Abstract
Population level survival of patients with myelodysplastic syndromes (MDS) treated with hypomethylating agents (HMA) is inferior to clinical trials. Using SEER-Medicare data, we identified 2,086 MDS patients diagnosed in 2004–13, aged ≥66 years at diagnosis, and receiving ≥1 HMA cycle. We used multivariate logistic regression and Cox proportional hazards models to assess the impact of provider experience on persistent HMA therapy and overall survival (OS), respectively.
Median number of HMA cycles was 4 and median OS was 10 months. 32% of patients were treated by providers with ≥1 prior HMA initiation in the last 2 years and were more likely to receive ≥4 cycles of HMA therapy (OR=1.29, 95%-CI: 1.06–1.57; P=.01). No significant association was found between MDS or HMA initiation volume and survival. In conclusion, while HMA initiation volume was associated with persistent HMA treatment, neither MDS nor HMA initiation volumes were associated with OS in older MDS patients.
Keywords: myelodysplastic syndrome, MDS, hypomethylating agent, outcome, SEER, Medicare
Introduction
Survival in the real-world setting for patients with higher risk myelodysplastic syndromes (MDS) treated with hypomethylating agents (HMAs) has been shown to be substantially lower than that observed in clinical trials. The median overall survival (OS) in multiple studies from the United States (US) and Europe was 11 to 16 months compared to 24.4 months in the landmark AZA-001 trial 1,2. The reasons behind this gap remain unclear. Some potential explanations include the selected nature of patients enrolled in clinical trials as well as potentially improved survival when care is provided by physicians experienced in the management of MDS patients 2. As higher risk MDS are rare neoplasms and generally associated with poor survival, it is unlikely that community physicians will have more than a handful of such patients under their care. The clinical use of HMAs is different than traditional chemotherapy. In contrast to conventional chemotherapy, therapeutic response to HMAs is often observed only after the administration of several cycles of therapy and it is necessary to continue therapy despite significant cytopenias 3. Given these unique characteristics, a greater provider experience with administration of HMAs would be expected to lead to optimization of HMA therapy and to improve patient outcomes. We assessed whether the experience of providers, as reflected by prior a) volume of MDS patients; b) number of patients initiated on HMAs; or c) practice in teaching hospitals/cancer centers, is associated with HMA therapy persistence and improved OS of HMA recipients.
Methods
Data source and study sample
In this retrospective cohort study, we used the Surveillance, Epidemiology and End Results (SEER)-Medicare database to identify Medicare beneficiaries diagnosed with MDS in 2004–2013 based on International Classification of Disease for Oncology, 3rd edition code (ICD-O-3: 9980, 9982, 9983, 9985, 9986, 9987, 9989). Inclusion criteria included age ≥66 years at diagnosis, continuous Medicare Parts A and B enrollment from 12 months before diagnosis until death or end of follow-up (12/31/2014), and at least one full HMA cycle, as defined in the following section. Only patients diagnosed with MDS and initiating HMA after 12/31/2005 were analyzed to allow a 2-year lookback to generate the MDS and HMA volume variables. The Yale Human Investigation Committee determined that this study did not directly involve human subjects.
Key outcomes
The key study outcomes were whether OS and persistence of HMA therapy are associated with a provider’s volume of MDS patients and initiation of HMA therapy. OS was measured from HMA initiation until death, with censoring at end of follow-up on 12/31/2014. HMA cycles were identified by claims for 3–10 treatment days within a 28-day period for the first cycle but no requirement for the number of days for the subsequent cycles. Subsequent cycles were distinguished by gaps of 14–90 days without treatment. We stopped counting HMA cycles if the gap was >90 days. HMA cycles were counted and a dichotomous indicator was created for persistent HMA therapy - receiving ≥4 cycles, which has been suggested by the National Comprehensive Cancer Network (NCCN) as the minimum treatment duration for patients treated with before response assessment 4.
Assessment of provider MDS volume and HMA volume
For each MDS patient in our study cohort, we identified the attending physician associated with initiating their HMA therapy and calculated the number of MDS patients that the physician encountered (MDS volume) or initiated on HMA therapy (HMA volume) within 24 months prior to their own HMA initiation. Based on the distribution of volumes and lack of significant differences in impact using multiple alternative cutoffs in sensitivity analyses, we dichotomized MDS volume as <15 or ≥15, while HMA volume was categorized as 0 versus ≥1. With our rolling lookback period, HMA and MDS volumes may vary for individual attending physicians over time as they treat different patients in the analytic cohort. Practice setting at HMA initiation was defined for each patient based on claim type (presence of hospital outpatient claims or outpatient claims associated with visit) and linked information on hospital characteristics as: 1) teaching hospital or cancer center (academic hospital outpatient department [HOPD]), 2) non-teaching hospital (non-academic HOPD), and 3) community-based practice.
Covariates
Our analyses controlled for patient sociodemographic characteristics, health status (comorbidity, disability status, MDS type and severity) and selected other MDS-related therapies. Patient characteristics included age, sex, race/ethnicity, marital status, Metropolitan Statistical Area size, Medicaid dual enrollment, and two linked census tract level measures – median household income and percentage of adults with high school education or less. Health status was measured based on the number of comorbid conditions within 12 months before the first HMA date using the approach developed by Elixhauser et al 5. Relevant diagnosis codes needed to be present in either a single inpatient claim or ≥2 physician or outpatient claims, with at least two that were ≥30 days apart for the comorbidity to be considered present. Disability status, a validated claims-based proxy for performance status, was assessed using claims one year prior to HMA initiation 6. Receipt of red blood cell (RBC) and/or platelet transfusions in the 8 weeks prior to HMA initiation were used as proxy for MDS severity, given that standard disease-specific measures of MDS severity such as cytogenetics and blood/bone marrow blast proportions were not available in our dataset. We measured other treatments such as erythropoietin stimulating agents (ESAs) from 12 months before through the date of HMA initiation, and allogeneic stem cell transplantation (alloSCT) during the entire observation period. All treatments and therapies were identified using the Healthcare Common Procedural Coding System (HCPCS, Supplementary Table 1).
Statistical analyses
We calculated frequency distributions to describe categorical variables, and median and interquartile range (IQR) for continuous variables. We used Chi-square tests to examine the association between HMA duration and level of MDS and HMA volume as well as by practice setting. Kaplan-Meier log-rank tests were used to examine the distribution of OS across volume levels and by practice setting. We estimated multivariable logistic regression models to examine the association between HMA volume, MDS volume and practice setting with persistent HMA therapy (≥4 cycles). Multivariable Cox proportional hazard models were used to examine the association between HMA volume/MDS volume and OS. Both models controlled for practice setting, and patient sociodemographic, health status and other treatment measures. We conducted sensitivity analyses using alternative cutpoints to categorize HMA and MDS volume, as well as testing for interactions between volume and practice setting. We used SAS version 9.4 (SAS Institute, Inc., Cary, NC) to conduct all analyses, with two-sided statistical tests and an alpha of 0.05.
Results
Study cohort
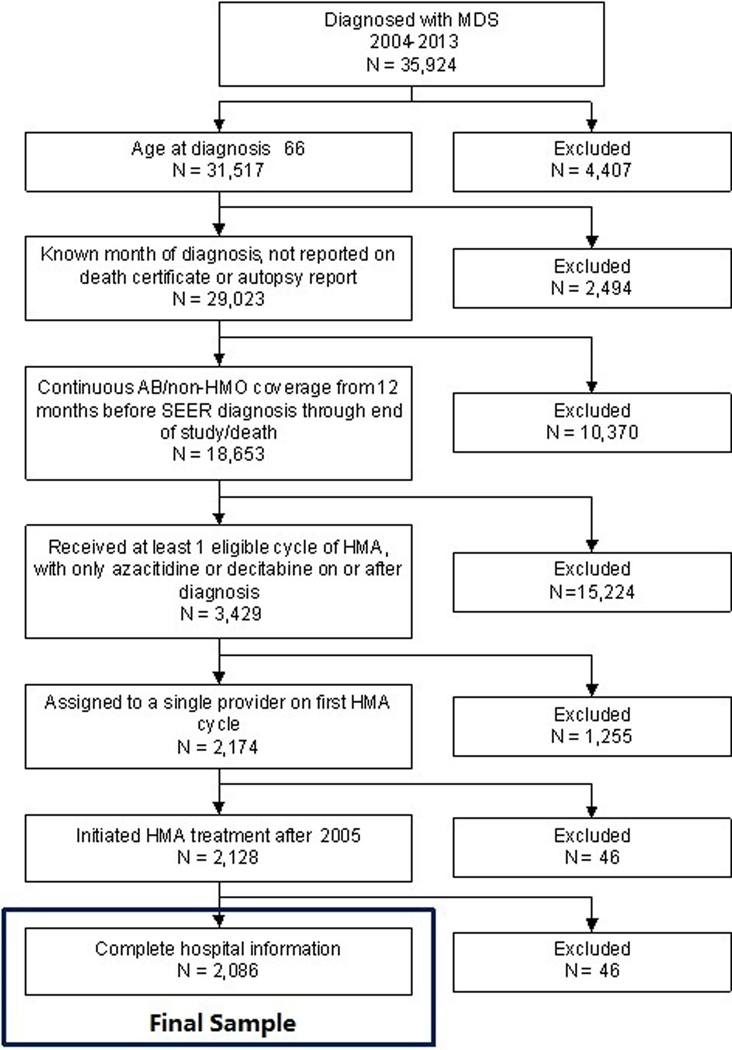
We identified 2,086 eligible MDS patients under the care of 1,142 providers [Figure 1]. The median age at diagnosis was 77 (IQR=72–82) years; 63.1% were male and 90.4% were non-Hispanic white [Table 1].
Figure 1: Selection of the study population.
35,924 patients with MDS were initially identified in the SEER-Medicare database. After excluding patients based on pre-specified exclusion criteria, 2,086 patients were included in the study.
Table 1.
Patient Characteristics (N=2,086)
| N or Median | % or [IQR] | |
|---|---|---|
| Total N | 2086 | 100.0% |
| Months followed from first HMA treatment (median, IQR) | 10 | [4, 19] |
| Months to First HMA (median, IQR) | 3 | [1, 13] |
| Dead by the end of study | ||
| No | 251 | 12.0% |
| Yes | 1835 | 88.0% |
| HMA volume (median, IQR) | 0 | [0, 1] |
| 0 | 1418 | 68.0% |
| 1 or more | 668 | 32.0% |
| MDS volume (median, IQR) | 8 | [5,13] |
| 0–14 | 1653 | 79.2% |
| 15 or more | 433 | 20.8% |
| Practice setting | ||
| Hospital-Not teaching or cancer center | 294 | 13.8% |
| Hospital-Teaching/cancer center | 491 | 23.1% |
| Community providers | 1301 | 61.1% |
| Cycles of HMA (median, IQR) | 4 | [2,8] |
| ≥ 4 cycles | 1196 | 57.3% |
| ≥ 6 cycles | 821 | 39.4% |
| MDS Subtype | ||
| 9980: refractory anemia (RA) | 109 | 5.2% |
| 9982: RA with ringed sideroblasts (RARS) | 124 | 5.9% |
| 9983: RA with excess blasts (RAEB) | 582 | 27.9% |
| 9985: refractory cytopenia with multilineage dysplasia (RCMD) | 165 | 7.9% |
| 9986: MDS associated with 5q deletion | 85 | 4.1% |
| 9987: therapy-related MDS | 20 | 1.0% |
| 9989: MDS, not otherwise specified | 1001 | 48.0% |
| MDS Risk Group | ||
| Lower Risk (RA, RARS, RCMD, 5q) | 483 | 23.2% |
| High Risk (RAEB) | 582 | 27.9% |
| Other (NOS, t-MDS) | 1021 | 48.9% |
| Year of first HMA | ||
| 2006 | 157 | 7.5% |
| 2007–2008 | 421 | 20.2% |
| 2009–2010 | 540 | 25.9% |
| 2011–2012 | 559 | 26.8% |
| 2013–2014 | 409 | 19.6% |
| Age at diagnosis, years (median, IQR) | 77 | [72, 82] |
| 66–69 | 289 | 13.9% |
| 70–74 | 476 | 22.8% |
| 75–79 | 536 | 25.7% |
| 80+ | 785 | 37.6% |
| Sex | ||
| Female | 770 | 36.9% |
| Male | 1316 | 63.1% |
| Race | ||
| White | 1885 | 90.4% |
| Other | 201 | 9.6% |
| Hispanic | ||
| Non-Hispanic | 1985 | 95.2% |
| Hispanic | 101 | 4.8% |
| Marital Status | ||
| Married | 1294 | 62.0% |
| Single/Divorced/Separated/Widowed/Unmarried | 611 | 29.3% |
| Unknown | 181 | 8.7% |
| Elixhauser Comorbidity Index | ||
| None | 298 | 14.3% |
| 1 to 2 | 790 | 37.9% |
| more than 3 | 998 | 47.8% |
| Disability | ||
| Not disabled | 1871 | 89.7% |
| Disabled | 215 | 10.3% |
| Red Blood Cell Transfusion Times Prior HMA (median, IQR) | 1 | [0, 2] |
| 0 | 721 | 34.6% |
| 1–2 | 530 | 25.4% |
| ≥ 3 | 825 | 39.5% |
| Platelet Transfusion Times Prior HMA (median, IQR) | 0 | [0,0] |
| 0 | 1729 | 82.9% |
| 1–2 | 182 | 8.7% |
| ≥ 3 | 175 | 8.4% |
| Percent of adults ≥25 years with ≤ High School education | ||
| <33% (area with low educational attainment) | 558 | 26.7% |
| 33%−66% | 1174 | 56.3% |
| ≥ 66% (area with higher educational attainment) | 231 | 11.1% |
| Income | ||
| <$33,000 | 332 | 15.9% |
| $33,000–40,000 | 310 | 14.9% |
| $40,000–50,000 | 452 | 21.7% |
| ≥ $50,000 | 869 | 41.7% |
| Metropolitan Statistical Area size | ||
| Metro | 1764 | 84.6% |
| Nonmetro | 322 | 15.4% |
| ESA Use pre HMA | ||
| No | 1059 | 50.8% |
| Yes | 1027 | 49.2% |
The median MDS volume for providers in the 2-year lookback period was 8 patients (IQR:5–13). The majority (79.2%) of the patients had their first HMA initiated by a provider who had a MDS volume of 0–14 [Table 1]. Only 32.0% of patients’ providers had one or more HMA initiations in the prior 24 months, and 10.8% of patients were treated by providers who had ≥3 HMA initiations (data not shown). Based on HMA claims from the first HMA cycle, 61.1% of the patients were treated in the community, 23.1% in academic HOPD, and 13.8% in non-academic HOPD. About half of the patients (49.2%) received ESA treatment before HMA initiation, and only 45 patients (2.2%) received alloSCT [Table 1].
HMA therapy persistence
The median number of HMA cycles was 4 (IQR=2–8), with 57.3% and 39.4% of patients receiving ≥4 or ≥6 HMA cycles, respectively. In unadjusted analyses, MDS patients whose provider had more experience in initiating HMA treatment were more likely to receive persistent HMA therapy. More specifically, if the patient’s provider initiated at least one HMA treatment in the prior two years, the patient was more likely to receive ≥ 4 cycles of HMA (P-value<0.01) [Table 2]. We did not observe an association between MDS patient volume or the practice setting and the probability of receiving ≥4 cycles of HMA therapy. In multivariable logistic regression analyses, we found that patients whose providers initiated at least one HMA treatment in the prior two years had 1.29 times greater odds of receiving ≥ 4 HMA cycles (95% Confidence Interval [CI]=1.06–1.57, P-value=0.01) [Table 3]. We did not observe an association between practice setting and odds of receiving ≥4 cycles; we observed a trend associated with higher MDS volume and odds of receiving ≥4 cycles (OR=1.20, 95% CI: 0.96–1.51, P-value=0.11), but it did not reach statistical significance.
Table 2.
Distribution of HMA duration overall and by HMA volume, MDS volume, and practice setting
| HMA cycles |
Chi-square P-value* | |||
|---|---|---|---|---|
| Sample N, row % | Overall 2086 (100%) | < 4 cycles 890 (43%) | ≥ 4 cycles 1196 (57%) | |
| Col % | Row % | Row % | ||
| HMA volume | ||||
| 0 | 1418 (68%) | 45% | 55% | <0.01 |
| 1 or more | 668 (32%) | 38% | 62% | |
| MDS volume | ||||
| 0–14 | 1653 (79%) | 44% | 56% | 0.05 |
| 15–43 | 433 (21%) | 38% | 62% | |
| Practice setting | ||||
| Non-Academic HOPD** | 294 (14%) | 42% | 58% | 0.38 |
| Academic HOPD | 491 (24%) | 45% | 55% | |
| Community/Physician | 1301 (62%) | 42% | 58% | |
Chi-square test of extended HMA cycles by HMA volume, MDS volume and practice setting
HOPD = Hospital outpatient department
Table 3.
Multivariate logistic regression for HMA duration ≥ 4 cycles
| Model with HMA volume |
Model with MDS volume |
|||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| HMA volume (Dichotomous) | .010 | |||
| 0 | Ref | |||
| 1 or more | 1.29 (1.06, 1.57) | |||
| MDS volume (Dichotomous) | .110 | |||
| 0–14 | Ref | |||
| 15 or more | 1.20 (0.96, 1.51) | |||
| Practice setting | .667 | .619 | ||
| Non-Academic HOPD | Ref | Ref | ||
| Academic HOPD | 0.89 (0.65, 1.21) | 0.89 (0.65, 1.21) | ||
| Community | 0.97 (0.74, 1.28) | 0.99 (0.75, 1.30) | ||
| MDS Subtype | .158 | .160 | ||
| 9980: refractory anemia (RA) | Ref | Ref | ||
| 9982: RA with ringed sideroblasts (RARS) | 1.25 (0.72, 2.16) | 1.21 (0.70, 2.10) | ||
| 9983: RA with excess blasts (RAEB) | 0.79 (0.51, 1.22) | 0.79 (0.51, 1.23) | ||
| 9985: refractory cytopenia with multilineage dysplasia (RCMD) | 0.62 (0.37, 1.03) | 0.61 (0.37, 1.02) | ||
| 9986: MDS associated with 5q deletion | 1.00 (0.55, 1.82) | 1.03 (0.56, 1.87) | ||
| 9987: therapy-related MDS | 0.91 (0.34, 2.47) | 0.91 (0.34, 2.47) | ||
| 9989: MDS, not otherwise specified | 0.94 (0.62, 1.43) | 0.94 (0.62, 1.43) | ||
| ESA Use | .676 | .588 | ||
| No | Ref | Ref | ||
| Yes | 0.96 (0.79, 1.17) | 0.95 (0.78, 1.15) | ||
| Year of HMA initiation | .827 | .001 | ||
| 2006 | Ref | Ref | ||
| 2007–2008 | 1.86 (1.27, 2.73) | 1.89 (1.29, 2.77) | ||
| 2009–2010 | 1.68 (1.15, 2.45) | 1.71 (1.17, 2.49) | ||
| 2011–2012 | 1.90 (1.30, 2.77) | 1.92 (1.32, 2.80) | ||
| 2013–2014 | 1.33 (0.89, 1.98) | 1.33 (0.90, 1.99) | ||
| Months to first HMA treatment | 1.00 (0.99, 1.01) | .827 | 1.00 (0.99, 1.01) | .927 |
| Age Group | .021 | .028 | ||
| 66–69 | Ref | Ref | ||
| 70–74 | 0.98 (0.72, 1.33) | 0.97 (0.72, 1.33) | ||
| 75–79 | 1.04 (0.77, 1.42) | 1.04 (0.77, 1.41) | ||
| 80+ | 0.75 (0.56, 1.00) | 0.76 (0.57, 1.01) | ||
| Sex | .605 | .611 | ||
| Male | Ref | Ref | ||
| Female | 0.95 (0.78, 1.16) | 0.95 (0.78, 1.16) | ||
| Race | .106 | .100 | ||
| White | Ref | Ref | ||
| Other | 0.78 (0.57, 1.06) | 0.78 (0.57, 1.05) | ||
| Hispanic | .570 | .430 | ||
| non-Hispanic | Ref | Ref | ||
| Hispanic | 0.85 (0.56, 1.30) | 0.85 (0.56, 1.28) | ||
| Marital Status | .337 | .309 | ||
| Married | Ref | Ref | ||
| Single/Divorced/Separate/Widowed/Unmarried | 0.89 (0.72, 1.10) | 0.88 (0.71, 1.09) | ||
| Unknown | 0.82 (0.59, 1.14) | 0.82 (0.59, 1.14) | ||
| Elixhauser Comorbidity Index (Pre HMA) | .063 | .043 | ||
| None | Ref | Ref | ||
| 1 to 2 | 1.05 (0.79, 1.40) | 1.04 (0.79, 1.38) | ||
| more than 3 | 0.82 (0.62, 1.09) | 0.81 (0.61, 1.08) | ||
| Disability | .055 | .056 | ||
| Not disabled | Ref | Ref | ||
| Disabled | 0.74 (0.55, 1.01) | 0.75 (0.55, 1.01) | ||
| Red Blood Cell Transfusion Group | .016 | .014 | ||
| 0 | Ref | Ref | ||
| 1–2 | 0.97 (0.76, 1.23) | 0.97 (0.76, 1.23) | ||
| ≥ 3 | 0.74 (0.60, 0.93) | 0.74 (0.60, 0.93) | ||
| Platelet Transfusion Group | .001 | .001 | ||
| 0 | Ref | Ref | ||
| 1–2 | 0.85 (0.62, 1.17) | 0.85 (0.62, 1.17) | ||
| ≥ 3 | 0.53 (0.38, 0.73) | 0.53 (0.38, 0.73) | ||
| Percent of adults ≥25 years of age in zip code with ≤ High School education | .681 | .685 | ||
| <33% | Ref | Ref | ||
| 33%−66% | 0.94 (0.74, 1.20) | 0.96 (0.75, 1.22) | ||
| ≥ 66% | 1.14 (0.74, 1.75) | 1.16 (0.76, 1.79) | ||
| Income | .554 | .541 | ||
| <$33,000 | Ref | Ref | ||
| $33,000–40,000 | 1.2 (0.84, 1.72) | 1.20 (0.84, 1.71) | ||
| $40,000–50,000 | 1.15 (0.8, 1.65) | 1.14 (0.80, 1.64) | ||
| ≥ $50,000 | 1.3 (0.89, 1.88) | 1.29 (0.89, 1.88) | ||
| Metropolitan Statistical Area size | .069 | .080 | ||
| Metro | Ref | Ref | ||
| Non-metro | 1.31 (0.98, 1.76) | 1.30 (0.97, 1.74) | ||
Increasing comorbidity and poor disability status prior to HMA initiation were associated with lower odds of persistent HMA, although with borderline significance. Patients who were more heavily RBC or platelet transfusion dependent were less likely to receive persistent HMA therapy [Table 3].
Overall survival
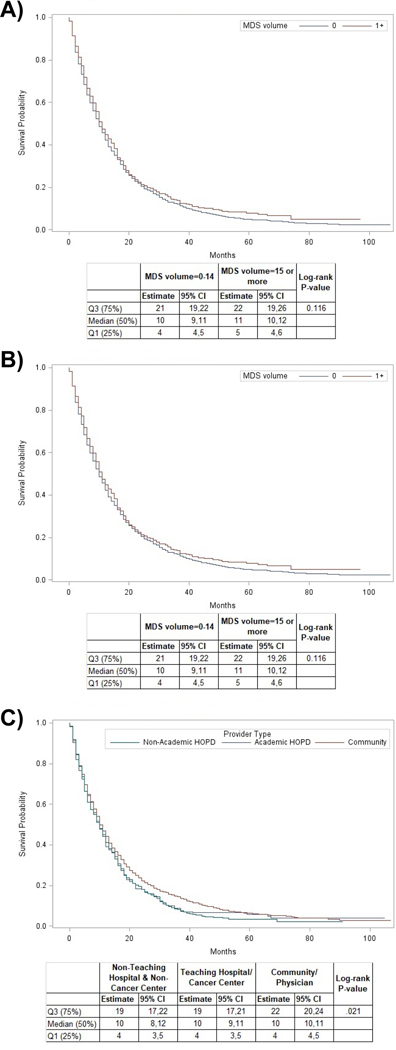
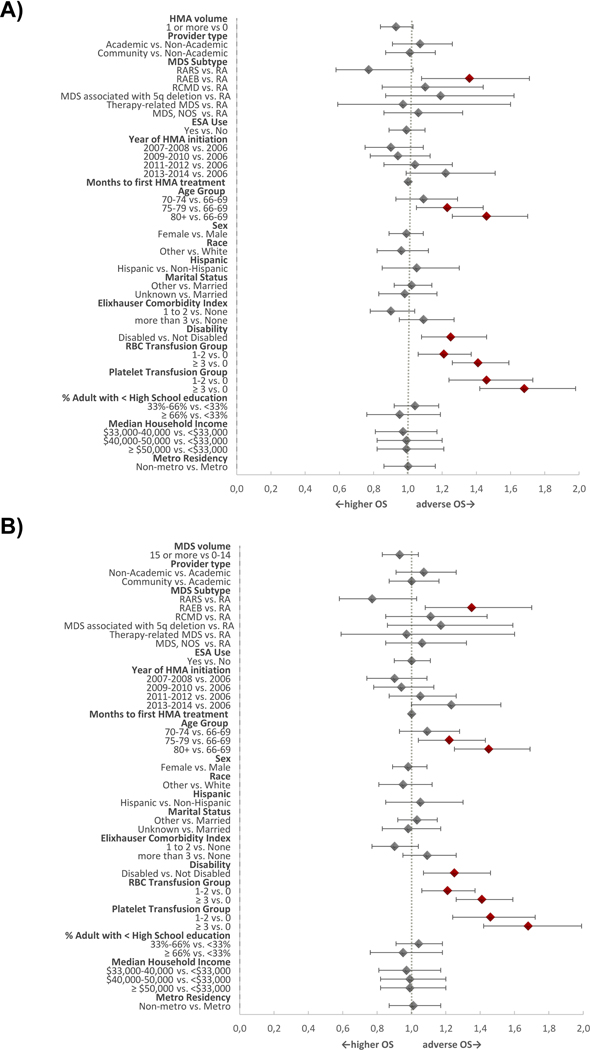
Median OS for the entire cohort from time of HMA initiation was 10 months (95% CI: 10–11 months, IQR: 4–21 months); two-year OS was 17.7%. In unadjusted analyses, we did not find differences in OS based on HMA initiation volume or MDS volume [Figure 2]. In contrast, practice setting in the community was associated with increased overall survival [Figure 2]. In adjusted analyses, we did not find significant associations between HMA volume (HR=0.93, 95% CI: 0.84–1.03, P-value=0.18), MDS volume (HR=0.93, 95% CI: 0.83–1.04, P-value=0.20), or practice setting with OS. As expected, patients with a higher pre-treatment RBC and platelet transfusion burden and patients with greater levels of disability had worse OS [Figure 3]. Patients receiving ≥4 cycles of HMA therapy had a statistically significantly better median OS and rate of 1-year survival compared to patients receiving <4 cycles (median OS: 16 months (95% CI: 15–17 months) vs 4 months (95% CI: 3–4 months); 1-year OS probability 61.9% vs 16%; p<0.01, respectively).
Figure 2: Kaplan Meier Survival Curves by HMA volume, MDS volume, and provider type.
(A) illustrates the survival of patients based on the volume of HMA initiation by the treating provider. In our study population there was no statistically significant difference in overall survival among patients treated by providers with 0 or ≥1 HMA initiations in the previous 24 months. (B) shows the survival of patients based on the volume of MDS patients treated by the provider. In our study population there was no statistically significant difference in overall survival among patients treated by providers with a higher volume of MDS patients compared to providers with a lower number of MDS patients. (C) illustrates the impact of practice setting on survival. Interestingly, patients treated by providers in the community had a better overall survival than patients treated at a hospital-based practice (both academic and non-academic). This difference reached statistical significance (log-rank test; p = 0.021)
Figure 3. Forest Plot for Overall Survival with HMA volume and MDS volume as primary predictors.
(A) illustrates adjusted analyses for OS with HMA volume as the primary predictor. Patients with a higher RBC and platelet transfusion burden as well as a greater level of disability had an adverse overall survival. (B) depicts adjusted analyses for OS with MDS volume as the primary predictor. Patients with a higher RBC and platelet transfusion burden as well as a greater level of disability had an adverse overall survival.
Abbreviation: RA= refractory anemia; RARS=RA with ringed sideroblasts; RAEB=RA with excess blasts; RCMD=refractory cytopenia with multilineage dysplasia.
Marital Status “Other” includes Single, Divorced, Separate, Widowed, and Unmarried.
Discussion
In this large population-based analysis of older adults with MDS, we found that care managed by more experienced HMA providers was associated with a higher likelihood of receiving persistent HMA therapy. In other words, patients whose providers initiated at least one HMA treatment in the prior two years had 29% increased odds of receiving ≥4 HMA cycles. However, more provider experience did not translate into improved survival in adjusted analysis.
The approval of HMAs for treatment of MDS has resulted in a major change in the management landscape of the disease. The landmark randomized phase 3 trial AZA-001 reported a median OS of 24.4 months with azacitidine among patients with higher risk MDS, which was significantly longer than the 15-month median OS observed with conventional care regimens (HR=0.58, P<0.0001) 1. However, several population-based analyses from Europe and the US reported median OS ranging from 11 to 17 months with HMA therapy 2,7,8. For example, the MDS Clinical Research Consortium study, which enrolled 632 higher risk MDS patients who received HMAs reported a median number of 5 administered cycles of HMAs, with 72% of patients receiving ≥4 cycles. The median OS for the overall cohort was 17 (95% CI: 15.8–18.4) months 7. In another report from the Spanish MDS Registry of patients with higher risk MDS patients, the median OS for 251 patients treated with azacitidine was 13.4 (95% CI: 11.8–16) months 8.
The differences in clinical outcomes between registry/population-level analyses and randomized trials are generally attributed to multiple factors. The unselected nature of patients in population-level analyses compared with highly selected individuals with better performance status and organ function enrolled in clinical trials could be one reason to explain the discrepancy between outcomes in real life setting and AZA-001. However, no published prospective study to date has replicated the median OS seen in AZA-001 2. Indeed, multiple subsequent clinical trials with azacitidine monotherapy arms reported a median OS of less than 20 months including the recently published North American Intergroup study which reported a median OS of 15 months for such patients 9–12. Therefore, better patient selection is unlikely to fully explain this difference.
Another factor that could explain this observed difference is the experience of providers who care for MDS patients. As MDS are rare and associated with poor survival, most community physicians will not have more than a handful of patients under their care at any time. The kinetics of response to HMA therapy differs from traditional chemotherapies and the NCCN recommends that a minimum of 4–6 cycles of HMA therapy should be completed before therapy is abandoned due to lack of response 4,13. Furthermore, continuation of HMA therapy despite significant cytopenias, might be challenging and uncomfortable for providers inexperienced with HMA use 13. However, previous studies have shown that the median time to first response was 2–3 cycles and up to 12 cycles can be required to obtain the optimal response 14,15. Additionally, discontinuation of therapy after maximal response is obtained and “therapy breaks”, which are both often practiced in solid oncology care, are not advised for patients experiencing a response to HMA therapy as loss of response usually occurs quickly and often cannot be reestablished with re-initiation of HMA therapy 16. Lastly, the 7-consecutive day administration of azacitidine was the only regimen associated with survival advantage for higher risk MDS. However, due to logistical reasons associated with weekend administration, the 7-day schedule is rarely used outside of academic centers 17. Providers in academic centers may initiate HMA therapy more often than community providers; they are therefore more likely to be aware of and adhere to recommended practices when using HMAs.
Therefore, it has been suggested that care by providers in the community setting who discontinue HMAs prematurely might be one of the reasons of worse clinical outcomes observed with HMAs in real-life analyses 2,18. In the current analysis, we indeed show that patients treated by experienced providers are more likely to persist on HMA therapy. However, the survival outcomes were independent of provider characteristics and were inferior compared to the AZA-001 results. The median OS observed in our study population, which was not limited to any MDS risk-category subgroup, was 10 months which is slightly lower but overall in line with other studies in the field that reported median OS of 11–18 months for patients with higher-risk MDS who are the primary candidates for therapy with HMAs 8,9,12,19.
While our study has important implications, some limitations exist. First, our volume measure was restricted to MDS patients and HMA initiations in the Medicare fee-for-service population and therefore should be viewed as a relative, rather than an absolute measure of provider MDS experience. Medicare is the largest purchaser of cancer care in the US and more than 80% of MDS patients are diagnosed after the Medicare-eligible age of 65 years. However, our findings might not apply to the minority of younger MDS patients or to practice patterns in Medicare Advantage plans. Secondly, reasons for HMA discontinuation and the appropriateness of discontinuation cannot be assessed in claims and could not be considered in the analysis. We were therefore unable to assess whether HMAs were discontinued due to progression to AML or lack of response. Thirdly, patients treated by higher volume providers or those in academic settings may have more severe disease, which may affect OS. The analysis adjusted for disease severity using measures of transfusion receipt and hospitalizations for infection and bleeding. However, there are differences in the baseline severity measures that are commonly used in the International Prognostic Scoring System (IPSS) and its revised version (IPSS-R), such as blast count, level of cytopenias, and cytogenetic information that are not included in the SEER-Medicare database and therefore might not be captured with the MDS classification or the severity measures we constructed. We instead used measures of RBC transfusion dependence, which has been repeatedly shown to correlate with poor survival in MDS and hospitalization for bleeding and infections which reflect functional consequences of severe thrombocytopenia and neutropenia as a measure of disease severity.20–25 Furthermore, the claims codes for MDS-RAEB (refractory anemia with excess blasts), which reflects ≥5% blasts in the bone marrow, was part of our severity measurements and has been strongly associated with poor survival and adverse outcomes in multiple studies.25,26
Additionally, we could not capture changes in severity over time, hence the estimated associations between volume and OS may be biased 27. Importantly, due to sample size consideration our analyses included all MDS patients and was not restricted to patients with higher risk disease who have the strongest evidence for OS advantage with HMA therapy.
Another limitation to note is that SEER-Medicare is not reporting additional clinical data on MDS risk stratification which might explain the high rate of MDS-NOS in our study and poses a risk for misclassification bias. This is an especially important limitation when comparing outcomes in well-characterized clinical trial patients, that often use risk stratification tools such as the International Prognostic Scoring System (IPSS) as inclusion criterion, with the heterogenous patient population in large claims-based datasets that lacks this clinically relevant information. Although we used transfusion frequency as a surrogate for MDS severity, we were unable to assess MDS disease risk for our patients. Finally, the provider of record and their experience is assigned at baseline but does not take into account possible changes in providers over time, for example, transfer of care from the community to an academic center, or even a consultation with an academic provider that may influence care and outcomes provided in the community. These limitations may have generated measurement error in the provider volume measures, which could bias estimates towards the null. Hence, our estimates should be considered to be at the lower bound.
Our findings further suggest that the median OS of 24.4 months associated with azacitidine use in the AZA-001 trial reflects a significant overperformance of azacitidine in the trial for yet unexplained reasons rather than an underperformance in the community setting due to suboptimal/inappropriate use. These observations argue against the discordance between the impressive overall survival observed in clinical trials and that supported by real-life data being largely related to the experience gap among health providers. Further research into the underlying reasons for this gap is warranted to improve patient outcomes at the population setting.
Supplementary Material
Acknowledgements:
Amer Zeidan is a Leukemia and Lymphoma Society Scholar in Clinical Research and is also supported by a NCI’s Cancer Clinical Investigator Team Leadership Award (CCITLA). Research reported in this publication was in part supported by the National Cancer Institute of the National Institutes of Health under Award Number P30 CA016359. This research was also partly funded by the Dennis Cooper Hematology Young Investigator Award (AZ). The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute (NCI)’s Surveillance, Epidemiology and End Results (SEER) Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement #U55/CCR921930–02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare and Medicaid Services; Information Management Services, Inc.; and the SEER Program tumor registries in the creation of the SEER-Medicare database. The interpretation and reporting of the SEER-Medicare data are the sole responsibility of the authors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Presentation: This work was presented in part in an oral session at the American Society of Hematology meeting in San Diego, California, December 2018.
A.M.Z. received research funding (institutional) from Celgene, Acceleron, Abbvie, Otsuka, Pfizer, Medimmune/AstraZeneca, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, and ADC Therapeutics. A.M.Z had a consultancy with and received honoraria from AbbVie, Otsuka, Pfizer, Celgene, Jazz, Ariad, Incyte, Agios, Boehringer-Ingelheim, Novartis, Acceleron, Astellas, Daiichi Sankyo, Cardinal Health, Seattle Genetics, BeyondSpring, Trovagene, and Takeda. A.M.Z received travel support for meetings from Pfizer, Novartis, and Trovagene. None of these relationships were related to the development of this manuscript. NAP received research funding (institutional) from Boehringer Ingelheim, Astellas Pharma, Daiichi Sankyo, Sunesis Pharmaceuticals, Celator, Pfizer, Astex Pharmaceuticals, CTI BioPharma, Genentech, LAM Therapeutics and Samus Therapeutics. NAP received research funding from Celgene. NAP had a consultancy with and received honoraria from Agios, Alexion and Pfizer. SFH received research funding (institutional) from Celgene, TG Therapuetics, DTRM, Genentech. SFH reports personal fees from Celgene, personal fees from Pharmacyclics, personal fees from Genentech, personal fees from Bayer, outside the submitted work; S.D.G. has consulted for and receives research funding from Celgene. AJD reports grants from Celgene during the conduct of the study; personal fees and other from Abbvie, grants from Boehringer-Ingelheim, grants from Pharmaceutical Research and Manufacturers of America Foundation outside of the submitted work. XM received research funding from Celgene Corp, which supported the development of this manuscript, and consulted for Celgene and Incyte.
Footnotes
Data sharing: SEER-Medicare data cannot be shared by the authors as directed by the SEER-Medicare data use agreement. Data may be requested directly from the National Cancer Institute. However, we are open to sharing our methodology and analytical approaches upon request.
Declaration of conflicts of interest:The other authors have no conflicts of interest to declare.
References:
- 1.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10(3):223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeidan AM, Stahl M, Sekeres MA, Steensma DP, Komrokji RS, Gore SD. A call for action: Increasing enrollment of untreated patients with higher-risk myelodysplastic syndromes in first-line clinical trials. Cancer. 2017;123(19):3662–3672. [DOI] [PubMed] [Google Scholar]
- 3.Zeidan AM, Stahl M, Komrokji R. Emerging biological therapies for the treatment of myelodysplastic syndromes. Expert Opin Emerg Drugs. 2016;21(3):283–300. [DOI] [PubMed] [Google Scholar]
- 4.Network NCC. NCCN Guidelines Version 2.2019: Myelodysplastic syndromes. Vol. 2018; 2019. [Google Scholar]
- 5.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. [DOI] [PubMed] [Google Scholar]
- 6.Davidoff AJ, Gardner LD, Zuckerman IH, Hendrick F, Ke X, Edelman MJ. Validation of disability status, a claims-based measure of functional status for cancer treatment and outcomes studies. Med Care. 2014;52(6):500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeidan AM, Sekeres MA, Garcia-Manero G, et al. Comparison of risk stratification tools in predicting outcomes of patients with higher-risk myelodysplastic syndromes treated with azanucleosides. Leukemia. 2016;30(3):649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernal T, Martinez-Camblor P, Sanchez-Garcia J, et al. Effectiveness of azacitidine in unselected high-risk myelodysplastic syndromes: results from the Spanish registry. Leukemia. 2015;29(9):1875–1881. [DOI] [PubMed] [Google Scholar]
- 9.Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20(10):2429–2440. [DOI] [PubMed] [Google Scholar]
- 10.Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106(8):1794–1803. [DOI] [PubMed] [Google Scholar]
- 11.Lubbert M, Suciu S, Baila L, et al. Low-dose decitabine versus best supportive care in elderly patients with intermediate- or high-risk myelodysplastic syndrome (MDS) ineligible for intensive chemotherapy: final results of the randomized phase III study of the European Organisation for Research and Treatment of Cancer Leukemia Group and the German MDS Study Group. J Clin Oncol. 2011;29(15):1987–1996. [DOI] [PubMed] [Google Scholar]
- 12.Sekeres MA, Othus M, List AF, et al. Randomized Phase II Study of Azacitidine Alone or in Combination With Lenalidomide or With Vorinostat in Higher-Risk Myelodysplastic Syndromes and Chronic Myelomonocytic Leukemia: North American Intergroup Study SWOG S1117. J Clin Oncol. 2017;35(24):2745–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Derissen EJ, Beijnen JH, Schellens JH. Concise drug review: azacitidine and decitabine. Oncologist. 2013;18(5):619–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silverman LR, McKenzie DR, Peterson BL, et al. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: studies 8421, 8921, and 9221 by the Cancer and Leukemia Group B. J Clin Oncol. 2006;24(24):3895–3903. [DOI] [PubMed] [Google Scholar]
- 15.Silverman LR, Fenaux P, Mufti GJ, et al. Continued azacitidine therapy beyond time of first response improves quality of response in patients with higher-risk myelodysplastic syndromes. Cancer. 2011;117(12):2697–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santini V, Prebet T, Fenaux P, et al. Minimizing risk of hypomethylating agent failure in patients with higher-risk MDS and practical management recommendations. Leuk Res. 2014;38(12):1381–1391. [DOI] [PubMed] [Google Scholar]
- 17.Grinblatt DL, Sekeres MA, Komrokji RS, Swern AS, Sullivan KA, Narang M. Patients with myelodysplastic syndromes treated with azacitidine in clinical practice: the AVIDA registry. Leuk Lymphoma. 2015;56(4):887–895. [DOI] [PubMed] [Google Scholar]
- 18.Steensma DP, Komrokji RS, Stone RM, et al. Disparity in perceptions of disease characteristics, treatment effectiveness, and factors influencing treatment adherence between physicians and patients with myelodysplastic syndromes. Cancer. 2014;120(11):1670–1676. [DOI] [PubMed] [Google Scholar]
- 19.Zeidan AM, Lee JW, Prebet T, et al. Comparison of the prognostic utility of the revised International Prognostic Scoring System and the French Prognostic Scoring System in azacitidine-treated patients with myelodysplastic syndromes. Br J Haematol. 2014;166(3):352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wehmeyer J, Zaiss M, Losem C, et al. Impact of performance status and transfusion dependency on outcome of patients with myelodysplastic syndrome, acute myeloid leukemia and chronic myelomonocytic leukemia treated with azacitidine (PIAZA study). Eur J Haematol. 2018;101(6):766–773. [DOI] [PubMed] [Google Scholar]
- 21.Kantarjian H, O’Brien S, Ravandi F, et al. Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original International Prognostic Scoring System. Cancer. 2008;113(6):1351–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malcovati L, Porta MG, Pascutto C, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol. 2005;23(30):7594–7603. [DOI] [PubMed] [Google Scholar]
- 23.Fletcher SA, Cronin AM, Zeidan AM, et al. Intensity of end-of-life care for patients with myelodysplastic syndromes: Findings from a large national database. Cancer. 2016;122(8):1209–1215. [DOI] [PubMed] [Google Scholar]
- 24.Bryan J, Jabbour E, Prescott H, Kantarjian H. Thrombocytopenia in patients with myelodysplastic syndromes. Semin Hematol. 2010;47(3):274–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malcovati L, Germing U, Kuendgen A, et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol. 2007;25(23):3503–3510. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120(12):2454–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenberg PL, Stone RM, Al-Kali A, et al. Myelodysplastic Syndromes, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15(1):60–87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.