In response to the coronavirus disease 2019 (COVID-19) pandemic many cardiac catheterization and electrophysiology laboratories canceled elective procedures to limit the burden on hospital resources. A major challenge in resuming elective procedures is the risk of exposing patients and health care personnel (HCP) to carriers of the virus. To facilitate procedure resumption, the Hospital of the University of Pennsylvania began testing all patients scheduled for elective and urgent catheterization and electrophysiology procedures for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on April 14, 2020. We report the results of testing for a 4-week period and describe results of patient and HCP surveys regarding their perceptions on universal testing.
When resuming elective procedures, the Hospital of the University of Pennsylvania required all patients undergoing elective or urgent cardiac procedures to wear surgical masks, prohibited visitors, and implemented universal preprocedure temperature, symptom, and polymerase chain reaction (PCR) screening, and undergo preprocedure PCR testing. Inpatients underwent PCR testing at the core laboratory. Outpatients were encouraged to undergo testing at one of 3 satellite drive-through facilities. Test turnaround time was 2 hours for same-day orders and 24 hours for off-site testing. During the first week, all patients were tested within 24 hours of the scheduled procedure, which was later changed to 48 hours for outpatients and 1 week for inpatients. Patients undergoing emergent procedures were tested, but the procedure was not delayed pending results. For HCPs, the Hospital of the University of Pennsylvania mandated use of surgical masks, eye protection, and gloves.
To evaluate patient perceptions regarding SARS-CoV-2 testing, all patients were contacted by telephone 2 weeks after the procedure. A separate electronic survey was distributed to HCPs working in the catheterization and electrophysiology laboratories during this period. The study was approved by the University of Pennsylvania Institutional Review Board; patients and staff provided informed consent before completing surveys. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Between April 14 and May 12, 2020, 215 patients underwent 252 elective or urgent procedures (128 catheterizations and 124 electrophysiology). Median age was 63 years and 62% were male; 111 procedures (41.0%) were performed on outpatients, of whom 53 (47.7%) underwent testing off-site. Overall, 17.1% of patients had either dyspnea, cough, or fever on the day of testing. Two asymptomatic patients (0.9%) tested positive for SARS-CoV-2. One presented for an outpatient atrial fibrillation ablation which was canceled; the second was an inpatient referred for right heart catheterization, which was performed with airborne isolation precautions, including N95 respirators. No patients who tested negative at the time of their procedure subsequently tested positive for SARS-CoV-2, and no staff members developed COVID-19 symptoms during this period.
When contacted by telephone, 55.8% of patients (n=120) agreed to participate in a survey. None reported testing positive for SARS-CoV-2 following the procedure. A majority (63.2%) reported that preprocedure testing did not change their comfort level in getting the procedure performed; 31.6% reported increased comfort.
Among 107 staff members invited to participate in the survey, 55 (51.4%) responded. All agreed that limiting visitors reduced risks, and a majority strongly agreed that universal testing reduced risk (Figure). A total of 61.9% reported a decrease in their level of concern for being infected at the workplace with universal testing.
Figure.
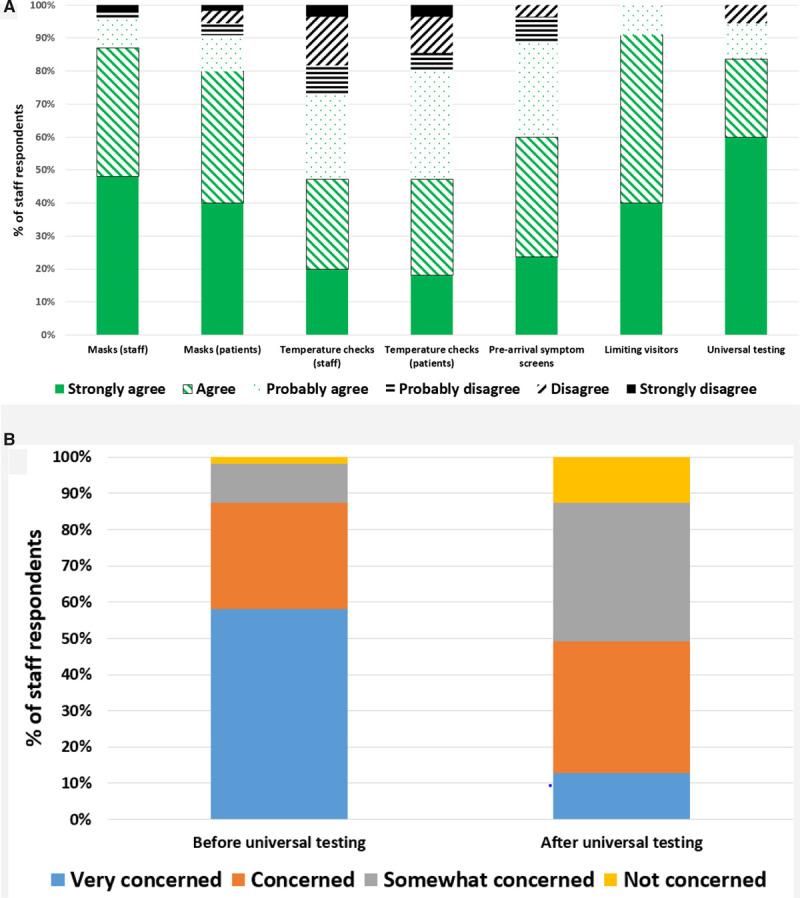
Staff members’ perceptions regarding severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) exposure. A, Staff members’ perceptions regarding effectiveness of various strategies to reduce SARS-CoV-2 exposure at the work place. B, Staff members’ level of concern regarding their risk of developing coronavirus disease 2019 (COVID-19) before and after universal preprocedure SARS-CoV-2 screening.
In this single-center experience, 0.9% of patients undergoing cardiac catheterization and electrophysiology procedures tested positive for active SARS-CoV-2 infection. Preprocedure testing, including off-site testing for outpatients, was feasible. Universal testing led to improvement in the comfort level of catheterization and electrophysiology laboratory HCPs.
In a recent report of universal screening for SARS-CoV-2 in 215 pregnant women in New York, 13.7% tested positive, of whom 87.9% had no symptoms.1 Fewer patients tested positive in our study, partly related to the lack of a catastrophic outbreak in Philadelphia during our study period. Moreover, prevalence in patients undergoing cardiac procedures was lower than pregnant women in Philadelphia, suggesting that cardiac patients may be more effectively isolating.2 Noncatastrophic community transmission of COVID-19 may persist in many regions for months or years, and strategies will be needed to continue necessary cardiovascular procedures. During the early phase of the COVID-19 pandemic, cardiovascular hospitalizations decreased, including for urgent and emergent conditions,3 leading to concerns that patients may be forgoing necessary care due to fears about the safety of coming to the hospital. Universal preprocedure SARS-CoV-2 testing improved perceptions of safety for some patients and a majority of HCPs. Increasing HCPs’ perception of safety is important and may also help health systems convincingly reassure patients that they can safely come to the hospital.
This study does have limitations. False-negative SARS-CoV-2 PCR tests might lead to underestimation of the number of infected patients. A 2-hour test turnaround time may not be universally feasible. Last, there was no control group to assess risk of transmission without universal testing.
SARS-CoV-2 PCR testing before catheterization and electrophysiology procedures was feasible and associated with improved staff perceptions of safety. These results may support universal testing as part of a multipronged approach to limit contagion and provide necessary cardiovascular procedure services.
Sources of Funding
None.
Disclosures
None.
Footnotes
Nonstandard Abbreviations and Acronyms
- COVID-19
- coronavirus disease 2019
- HCP
- health care personnel
- PCR
- polymerase chain reaction
- SARS-CoV-2
- severe acute respiratory syndrome coronavirus 2
For Sources of Funding and Disclosures, see page 408.
Contributor Information
Naga Venkata K. Pothineni, Email: Naga.Pothineni@Pennmedicine.upenn.edu.
Samantha Starkey, Email: samantha.starkey@pennmedicine.upenn.edu.
Kristine Conn, Email: kristine.conn@pennmedicine.upenn.edu.
Christina Evans, Email: christina.evans@pennmedicine.upenn.edu.
Ronak Shah, Email: ronak.shah@uphs.upenn.edu.
Matthew C. Hyman, Email: Matthew.Hyman@uphs.upenn.edu.
David S. Frankel, Email: David.Frankel@pennmedicine.upenn.edu.
Rim Halaby, Email: halabyrim@gmail.com.
Hillary A. Johnston-Cox, Email: hjcox@bu.edu.
Katherine Kunkel, Email: Katherine.J.Kunkel@gmail.com.
Ashwin S. Nathan, Email: ashwin.nathan@gmail.com.
Matthew E. Seigerman, Email: matthew.seigerman@pennmedicine.upenn.edu.
Howard C. Herrmann, Email: howard.herrmann@uphs.upenn.edu.
Jay Giri, Email: giri.jay@gmail.com.
Francis E. Marchlinski, Email: francis.marchlinski@uphs.upenn.edu.
Pasquale Santangeli, Email: pasquale.santangeli@gmail.com.
References
- 1.Sutton D, Fuchs K, D’Alton M, Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med. 2020;382:2163–2164. doi: 10.1056/NEJMc2009316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flannery DD, Gouma S, Dhudasia MB, Mukhopadhyay S, Pfeifer MR, Woodford EC, Gerber JS, Arevalo CP, Bolton MJ, Weirick ME, et al. SARS-CoV-2 seroprevalence among parturient women in Philadelphia. Sci Immunol. 2020;5:eabd5709 doi: 10.1126/sciimmunol.abd5709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solomon MD, McNulty EJ, Rana JS, Leong TK, Lee C, Sung SH, Ambrosy AP, Sidney S, Go AS. The Covid-19 pandemic and the incidence of acute myocardial infarction. N Engl J Med. 2020;383:691–693. doi: 10.1056/NEJMc2015630 [DOI] [PubMed] [Google Scholar]


