Proactively preparing for climate change produces more effective ocean plans and imposes few trade-offs.
Abstract
Societies increasingly use multisector ocean planning as a tool to mitigate conflicts over space in the sea, but such plans can be highly sensitive to species redistribution driven by climate change or other factors. A key uncertainty is whether planning ahead for future species redistributions imposes high opportunity costs and sharp trade-offs against current ocean plans. Here, we use more than 10,000 projections for marine animals around North America to test the impact of climate-driven species redistributions on the ability of ocean plans to meet their goals. We show that planning for redistributions can substantially reduce exposure to risks from climate change with little additional area set aside and with few trade-offs against current ocean plan effectiveness. Networks of management areas are a key strategy. While climate change will severely disrupt many human activities, we find a strong benefit to proactively planning for long-term ocean change.
INTRODUCTION
The coastal ocean is a crowded landscape that supports diverse and expanding human uses, from fishing and recreation to energy development, transportation, aquaculture, and conservation (1–3). Governance that historically focused on individual activities or species has often allowed substantial and negative cumulative impacts on ocean ecosystems, including the decline of coral reefs and the collapse of both fishery and non-fishery species (1, 4, 5). In addition, many ocean and coastal uses affect and conflict with each other, such as scenic views and wind turbines or conservation and fishing (2, 6). As a result, ecosystem-based management efforts to coordinate among marine activities have become common, often expressed as coastal and marine spatial planning or ocean planning (1, 2, 4, 7).
Ecological principles for ocean planning are built upon the spatial distribution of species, habitats, and ecological communities (8, 9). Even though species and biogenic habitats are rapidly shifting geographically as climate changes (10) and despite calls for greater consideration of these climate change impacts (7, 11), species redistributions are not a central consideration in the current principles, legal frameworks, or examples of ocean planning (7, 11, 12). A major impediment has been uncertainty about the difficulty of and trade-offs required for incorporating long-term change into multisector ocean plans (3, 12).
Periodic revisions of ocean plans could enable climate adaptation over time, although revisions are challenging given the substantial negotiations among stakeholders inherent to ocean planning and the long-term legal agreements and impacts involved in offshore energy, mineral extraction, and other development or habitat-modifying activities (13). Alternatively, ocean plans could be designed around climate change impacts from the start (14), but the extent to which advance planning across multiple sectors can help in this regard remains unclear. One proposal in the context of conservation alone has been to identify areas that are likely to be consistently important through time (15). It is unknown whether planning for the future requires setting aside substantially more area for ocean plans or whether there are strong trade-offs between plans that are effective in the near term versus those that are effective in the long term. One heuristic approach for climate adaptation may be to designate networks of management areas that could act like stepping-stones as species shift (14). The extent to which networks can help in this regard, however, has not been quantified.
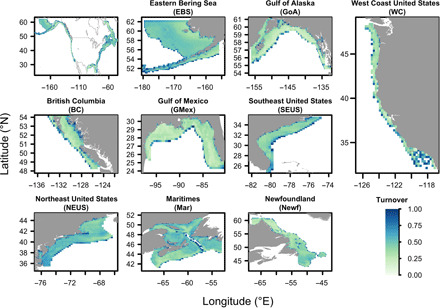
Here, we use nine regions on the continental shelves of North America (Fig. 1) to study these issues. Ocean planning efforts have occurred and are under way to varying degrees across this geography (6). We simulated the multisector ocean planning process to site zones for conservation, fishing, or energy development within each region. Inspired by the Convention on Biological Diversity’s (CBD’s) Aichi Target 11, we designed conservation zones to protect at least 10% of the locations with occurrences of each species in a region. In contrast, we designed fishery zones to include locations that had, in sum, at least 50% of the biomass of each of the top 10 fishery species in each region. Energy zones included at least 20% of the value from wind and wave energy resources, consistent with the ~20% of offshore energy potential proposed to be captured as part of a roadmap to 100% clean energy (16, 17).
Fig. 1. Study areas for simulating the ocean planning process, shown with projected species turnover (Sørenson dissimilarity) 2007–2100 on the continental shelf.
RESULTS AND DISCUSSION
We first developed myopic “present-only” plans that only considered species’ current geographic distributions for evaluating whether conservation and fishery zones met their goals. We then evaluated these plans against 11,776 projections of future species habitat distributions through time: 736 species across eight climate models following a low [Representative Concentration Pathway (RCP) 2.6] and a high (RCP8.5) greenhouse gas emissions scenario. This evaluation revealed substantial declines in effectiveness of the present-only plans that implied difficulty meeting societal targets for fishing and conservation (Fig. 2). By the middle of the 21st century (2041–2060), an average of 63 ± 16% (±1 SD across climate models and scenarios) of goals were met (Fig. 2). Only 50 ± 18% of goals were met, on average, by the end of this century (2081–2100) under a high greenhouse gas emissions scenario (64 ± 16% under a low emissions scenario). Plans were especially sensitive to species habitat redistribution in the Eastern Bering Sea, Northeast United States, and the Canadian Maritimes (Fig. 2), where plans met less than half of the goals by the end of this century.
Fig. 2. Comparison of present-only plans that only consider current conditions (orange) and “proactive” ocean plans that also consider species redistributions (purple).
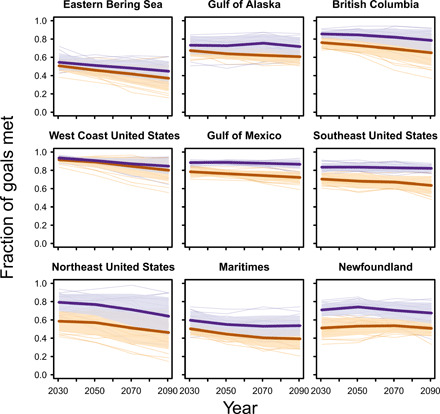
Success is expressed as the fraction of planning goals that are met by each plan at a given time. Thick lines show averages, thin lines show individual projections, and shading shows ±1 SD across the projections from eight global climate models used for testing and two greenhouse gas emissions scenarios (RCP2.6 and RCP8.5).
We contrasted these results with a “proactive” approach that explicitly planned for future species redistributions. The plans were developed to meet the conservation, fishing, and energy goals both under current species habitat distributions and future habitat distributions (see Materials and Methods). The species projections that were used for planning were not used for plan evaluation. Compared to present-only plans, proactive plans were substantially different and changed the zone designation for 22 ± 7% of the area across the nine regions (Fig. 3). However, proactive solutions included only marginally more area (0 to 7% more per region, mean 2 ± 0.07% SE) in conservation, fishing, or energy zones than did present-only plans (Fig. 3). Ocean plans that require less area also leave more space (more opportunities) available for other ocean uses, both including and beyond the three activities we considered. The small increase in area required for the proactive plans implies that there was relatively little opportunity cost of planning for the future. In contrast, some ocean plans have high opportunity costs. An inefficient designation of marine conservation areas in South Australia, for example, has been described as an opportunity cost that may impede the expansion of marine conservation (18).
Fig. 3. Cost and impact on ocean plans that result from planning for future shifts in species distributions, as opposed to planning only for the present ocean state.
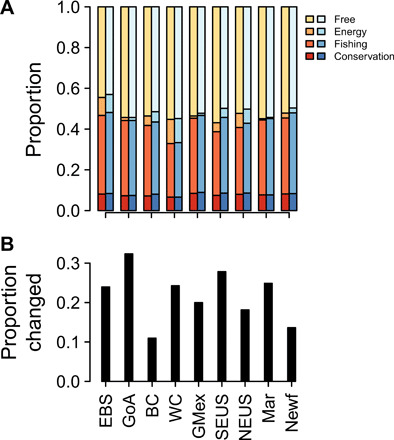
(A) Fraction of each region included in conservation, fishing, or energy zones for proactive plans (blue colors) is only slightly higher than under present-only plans (warm colors). (B) Despite similar total areas, a substantial fraction of planning grids change zones between the two plans. Region abbreviations are defined in Fig. 1.
We then evaluated the proactive plans under 16 sets of redistribution projections (eight climate models across two emissions scenarios) that had not been used in planning. Despite this constraint, the plans met 75 ± 15% (±1 SD) of goals by the middle of the century (Fig. 2). Under a high greenhouse gas emissions scenario, the plans met 64 ± 19% of goals by the end of the century or 76 ± 14% under low emissions scenario (Fig. 2). This was significantly more goals met than the present-only plans [odds ratio: 1.9 (95% confidence interval: 1.86 to 1.97), P = 2 × 10−16, n = 1440, generalized linear mixed-effects model with binomial errors]. Some conservation and fishing goals could not be met by the end of this century even with careful planning because species were expected to be extirpated from a region by then. Proactive plans, however, were also relatively robust to uncertainty in species redistributions across emissions scenarios and global climate models. With a proactive plan, we found a 42% chance of not meeting at least 7 in every 10 planning goals by the end of the 21st century across regions. In contrast, present-only plans had a 72% chance of not meeting at least 7 in every 10 planning goals by the end of this century.
Many of the benefits of proactive planning as compared to present-only plans appeared well before the end of this century (Fig. 2), consistent with substantial spread in species distribution projections under different global climate models in all time windows (19). Planning for long-term species redistribution therefore appears to have the added benefit of hedging against near-term uncertainty.
To more explicitly examine trade-offs, we plotted trade-off curves (6, 20) for ocean plans in terms of their ability to meet conservation (10% of all species’ occurrences) and fishing (50% of fishery species biomass) goals in the present time versus goals at the end of the century. Trade-off curves, also called constraint envelopes or Pareto efficiency frontiers, are visualization tools from microeconomics that represent the maximum extent to which one goal can be met for a given value of another goal, and vice versa, subject to constraints like a limited budget (20). The shape of the curve indicates the type of trade-off between two goals, which, in our case, are goals for the present and for the future (Fig. 4). A plan that designates larger conservation and fishing zones in effect costs more because it restricts ocean uses across a wider area, so we defined the budget in terms of the total area used for the ocean plan. For plotting the trade-off curves, we then limited the plans to only use 75% of the total area that would have been needed to meet all of the ocean plan goals. The curves revealed little to no trade-off between present and future (Fig. 4). In four regions (Gulf of Alaska, West Coast United States, Maritimes, and Newfoundland), right-angle lines on the curves indicated that present and future goals did not interact (no trade-off) and that plans could maximize both future and present goals at the same time. In the other five regions, small, angled corners indicated a minimal trade-off among future and present goals. The largest trade-off was in the Northeast United States, where 9% more future goals could be met in exchange for a 9% decrease in present goals met, or vice versa (Fig. 4). Trade-off curves for plans with areas limited to 50 or 90% of the area needed to meet all goals revealed similarly small trade-offs (fig. S1).
Fig. 4. Curves that delineate the trade-off between the ability to meet planning goals in the present (x axis) or in the future (y axis).
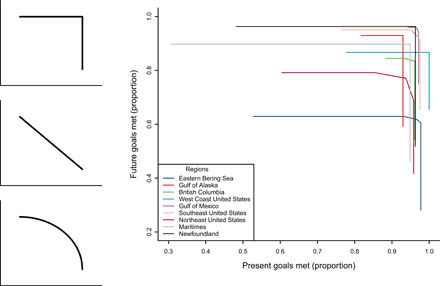
Left-hand images illustrate (from top to bottom) curves that have no trade-off, a direct trade-off, and a concave trade-off (20). The main figure shows curves for the nine regions around North America. Points on each line are generated by weighting present versus future goals to a greater or lesser extent with a limited total plan area (75% of the area needed to meet all present and future goals at once). The ends of each line indicate no attempt to meet one of the goal types. Areas of 50 or 90% are shown in fig. S1.
We also examined the benefits of heuristic planning approaches such as designing management zones in spatial networks, a concept that has been applied to date through networks of protected areas (4, 14). We found that existing marine spatial management areas are expected to experience substantial change in species composition by the end of this century, including the extirpation of 29 ± 7% of existing species and overall 84 ± 2% species dissimilarity (Fig. 5) under a high greenhouse gas emissions scenario (RCP8.5). However, networks of management zones were expected to experience half the loss of species (16 ± 4%) and substantially less species turnover (11 ± 3% dissimilarity), as compared to individual management areas under the RCP8.5 scenario (P = 2 × 10−16, paired Mann-Whitney U test, n = 32; Fig. 5). Each network spanned a range of temperatures, and species often shifted within rather than into or out of a network. Simulated networks revealed that network size and thermal range were both important for minimizing turnover (fig. S2). While corridors are central to conservation on land, stepping-stones of MPAs are important in the ocean because many species disperse as larvae in the water column.
Fig. 5. Average ecological turnover across existing individual management areas or in networks by 2081–2100.
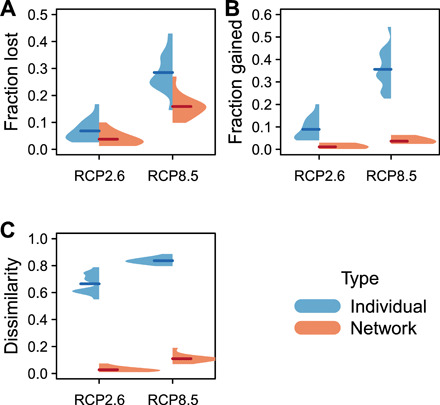
Panels show turnover as (A) fraction of species lost, (B) fraction of species gained, and (C) Sørenson dissimilarity. Beanplots show density distributions across projections from 16 global climate models under a low (RCP2.6) or a high (RCP8.5) emissions scenario. Thick lines show means within each group.
While the reduction of local stressors can delay extirpation of local populations, such measures cannot maintain populations pushed far beyond their thermal tolerances. Instead, updating local conservation and management goals to adapt to change will often be necessary. Our results suggest that explicit consideration of future species distributions, even in static ocean plan designs, can be an effective approach to adapt to shifting species. In particular, our finding that proactive plans require little additional designated area suggests that proactive planning need not involve substantial trade-offs for other ocean users or substantial opportunity costs in terms of additional ocean plan areas, thereby lowering potential barriers to implementation. An additional benefit of planning for long-term shifts in species distributions is that such plans may also be helpful for coping with seasonal, annual, and decadal shifts (21).
Our study tests the value of proactive planning from a biophysical perspective but does not represent all relevant steps or considerations for ocean planning, including stakeholder interactions or adaptive management to learn from experience, to address non–climate-driven changes in ocean biodiversity, or to address changing societal goals, technologies, and ocean uses (2, 13). Our evaluation also considers only three of the many (and growing) human ocean activities (22), although we note that the value of ocean planning often increases as more activities are considered (6). Even with proactive planning, ecological and social surprises are inevitable and will require resilient and adaptive systems informed by ongoing monitoring, evaluation, and anticipation (13, 23, 24). For example, changes in ship traffic, water quality, habitat availability, population abundance, and other factors will also alter species ranges in the future, in addition to climate change impacts. Some of these may be predictable in a way that allows proactive planning similar to what we demonstrate for climate, while others will be surprises for which adaptive management, such as through dynamic ocean management, is the best or only realistic approach. We also note that the species habitat distribution projections that we used capture the major changes in biogeographic patterns that are expected in each region and exhibit good out-of-sample predictive skill (19), but do not reflect evolutionary processes, acclimation, or potential changes in species interactions that may cause species to occupy new thermal conditions or disappear from previously occupied conditions. The projections also do not consider changes in salinity, oxygen, acidification, or primary productivity that may further contract and fragment species geographic ranges (25). Global climate models do not resolve fine-scale oceanographic features that may be important for modulating oceanographic changes in some regions, particularly upwelling regions like the West Coast of the United States (26). However, ensembles of global climate models help to bracket uncertainty in regional climate responses (26).
The ocean is changing rapidly, and warming is expected to continue (27). Climate change mitigation can substantially reduce the impact on ocean ecosystems and human activities, including the probability and magnitude of undesirable outcomes (27). However, major questions also surround how to adapt human activities—from coastal infrastructure to shipping, aquaculture, conservation, fisheries, and other uses—to expected changes over the coming decades. Resistance to proactive adaptation, however, can become the default when the benefits and costs are unclear. Our demonstration that ocean plans are more effective and can require few trade-offs among ocean activities when they consider shifting species distributions is a timely contribution to ongoing adaptation efforts and the transition toward ecosystem-based management. While complete climate-proofing is impossible, proactively planning for long-term ocean change across a wide range of sectors is likely to provide substantial benefits.
MATERIALS AND METHODS
Our overall approach was to simulate the ocean planning process for conservation, fishery, and offshore energy goals and then evaluate these goals against future shifts in species habitat distributions. We conducted planning that only considered species’ current distributions (present-only) and planning that considered species current and future distributions (proactive). The sections below describe the input data (Resource distribution data), the planning and evaluation process (Marine spatial planning), and a comparison of networks of spatial management zones against shifts in species distributions (Analysis of management area networks).
Resource distribution data
Marine spatial planning integrates across sectors, and so, our methods are, by necessity, interdisciplinary. For simulating the marine spatial planning process, we used information on species distributions and on the distribution of wave and wind energy resources. Species habitat distributions were used for conservation goals (species presence or absence) and fishery goals (species biomass), while wave and energy spatial distributions were used for energy goals.
Species habitat distributions
For species habitat distributions, we used an existing set of distribution projections for fish and invertebrates on the continental shelves of North America (19). The species habitat distribution models had been fit to species biomass data from 136,044 sampling events 1963–2015 during scientific surveys in Canada and the United States by considering seasonal bottom and surface temperatures, annual minimum and maximum temperatures, seafloor rugosity, and sediment grain size. Model selection procedures had been used to trim the number of explanatory variables used for each species. The models consisted of two parts: a first part that projected probability of species occurrence and a second part that projected species biomass conditional on presence. The product of the two parts provided projections of biomass (19).
The species distribution models had been projected at a grid size of 0.05° longitude × 0.05° latitude under a low and a high greenhouse gas emissions scenario (RCP2.6 and RCP8.5) using ocean temperature projections from 16 global climate models (19). We randomly selected eight of the climate models, averaged them into ensemble means for present (2007–2020, both RCP2.6 and RCP8.5) and end-of-century (2081–2100, only RCP8.5) time periods, and used the ensemble means for ocean planning (table S1). We set aside projections under the other eight climate models across each of two RCPs for evaluating the ocean plans (table S1).
To match the spatial scale of the projections to the 0.25° ocean planning grid, we averaged probabilities of occurrence (for conservation goals) or biomass (for fishery goals). We then converted probability of occurrence into projections of species presence and absence by applying a species-specific threshold that maximized Cohen’s kappa (28). Kappa measures the extent to which the agreement between observed and projected values is higher than expected by chance alone, considering both omission and commission errors (28).
Wave and wind energy
We used the InVEST (Integrated Valuation of Ecosystem Services and Trade-offs) 3.7.0 toolkit (29) to calculate the spatial distribution of offshore wind and wave power in each region. InVEST is a decision-support tool for ecosystem services that was developed for and is commonly used in marine spatial planning efforts (12, 29), including for wave and wind energy (30–32).
The InVEST Offshore Wind Energy Production tool estimates wind power density from data on wind statistics (a probability density of wind speeds) at each location and then uses wind turbine characteristics (hub height, cut-in wind speed, cut-out wind speed, rated power, rated wind speed, etc.) to calculate the harvestable energy (table S3) (30). We used global wind statistics at 30–arc min spatial resolution that are distributed with InVEST. These statistics were calculated from a global WAVEWATCH III hindcast reanalysis of winds globally for 1999–2012 (30). We did not consider changes in energy resources over the 21st century because the anthropogenic climate change signal appears small relative to natural variability (33).
Harvestable energy was calculated for wind farms composed of 16 turbines of 5.0 MW each. While wind farm designs can vary greatly in size and design (31), we chose a standard design to ensure comparability across different locations (table S3). A size of 16 turbines was chosen to achieve a density of approximately two per square kilometer, following proposals of this magnitude in the United States (6). Turbines were sited in locations 0 to 200 km from shore and 3 to 60 m depth using the ETOPO1 depth dataset (34) and a high-resolution global shoreline dataset (35). We used the default turbine design parameters distributed with InVEST for a 5.0-MW turbine. Last, installation and maintenance costs as well as electricity prices were used to calculate the net present value (NPV) of offshore wind at each location, following (31). The default costs included in InVEST were based on a detailed review of stated project costs from existing offshore wind development (31). Energy prices were set at $0.161/kilowatt-hour to match approximate wholesale energy prices in the United States, as has been used for other wind energy planning calculations (6). Overall, the offshore wind tool outputs a raster map of NPV across the continental shelf. The absolute values of these calculations are not of interest, but rather the relative value of one location compared to another so that areas particularly valuable to energy production can be designated for such uses.
Similarly, the InVEST Wave Energy Production tool estimates potential wave power from data on significant wave height and peak wave period and then calculates harvested wave energy from information on the performance of wave energy conversion devices (32, 36). We used global wave statistics at 30–arc min spatial resolution that are also distributed with InVEST and that had been calculated from a global WAVEWATCH III reanalysis (36). We then calculated harvested energy from wave farms composed of 100 attenuator-type Pelamis wave energy conversion devices (table S3) (36). These devices are in a relatively advanced stage of development (36), and so, they provide a consistent method for comparing wave energy potential across different locations. The number of devices was based on recommended densities in the literature (32). The tool then calculates the NPV of a wave energy conversion facility using information on the price of electricity, discount rate, and costs that had been derived for a wave energy planning project on the West Coast of Vancouver Island (36). Because no commercial-scale wave energy projects currently exist, the economic parameters are uncertain (32, 36). However, the calculations are useful for comparing the relative (not absolute) value of different ocean locations for wave energy capture, which is what we need for this ocean planning exercise.
Calculated NPV values for wind and wave energy were averaged separately within planning grid cells for incorporation into marine spatial planning. We then summed positive NPV values across wind and wave energy for a combined offshore energy NPV for each planning grid cell.
Marine spatial planning
Plan development
Marine spatial planning is a multi-stakeholder, multi-objective process by which areas of the ocean are designated for different uses. Here, we simulated that process by defining three types of planning zones for our North American case study: fishery, conservation, and energy development. Fishery, conservation, and energy zones each had their own planning goals. Our approach implicitly assumed that fishery, conservation, and energy development are mutually exclusive ocean uses, although in reality, not all ocean uses are incompatible (2). Our planning units consisted of 14,588 grid cells at 0.25° latitude × 0.25° longitude resolution across the continental shelves (Fig. 1). We divided these into nine regions (Fig. 1 and table S2), because ocean planning is typically conducted at a regional scale (2).
We set conservation zone goals to protect at least 10% of the occurrences of each species in a region, in line with the CBD’s Aichi Target 11 to protect at least 10% of coastal and marine areas by 2020. We set conservation goals for all species present in at least 5% of the area of each region, which resulted in 29 to 165 conservation goals per region (table S2). We set fishery zone goals to protect at least 50% of the biomass of each of the top 10 fishery species in each region, inspired by simple fishery models that estimate maximum sustainable yield at 50% of unfished biomass. We defined the top fishery species in each region using fishery landings for 1995–2014 by Large Marine Ecosystem (table S4) (37). Large fishery landings not only are a useful indicator of importance to fisheries but also identify species caught incidentally in large quantities, like arrowtooth flounder (Atheresthes stomias). In the energy zone, the goal was to include at least 20% of the total NPV from wind and wave resources in each region. This goal was inspired by the projection that the United States needs 781 GW of offshore wind turbines installed (of 4200 GW potential, i.e., ~20%) as part of a roadmap to 100% clean energy (16, 17).
We simulated two different planning approaches. In the present-only approach, we developed plans that met our goals for the current distributions of marine animals. For consistency with our proactive approach (described next), we used species distributions (occurrence and biomass; see the “Resource distribution data” section) projected onto 2007–2020 temperatures as our current distributions. Occurrence information was used for conservation goals, biomass information was used for fishery goals, and the combined NPV of wind and wave energy was used for meeting energy goals.
In the proactive planning approach, we set goals for both the current (2007–2020) and the end-of-century (2081–2100) species distributions. We used ensemble projections under the RCP8.5 greenhouse gas emissions scenario (a high emissions scenario). The proactive approach doubled the number of goals to be met in the conservation and fishery zones (i.e., both current and future distributions for each species). We kept the energy goals the same because we did not project future wind or wave conditions.
After defining the input data and goals, we then solved the “minimum set problem” of allocating grid cells to conservation, fishery, or energy zones to meet the goals while minimizing the area of each zone. We solved the problem using prioritzr (38) in R v3.5.3 (39) with the Gurobi solver v8.1.1 (40). Prioritizr uses integer linear programming (ILP) techniques to solve spatial planning problems. It is guaranteed to find optimal solutions given sufficient time and supports multiple zones. We specified an efficiency gap of 1% (following the program’s recommendations) and specified a uniform cost of including any planning grid cell in a zone. This choice was equivalent to assuming that the primary concern was minimizing the area included in conservation, fishing, or energy zones.
Plan evaluation
We then evaluated the present-only and the proactive marine spatial plans in each region by testing whether each zoning goal (species representation goals in conservation zones, percent of biomass in fishing zones, and percent of NPV in energy production zones) was met in future time periods as species habitat distributions shifted. We evaluated each of the future climate scenarios in each time period independently against the same single set of goals (i.e., we tested whether each plan met conservation, fishing, and energy goals in a given time period). We considered a wide range of future scenarios in each region by using the 16 projected distributions for each species (i.e., for each of two RCPs in each of the eight global climate models reserved for testing; table S1) for 2021–2040, 2041–2060, 2061–2080, and 2081–2100. This analysis approach allowed us to consider uncertainty in both emissions scenarios and climate models.
We used a generalized linear mixed model with binomial errors to test whether the proactive planning approach met more goals than the present-only approach
The response variable was the proportion of goals met (coded as the number of goals met, numgoalsmet, and the total number of goals, numtotalgoals). The fixed effect was the planning approach (plantype). Random effects were time period (period) nested within climate model (model), nested within RCP (rcp), nested within region (region). We used the lme4 package v1.1-21 in R 3.6.1 to fit the model (39, 41).
Trade-off curves
We also calculated trade-off curves (Pareto efficiency frontiers) (20) between present and future planning goals for conservation and fishing by setting a constrained plan area such that all present and future goals could not be met. We set the constrained area (the “budget”) as 50, 75, or 90% of the total area needed to meet all conservation and fishing goals. The input data were the same as for the proactive planning approach described in the “Plan development” section, although for simplicity, we did not include energy goals. In other words, we used the ensemble mean species occurrence and biomass information for 2007–2020 and 2081–2100 (see the “Species habitat distributions” section).
We then used prioritizr to solve the “fixed budget problem” of meeting as many goals as possible, subject to the constrained area. We ran prioritizr multiple times, each time applying a different set of weights to either future goals or present goals. The weights specified how important it was to meet future versus present goals. Weights for present goals were varied from 0 (no attempt to meet present goals) to 100, while weights for future goals were set as 100 minus the weight assigned to present goals.
Analysis of management area networks
Existing management areas
To evaluate the climate sensitivity of existing marine spatial plans and the value of networks, we examined marine designations within the August 2019 version of the World Database on Protected Areas (WDPA) (42). These are not formal marine spatial plans, but they do represent areas of the ocean that have been set aside for particular purposes. The management areas included in this database have been designated for a wide range of purposes, including fishery management or conservation. The Greater Farallones National Marine Sanctuary in California, for example, regulates construction, discharge, and research activities but does not restrict fishing activities. The Great South Channel Restricted Gillnet Area in the Northeast United States restricts gillnet fishing gear in certain seasons but allows other kinds of fishing. The full set of areas, therefore, provides an example of regions of the ocean set aside for spatial management and helps provide an example of existing (although largely uncoordinated) efforts toward marine spatial planning in North America.
We then compared ecological turnover within individual management areas and within networks of management areas driven by shifting species distributions. Within each management area, we evaluated the fraction of species habitats that were lost from the initial (2007–2020) to final (2081–2100) time period, the fraction gained, and Sørenson’s similarity index between the initial and final species assemblages within each management area. Our input data were the high-resolution distribution projections described in the “Species habitat distributions” section above (0.05° × 0.05° for each global climate model and RCP).
We took a probability-based approach to these calculations to account for potential differences in scale between the projections and the reserves (43). We first calculated the probability (pi,t) of each species i appearing in each management area in time period t, accounting for the fact that a given management area might span portions of multiple species projection grid cells
where pi,t,x was the probability of species i being present in time period t in grid cell x, rx was the fraction of grid cell x contained within the management area, and X was the total number of grid cells overlapping the management area. The logic of this equation combines two ideas. First, the probability of a species being present in an area smaller than a grid cell is equal to the proportion of the grid cell covered by the smaller area (rxpi,t,x) (43). Second, the combined probability of presence across multiple grid cells is the complement of the probability that the species is not present in any of the grid cells.
To test this approach, we compared our calculations against data on whether or not species had been observed in each management area during bottom trawl surveys 2016–2018 [i.e., data that were not used to fit species distribution models by (19)]. The trawl data were downloaded from OceanAdapt version 25 March 2019 (44). We calculated the fraction of management areas that were observed to have a species when it was predicted to be present [positive predictive value (PPV), also called precision] and the fraction of management areas that were not observed to have a species when it was predicted to be absent [negative predictive value (NPV)] (28, 45). We bootstrapped across management areas and species to derive SEs. We found relatively high values of both quantities, with PPV = 0.51 ± 0.007 (±1 SE) and NPV = 0.81 ± 0.009. These values compare favorably to distribution models for phytoplankton (PPV 0.15 to 0.77 and NPV 0.7 to 1) and trees (PPV 0 to 0.6) (45, 46). Most management areas had only four or fewer sampling events (trawl tows) in our dataset (fig. S3A), increasing the chance that some observed absences were, in fact, presences (i.e., false absences). When we trimmed out management areas with few sampling events, PPV increased toward 0.8, although NPV also decreased somewhat (fig. S3B).
We then calculated the probabilistic number of species gained, lost, or shared within individual management areas between the first (2007–2020) and second (2081–2100) time period
where n is the total number of species. From these, we then calculated the fraction of species lost, the fraction of species gained, and the Sørenson dissimilarity index
We then repeated these calculations of turnover statistics (gain, loss, and similarity) for networks of management areas. We defined three networks from the WDPA database: (i) areas in California managed by the California Department of Fish and Game (n = 55), (ii) areas in U.S. state waters east of 100°W (Atlantic Coast, n = 342), and (iii) areas in Alaska state waters (Alaska, n = 35). The California areas are managed together as a network (4), while we defined the other networks as illustrative sets potentially connected through species dispersal or range shifts.
For a statistical test of similarity within networks and within the individual management areas of those networks, we first averaged similarity within each management area or network across the RCPs and climate models. We then conducted a nonparametric Mann-Whitney (Wilcoxon two-sample signed rank) test of the null hypothesis that the two distributions share the same location with wilcox.test() in R 3.6.3 (39).
Simulated networks
Last, we simulated management networks in each region by randomly choosing 0.25° × 0.25° grid cells within each region to designate. The simulated networks were constrained to cover only a designated area (11 levels from 1 to 50% of the grid cells in each region) and to span a limited range of temperatures (11 levels from 1 to 100% of the thermal range in a region). To define the thermal range and to guide site selection, we used the bottom temperature climatology for the North American continental shelf developed by Morley et al. (19). This climatology integrated data from the Simple Ocean Data Assimilation reanalysis product (47). We repeated the process of randomly selecting areas with a network three times at each combination of area and thermal range constraints, for a total of 363 random networks in each of the nine regions. We then evaluated ecological similarity between the beginning and end of the 21st century following the procedure in the “Existing management areas” section.
Supplementary Material
Acknowledgments
We thank the many people at the National Marine Fisheries Service, Department of Fisheries and Oceans and affiliated organizations that maintained the continental shelf survey data used in this project, particularly H. Benoît, B. Brodie, D. Clark, J. Hare, B. Horness, B. Lauth, S. Lucey, and J. Rester. We thank M. Fogarty, R. Griffin, K. Holsman, S. Levin, M. Payne, M. Ruckelshaus, J. Samhouri, J. Sarmiento, R. Seagraves, C. Stock, M. Tingley, M. Watts, and D. Wilcove for helpful conversations and suggestions during our research. We acknowledge the World Climate Research Programme’s Working Group on Coupled Modelling and thank the groups that contributed. Funding: M.L.P. acknowledges support from a David H. Smith Conservation Research Fellowship, an Alfred P. Sloan Research Fellowship (BR2014-044), U.S. NSF OCE-1426891 and DEB-1616821, the Pew Charitable Trusts (no. 28295), the Lenfest Ocean Program (00032755), the Mid-Atlantic Fisheries Management Council, NOAA’s Climate Program Office (OAR-CPO-2014-2004106), NOAA’s Office of Science and Technology (NA14OAR4320158), the Nippon Foundation–University of British Columbia Nereus Program, GreenMAR (Nordforsk no. 61582), and an sDiv synthesis sabbatical fellowship from the German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig (funded by DFG FZT 118) during the course of this research. L.A.R. acknowledges support from the Gordon and Betty Moore Foundation and NOAA’s North Pacific Climate Regimes and Ecosystem Productivity program. T.L.F. acknowledges financial support from the Swiss National Science Foundation (PP00P2_170687) and the European Union’s Horizon 2020 research and innovation programme under grant agreement no. 820989 (project COMFORT, Our common future ocean in the Earth system—quantifying coupled cycles of carbon, oxygen, and nutrients for determining and achieving safe operating spaces with respect to tipping points). J.W.M. was supported by North Carolina Sea Grant (projects: R/18-SFA-2; 2016-R/16-HCE-3) and the North Carolina Policy Collaboratory. This research is contribution EcoFOCI-0871 to NOAA’s Ecosystems and Fisheries-Oceanography Coordinated Investigations. The findings and conclusions in the paper are those of the authors and do not necessarily represent the views of the National Marine Fisheries Service or the Lenfest Ocean Program. Author contributions: Conceptualization: M.L.P. Methodology: M.L.P., L.A.R., and J.W.M. Software: M.L.P., L.A.R., and J.W.M. Formal analysis: M.L.P. Resources: T.L.F. and J.W.M. Data curation: M.L.P. and J.W.M. Writing—original draft: M.L.P. Writing—review and editing: M.L.P., L.A.R., T.L.F., and J.W.M. Visualization: M.L.P. Project administration: M.L.P. Funding acquisition: M.L.P., T.L.F., J.W.M., and L.A.R. Competing interests: M.L.P. serves as an Oceana science advisor and is a visiting research collaborator at Princeton University and a guest researcher at the University of Oslo. The other authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Data and code from our analyses are available at https://doi.org/10.5281/zenodo.3991884. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/50/eabb8428/DC1
REFERENCES AND NOTES
- 1.Crowder L. B., Osherenko G., Young O. R., Airamé S., Norse E. A., Baron N., Day J. C., Douvere F., Ehler C. N., Halpern B. S., Langdon S. J., McLeod K. L., Ogden J. C., Peach R. E., Rosenberg A. A., Wilson J. A., Resolving mismatches in U.S. ocean governance. Science 313, 617–618 (2006). [DOI] [PubMed] [Google Scholar]
- 2.C. N. Ehler, F. Douvere, Marine Spatial Planning: A step-by-step approach toward ecosystem-based management (UNESCO, 2009).
- 3.Halpern B. S., Diamond J., Gaines S., Gelcich S., Gleason M., Jennings S., Lester S., Mace A., McCook L., McLeod K., Napoli N., Rawson K., Rice J., Rosenberg A., Ruckelshaus M., Saier B., Sandifer P., Scholz A., Zivian A., Near-term priorities for the science, policy and practice of Coastal and Marine Spatial Planning (CMSP). Mar. Policy 36, 198–205 (2012). [Google Scholar]
- 4.Ruckelshaus M. H., Klinger T., Knowlton N., DeMaster D. P., Marine ecosystem-based management in practice: Scientific and governance challenges. Bioscience 58, 53–63 (2008). [Google Scholar]
- 5.Lester S. E., McLeod K. L., Tallis H., Ruckelshaus M., Halpern B. S., Levin P. S., Chavez F. P., Pomeroy C., McCay B. J., Costello C., Gaines S. D., Mace A. J., Barth J. A., Fluharty D. L., Parrish J. K., Science in support of ecosystem-based management for the US West Coast and beyond. Biol. Conserv. 143, 576–587 (2010). [Google Scholar]
- 6.White C., Halpern B. S., Kappel C. V., Ecosystem service tradeoff analysis reveals the value of marine spatial planning for multiple ocean uses. Proc. Natl. Acad. Sci. U.S.A. 109, 4696–4701 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frazão Santos C., Agardy T., Andrade F., Barange M., Crowder L. B., Ehler C. N., Orbach M. K., Rosa R., Ocean planning in a changing climate. Nat. Geosci. 9, 730 (2016). [Google Scholar]
- 8.Foley M. M., Halpern B. S., Micheli F., Armsby M. H., Caldwell M. R., Crain C. M., Prahler E., Rohr N., Sivas D., Beck M. W., Carr M. H., Crowder L. B., Duffy J. E., Hacker S. D., McLeod K. L., Palumbi S. R., Peterson C. H., Regan H. M., Ruckelshaus M. H., Sandifer P. A., Steneck R. S., Guiding ecological principles for marine spatial planning. Mar. Policy 34, 955–966 (2010). [Google Scholar]
- 9.Crowder L. B., Norse E. A., Essential ecological insights for marine ecosystem-based management and marine spatial planning. Mar. Policy 32, 772–778 (2008). [Google Scholar]
- 10.Poloczanska E. S., Brown C. J., Sydeman W. J., Kiessling W., Schoeman D. S., Moore P. J., Brander K., Bruno J. F., Buckley L. B., Burrows M. T., Duarte C. M., Halpern B. S., Holding J., Kappel C. V., O’Connor M. I., Pandolfi J. M., Parmesan C., Schwing F., Thompson S. A., Richardson A. J., Global imprint of climate change on marine life. Nat. Clim. Change 3, 919–925 (2013). [Google Scholar]
- 11.Craig R. K., Ocean governance for the 21st century: Making marine zoning climate change adaptable. Harv. Environ. Law Rev. 36, 305–350 (2012). [Google Scholar]
- 12.Gissi E., Fraschetti S., Micheli F., Incorporating change in marine spatial planning: A review. Environ. Sci. Policy 92, 191–200 (2019). [Google Scholar]
- 13.Douvere F., Ehler C. N., The importance of monitoring and evaluation in adaptive maritime spatial planning. J. Coast. Conserv. 15, 305–311 (2011). [Google Scholar]
- 14.McLeod E., Salm R., Green A., Almany J., Designing marine protected area networks to address the impacts of climate change. Front. Ecol. Environ. 7, 362–370 (2009). [Google Scholar]
- 15.Makino A., Yamano H., Beger M., Klein C. J., Yara Y., Possingham H. P., Spatio-temporal marine conservation planning to support high-latitude coral range expansion under climate change. Divers. Distrib. 20, 859–871 (2014). [Google Scholar]
- 16.Jacobson M. Z., Delucchi M. A., Bazouin G., Bauer Z. A. F., Heavey C. C., Fisher E., Morris S. B., Piekutowski D. J. Y., Vencill T. A., Yeskoo T. W., 100% clean and renewable wind, water, and sunlight (WWS) all-sector energy roadmaps for the 50 United States. Energy Environ. Sci. 8, 2093–2117 (2015). [Google Scholar]
- 17.A. Lopez, B. Roberts, D. Heimiller, N. Blair, G. Porro, U.S. renewable energy technical potentials: A GIS-based analysis. Technical Report NREL/TP-6A20–51946 (National Renewable Energy Laboratory, 2012).
- 18.Stewart R. R., Noyce T., Possingham H. P., Opportunity cost of ad hoc marine reserve design decisions: An example from South Australia. Mar. Ecol. Prog. Ser. 253, 25–38 (2003). [Google Scholar]
- 19.Morley J. W., Selden R. L., Latour R. J., Frölicher T. L., Seagraves R. J., Pinsky M. L., Projecting shifts in thermal habitat for 686 species on the North American continental shelf. PLOS ONE 13, e0196127 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lester S. E., Costello C., Halpern B. S., Gaines S. D., White C., Barth J. A., Evaluating tradeoffs among ecosystem services to inform marine spatial planning. Mar. Policy 38, 80–89 (2013). [Google Scholar]
- 21.Tommasi D., Stock C. A., Hobday A. J., Methot R., Kaplan I. C., Eveson J. P., Holsman K., Miller T. J., Gaichas S., Gehlen M., Pershing A., Vecchi G. A., Msadek R., Delworth T., Eakin C. M., Haltuch M. A., Séférian R., Spillman C. M., Hartog J. R., Siedlecki S., Samhouri J. F., Muhling B., Asch R. G., Pinsky M. L., Saba V. S., Kapnick S. B., Gaitan C. F., Rykaczewski R. R., Alexander M. A., Xue Y., Pegion K. V., Lynch P., Payne M. R., Kristiansen T., Lehodey P., Werner F. E., Managing living marine resources in a dynamic environment: The role of seasonal to decadal climate forecasts. Prog. Oceanogr. 152, 15–49 (2017). [Google Scholar]
- 22.McCauley D. J., Pinsky M. L., Palumbi S. R., Estes J. A., Joyce F. H., Warner R. R., Marine defaunation: Animal loss in the global ocean. Science 347, 1255641 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Gunderson L., Resilience, flexibility and adaptive management—Antidotes for spurious certitude? Conserv. Ecol. 3, 7 (1999). [Google Scholar]
- 24.Boyd E., Nykvist B., Borgström S., Stacewicz I. A., Anticipatory governance for social-ecological resilience. Ambio 44, 149–161 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McHenry J., Welch H., Lester S. E., Saba V., Projecting marine species range shifts from only temperature can mask climate vulnerability. Glob. Change Biol. 25, 4208–4221 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Alexander M. A., Shin S.-i., Scott J. D., Curchitser E., Stock C., The response of the Northwest Atlantic Ocean to climate change. J. Climate 33, 405–428 (2019). [Google Scholar]
- 27.Gattuso J.-P., Magnan A., Billé R., Cheung W. W. L., Howes E. L., Joos F., Allemand D., Bopp L., Cooley S. R., Eakin C. M., Hoegh-Guldberg O., Kelly R. P., Pörtner H.-O., Rogers A. D., Baxter J. M., Laffoley D., Osborn D., Rankovic A., Rochette J., Sumaila U. R., Treyer S., Turley C., Contrasting futures for ocean and society from different anthropogenic CO2 emissions scenarios. Science 349, aac4722 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Liu C., White M., Newell G. R., Measuring and comparing the accuracy of species distribution models with presence absence data. Ecography 34, 232–243 (2011). [Google Scholar]
- 29.Guerry A. D., Ruckelshaus M. H., Arkema K. K., Bernhardt J. R., Guannel G., Kim C.-K., Marsik M., Papenfus M., Toft J. E., Verutes G., Wood S. A., Beck M., Chan F., Chan K. M. A., Gelfenbaum G., Gold B. D., Halpern B. S., Labiosa W. B., Lester S. E., Levin P. S., Field M. M., Pinsky M. L., Plummer M., Polasky S., Ruggiero P., Sutherland D. A., Tallis H., Day A., Spencer J., Modeling benefits from nature: Using ecosystem services to inform coastal and marine spatial planning. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 8, 107–121 (2012). [Google Scholar]
- 30.Griffin R., Chaumont N., Denu D., Guerry A., Kim C.-K., Ruckelshaus M., Incorporating the visibility of coastal energy infrastructure into multi-criteria siting decisions. Mar. Policy 62, 218–223 (2015). [Google Scholar]
- 31.Griffin R., Buck B., Krause G., Private incentives for the emergence of co-production of offshore wind energy and mussel aquaculture. Aquaculture 436, 80–89 (2015). [Google Scholar]
- 32.Plummer M. L., Feist B. E., Capturing energy from the motion of the ocean in a crowded sea. Coast. Manag. 44, 464–485 (2016). [Google Scholar]
- 33.Pryor S. C., Barthelmie R. J., Climate change impacts on wind energy: A review. Renew. Sustain. Energy Rev. 14, 430–437 (2010). [Google Scholar]
- 34.C. Amante, B. W. Eakins, ETOPO1 1 arc-minute global relief model: Procedures, data sources, and analysis. NOAA Technical Memorandum NESDIS NGDC-24 (National Geophysical Data Center, NOAA, 2009).
- 35.Wessel P., Smith W. H. F., A global self-consistent, hierarchical, high-resolution shoreline database. J. Geophys. Res. Solid Earth 101, 8741–8743 (1996). [Google Scholar]
- 36.Kim C.-K., Toft J. E., Papenfus M., Verutes G., Guerry A. D., Ruckelshaus M. H., Arkema K. K., Guannel G., Wood S. A., Bernhardt J. R., Tallis H., Plummer M. L., Halpern B. S., Pinsky M. L., Beck M. W., Chan F., Chan K. M. A., Levin P. S., Polasky S., Catching the right wave: Evaluating wave energy resources and potential compatibility with existing marine and coastal uses. PLOS ONE 7, e47598 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D. Pauly, D. Zeller, Sea Around Us (2019); http://seaaroundus.org [accessed 25 October 2019].
- 38.Beyer H. L., Dujardin Y., Watts M. E., Possingham H. P., Solving conservation planning problems with integer linear programming. Ecol. Model. 328, 14–22 (2016). [Google Scholar]
- 39.R Core Team, R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2019); www.R-project.org/.
- 40.Gurobi Optimization LLC, Gurobi optimizer reference manual (Gurobi Optimization, LLC, 2019); www.gurobi.com.
- 41.Bates D., Mächler M., Bolker B., Walker S., Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015). [Google Scholar]
- 42.UNEP-WCMC, World Database on Protected Areas user manual 1.5 (UNEP-WCMC, 2015).
- 43.Alagador D., Martins M. J., Cerdeira J. O., Cabeza M., Araújo M. B., A probability-based approach to match species with reserves when data are at different resolutions. Biol. Conserv. 144, 811–820 (2011). [Google Scholar]
- 44.M. L. Pinsky, M. R. Stuart, OceanAdapt update2019 (Rutgers University, 2019); 10.5281/zenodo.3890214. [DOI]
- 45.Brun P., Kiørboe T., Licandro P., Payne M. R., The predictive skill of species distribution models for plankton in a changing climate. Glob. Change Biol. 22, 3170–3181 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Drake J. M., Randin C., Guisan A., Modelling ecological niches with support vector machines. J. Appl. Ecol. 43, 424–432 (2006). [Google Scholar]
- 47.Carton J. A., Chepurin G. A., Chen L., SODA3: A new ocean climate reanalysis. J. Climate 31, 6967–6983 (2018). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/50/eabb8428/DC1


