | — | >100 | — | — | — | — | — |
| AZTc | 0.0026 | 0.010 | >100 | 14.3 | 56.0 | — | — | — |
| 2-C-Me-Cc | — | — | — | — | — | 1.3 | 5.4 | >10 |
The anti-HIV activity of lodenosine (2′-β-FddA) 1 in PBM cells was taken from literature [23] and presented for comparison.
Toxicity in Huch-7 Replicon cells.
AZT and 2′-C-Me C were used as positive controls for the HIV and HCV assays.
It should be stressed that nucleoside 20 as an analogue of lodenosine 1 displays higher antiviral activity than 2′-β-FddA[23] with similar cytotoxic effect in the same cell system (Table 5). None of the compounds were effective against HCV replication in a Huh-7 based replicon system when tested at 10 μM. The methodologies for evaluating the antiviral activity against HIV, HCV and cytotoxicity have been published elsewhere.[24, 25]
Considering that 9-(2′,3′-dideoxy-2′,3′-difluoro-β-d-arabinofuranosyl) adenine (20) as new closely related nucleoside analogue of known anti-HIV agent, lodenosine 1, both exhibit anti-HIV activity in vitro tests, it is interesting to compare the conformational peculiarities of the both 2′-β-fluoro nucleosides possessing antiviral activity, namely, N/S equilibrium of their pentofuranose rings in solution.
Conformational analysis of the pentofuranose ring of the adenine β-nucleoside 20 was performed employing the PSEUROT 6.3 program[26] and the results have been compared with those for closely related deoxyfluoro nucleoside 1[22] (Table 6). All calculations have been performed aiming at achieving: (i) the minimal rms and |ΔJ| values, and (ii) maximal correspondence of the pseudorotational parameters obtained using only four vicinal [H,F] couplings and simultaneous analysis of seven [H,H] and [H,F] couplings.[27,28] Scale factors for the 3J H,H and 3J H,F were 1.0 and 0.2, respectively.
TABLE 6.
Pseudorotational parameters of adenine nucleosides 20 and 1
| Pseudorotational parameters of nucleosidesa | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ΔGeffc) | |||||||||||
| Compound | PN (°) | ψN (°) | PS (°) | ψS (°) | Rms (Hz) | |ΔJ| (Hz) | l′−2′ | 2′−3′ (2′F) | 2′−3′ (3′F) | 3′−4′ | %S |
| 20 (CD3OD) | −15.9 (2E) | 48 | 137.7 (1T2) | 32 | .11 | .13 (H,H) .20 (H,F) | −8.370 | .372 | −3.906 | 2.700 | 77 |
| Using 4 vicinal [H,F] coupling constants | −20.4 (2E) | 48 | 139.8 (1T2) | 32 | .02 | .03 | 76 | ||||
| 1b (CD3OD) | −13.7 (2E) | 29.3 | 129.3 (1E) 145.9 (1T2) | 43.1 39.9 | .06 | .12 | 71 | ||||
All data resulted from analysis of the [H,H] and [H,F] coupling constants, if is not specified. The ψ values given in italics and underlined are kept fixed during the final minimization.
The pseudorotational parameters of lodenosine 1 have been calculated previously[22] and included in the table for comparison of stereochemistry of adenine nucleosides.
ΔGeff = − 3.72[(αFCC + αHCC)/2 – 110] along a given C—C bond.[27]
According to the data of PSEUROT analysis for nucleosides 20 and 1 with application of 1H NMR data prepared in the same deuterated solvent it can be concluded that pentofuranose rings of the both purine nucleosides are predominantly in the S-type conformations, the degree of populations being very similar (76% and 71%). It is noteworthy that the dominating conformations for N/S equilibrium of the pentofuranose rings 20 and 1 occupy a narrow close segment of pseudorotational wheel for these nucleosides (Table 6).
Further, comparison of N↔S pseudorotational equilibrium of 2′-β-FddA 1 and difluoride 20 possesing anti-HIV activity with the one reported earlier[13,27,29] for isomeric adenine nucleosides with 2′-α-fluoro atom and without antiviral activity, 9-(2′,3′-dideoxy-2′-fluoro-β-d-erythro-pentofuranosyl)adenine (2′-α-FddA) and 2′,3′-dideoxy-2′,3′-difluoro–adenosine, permits us to note that the conformation of pentofuranose rings of 2′-β-fluoro anti-HIV nucleosides in solution differ from the stereochemistry of inactive adenine nucleosides by pseudorotational parameters.
In conclusion, the preferred conformation of adenine 2′-β-fluoro nucleosides 20 and 1 appear to play an important role in their anti-HIV activity. The synthesis of new purine dideoxydifluoro nucleosides with β-d-arabino-configuration is of interest for the development of novel nucleoside analogues with potential antiviral activity and for the continuing search for a stereochemical rationale[30] for the activity of anti-HIV nucleosides fluorinated on carbohydrate moiety.
EXPERIMENTAL
Column chromatography was performed on silica gel 60 H (70–230 mesh; Merck, Germany). TLC: aluminium-backed silica gel 60 F254 sheets (Merck, Germany); eluents: hexane/AcOEt 3:1 (A), hexane/AcOEt 1:2 (B), CHCl3/MeOH 4:1 (C). All the anhydrous solvents were distilled over CaH2, P2O5 or sodium prior to the reaction. The UV and CD spectra were recorded on Specord M-400 (Carl Zeiss, Germany) and a J-20 (JASCO, Japan) spectropolarimeter, respectively.1H, 13C, and 19F NMR Spectra were recorded in CDCl3, CD3OD and (D6)DMSO with Bruker Avance-500-DRX spectrometer at 500.13, 126.76, and 470.593 MHz, respectively. NMR (δ values) are in ppm downfield from internal SiMe4 (1H, 13C) or external CFCl3 (19F). J values are reported in Hz. All NMR assignments were confirmed by two-dimensional (1H, 1H and 1H, 13C) correlation spectroscopy. Mass spectra were recorded on a chromatomass spectrometer with HPLC-Accela and LCQ Fleet mass detector (Thermo Electron Corporation, USA).
Methyl 5-O-benzoyl-3-deoxy-3-fluoro-2-O-[(4-methylphenyl)sulfonyl]-α-d-ribofuranoside (6)
Standard tosylation of 3 (1.49 g, 5.83 mmol) with TsCl in pyridine followed by column chromatography on silica gel (80 mL), using for elution mixture EtOAc/hexane 1:3 and 1:1, afforded tosylate 4 (2.0 g, 84%) as a syrup. Rf 0.31 (A). 1H NMR (CDCl3): 7.78 (d, 2H, CH3C6H4SO2-), 7.22–7.35 (m, 7H, C6H5CH2-, CH3C6H4SO2-), 4.89 (d, 1H, H-1, J 1,2 = 4.8, H-1), 4.80 (ddd, 1H, J 3,2 = 4.8, J 3,4 = 1.2, J 3,F = 60.0, H-3), 4.73 (dm, 1H, J 2,F = 19.2, H-2), 4.55 (d,1H, PhCH2), 4.45 (d,1H, PhCH2), 4.38 (dm, 1H, H-4, J 4,F = 25.8), 3.58 (dd, 1H, H-5, J 5,4 = 2.5, J 5,5′ = 10.8), 3.53 (dd, 1H, H-5′, J 5,4 = 2.9), 3.33 (s, 3H, OCH3), 2.43 (s, 3H, CH3C6H4SO2-). 13C (CDCl3): 145.4, 137.5, 129.9, 129.3, 128.7, 128.3, 128.1 and 127.8 (s, Bzl and CH3C6H4SO2-),101.3 (s, C-1), 89.1 (d, J C-3,F = 192.3, C-3), 81.8 (d, J C-2, F = 23.6, C-2), 75.5 (d, J C-4, F = 14.7, C-4), 73.8 (s, −CH2C6H5), 69.1 (d, J C-5, F = 9.5, C-5), 55.9 (s, OCH3), 21.8 (s, CH3C6H4SO2-). Anal. calc. for C20H23FO6S: C, 58.52; H, 5.65; Found : C, 58.63; H, 5.58. 19F (CDCl3): −193.45 (dt, F-3).
To a solution of 4 (1.8 g, 4.38 mmol) in 85 mL anhydrous ethanol, 20% Pd(OH)2/C (3.26 g) and 85 mL cyclohexene was added. The reaction mixture was refluxed for 150 minutes, catalyst was filtered off and washed with EtOH (100 mL). The filtrate was evaporated and coevaporated with toluene (2 × 30 mL). Standard benzoylation of oil product (1.5 g) followed by silica gel (90 mL) column chromatography, using for elution mixture EtOAc/hexane 1:6, 1:2, and 1:1, afforded benzoyl derivative 6 (1.3 g, 70%) as syrup. Rf 0.32 (A). 1H NMR (CDCl3): 8.04 (d, 2H, Bz), 7.80 (d, 2H, J = 8.1, CH3C6H4SO2-), 7.63 (m, 1H, Bz), 7.48 (m, 2H, Bz), 7.30 (d, 2H, J = 8.1, CH3C6H4SO2-),4.94 (d, 1H, H-1, J 1,2 = 4.6), 4.91 (ddd, 1H, J 3,2 = 5.4, J 3,4 = 1.2, J 3,F = 56.4, H-3), 4.76 (ddd, 1H, J 2,F = 22.8, H-2), 4.60 (dm, 1H, H-4, J 4,F = 25.2), 4.51 (dd, 1H, H-5, J 5,4 = 3.5, J 5,5′ = 12.0), 4.43 (dd, 1H, H-5′, J 5,4 = 3.6), 3.39 (s, 3H, OCH3), 2.44 (s, 3H, CH3C6H4SO2-). 13C (CDCl3): 165.9 (s, C = O, Bz), 145.6, 133.7, 132.9, 130.0, 129.8, 129.3, 128.8 and 128.2 (s, Bz and CH3C6H4SO2-), 101.2 (s, C-1), 88.4 (d, J C-3, F = 194.6, C-3), 80.3 (d, J C-2, F = 25.0, C-2), 75.2 (d, J C-4, F = 15.0, C-4), 63.5 (d, J C-5, F = 9.1, C-5), 56.0 (s, OCH3), 21.8 (s, CH3C6H4SO2-). Anal. calc. for C20H21FO7S : C, 56.59; H, 4.99; Found : C, 56.63; H, 5.08. 19F (CDCl3): −192.97 (ddd, F-3).
Methyl 5-O-benzoyl-3-deoxy-3-fluoro-2-O-(imidazolylsulfonyl)-α-d-ribofuranoside (7)
Method A.
Sulfuryl chloride (0.8 mL, 9.87 mmol) was added dropwise to a solution of fluoride 3 (1.24 g, 4.84 mmol) in anhydrous CH2Cl2 (15 mL) at −40°C. The reaction mixture was stirred at this temperature during 1 hour and then temperature was gradually raised to room temperature for 3 hours. After cooling to 0°C, imidazole (3.35 g, 49.1 mmol) was added to prepared solution and the reaction mixture was stirred at room temperature for 18 hours. The solution was diluted CH2Cl2 (20 mL), washed cold water (10 mL), the aqueous phase was extracted with CH2Cl2 (3 × 30 mL). The combined organic extracts were dried over anh. Na2SO4 and evaporated to dryness. The residue was chromatographed on a silica gel (70 mL), using a linear gradient of EtOAc (0→50%, v/v; 500 mL) in hexane, to afford syrupy 5 (1.2 g, 64%). Rf 0.73 (B). 1H NMR (CDCl3): 7.16–7.90 (m, 8H, Ar-H), 4.89 (d, 1H, H-1, J 1,2 = 4.6), 4.82 (ddd, 1H, J 3,2 = 5.3, J 3,4 = 1.0, J 3,F = 59.6, H-3), 4.81 (dt, 1H, J 2,F = 21.3, H-2), 4.57 (d,1H, PhCH2), 4.48 (d,1H, PhCH2), 4.43 (dm, 1H, H-4, J 4,F = 25.4), 3.63 (dd, 1H, H-5, J 5,4 = 2.4, J 5,5′ = 10.7), 3.59 (dd, 1H, H-5′, J 5,4 = 1.8), 3.37 (s, 3H, OCH3). 13C (CDCl3): 137.5, 137.1, 131.4, 128.8, 128.7, 128.3, 127.9 and 118.4 (s, C6H5CH2- and C3H3N2SO2-), 100.4 (s, C-1), 88.5 (d, J C-3, F = 194.3, C-3), 81.8 (d, J C-2,F = 23.5, C-2), 78.4 (d, J C-4,F = 14.5, C-4), 73.9 (s, −CH2C6H5), 69.0 (d, JC-5, F = 9.5, C-5), 55.8 (s, OCH3). 19F (CDCl3): −193.83 (ddd, F-3). Anal. calc. for C16H19FN2O6S: C, 49.73; H, 4.96; N, 7.25; Found: C, 49.70; H, 5.02; N, 7.35.
To a solution of imidazolylsulfonate 5 (1.2 g, 3.12 mmol) in 57 mL anhydrous ethanol, 20% Pd(OH)2/C (2.4 g) and 57 mL cyclohexene was added and the reaction mixture was refluxed for 150 minutes, catalyst was filtered off and washed with EtOH (100 mL). The filtrate was evaporated and coevaporated with toluene (2 × 30 mL). Standard benzoylation of oil product (1.2 g) followed by silica gel (70 mL) column chromatography afforded benzoyl derivative 7 (0.75 g, 64%) as syrup. Rf 0.79 (B). 1H NMR (CDCl3): 7.10–7.98 (m, 8H, Ar-H), 4.97 (ddd, 1H, J 3,2 = 5.7, J 3,4 = 1.8, J 3,F = 56.4, H-3), 4.91 (d, 1H, H-1, J 1,2 = 4.6), 4.78 (dt, 1H, J 2,F = 20.0, H-2), 4.62 (dm, 1H, H-4, J 4,F = 24.8), 4.57 (dd, 1H, H-5, J 5,4 = 3.4, J5,5′ = 12.2), 4.43 (dd, 1H, H-5′, J 5,4 = 3.2), 3.38 (s, 3H, OCH3). 13C (CDCl3): 165.8 (s, C = O, Bz), 137.1, 133.9, 131.5, 129.6, 129.2, 128.9 and 118.3 (s, C6H5CO- and C3H3N2SO2-), 100.2 (s, C-1), 87.8 (d, J C-3,F = 196.7, C-3), 80.1 (d, J C-2,F = 24.9, C-2), 78.0 (d, J C-4, F = 14.9, C-4), 63.1 (d, J C-5,F = 8.3, C-5), 56.0 (s, OCH3). 19F (CDCl3): −193.55 (dt, F-3). Anal. calc. for C16H17FN2O7S : C, 48.00; H, 4.28; N, 6.99; Found : C, 48.09; H, 4.32; N, 7.15.
Method B.
Sulfuryl chloride (0.15 ml, 1.85 mmol) was added dropwise to a solution of fluorosugar 11 (0.17 g, 0.63 mmol) in anhydrous CH2Cl2 (2 mL) and DMF (0.53 ml) at −40°C. The reaction mixture was stirred at this temperature during 50 minutes and then for 90 minutes at room temperature. After cooling to 0°C, 0.55 g (8.08 mmol) imidazole was added to prepared solution and the reaction mixture was stirred at room temperature for 18 hours. After standard work-up and removal of solvent, the residue was chromatographed on a silica gel (25 mL), using for elution mixture EtOAc/hexane 1:3, 1:2, and 2:1, to afford syrupy 7 (0.232 g, 92%) which was identical to imidazolylsulfonate 7 prepared from fluoride 3.
Methyl 5-O-benzoyl-2,3-dideoxy-2,3-difluoro-α-d-arabinofuranoside (9)
Method A.
To a stirred solution of 6 (1.2 g, 2.83 mmol) in anhydrous DMSO (8 ml) and HMPA (0.7 mL) was added freshly dried cesium fluoride (2.3 g, 15.1 mmol). The reaction mixture was stirred at 190°C for 70 minutes. After cooling to room temperature the reaction mixture was poured into water (30 ml), and extracted with EtOAc (4 × 90 mL). The combined organic extracts was dried over anhydrous Na2SO4 and evaporated to dryness. The residue was chromatographed on a silica gel (200 mL), using a linear gradient of EtOAc (0→33%, v/v; 600 mL) in hexane, to afford syrupy difluoride 9 (0.08 g, 10%). Rf 0.74 (A). 19F (CDCl3): −195.78 (F-2, m), −194.28 (F-3, m, J F-2,F-3 = 8.08). HPLC/APCI-MS, m/z 272 M+, 241 (M-OCH3)+. Anal. calc. for C13H14F2O4: C, 57.35; H, 5.18. Found: C, 56.90; H, 5.02.
Vinyl fluoride 8 (0.183 g, 26%) as syrup. Rf 0.65 (A). 1H NMR (CDCl3): 8.04 (d, 2H, Bz), 7.59 (m, 1H, Bz), 7.48 (m, 2H, Bz), 5.79 (m, 1H, H-2, J 2,1 = 1.3, J 2,4 = J 2,F = 4.2), 5.35 (t, 1H, H-1, J 1,F < 1.0, J 1,4 = 1.3), 5.09 (m, H-4), 4.65 (dd, 1H, H-5, J 5,4 = 2.8, J5,5′ = 12.1), 4.41 (dd, 1H, H-5′, J 5′,4 = 4.0), 3.44 (s, 3H, OCH3). 13C(CDCl3): 166.2 (s, C=O, Bz), 161.5 (d, J C-3,F = 287.0, C-3), 133.4, 129.9, 129.8, 128.6 (s, C6H5CO-), 106.4 (d, J C-2,F = 15.1, C-2), 101.8 (d, J C-1,F = 7.2, C-1), 76.6 (d, J C-4,F = 25.4, C-4), 63.4 (d, J C-5,F = 1.9, C-5), 54.3 (s, OCH3). 19F (CDCl3): −131.6 (m, F-3). HPLC/APCI-MS, m/z 252 M+. Anal. calc. for C13H13FO4: C, 61.90; H, 5.19. Found: C, 62.10; H, 5.27.
Method B.
To a solution of 7 (0.75 g, 1.87 mmol) in freshly distilled 2,3-butanediol (7.2 mL) was added KHF2 (0.61 g, 7.8 mmol) and suspension was stirred under argon at 160°C for several minutes, and then HF (0.36 mL, 46% in H2O) was added to this mixture. The reaction mixture was stirred at 160°C during 50 minutes. After the standard work up and removal of solvent, the residue was chromatographed on a silica gel (100 mL), using for elution mixture EtOAc/hexane 1:8, 1:6 to afford syrup difluoride 9 (0.25 g, 49%) which was identical to the one described above.
Method C.
To a solution of xyloside 10 (0.3 g, 1.12 mmol) in anhydrous CH2Cl2 (6.5 mL) at room temperature was added under argon (0.82 ml, 6.19 mmol) DAST and the reaction mixture was stirred at this temperature during 150 minutes and then for 18 hours at 27–29°C under argon. After cooling, the reaction mixture was poured into saturated cooled aqueous NaHCO3 (75 mL). When evolution of gas ceased, it extracted with CH2Cl2 (3 × 30 mL). The combined organic extracts were dried over anh. Na2SO4 and evaporated to dryness. The residue was chromatographed on silica gel (90 mL), using for a linear gradient of hexane/EtOAc/2:1 (v/v; 500 mL) in hexane/EtOAc/8:1, to afford difluoride 9 (0.103 g, 34%) and fluoride 11 (0.058 g, 19%) which was identical to the one prepared earlier.[15] 19F for 11 (CDCl3): −195.58 (dt, F-3).
1-O-Acetyl-5-O-benzoyl-2,3-dideoxy-2,3-difluoro-α/β-d-arabinofuranoside (12)
Concentrated H2SO4 (0.1 mL) was added to a solution of fluoride 9 (0.337 g, 1.24 mmol) in acetic acid (2.33 mL) and acetic anhydride (0.59 mL) at 0°C. The reaction mixture was stirred at this temperature during 30 minutes and left at +4°C for 18 hours, then it was poured into mixture ice/water. After ice melted, the aqueous phase was extracted with CH2Cl2 (3 × 40 mL). The combined organic extracts was washed aqueous NaHCO3, dried over anh. Na2SO4 and evaporated to dryness. The residue was chromatographed on a silica gel (55 mL), using for elution mixture EtOAc/hexane 1:3 and 1:1 to afford 12 (0.286 g, 77%) as syrup. Rf 0.48 (A). 1H NMR (CDCl3): (α, β ratio ca. 3:1), 7.43–8.08 (m, ArH), 6.42 (d, H-1 α, J 1,F-2 = 10.57), 6.39 (d, H-1 β, J 1,2 = 4.7), 5.29–5.49 (m, H-2 β and H-3 β), 5.21 (dd, H-2 α), 5.20 (ddd, H-3 α), 4.70 (ddt, H-4 α), 4.40–4.60 (m, H-5 α, β and H-5′ α, β, H-4 β), 2.12 (s, OAc α), 2.00 (s, OAc β). HPLC/APCI-MS, m/z 241 (M-CH3COO)+. 19F (CDCl3, for α-anomer): −195.3 (F-2, m), −192.9 (F-3, m, J F-2,F-3 = 8.28). Anal. calc. for C14H14F2O5: C, 56.00; H, 4.70; Found : C, 56.11; H, 4.65.
9-(2,3-dideoxy-2,3-difluoro-β-d-arabinofuranosyl)adenine (20) and its α-anomer (21)
TMSiBr (0.2 ml, 1.5 mmol) was added to a solution of 12 (0.19 g, 0.64 mmol) in CDCl3 (1.4 mL) at 0°C and the mixture was stirred at this temperature for 30 minutes. After standing at room temperature for 14 days, the reaction mixture was evaporated, and coevaporated with anhydrous toluene (3 × 4 mL) and 13 (content ≈50% according to TLC data and 1H NMR data, 6.55 ppm, d, J 1,F = 12.6, H-1 and 4.85 ppm, ddt, H-4 for 1-α-bromo sugar 13) was used in the next step without purification.
The solution of 13 in anhydrous THF (10 mL) was added to sodium salt of N6-benzoyladenine, prepared from 0.098 g (0.40 mmol) N6-benzoyladenine and NaH in oil (14 mg of 80% in oil, 0.46 mmol). The reaction mixture was refluxed for 5 hours, filtered off, and washed with CH2Cl2. After evaporation of combined filtrates, the residue was chromatographed on silica gel (120 mL), using a linear gradient of EtOAc (0→66%, v/v; 500 mL) in hexane, to afford syrupy nucleoside 15 (14 mg, 9%). Rf 0.44 (B). 1H NMR (CDCl3): 9.09(s, 1H, NH), 8.84 (s, 1H, H-2), 8.15 (s, 1H, H-8), 7.48–8.08 (5m, 10H, 2Bz), 6.55 (dd, 1H, J 1′,2′ = 1.1, J 1′,F-2′ = 15.6, H-1′), 6.03 (ddm, 1H, H-2′), 5.46 (ddm, 1H, H-3′), 5.11 (dm, 1H, H-4′), 4.64 (dd, 1H, H-5′), 4.60 (dd, 1H, H-5″). HPLC/APCI-MS, m/z 479 M+.
14 (36 mg, 23%) as syrup. Rf 0.33 (B) 1H NMR (CDCl3): 9.16 (s, 1H, NH), 8.14 (s, 1H, H-2), 8.25 (d, 1H, J F2′,H8 = 2.9, H-8), 7.47–8.08 (5m, 10H, 2Bz), 6.68 (dt, 1H, J 1′,F-2′ = 22.3, J 1′,2′ = J 1′,F-3′ = 2.67, H-1′), 5.47 (dm, 1H, H-2′), 5.38 (ddd, 1H, H-3′), 4.62–4.75 (m, 3H, H-4′, 2H-5′). HPLC/APCI-MS, m/z 479 M+.
Deprotection of 14 and 15 with methanol saturated at 0°C by ammonia and subsequent chromatographic purification on silica gel using for elution mixture CHCl3:MeOH-20:1 and 6:1 afforded adenine nucleosides 20 (18 mg, 88%) and 21 (6.4 mg, 81%), respectively. Compound 20. Rf 0.64 (C). m.p. 166–169°C (from EtOH/ether); UV (EtOH) λmax, nm (ε): 259 (14000), λmin, nm (ε): 227 (2000). CD (EtOH), λ, nm ([θ]·10−3): 205 (+6.4), 214(0), 218 (−2.2), 226 (0), 248 (+1.3), 252 (0), 263 (−1.7), 288 (0). HPLC/APCI-MS, m/z 271 M+. 19F (CD3OD):−204.6 (m, F-C2′ or F-C3′, J F-2′,F-3′ is not determined), −195.8 (m, F-C2′ or F-C3′). Anal. calc. for C10H11F2N5O2: C, 44.28; H, 4.09; Found: C, 44.19; H, 4.00.
Compound 21. Rf 0.64 (C). m.p. 96–100°C (from EtOH/ether); UV (EtOH) λmax, nm (ε): 259 (14300), λmin, nm (ε): 227 (2100). CD (EtOH)), λ, nm ([θ]·10−3): 205 (+6.2), 214(0), 217 (−4.7), 220 (0), 230 (+1.9), 255 (+0.8), 260 and 288 (0). HPLC/APCI-MS, m/z 271 M+. 19F (CD3OD):−187.29 (m, F-C2′ or F-C3′), −183.08 (m, F-C2′ or F-C3′, J F-2′,F-3′ is not determined). Anal. calc. for C10H11F2N5O2 : C, 44.28; H, 4.09; Found: C, 44.35; H, 4.17.
2-Chloro-9-(2,3-dideoxy-2,3-difluoro-β-d-arabinofuranosyl) adenine (22) and its α-anomer (23)
A suspension of 2,6-dichloropurine (0.064 g, 0.332 mmol) in anhydrous acetonitrile (3.0 mL) at room temperature was treated NaH (11.0 mg of 80% in oil, 0.034 mmol), and the mixture was stirred for 30 minutes under argon. To this stirred suspension, a solution bromide 13 prepared from 12 (0.19 g, 0.64 mmol) as described above was added in anhydrous acetonitrile (4 mL) and the reaction mixture was stirred at room temperature overnight. Insoluble material was removed by filtration and washed with acetonitrile (5 mL). The combined filtrate and washings were evaporated and the residue was chromatographed on silica gel (130 mL) using for elution mixture EtOAc/hexane 1:3 and 2:3 to afford mixture nucleosides 16 and 17 (0.066 g, 48%) as syrup. Rf 0.48 (A). 1H NMR (CDCl3): 8.32 (d, H-8β, J F2′,H8 = 2.9, 2.9H), 8.26 (s, H-8α, 1H), 7.44–8.06 (m, 4Bz), 6.59 (dt, 1H, J 1′,F-2′ = 21.97, J 1′,2′ = J 1′,F-3′ = 2.6, H-1′ β), 6.56 (br. d, 1H’α, J 1′,2′ < 1.0, J 1′,F-2′ = 19.1, H-1′), 5.75–5.93 (m, H-2′α and H-3′α), 5.46 (dd, H-2′β), 5.36(ddd, H-3′β), 5.09 (dm, H-4′α), 4.52–4.75 (m, 2H-5′α, 2H-5′β and H-4′β). (UV (EtOH) λmax, nm (ε): 274 (5660), 231 (7300).
A solution of mixture nucleosides 16 and 17 (0.066 g, 0.154 mmol) in anhydrous 1,2-dimethoxyethane (10 mL) was saturated by dry ammonia for 4 hours and then left for 18 hours at room temperature. The reaction mixture was filtered off and washed with CH2Cl2. The combined filtrate and washings were evaporated and the residue was chromatographed on silica gel (130 mL), using a linear gradient of EtOAc (0→66%, v/v; 500 mL) in hexane, to afford nucleoside 18 as foam (42 mg, 67%). Rf 0.60 (B). 1H NMR (CDCl3): 7.45–8.06 (3m, 5H, Bz), 7.99 (d, 1H, J F2′,H8 = 3.0, H-8), 6.52 (dt, 1H, J 1′,F2′ = 12.5, H-1′), 6.32 (br. s, 2H, NH2), 5.41 (dd, H-2′), 5.31 (ddd, H-3′), 4.54–4.70 (m, 2H-5′and H-4′). HPLC/APCI-MS, m/z, 410 and 412, Cl35/Cl37 ratio ≈ 3:1, M+. Nucleoside 19 (16 mg, 25%) as foam. Rf 0.46 (B). 1H NMR (CDCl3): 7.47–8.10 (3m, 5H, Bz), 7.97 (s, 1H, H-8), 6.48 (d, 1H, J 1′,F2′ = 14.9, H-1′), 6.18 (br. s, 2H, NH2), 5.88 (dm, 1H, H-2′), 5.44 (dm, 1H, H-3′), 5.07 (dm, 1H, H-4′), 4.63 (dd, 1H, H-5′), 4.58 (dd, 1H, H-5″). HPLC/APCI-MS, m/z 410 and 412, Cl35/Cl37 ratio ≈ 3:1, M+.
Solution of nucleoside 18 (36 mg, 0.088 mmol) in 10 mL methanol saturated at 0°C by ammonia was kept during 195 minutes at room temperature and then was evaporated. The residue was chromatographed on silica gel (40 mL) using for elution mixture CHCl3:MeOH-20:1 and 15:1 to afford nucleoside 22 (20 mg, 75%). Rf 0.75 (c). m.p. 171–173°C (CHCl3/EtOH). UV λmax, nm (ε): 263 (14900) at pH 7, 263 (15000) at pH 13, 263 (13900) at pH 1. CD (EtOH), λ, nm ([θ]·10−3): 207 (+12.9), 212(0), 217 (−4.0), 231 (0), 260 (−1.7), 288 (0). HPLC/APCI-MS, m/z 307 and 309, Cl35/Cl37 ratio ≈ 3:1, (M+H)+. 19F (DMSO-d6): −202.47 (m, F-C2′), −196.40 (m, F-C3′, J F-2′,F-3′ = 8.48). Anal. calc. for C10H10F2ClN5O2: C, 39.29; H, 3.30; Cl, 11.60; Found : C, 39.40; H, 3.47; Cl, 11.72.
In a similar way, nucleoside 23 (6 mg, 80%) was prepared starting from nucleoside 19 (10 mg, 0.024 mmol). Rf 0.75 (C). m.p. 176–178°C (CHCl3/EtOH). UV λmax, nm (ε): 263 (14500) at pH 7, 263 (14700) at pH 13, 263 (13900) at pH 1. CD (EtOH), λ, nm ([θ]·10−3): 205 (+11.6), 212(0), 217 (−8.5), 228 (0), 255 (+1.1), 269 (0). HPLC/APCI-MS, m/z 307 and 309, Cl35/Cl37 ratio ≈ 3:1, (M + H)+. 19F (DMSO-d6): −195.21 (m, F-C2′, J F-2′,F-3′ = 8.48), −195.76 (m, F-C3′). Anal. calc. for C10H10F2ClN5O2: C, 39.29; H, 3.30; Cl, 11.60; Found : C, 39.38; H, 3.20; Cl, 11.69.
Acknowledgments
This work was supported in part by NIH grant 2P30-AI-50409 (RFS), 5R37-AI-041980 (RFS) and Department of Veterans Affairs (RFS) and Belarus State Program of FOI “Physiological Active Compounds” (Grant 2.04). R.F.S. is the Founder and Director of RFS Pharma, LLC, and received no compensation for performing this work.
REFERENCES
- 1.Ray AS; Schinazi RF; Murakami E; Basavapathruni A; Shi J; et al. Probing the mechanistic consequences of 5-fluorine substitution on cytidine nucleotide analogue incorporation by HIV-1 reverse transcriptase. Antiv. Chem. Chemother 2003, 14, 115–125. [DOI] [PubMed] [Google Scholar]
- 2.Lee K; Choi Y; Gumina G; Zhou W; Schinazi RF; Chu CK Structure-Activity Relationships of 2′-fluoro-2′,3′-unsaturated d-nucleosides as anti-HIV-1 agents. J. Med. Chem 2002, 45, 1313–1320. [DOI] [PubMed] [Google Scholar]
- 3.Clarck JL; Hollecker L; Mason JC; Stuyver LJ; Tharnish PM; et al. Design, synthesis, and antiviral activity of 2′-deoxy-2′-fluoro-2′-C-methylcytidine, a potent inhibitor of hepatitis C virus replication. J. Med. Chem 2005, 48, 5504–5508. [DOI] [PubMed] [Google Scholar]
- 4.Shi J; Du J; Ma T; Pankiewicz KW; Patterson SE; et al. Synthesis and antiviral activity of a series of D- and L-2′-deoxy-2′-fluororibonucleosides in the subgenomic HCV replicon system. Bioorg. Med. Chem 2005, 13, 1641–1652. [DOI] [PubMed] [Google Scholar]
- 5.Martin JA; Busnell DJ; Duncan IB; Dunsdon SJ; Hall MJ; et al. Synthesis and antiviral activity of monofluoro and difluoro analogues of pyrimidine deoxyribonucleosides against human immunodeficiency virus (HIV-1). J. Med. Chem 1990, 33, 2137–2145. [DOI] [PubMed] [Google Scholar]
- 6.Herdewijn P; Pauwels R; Baba M; Balzarini J; De Clercq E Synthesis and anti-HIV activity of various 2′- and 3′-substituted 2′,3′-dideoxyadenosines: A structure-activity analysis. J. Med. Chem 1987, 30, 2131–2137. [DOI] [PubMed] [Google Scholar]
- 7.Marquez VE; Tseng CK-H; Kelly JA; Mitsuya H; Broder S; et al. 2′,3′-Dideoxy-2′-fluoro-ara-A. An acid-stable purine nucleoside active against human immunodeficiency virus (HIV). Biochem. Pharmacol 1987, 36, 2719–2722. [DOI] [PubMed] [Google Scholar]
- 8.Bonate PL; Arthaud L; Cantrell WR; Stephenson K; Secrist JA III; Weitman S Discovery and development of clofarabine: a nucleoside analogue for treating cancer. Nature Reviews 2006, 5, 855–863. [DOI] [PubMed] [Google Scholar]
- 9.Sivets GG; Kalinichenko EN; Mikhailopulo IA Synthesis of 9-(2,3-dideoxy-2,3-difluoro-β-d-arabinofuranosyl)adenine. Nucleosides Nucleotides Nucleic Acids. 2007, 26, 1387–1389. [DOI] [PubMed] [Google Scholar]
- 10.Mikhailopulo IA; Sivets GG; Poopeiko NE; Khripach NB Oxidation-reduction sequence for the synthesis of peracylated fluorodeoxy pentofuranosides. Carbohyd. Res 1995, 278, 71–89. [Google Scholar]
- 11.Hanessian S; Vatele J-M Design and reactivity of organic functional groups: imidazolylsulfonate (imidazylate)—an efficient and versatile leaving group. Tetrahedron Lett. 1981, 22, 3579–3582. [Google Scholar]
- 12.Su T-L; Klein RS; Fox JJ Improved synthesis of α-d-ribofuranosides via stereoselective alkylation of a dibutylstannylene derivative for ready access to the 2-substituted 2-deoxyarabinofuranosides. J. Org. Chem 1981, 47, 1506–1509. [Google Scholar]
- 13.Sivets GG; Kalinichenko EN; Mikhailopulo IA Synthesis and conformational analysis of 1′-and 3′-substituted 2-deoxy-2-fluoro-d-ribofuranosyl nucleosides. Helv. Chim. Acta 2007, 90, 1818–1836. [Google Scholar]
- 14.Tann CH; Brodfuehrer PR; Brundidge SP; Sapino C Jr.; Howell HG An efficient synthesis of 1-(2-deoxy-2-fluoro-β-d-arabinofuranosyl)-5-iodouracil (β-FIAU) and 1-(2-deoxy-2-fluoro-β-d-arabinofuranosyl) thymine (β-FMAU). J. Org. Chem 1985, 50, 3644–3647. [Google Scholar]
- 15.Mikahilopulo IA; Sivets GG A Novel route for the synthesis of deoxy fluoro sugars and nucleosides. Helv. Chim. Acta 1999, 82, 2052–2065. [Google Scholar]
- 16.Gillard JW; Israel M Trimethylsilyl bromide as a mild, stereoselective anomeric brominating agent. Tetrahedron Lett. 1981, 22, 513–516. [Google Scholar]
- 17.Kazimierczuk Z; Cottam HB; Revankar GR; Robins RK Synthesis of 2′-Deoxytubercidin, 2′-deoxyadenosine, and related 2′-deoxynucleosides via a novel direct stereospecific sodium salt glycosylation procedure. J. Am. Chem. Soc 1984, 106, 6379–6382. [Google Scholar]
- 18.Zaitseva GV; Sivets GG; Kazimierczuk Z; Vilpo JA; Mikahilopulo IA Convergent syntheses and cytostatic properties of 2-chloro-2′-deoxy-2′-fluoroadenosine and its N7-isomer. Bioorg. Med. Chem. Lett 1995, 5, 2999–3002. [Google Scholar]
- 19.Mikhailopulo IA; Poopeiko NE; Pricota TI; Sivets GG; Kvasyuk EI; Balzarini J; De Clercq E Synthesis and antiviral and cytostatic properties of 3′-deoxy-3′-fluoro- and 2′-azido-3′-fluoro-2′,3′-dideoxy-d-ribofuranisides of natural heterocyclic bases. J. Med. Chem 1991, 34, 2195–2202. [DOI] [PubMed] [Google Scholar]
- 20.Tennilä T; Azhayeva E; Vepsäläinen J; Laatikainen R; Azhayev A; Mikhailopulo IA Oligonucleotides containing 9-(2-deoxy-2-fluoro-β-d-arabinofuranosyl)-adenine and -guanine: synthesis, hybridization and antisense properties. Nucleosides Nucleotides Nucleic Acids. 2000, 19, 1861–1884. [DOI] [PubMed] [Google Scholar]
- 21.Michalik M; Hein M; Frank M NMR spectra of fluorinated carbohydrates. Carbohyd. Res 2000, 327, 185–218. [DOI] [PubMed] [Google Scholar]
- 22.Sivets GG; Kalinichenko EN; Mikahilopulo IA Synthesis of C2′-β-fluoro-substituted adenine nucleosides via pivaloyl derivatives of adenosine and 3′-deoxyadenosine. Lett. Org. Chem 2006, 3, 402–408. [Google Scholar]
- 23.De Clercq E HIV inhibitors targeted at the reverse transcriptases. AIDS Res. Human Retroviruses 1992, 8, 119–134. [DOI] [PubMed] [Google Scholar]
- 24.Schinazi RF; Sommadossi JP; Saalmann V; Cannon DL; Xie M-W; et al. Activities of 3′-azido-3′-deoxythymidine nucleotide dimers in primary lymphocytes infected with human immunodeficiency virus type 1. Antimicrob. Agents Chemother 1990, 34, 1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stuyver LJ; Lostia S; Adams M; Mathew J; Pai BS; et al. Antiviral activities and cellular toxicities of modified 2′,3′-dideoxy-2′,3′-didehydrocytidine analogues. Antimicrob. Agents Chemother 2002, 46, 3854–3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Wijk J; Haasnot CAG; de Leeuw FAAM; Huckriede BD; Westra Hoekzema AJA; Altona C PSEUROT 6.3. A program for the conformational analysis of five-membered rings, Leiden Institute of Chemistry: Leiden, the Netherlands, 1999. [Google Scholar]
- 27.Mikahilopulo IA; Pricota TI; Sivets GG; Altona C 2′-Chloro-2′,3′-dideoxy-3′-fluoro-d-ribofuranosides: synthesis, stereospecificity, some chemical transformations, and conformational analysis. J. Org. Chem 2003, 68, 5897–5908. [DOI] [PubMed] [Google Scholar]
- 28.Thibaudeau C; Plavec J; Chattopadhyaya J A new generalized Karplus-type equation relating vicinal proton-fluorine coupling constants to H-C-C-F torsion angles. J. Org. Chem 1998, 63, 4967–4984. [Google Scholar]
- 29.Ford H; Dai F; Mu L; Siddiqui MA; Nicklaus MC; et al. Adenosine deaminase prefers a distinct sugar ring conformation for binding and catalysis: kinetic and structural studies. Biochemistry 2000, 39, 2581–2592. [DOI] [PubMed] [Google Scholar]
- 30.Barchi JJ; Karki RG; Nicklaus MC; Siddiqui MA; Clifford G; et al. Comprehensive structural studies of 2′,3′-difluorinated nucleosides: comparison of theory, solution and solid state. J. Am. Chem. Soc 2008, 130, 8048–8057. [DOI] [PMC free article] [PubMed] [Google Scholar]


