Abstract
Background
E-cigarette or vaping product use–associated lung injury (EVALI) is a complex inflammatory syndrome predominantly seen in adolescents and young adults. The clinical and laboratory profile can easily mimic infectious and noninfectious conditions. The exclusion of these conditions is essential to establish the diagnosis. Recently, the novel coronavirus disease 2019 (COVID-19) pandemic introduced the multisystem inflammatory syndrome in children (MIS-C). MIS-C knowledge is evolving. The current criteria to establish the diagnosis are not specific and have overlapping features with EVALI, making the accurate diagnosis a clinical challenge during continued COVID-19 transmission within the community.
Case Report
Three young adults evaluated at our emergency department for prolonged fever and gastrointestinal and respiratory symptoms were initially assessed for possible MIS-C due to epidemiologic links to COVID-19 and were eventually diagnosed with EVALI. The clinical, laboratory, and radiologic characteristics of both entities are explored, as well as the appropriate medical management.
Why Should an Emergency Physician Be Aware of This?
Physician awareness of overlapping and differentiating EVALI and MIS-C features is essential to direct appropriate diagnostic evaluation and medical management of adolescents and young adults presenting with systemic inflammatory response during the unfolding pandemic of COVID-19.
Keywords: E-cigarette or vaping product use-associated lung injury, novel coronavirus disease 2019, multisystem inflammatory syndrome in children, severe acute respiratory syndrome coronavirus 2
Introduction
In mid-2019, the United States experienced a surge in e-cigarette or vaping product use–associated lung injury (EVALI) hospitalizations or deaths. As of February 18, 2020, more than 2800 cases from 50 states were reported to the Centers for Disease Control and Prevention, half of which were in patients 13–24 years old (1). By March 2020, novel coronavirus disease 2019 (COVID-19) had spread within the United States, with the initial epicenter of disease in New York City. During the next few months, the COVID-19–associated multisystem inflammatory syndrome in children (MIS-C) emerged as a new condition (2). Reports from Europe described the initial wave of cases presenting with clinical findings reflecting a spectrum of inflammatory effects on multiple organ systems in children (3,4). Recently, the COVID-19–associated MIS in adults extended the definition of MIS-C to adults older than 21 years (5).
The overlap of symptomatology and laboratory findings constitutes a diagnostic challenge to frontline pediatricians, especially during continued COVID-19 community transmission. Our knowledge of both conditions is evolving. Early recognition is imperative to direct appropriate management. We report on 3 patients evaluated for possible MIS-C and subsequently diagnosed with EVALI.
Case Reports
Case 1
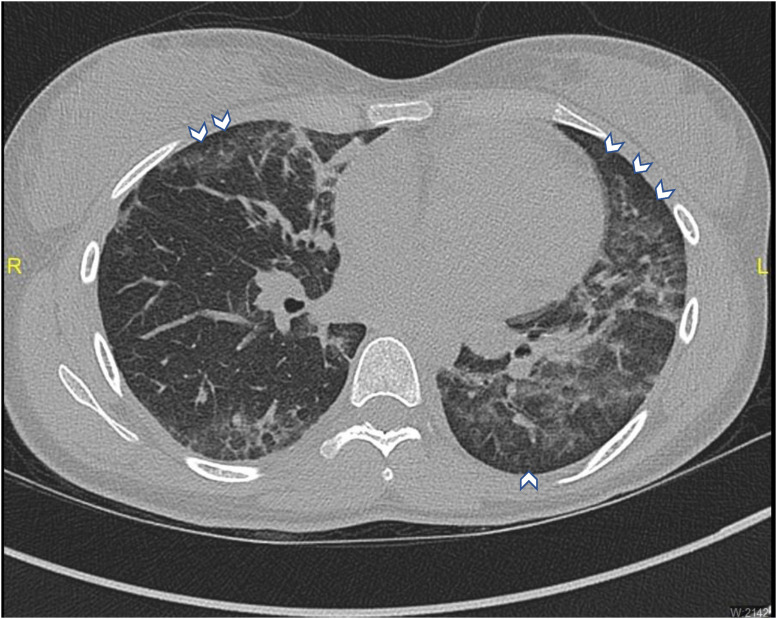
A healthy 19-year-old woman presented to the pediatric emergency department (PED) reporting 1 week of abdominal pain, vomiting, and diarrhea, with 3 days of fevers up to 38.3°C (Table 1 ). She had been taking trimethoprim and sulfamethoxazole for 3 days after an urgent care visit for a presumed urinary tract infection. She denied dysuria, cough, shortness of breath, rash, sore throat, or ear pain. Her boyfriend tested positive for COVID-19 approximately 2 weeks prior. Her physical examination revealed fever, tachycardia, tachypnea, and diffuse abdominal tenderness, worse in both upper quadrants, but without guarding or rebound. A workup for MIS-C was initiated. Her laboratory results were significant for ketonuria, elevated white blood cell count (WBC), and neutrophilia. Inflammatory markers (C-reactive protein and erythrocyte sedimentation rate), D-dimer, and fibrinogen were elevated. COVID-19 polymerase chain reaction (PCR) and serology were both negative. An abdominal sonogram was unremarkable. The decision was made to admit her for further evaluation and intravenous fluid hydration. On admission, a history of vaping tetrahydrocannabinol (THC) was elicited. On the first night of admission, the patient had an episode of shortness of breath with transient oxygen desaturation to 92% after a vomiting episode. A chest x-ray (CXR) study revealed a mild left perihilar opacity. Given her vaping history, a chest computed tomography (CT) scan was performed, which showed bilateral ground-glass opacities, with consolidation in the left lower lobe (Figure 1 ). The patient was started on methylprednisolone, ceftriaxone, and azithromycin. The patient was discharged 3 days after admission with a complete tapering course of prednisone and azithromycin. Follow-up with the pediatric pulmonology clinic confirmed the rapid resolution of symptoms and improvement in inflammatory markers. Outpatient pulmonary function testing revealed a mild lung restriction with moderate diffusion limitation, with improvement after 2 months.
Table 1.
Clinical, Laboratory, and Pulmonary Function Test Characteristics of Patients With E-Cigarette or Vaping–Associated Lung Injury
| Characteristic | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| Age (years) | 19 | 19 | 21 |
| Sex | Female | Male | Male |
| Ethnicity | Hispanic | White | Hispanic |
| Coexisting conditions | None | None | None |
| Social history | Vaping synthetic THC | Vaping synthetic THC | Vaping synthetic THC |
| COVID-19 sick contact | Yes | No | Yes |
| Symptoms at presentation | |||
| Constitutional | Fever | Lightheadedness, dizziness | Fever, body aches |
| Gastrointestinal | Diarrhea, vomiting, epigastric abdominal pain | Dry heaving, nausea, abdominal pain | Abdominal pain, nausea, throat pain |
| Respiratory | None | Shortness of breath | Cough |
| Vital signs at ED triage | |||
| Temperature (°C) | 38.6 | 38.9 | 39.5 |
| Heart rate (beats/min) | 123 | 116 | 123 |
| Respiratory rate (breaths/min) | 28 | 30 | 26 |
| Pulse oximetry (%) | 96 | 92 | 94 |
| Blood pressure (mm Hg) | 124/80 | 139/83 | 114/73 |
| Laboratory (normal range) | |||
| WBC (4.8–10.8 K/μL) | 18.20 | 15.20 | 12.7 |
| Neutrophils (45–75%), % | 89 | 87.5 | 86 |
| Lymphocytes (20–50%), % | 5 | 8.60 | 9.7 |
| ESR (0–20 mm) | 94 | > 130 | > 130 |
| CRP (0.03–0.49 mg/dL) | 22 | 28 | 40 |
| D-dimer (0–229 ng/mL) | 1831 | NA | 167 |
| Procalcitonin (>0.5 ng/mL) | 0.39 | 0.2 | 0.61 |
| Fibrinogen (250-490 mg/dL) | 1152 | NA | 1350 |
| Lactate dehydrogenase (135–225 U/L) | 362 | 434 | 402 |
| NT-proBNP (0–125 pg/mL) | 5 | 7 | < 5 |
| Ferritin (30–400 ng/mL) | 65 | 451 | 549 |
| Troponin T (0.01–0.03 ng/mL) | < 0.01 | < 0.01 | < 0.01 |
| Total bilirubin (0–.2 mg/dL) | 0.2 | 2.4 | 0.4 |
| SARS-CoV-2 RT-PCR | Negative | Negative | Negative |
| SARS-CoV-2 antibody | Negative | NA | Negative |
| Respiratory viral panel | Negative | NA | Negative |
| Gastrointestinal viral panel | Negative | NA | Negative |
| Cultures | |||
| Blood | Negative | Negative | Negative |
| Urine | Negative | Negative | Negative |
| Pulmonary function testing (% predicted) | |||
| FEV1 (L) | 2.66 (94) | NA | 3.94 (105) |
| TLC (L) | 2.99 (69) | NA | 5.50 (94) |
| DLCO (mL/min × mm Hg) | 11.71 (46) | NA | 17.5 (57) |
COVID-19 = coronavirus disease 2019; CRP = C-reactive protein; DLCO = diffusing capacity of the lungs carbon monoxide; ED = emergency department; ESR = erythrocyte sedimentation rate; FEV1 = forced expiratory volume in 1 s; NA = not applicable; NT-proBNP = N-terminal pro-B-type natriuretic peptide; RT-PCR = real-time polymerase chain reaction; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; THC = tetrahydrocannabinol; TLC = total lung capacity; WBC = white blood cell.
Figure 1.
Chest computed tomography scan revealing bilateral ground-glass opacities with subpleural sparing (white arrowheads), and consolidation in the left lower lobe.
Case 2
A 19-year-old man with a history of asthma presented with 1 week of nausea, lightheadedness, and anorexia. He had a mild sore throat and cough a few days prior, which resolved spontaneously. He denied vomiting, diarrhea, abdominal pain, shortness of breath, fever, or COVID-19 exposure. He admitted vaping both nicotine and THC oils. Clinically, the patient was febrile, tachycardic, tachypneic, and hypoxic (Table 1). He had a mildly erythematous posterior oropharynx and dry mucous membranes. Chest auscultation revealed a bilateral decrease in breath sounds without accessory muscle use. In addition, the patient experienced tenderness and guarding in the right lower abdomen.
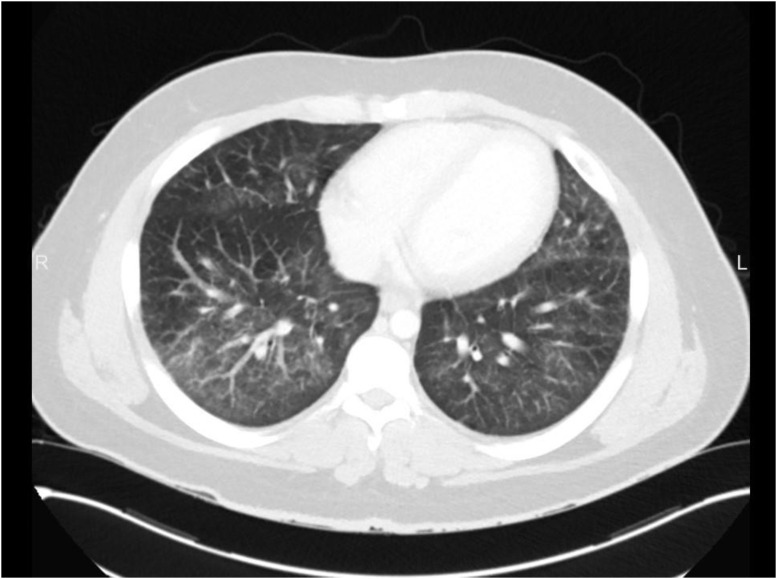
The differential diagnosis at that time included EVALI, pneumonia, appendicitis, and MIS-C. Laboratory results were significant for elevated WBC, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP). His COVID-19 PCR was negative. A rapid Group A streptococcus test was positive. CXR study revealed mildly prominent interstitial lung markings, worse on the right. A contrast-enhanced CT of the abdomen and pelvis was performed to evaluate for appendicitis. The lung bases on this scan had bilateral ground-glass opacities with reticulation and interlobular septal thickening, most prominently at the right lower lobe and left lingula (Figure 2 ). The appendix was normal. While in the PED, the patient became hypoxic to 88% and remained tachypneic; he was given 4 L of O2 via nasal cannula. He was started on ceftriaxone, methylprednisolone, and azithromycin and was admitted to the inpatient floor. He was weaned to room air within 3 days of hospitalization, after which he signed out against medical advice with a complete course of azithromycin and prednisone. This patient did not follow-up after leaving the hospital.
Figure 2.
Contrast computer tomography of abdomen and pelvis revealed bilateral ground-glass opacities of lung basis with reticulation and interlobular septal thickening, most prominently at the right lower lobe and left lingula.
Case 3
A healthy 21-year-old man presented with 1 week of fevers up to 38.8°C, with associated body aches, nausea, throat pain, and epigastric abdominal pain radiating to the chest. He had a sick contact with COVID-19 two weeks before the presentation and had a negative COVID-19 PCR swab 1 week prior. He denied cough, shortness of breath, vomiting, diarrhea, or rash. He initially denied any use of tobacco, alcohol, or drugs. On physical examination, he was febrile, tachycardic, and tachypneic (Table 1). He had some mild posterior pharyngeal erythema and mild epigastric tenderness to palpation. Due to the prolonged fevers and recent sick contact with COVID-19, an evaluation for MIS-C was initiated. Laboratory results were significant for a slightly elevated WBC and markedly elevated CRP, ESR, and fibrinogen. COVID-19 PCR and serology were negative. Contrast-enhanced CT of the abdomen and pelvis was obtained to evaluate for possible intra-abdominal abscess and showed mild mosaic attenuation at the lung bases, suggestive of possible airway inflammation. Urine toxicology was positive for cannabinoids and, at that time, the patient admitted to vaping THC oils. A CT of the lungs showed bilateral subtle scattered ground-glass opacities. This patient was hospitalized to manage EVALI with methylprednisolone, ceftriaxone, and azithromycin. The patient was discharged 2 days after to complete a course of azithromycin and prednisone. Follow-up with the pediatric pulmonology clinic confirmed the resolution of symptoms and improvement in inflammatory markers within 10 days. Outpatient pulmonary function testing revealed only a mild diffusion limitation.
Discussion
The human inflammatory response can be triggered by multiple factors (e.g., infection, trauma, malignancy, environmental exposures). Although this response is a valuable defense mechanism, hyperinflammation can lead to multiorgan dysfunction or shock. Lung tissue damage from infections or chemical exposures can trigger a localized hyperinflammation (6,7). This local hyperinflammation can set off cytokine overproduction (cytokine storm), leading to systemic inflammatory response syndrome. Multiple organ systems can be affected by systemic hyperinflammation, and once a specific set of characteristic features are measured clinically, the term multisystem inflammatory syndrome is applied.
During the COVID-19 pandemic, an inflammatory syndrome was recognized that affects multiple organ systems in children. Health organizations worldwide evaluated the initial reports to create sensitive criteria based on clinical presentations and laboratory evaluation to establish the diagnosis (Table 2 ) (8,9). Six months prior, the United States experienced the EVALI outbreak. Studies suggested that e-cigarette or vaping products containing vitamin E acetate, usually from informal sources (e.g., family, friends, or drug dealers), can trigger lung injury and systemic inflammatory response (10,11). Patients with EVALI usually experience fever, cough, shortness of breath, nausea, vomiting, diarrhea, or abdominal pain. Hypoxia, tachypnea, or tachycardia are also common (Table 3 ). Patients with MIS-C also present with similar symptoms. Ninety percent of MIS-C patients experience gastrointestinal symptoms and > 60% experience respiratory system abnormalities (cough, shortness of breath, pneumonia, or acute respiratory distress syndrome), which could indicate a mixed illness of acute COVID-19 and MIS-C (12). The similarities of the clinical picture between COVID-19 and EVALI and the emergence of MIS-C with overlapping clinical features can make a clinical diagnosis challenging (13).
Table 2.
Clinical Characteristics of Multisystem Inflammatory Syndrome in Children: All Six Criteria Must Be Met
| Characteristic | Variable |
|---|---|
| Age | < 21 years |
| Fever | Documented fever > 38.0°C (100.4°F) for ≥ 24 h or subjective fever lasting ≥ 24 h |
| Laboratory evidence of inflammation | Elevated C-reactive protein |
| Elevated erythrocyte sedimentation rate | |
| Elevated fibrinogen | |
| Elevated procalcitonin | |
| Elevated D-dimer | |
| Elevated ferritin | |
| Elevated lactate dehydrogenase | |
| Elevated interleukin 6 | |
| Elevated neutrophil count | |
| Reduced lymphocyte count | |
| Low albumin level | |
| Multisystem involvement | Involvement of 2 or more of the following organ systems: |
| Cardiovascular: elevated cardiac enzymes, abnormal echocardiogram | |
| Respiratory: pneumonia, pulmonary embolism, ARDS | |
| Renal: renal failure | |
| Neurologic: seizures, aseptic meningitis, stroke | |
| Hematologic: coagulopathy | |
| Gastrointestinal: abdominal pain, vomiting, diarrhea, elevated liver enzymes | |
| Dermatologic: erythroderma, mucositis, any other rash. | |
| Lack of alternative diagnosis | |
| Recent or current SARS-CoV-2 infection or exposure | Any of the following findings: |
| Positive SARS-CoV-2 RT-PCR | |
| Positive serology | |
| Positive antigen test | |
| COVID-19 exposure within 4 weeks prior to onset of symptoms |
ARDS = acute respiratory distress syndrome; COVID-19 = coronavirus disease 2019; RT-PCR = real-time polymerase chain reaction; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Table 3.
Clinical Characteristics of E-Cigarette or Vaping–Associated Lung Injury
| Characteristic | Data |
|---|---|
| Age (years), median (range) | 22 (13–71) |
| Behavioral | Recent use (within past 90 days) of e-cigarettes or vaping products |
| Substances used: Δ-9-tetrahydrocannabinol, cannabis, nicotine | |
| Multiple psychosocial stressors | |
| Symptoms | Constitutional: fever, chills, malaise, weight loss |
| Respiratory: cough, chest pain, shortness of breath, dyspnea on exertion | |
| Gastrointestinal: abdominal pain, nausea, vomiting, diarrhea | |
| Vital signs | Oxygen saturation < 95% while breathing room air |
| Tachycardia (heart rate > 100 beats/min) | |
| Tachypnea (respiratory rate > 20 breaths/min) | |
| Diagnostic results | CXR or CT scans: bilateral diffuse ground-glass opacities |
| Elevated C-reactive protein | |
| Elevated erythrocyte sedimentation rate | |
| Positive urine drug screen for Δ-9-tetrahydrocannabinol or its metabolites | |
| Exclusion of viral or bacterial etiology |
CT = computed tomography; CXR = chest x-ray.
Laboratory test results can complicate the picture further. EVALI and MIS-C can present with elevated WBC counts or neutrophilia. Inflammatory markers (Table 4 ) like CRP and ESR are usually elevated. Furthermore, our patients had elevations in other inflammatory markers (i.e., fibrinogen, ferritin, lactate dehydrogenase, and D-dimer) not commonly used to evaluate for EVALI.
Table 4.
Common Laboratory Markers of Inflammation
| Marker |
|---|
| C-reactive protein |
| Erythrocyte sedimentation rate |
| Fibrinogen |
| Procalcitonin |
| D-dimer |
| Ferritin |
| Lactic acid dehydrogenase |
| Interleukin 6 |
Obtaining a social history of vaping or e-cigarettes use can be critical to consider EVALI in the initial clinical evaluation (14). In 2 of our patients, EVALI was not considered in the initial differential as the exposure history was obtained later in the course. Other discriminatory features between the two conditions include cardiovascular involvement, as 86% of MIS-C patients experience a form of cardiac injury (e.g., shock, abnormal blood levels of troponin, or pro-brain natriuretic peptide) (9). Our patients had normal cardiac enzymes, electrocardiography, and unremarkable cardiac imaging. Patients with MIS-C usually test positive on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) real-time PCR or serology (SARS-CoV-2 IgG antibody). None of the patients in this series experienced such results. Two patients in this report (patients 1 and 3) had an epidemiologic link to a confirmed COVID-19 household member. This link would increase the suspicion for MIS-C if SARS-CoV-2 serology and PCR tests were not available. Evaluation for systemic infections is essential, and establishing diagnoses of both conditions depends on excluding other mimicking infectious conditions.
Chest imaging can be a useful tool for differentiating between the two illnesses (15). CXR study can be unremarkable or with patchy ground-glass opacities in COVID-19. In comparison, EVALI CXR study might show bilateral symmetrical multifocal ground-glass opacities or consolidation with subpleural sparing. Furthermore, a chest CT scan can accurately detect involvement of subpleural lung spaces (COVID-19) or sparing (EVALI). A case discussion with the radiologist is imperative to help differentiate between the two conditions.
It is critical to make the diagnosis of EVALI early on in order to guide management. The current evidence supports the initiation of systemic corticosteroids to treat EVALI. Clinicians should also consider antimicrobial treatment due to overlapping clinical presentations. The treatment for MIS-C is supportive, with the initiation of intravenous immunoglobulins. Other experimental treatments include systemic glucocorticoids or interleukin inhibitors. Systemic broad-spectrum antimicrobials to treat infections with a similar presentation and anticoagulation for patients with elevated D-dimer or fibrinogen should also be considered. Intravenous fluids or vasopressors can be instrumental in the treatment of MIS-C cardiovascular dysfunction or shock.
Frontline emergency physicians need to stay current with the evolving knowledge of these entities. The overlapping clinical picture can lead to delays in diagnosis and management plans. Appropriate history (e-cigarette use or vaping), physical examination, laboratory, and radiologic interpretation with the absence of any evidence of COVID-19 infection can be the blueprints for EVALI diagnosis in the era of MIS-C.
Why Should an Emergency Physician Be Aware of This?
The COVID-19 pandemic is still unfolding. Emergency physician's awareness of the overlapping and differentiating features between EVALI and MIS-C is essential for diagnostic and treatment plan formulation. The COVID-19 epidemiologic link or abnormal inflammatory markers can cloud the initial evaluation for patients with EVALI. History of vaping, SARS-CoV-2 PCR, SARS-CoV-2 serology, and subpleural sparing or involvement on chest imaging might be the road to diagnose EVALI during this unprecedented pandemic of COVID-19.
References
- 1.Centers for Disease Control and Prevention Outbreak of lung injury associated with the use of e-cigarette, or vaping, products. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html#epi-chart Available at: Updated February 25, 2020.
- 2.Feldstein L.R., Rose E.B., Horwitz S.M. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–336. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verdoni L., Mazza A., Gervasoni A. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morris S.B., Schwartz N.G., Patel P. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection—United Kingdom and United States, March-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1450–1456. doi: 10.15585/mmwr.mm6940e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., Katze M.G. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christiani D.C. Vaping-induced acute lung injury. N Engl J Med. 2020;382:960–962. doi: 10.1056/NEJMe1912032. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19) https://emergency.cdc.gov/han/2020/han00432.asp Available at: Published May 14, 2020.
- 9.World Health Organization Multisystem inflammatory syndrome in children and adolescents with COVID-19. https://www.who.int/publications/i/item/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 Available at: Published May 15, 2020.
- 10.Blount B.C., Karwowski M.P., Shields P.G. Lung Injury Response Laboratory Working Group. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382:697–705. doi: 10.1056/NEJMoa1916433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegel D.A., Jatlaoui T.C., Koumans E.H. Update: interim guidance for health care providers evaluating and caring for patients with suspected e-cigarette, or vaping, product use associated lung injury–United States, October 2019. MMWR Morb Mortal Wkly Rep. 2019;68(41):919–927. doi: 10.15585/mmwr.mm6841e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godfred-Cato S., Bryant B., Leung J. COVID-19-associated multisystem inflammatory syndrome in children–United States, March–July 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1074–1080. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson K.R., Villafranco N., Cameron L.H. A 16-year-old boy with cough and fever in the era of COVID-19 [published online ahead of print August 12, 2020] Pediatrics. 2020 doi: 10.1542/peds.2020-008235. [DOI] [PubMed] [Google Scholar]
- 14.Armatas C., Heinzerling A., Wilken J.A. Notes from the field: e-cigarette, or vaping, product use-associated lung injury cases during the COVID-19 response–California, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:801–802. doi: 10.15585/mmwr.mm6925a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foust A.M., Winant A.J., Chu W.C., Das K.M., Phillips G.S., Lee E.Y. Pediatric SARS, H1N1, MERS, EVALI, and now coronavirus disease (COVID-19) pneumonia: what radiologists need to know. AJR Am J Roentgenol. 2020;215:736–744. doi: 10.2214/AJR.20.23267. [DOI] [PubMed] [Google Scholar]