Atherosclerosis is a chronic inflammatory disease, occurring preferentially in branches and curves in arteries exposed to disturbed blood flow (d-flow). In contrast, straight arteries are exposed to stable flow (s-flow) and are protected from atherosclerosis development.1–3 D-flow is characterized by low, oscillatory shear stress (OS) whereas s-flow is associated with unidirectional (either pulsatile or nonpulsatile) and relatively high level of laminar shear stress. Flow regulates these atherogenic responses by regulating gene expression and signaling pathways in endothelial cells (ECs). Mechanosensors (also known as mechanoreceptors) detect changes in shear stress direction and magnitude with respect to time in ECs. Once detected, the mechanosensors convert the mechanical force into biochemical signals, triggering cell signaling pathways, and changes in cell functions. D-flow induces, while s-flow prevents, endothelial dysfunction including inflammation, endothelial-mesenchymal transition, thrombosis, vasoconstriction, and barrier dysfunction, which in turn lead to atherosclerosis in conjunction with additional risk factors such as hypercholesterolemia.1,4 While these flow-dependent changes are relatively well described, the cell and molecular mechanisms by which d-flow and s-flow induce these different endothelial responses are still unclear. Are there specialized mechanosensors that detect d-flow versus s-flow, or does one mechanosensor respond differently to different flow conditions? In the current edition of Circulation Research, Zhang et al define a novel molecular mechanism by which d-flow induces inflammation and atherosclerosis by activating integrin α5 through interaction with ANXA2 (annexin A2) by a Piezo1-dependent mechanism.
ECs are equipped with numerous mechanosensors located on the luminal and basal surfaces as well as in the cell-cell junction and cytoskeleton. Some of the well-characterized mechanosensors are the PECAM-1 (platelet endothelial cell adhesion molecule 1), VE-cadherin (vascular endothelial-cadherin), and VEGFR-2 (vascular endothelial growth factor receptor) mechanosensory complex, PlxnD1 (Plexin D1), Piezo1, integrins, and NOTCH1.4–7 Purinergic receptor calcium channels, G-proteins, glycocalyx, primary cilia, cytoskeleton, and caveolae can also function as mechanosensors.4–8 The PECAM-1, VE-cadherin, and VEGFR-2 mechanosensory complex transduces laminar shear stress into the activation of PI3K (phosphoinositide 3-kinase) and AKT, stimulating nitric oxide (NO) production from eNOS (endothelial NO synthase). LS (laminar shear stress or s-flow) also induces integrin αvβ3 activation, which leads to endothelial alignment to the direction of flow (Figure).8,9,10 Whether the PECAM-1 complex also responds to OS is unknown.
Figure. Disturbed blood flow (D-flow) and stable flow (s-flow) induce differential mechanosensing and mechanotransduction.
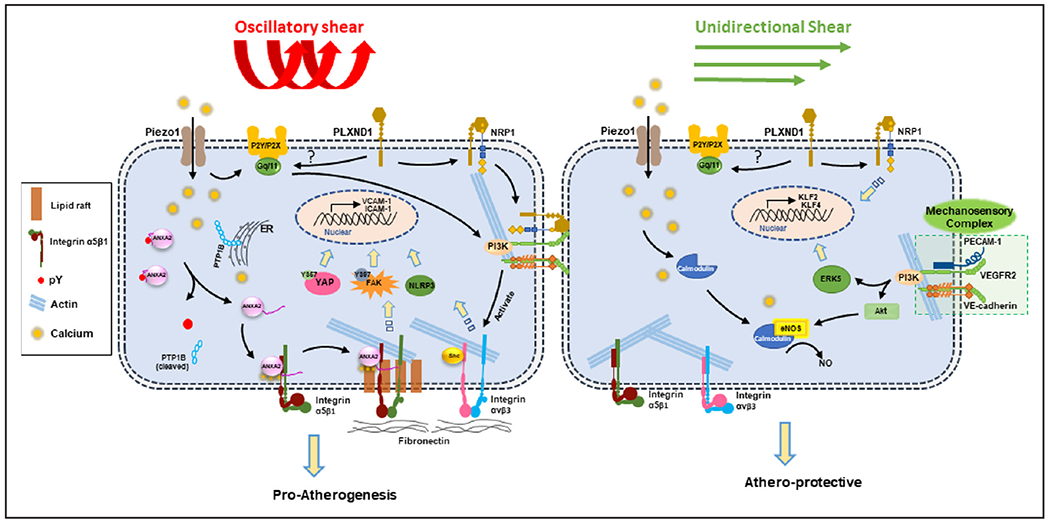
D-flow is sensed by Piezo1 and Plexin D1, which lead to activation of integrins and proatherogenic signaling responses. D-flow induces calcium influx through Piezo1, which activates PTP1B (protein-tyrosine phosphatase 1B)-dependent ANXA2 (annexin A2) dephosphorylation. ANXA2 then binds integrin α5 which is translocated to the lipid raft and activated, leading to stimulation of FAK (focal adhesion kinase)-dependent endothelial inflammation and atherosclerosis.
Two mechanosensors, Piezo1 and PlxnD1, have been identified to respond to both laminar shear stress (s-flow) and OS (d-flow) differentially. PlxnD1 is a semaphorin guidance receptor which responds to both flow conditions.11 EC-specific loss of PlxnD1 inhibits the site-specific atherosclerosis development in the aortic arch exposed to d-flow while increasing the plaque development in the straight thoracic aortas exposed to s-flow.11 This result suggests dual roles of PlxnD1 serving as a mechanosensor for both s-flow and d-flow. D-flow induces a conformational change of PlxnD1, which triggers complex formation with neuropilin-1 and VEGFR-2 to mediate the AKT, ERK (extracellular signal-regulated kinase), FAK (focal adhesion kinase), and eNOS activation pathway. This suggests that PlxnD1 interacts with the PECAM-1/VEGFR-2/VE-cadherin mechanosensory complex to mediate the s-flow effect, but it is unclear if this is also involved in the d-flow response. Interestingly, the PlxnD1 flow response is dependent on the Gq/G11 pathway, but not on Piezo1. This implicates Gq/G11 as a possible hub where the 2 different mechanosignaling pathways diverge in response to various shear stress profiles.
Piezo1 is a calcium-permeable, nonselective cation channel that has been well-characterized as a mechanosensor responding to both s-flow and d-flow.6, 7 Upon activation by either flow condition, Piezo1 triggers the same initial mechanosignaling response of an influx of calcium, which in turn activates Gq/G11-mediated endothelial responses.6 However, depending on the flow pattern, ECs read these initial mechanosensing and mechanotranduction responses as either atheroprotective signaling by activating the PI3K-eNOS pathway or as proatherogenic signaling through activation of the integrin, FAK-mediated NF-κB (nuclear factor-κB) pathway (Figure).10, 12 Recent studies have shown that Piezo1 predominantly responds to OS to increase calcium influx, which leads to the Gq/G11-dependent activation of integrin α5.6 Integrin α5 translocates into lipid rafts in response to OS, a process which is necessary for integrin activation and ligation into α5β1.13 Once activated, integrin α5β1 promotes the FAK-dependent NF-κB activation and expression of proinflammatory cell adhesion proteins VCAM-1 and ICAM-1.6, 13 However, it was unknown how OS causes translocation and activation of integrin α5β1.
Zhang et al demonstrate that the translocation and activation of integrin α5β1 induced by d-flow are mediated by Piezo 1-dependent activation of ANXA2, leading to endothelial inflammation and atherosclerosis.14 For this study, the authors employed a series of elegant and comprehensive approaches using proteomics, biophysics, genetic models, and novel in vivo EC-targeted gene modification methods. They first performed a proteomics study to identify proteins that bind to integrin α5 and identified ANXA2 as a potential binding protein. Subsequent validation studies clearly established that ANXA2 indeed binds to integrin α5, and this interaction was essential in mediating the translocation and activation of the integrin into the lipid rafts in response to OS in ECs. Through a series of biophysical and molecular imaging studies using various constructs of ANXA2 and integrin α5, they showed that ANXA2 undergoes conformational changes in a phosphorylation/dephosphorylation-dependent manner in response to OS. ANXA2 is in a closed conformation when it is phosphorylated at Tyr-24 (pY24) but opens when dephosphorylated by PTP1B (protein-tyrosine phosphatase 1B) in response to OS. Upon dephosphorylation at Y24, the C-terminal domain of ANXA2 is released from the N-terminus, allowing the cytoplasmic domain of the integrin α5 to bind to the C-terminal of ANXA2. The binding of ANXA2 to integrin α5 then triggers its translocation to the lipid rafts, where the integrin is activated and stimulates the FAK-dependent endothelial inflammation pathway.
The mechanism by which ANXA2 is dephosphorylated by PTP1B activation is mediated by calcium influx through Piezo1 in response to OS. Since ANXA2 and PTP1B were known to be calcium sensitive, they tested the role of calcium influx through the mechanosensor Piezo1. In studies using various siRNAs and the Piezo1 activator YODA1, they showed that OS activated PTP1B through Piezo1-mediated calcium flux. Through detailed biochemical studies including a mass spectrometry, they confirmed the dephosphorylation of ANXA2 at Y24 by PTP1B.
To validate the in vitro observations regarding the role of PTP1B, ANXA2, and integrin α5 in endothelial inflammation and atherosclerosis, they performed several elegant and innovative in vivo studies using genetic and molecular interventions at various points in this pathway. For these studies, they used the partial carotid ligation model of atherosclerosis.15 In this model, ligation of 3 of 4 distal branches of common carotid artery induces d-flow, which leads to robust endothelial dysfunction and atherosclerosis within 2 weeks.15 They first showed that the loss of ANXA2 inhibits atherosclerosis development using ApoE_ANXA2 double knockout mice. Furthermore, they showed that the atheroprotective effect of ANXA2 knockout was reversed by infecting the carotids with lentiviral-ANXA2-wild type. Moreover, infection with constitutively dephosphorylated lentiviral-ANXA2Y24F mutant made the atherosclerosis even worse than the wild type, consistent with the in vitro finding. In addition, infection of the carotids with lentiviral-PTP1B shRNA dramatically knocked down endothelial level of PTP1B, resulting in significant prevention of atherosclerosis in ApoE knockout, but not in ApoE_ANXA2 double knockout mice. These results clearly validate the role of PTP1B and ANXA2 in d-flow-induced endothelial inflammation and atherosclerosis.
It is noteworthy to highlight the critical technical advancement and innovative approaches developed and demonstrated in this study. The use of lentiviral constructs to overexpress and knockdown key target genes, ANXA2 wild type and Y24F mutants and PTP1B shRNA in carotid artery ECs in the partial carotid ligation model of flow-induced atherosclerosis is remarkable in its tissue-targetability and overexpression and knockdown efficiencies. These approaches should be helpful to other investigators in the field in studying the role of many other genes, especially flow-sensitive genes, in endothelial function and atherosclerosis in vivo.
In summary, the report by Zhang et al demonstrates the novel mechanism by which d-flow induces integrin α5-dependent activation through the Piezo1-PTP1 B-ANXA2 pathway, underlining the importance of this process in mechanotransduction. While these new insights provides deeper understanding of the differential activation mechanisms by which s-flow and d-flow lead to atheroprotective and proatherogenic responses, respectively, there still remain several outstanding unresolved questions. While s-flow and d-flow both stimulate initial calcium influx via Piezo1, what determines the divergence of proatherogenic and antiatherogenic pathways such as the activation of PTP1B or protein kinases regulating ANXA2? Although there are multiple mechanosensors, only a few have been tested for their ability to respond to d-flow versus s-flow. While it is likely that many mechanosensors respond in a coordinated manner, it still remains an open question. New insights provided by the current study provided by the current study also illustrate novel therapeutic opportunities for atherosclerosis.
Acknowledgments
Sources of Funding
This work was supported by funding from the National Institutes of Health (NIH) grants HL119798 and HL095070 to H. Jo. D. Williams was supported by the NIH F31 HL145974 grant. H. Jo was also supported by Wallace H. Coulter Distinguished Faculty Professorship.
Footnotes
Disclosures
None.
REFERENCES
- 1.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91:327–387. doi: 10.1152/physrev.00047.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwak BR, Bäck M, Bochaton-Piallat ML, Caligiuri G, Daemen MJ, Davies PF, Hoefer IE, Holvoet P, Jo H, Krams R, et al. Biomechanical factors in atherosclerosis: mechanisms and clinical implications. Eur Heart J. 2014;35:3013–3020, 3020a. doi: 10.1093/eurheartj/ehu353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tarbell JM, Shi ZD, Dunn J, Jo H. Fluid mechanics, arterial disease, and gene expression. Annu Rev Fluid Mech. 2014;46:591–614. doi: 10.1146/annurev-fluid-010313-141309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demos C, Tamargo I, Jo H, Chapter 1 - Biomechanical regulation of endothelial function in atherosclerosis In: Ohayon J, Finet G, Pettigrew RI, eds. Biomechanics of Coronary Atherosclerotic Plaque. London, England: Academic Press; 2020:3–50. [Google Scholar]
- 5.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952 [DOI] [PubMed] [Google Scholar]
- 6.Albarrán-Juárez J, Iring A, Wang S, Joseph S, Grimm M, Strilic B, Wettschureck N, Althoff TF, Offermanns S. Piezo1 and Gq/G11 promote endothelial inflammation depending on flow pattern and integrin activation. J Exp Med. 2018;215:2655–2672. doi: 10.1084/jem.20180483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, Hou B, Tumova S, Muraki K, Bruns A, Ludlow MJ, Sedo A, Hyman AJ, McKeown L, Young RS,et al. Piezo1 integration of vascular architecture with physiological force. Nature. 2014;515:279–82. doi: 10.1038/nature13701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto K, Korenaga R, Kamiya A, Qi Z, Sokabe M, Ando J. P2X4 receptors mediate ATP-induced calcium influx in human vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2000;279:H285–H292. doi: 10.1152/ajpheart.2000.279.1.H285 [DOI] [PubMed] [Google Scholar]
- 9.Conway DE, Breckenridge MT, Hinde E, Gratton E, Chen CS, Schwartz MA. Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr Biol. 2013;23:1024–1030. doi: 10.1016/j.cub.2013.04.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orr AW, Ginsberg MH, Shattil SJ, Deckmyn H, Schwartz MA. Matrix-specific suppression of integrin activation in shear stress signaling. Mol Biol Cell. 2006;17:4686–4697. doi: 10.1091/mbc.e06-04-0289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta V, Pang KL, Rozbesky D, Nather K, Keen A, Lachowski D, Kong Y, Karia D, Ameismeier M, Huang J, et al. The guidance receptor plexin D1 is a mechanosensor in endothelial cells. Nature. 2020;578:290–295. doi: 10.1038/s41586-020-1979-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhullar IS, Li YS, Miao H, Zandi E, Kim M, Shyy JY, Chien S. Fluid shear stress activation of IkappaB kinase is integrin-dependent. J Biol Chem. 1998;273:30544–30549. doi: 10.1074/jbc.273.46.30544 [DOI] [PubMed] [Google Scholar]
- 13.Sun X, Fu Y, Gu M, Zhang L, Li D, Li H, Chien S, Shyy JY, Zhu Y. Activation of integrin α5 mediated by flow requires its translocation to membrane lipid rafts in vascular endothelial cells. Proc Natl Acad Sci U S A. 2016;113:769–774. doi: 10.1073/pnas.1524523113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang C, Zhou T, Chen Z, Yan M, Li B, Lv H, Wang C, Xiang S, Shi L, Zhu Y, et al. Coupling of integrin α5 to annexin A2 by flow drives endothelial activation. Circ Res. 2020;127:1074–1090. doi: 10.1161/CIRCRESAHA.120.316857 [DOI] [PubMed] [Google Scholar]
- 15.Nam D, Ni CW, Rezvan A, Suo J, Budzyn K, Llanos A, Harrison D, Giddens D, Jo H. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol. 2009;297:H1535–H1543. doi: 10.1152/ajpheart.00510.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]


