Abstract
AIM
To compare the computed tomography (CT) features of Sars-CoV-2 pneumonia between the two sexes and among different age groups.
MATERIALS AND METHODS
Consecutive patients (n=331) who presented to the emergency department and underwent chest CT and reverse transcription polymerase chain reaction (RT-PCR) with a time interval <7 days, which were subsequently found to be consistent with Sars-CoV-2 infection, were enrolled retrospectively. Two experienced radiologists evaluated the images in consensus, recording the number of pulmonary lobes with ground-glass opacities and with consolidation. A CT score was subsequently calculated based on the percentage involvement of each lobe. Clinical symptoms, comorbidities, and level of required hospitalisation were noted. In-hospital mortality was recorded and analysed via the Kaplan–Meier estimator.
RESULTS
Males and females had the same age distribution. No statistically significant difference was found in the analysed CT features and in the CT score (p=0.31) between the sexes. More females were affected by two or more comorbidities (17.1% versus 7.5%, p=0.024), all comorbidities except diabetes were more prevalent in females. Women had a higher probability to be discharged home and a lower probability to be admitted to the intensive care unit (ICU; p=0.008), in-hospital mortality was inferior (13.5% versus 22%).
CONCLUSION
Despite more comorbidities, women had lower hospital admission and mortality, which was independent of CT findings between both sexes.
Highlights
-
•
No differences in CT imaging existed in COVID-19 affected males and females.
-
•
More females were affected by 2 or more comorbidities (17.1% vs. 7.5%, p=0.024).
-
•
All comorbidities but diabetes were more prevalent in the female sex.
-
•
Women had a higher probability to be discharged home (p=0.008).
-
•
Females had a lower probability to be admitted in ICU (p=0.008).
Introduction
Coronavirus disease 2019 (COVID-19) is a multisystemic disease caused by a new strain of coronavirus identified on 9 January 2020 called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 , 2 The clinical expression of the disease involves prevalently the respiratory system, with symptoms that range from flu-like to respiratory failure and diffuse alveolar damage.3 There has been a high rate of hospital and intensive care unit (ICU) admissions among COVID 19 patients in all the nations affected by the pandemic.4 , 5
The syndrome seems to be more prevalent in the male sex. A first observational and multicentric study performed in China6 showed that the prevalence is 0.31/100,000 in males versus 0.27/100,000 in females. In a recent report,7 females comprised 39.7% of 5700 patients hospitalised in the New York City area. The authors reported that 14.1% of the patients required treatment in an ICU, but only 33.5% of them were female. In a late account8 of 1099 COVID-19 patients in 552 Chinese hospitals, 58% of the affected were men. On the other hand, other two studies that included Sars-CoV-2-positive patients regardless of the severity of the disease found no significant differences between the sexes9 , 10
In Italy, data originating from 30 April onwards from the Istituto Superiore di Sanità reveal that the lethality of the disease was always higher in males regardless of the considered age group (16.6% in men versus 9.1% in women). Additionally, the prevalence of the illness was higher in males in all age groups except those >90 years.11
Eventually, a large study conducted on 8910 patients12 demonstrated that 60% of the affected were male and that non-survivors were more likely to be male (75.2% versus 34.8%). Additionally, non-survivors were older and more affected by comorbidities such as diabetes mellitus, hyperlipidaemia, coronary artery disease and heart failure.
A large percentage of the adult patients clinically suspected of COVID-19 infection presenting to the emergency department (ED) during the period of data collection underwent computed tomography (CT). During that challenging and unprecedented scenario, with limited available resources, the clinicians considered CT as a useful tool to assess patients rapidly, in particular to decide whether to isolate them.
The purpose of the present single-centre retrospective analysis of patients with a confirmed diagnosis of COVID-19 obtained with a positive real-time reverse-transcriptase-polymerase chain reaction (RT-PCR), was to compare the severity of the infection between the two sexes and among different age groups, assessing the possible differences in the CT features and in the level of hospitalisation required and mortality rate. The presence of comorbidities was also taken into account.
Materials and methods
Patient population and study design
The present study was approved by the internal review board of the institution (ref. no. CE 122/20).
A cohort of 331 consecutive patients was analysed from 4 March to 30 April. Inclusions criteria were suspected Sars-CoV-2 infection (either fever >37.5°, cough or dyspnoea); unenhanced chest CT in the ED suggestive of Sars-CoV-2 lung infection; one or two RT-PCR tests positive for Sars-CoV-2 RNA with a time interval from the chest CT no longer than 7 days. Exclusion criteria were severe motion artefacts on the CT images; CT not suggestive of Sars-CoV-2 lung infection; time interval of >7 days between chest CT and an RT-PCR test or unknown RT-PCR date; RT-PCR test negative for Sars-Cov-2 RNA.
Of note, the included patients presented autonomously to the ED during various stages of the disease. The time of onset of the clinical signs, although significant, was therefore not available for analysis.
Personal data
For each patient, personal information such as sex, date of birth, RT-PCR test date, and chest CT date were recorded. Enrolled patients were assigned an identification number (ID), which was subsequently used to identify the study members and guarantee anonymity and protection of sensitive data.
CT acquisition technique and image analysis
All chest CT were performed during a single full inspiratory breath-hold in the supine position on a 128-slice CT (Philips Ingenuity Core, Philips Healthcare, Netherlands). The imaging parameters were 120 kV tube voltage; 226 mAs tube current modulation; 1.08 spiral pitch factor; 0.625 collimation width, 512 (mediastinal window) and 768 (lung window) matrix. All images were reconstructed with a section thickness of 1 mm.
DICOM data were transferred to the picture archiving and communication system (PACS) of the institution and then analysed on a workstation equipped with two 35 × 43 cm monitors produced by Eizo, with a 2048 × 1536 matrix.
Two radiologists both with >5 years of thoracic imaging experience, evaluated the images in consensus, without knowing the RT-PCR results. The patients' CT images were defined as either COVID-19 positive or negative based on the STR/ACR/RSNA consensus statement13; in particular, images with “typical” or “indeterminate” characteristics were considered positive, while scans with “atypical” or “negative” features were considered negative.
The following CT aspects were recorded following the current literature, in particular the article written by Chung et al. 14: (a) number of lobes with ground-glass opacities (GGOs); (b) number of lobes with consolidation; (c) presence of crazy paving; (d) presence of reversed halo sign; (e) bilateral distribution; (f) lymphadenopathy, defined as at least one lymph node with short axis >10 mm; and (g) pleural or or (h) pericardial effusion.
The disease involvement in each lobe was evaluated in consensus by the two radiologists using a visual assessment scale on the model of the paper written by Moradi et al. 15 They assigned zero points if the lobe was free of disease, one point if the lobe involvement was <5%, two points if the involvement ranged between 5–24%, three points for 25–49%, four points for 50–74%, and five if the CT alteration occupied >75% of the lung parenchyma in the examined lobe. A total CT score was then calculated adding up the points of the five pulmonary lobes, which consequently ranged from 0 to 25.
Clinical data
Clinical symptoms on the day of admission to the ED, such as fever, dyspnoea, cough, and diarrhoea were noted. Significant comorbidities were recorded for each patient. In particular, the presence or absence of coronary artery disease, hypertension, diabetes mellitus, chronic obstructive pulmonary disease (COPD), non-COPD lung disease, neurological disorders, neoplastic diseases, chronic kidney disease (CKD), hepatic failure, and rheumatoid arthritis was noted. During statistical analysis, comorbidities had been categorised into three groups: (1) no comorbidities, (2) 1–2 comorbidities, (3) >2 comorbidities.
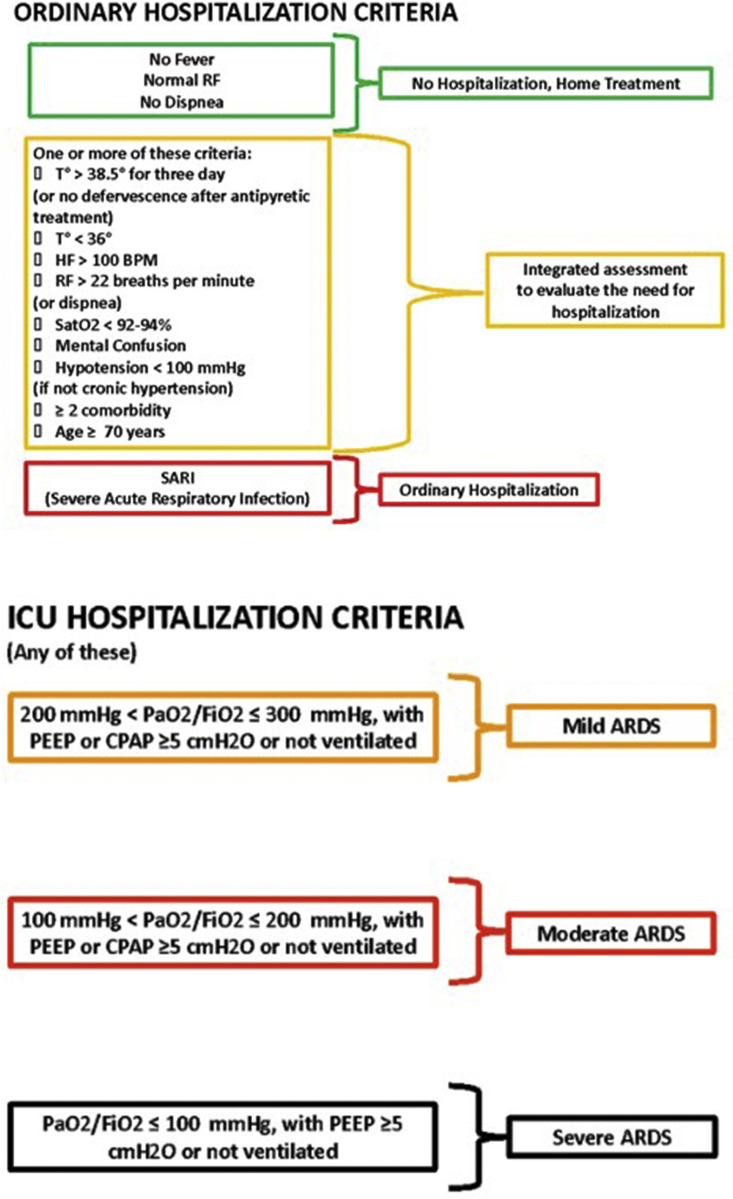
Whether the patients were hospitalised or deferred for home treatment was documented. When hospitalisation was necessary, invasive or non-invasive high-flow ventilation use in the ICU or hospitalisation on a general ward with non-invasive low-flow ventilation was recorded. ICU admission in the hospital was regulated by precise institutional guidelines, shown in Fig 1 .
Figure 1.
Hospitalisation criteria. When PaO2 is not available, SpO2/FiO2 ≤315 suggests the presence of acute respiratory distress syndrome (ARDS) (including not ventilated patients). ICU, intensive care unit; RF, respiratory frequency; HF, heart frequency; BPM, beats per minute; T, temperature; PEEP, positive end-expiratory pressure; CPAP, continuous positive airway pressure.
In order to perform multivariate regression analysis, the clinical outcomes were dichotomously divided: referral to home treatment and hospitalisation on a general ward were denoted positive outcomes, whereas ICU admission and death were negative outcomes. In-hospital mortality and the date of the discharge or of the demise were recorded.
Statistical analysis
Continuous variables were reported as mean ± standard deviation; categorical variables were noted down as counts and percentages. The distribution of continuous variables within the groups was evaluated by the unpaired t-test (two groups) or by the analysis of variance (ANOVA) model with Bonferroni's correction (more than two groups). Chi-squared tests or Fisher's exact test were used to compare categorical variables, as appropriate. Each variable predictive of the outcome was analysed with univariate logistic regression. The variables selected (p<0.10) by every univariate analysis were entered into a logistic regression model with the use of a forward stepwise elimination algorithm (terms with p>0.05 were eligible for removal). The survival function from lifetime data was reported through the Kaplan–Meier estimator. A two-sided alpha of <0.05 was considered statistically significant. All analyses were performed with the use of STATA, version 15.0.
Results
A total of 535 chest CT examinations were performed over the study period in patients with suspected Sars-CoV-2 lung infection. Of those, 147 patients were excluded because CT features were not suggestive of Sars-Cov 2 lung infection, 124 also had a negative RT-PCR while 23 had a positive RT-PCR. Eighteen patients were excluded because of severe motion artefacts; 39 were eliminated because the CT diagnosis of COVID-19 was not confirmed by the RT-PCR.
The study included 214 (64.7%) males and 117 (35.3%) females, for a total of 331 study participants. Mean age of the population was 62.3 ± 16.2. Mean age of the male and the female subgroups were 61.9 ± 16.2 and 62.7 ± 16.1, respectively. Regarding the age groups 39 participants (11.8%), 10 females (8.5%) and 29 males (13.5%), were ≤39 years. One hundred and thirteen patients (34.1%), 44 women (37.6%) and 69 males (32.3%), were 40 and 60 years, while 179 patients (54.1%), 63 females (53.9) and 116 males (54.2%), were ≥61 years (Table 1 ). The age distribution was not significantly different between the sexes.
Table 1.
Demographic characteristics.
| Characteristics | Results |
|---|---|
| General population | 331 (100) |
| Mean age | 62.3 ± 16.2, range 20–99 |
| 0–39 | 39 (11.8) |
| 40–60 | 113 (34.1) |
| ≥ 61 | 179 (54.1) |
| Males | 214 (64.7) |
| Mean age | 61.9 ± 16.2, range 20–99 |
| 0–39 | 29 (13.5) |
| 40–60 | 69 (32.3) |
| ≥ 61 | 116 (54.2) |
| Females | 117 (35.3) |
| Mean age | 62.7 ± 16.1, range 24–91 |
| 0–39 | 10 (8.5) |
| 40–60 | 44 (37.6) |
| ≥ 61 | 63 (53.9) |
Stratifying groups based on symptoms, no statistically significant difference in the number of patients suffering from cough and dyspnoea was found between the two sexes. On the other hand, in the present study population women were more likely to be affected by diarrhoea (p=0.014) and less likely to be feverish (p=0.001; Table 1).
An analysis of the different comorbidities between sexes was performed, showing a statistically significant difference (p=0.024) between males and females. In particular, females were more likely to have two or more diseases in their medical history: in this category were 16 males (7.5%) and 20 females (17.1%; Table 2 ). Nevertheless, a higher percentage of males were affected by one or two comorbidities (54.2% versus 46.2%). As expected, dyspnoea was more prevalent in patients ≥61 years (p=0.022) without differences between the sexes (Table 3 ).
Table 2.
Analysis of comorbidities between the sexes.
| General population | No. of comorbidities | p-Value | ||
|---|---|---|---|---|
| Sex | 0 | 1–2 | >2 | |
| Males | 82 (38.3) | 116 (54.2) | 16 (7.5) | 0.024 |
| Females | 43 (36.8) | 54 (46.2) | 20 (17.1) | |
| Type of comorbidities |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | CAD | HT | DM | COPD | NO COPD | NEUR D | K | CKD | HF | RA |
| Males | 35 (16.3) | 43 (20.1) | 33 (15.4) | 15 (7) | 5 (2.3) | 11 (5.1) | 17 (7.9) | 16 (7.4) | 3 (1.4) | 1 (0.4) |
| Females | 21 (17.9) | 55 (47) | 17 (14.5) | 10 (8.5) | 4 (3.4) | 14 (11.9) | 12 (10.2) | 13 (11.1) | 4 (3.4) | 5 (4.2) |
| Tot. | 56 (16.9) | 98 (29) | 50 (15.1) | 25 (7.5) | 9 (2.7) | 25 (7.5) | 29 (8.7) | 29 (8.7) | 7 (2.1) | 6 (1.8) |
Data are patients with percentages in parentheses.
CAD, coronary artery disease; HT, hypertension; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; NEUR D, neurological disorders; K, neoplastic diseases; CKD, chronic kidney disease; HF, hepatic failure; RA, rheumatoid arthritis.
Table 3.
Analysis of age group.
| Age group |
p-Value | |||
|---|---|---|---|---|
| ≤ 39 | 40–60 | ≥ 61 | ||
| Treatment level | ||||
| Home treatment | 18 (46.2) | 31 (27.4) | 7 (3.9) | <0.001b |
| Ordinary hospitalisation | 18 (46.2) | 65 (57.5) | 144 (80.4) | <0.001c |
| ICU | 7 (3.9) | 17 (15) | 28 (15.6) | <0.001b |
| CT findings | ||||
| CT score | 6.18 ± 5.96 | 9.20 ± 4.59 | 12.65 ± 5.17 | <0.001a |
| No. of GGO lobes | 2.8 ± 1.66 | 4.23 ± 1.25 | 4.53 ± 0.97 | <0.001a |
| No. of consolidation lobes | 1.87 ± 1.79 | 2.72 ± 1.62 | 2.9 ± 1.68 | 0.002a |
| Bilateral alteration | 23 (59) | 104 (92) | 172 (96.1) | < 0.001c |
| Crazy paving | 3 (2.6) | 40 (35.1) | 71 (62.3) | 0.001b |
| Reversed halo sign | 4 (10.3) | 14 (12.4) | 13 (7.3) | 0.352b |
| Lymph nodes | 5 (12.8) | 19 (16.8) | 44 (24.6) | 0.138b |
| Pleural effusion | 0 (0) | 1 (0.9) | 16 (8.9) | 0.020b |
| Pericardial effusion | 0 (0) | 4 (3.5) | 3 (1.7) | 0.347b |
| Clinical findings | ||||
| Fever | 24 (77.4) | 79 (82.3) | 115 (79.9) | 0.797c |
| Cough | 15 (38.5) | 50 (44.3) | 82 (47.4) | 0.579c |
| Dyspnoea | 23 (59) | 61 (54) | 120 (69.8) | 0.022c |
| Diarrhoea | 4 (10.2) | 10 (8.9) | 12 (7) | 0.645b |
Data are patients with percentages in parentheses. Age, GGO and consolidation are mean ± standard deviation.
GGO, ground glass opacity; LNs, lymph nodes; ICU, intensive care unit.
One way ANOVA.
Fisher's exact test.
Chi-squared test.
The most common comorbidity was hypertension, which affected 29% of the study participants, followed by coronary artery disease (16.9%) and diabetes mellitus (15.1%). All comorbidities but diabetes were more prevalent in women, as shown in Table 1.
Concerning the CT imaging findings, no statistically relevant difference was observed between the sexes, as shown in Table 4 . Some examples of analogous CT findings in men and women are shown in Fig 2 . Twenty-three patients, 11 females and 12 males, were affected by COVID-19 as demonstrated by RT-PCR, but had non-suggestive CT features. Ten patients (43.5%) had a completely negative CT, six were females and four were males. The other 13 patients had confounding conditions that are congestive heart failure (four patients), severe COPD (three patients), bacterial pneumonia (three patients), other interstitial pneumonia (one patients), or cancer (two patients).
Table 4.
Computed tomography (CT) findings of 331 chest scans.
| Characteristics | Males | Females | p-Value |
|---|---|---|---|
| CT findings | |||
| Score CT | 10.94 ± 5.62 | 10.29 ± 5.52 | 0.241a |
| No. of lobes with GGO | 4.22 ± 1.3 | 4.24 ± 1.26 | 0.447a |
| No. of lobes with consolidation | 2.71 ± 1.74 | 2.74 ± 1.64 | 0.459a |
| Bilateral alteration | 191 (89.3) | 108 (92.3) | 0.439b |
| Crazy paving | 76 (35.5) | 38 (32.5) | 0.578c |
| Reversed halo sign | 18 (8.4) | 13 (11.1) | 0.420c |
| Lymph nodes | 45 (21) | 23 (19.7) | 0.768c |
| Pleural effusion | 9 (4.2) | 8 (6.8) | 0.124b |
| Pericardial effusion | 4 (1.9) | 3 (2.6) | 0.674b |
| Clinical findings | |||
| Fever | 148 (87.1) | 72 (71.3) | 0.001c |
| Cough | 99 (47.4) | 48 (41.4) | 0.299c |
| Dyspnoea | 128 (61.2) | 76 (66.1) | 0.388c |
| Diarrhoea | 11 (5.3) | 15 (13) | 0.014c |
Data are patients with percentages in parentheses. Age, GGO and consolidation are mean ± standard deviation.
GGO, ground glass opacity.
Unpaired Student's t-test.
Fisher's exact test.
Chi-squared test.
Figure 2.
Chest CT images of patients with COVID-19 pneumonia (coronal view). (ai) A 32-year-old man with bilateral patchy areas of GGO. (aii) A 37-year-old woman with similar CT features but with some small areas of consolidation. (bi) A 53-year-old man with bilateral diffuse areas of consolidation and small areas of GGO. (bii) A 45-year-old woman with similar CT features, but more extensive GGO. (ci) A 73-year-old man with bilateral GGO and consolidation that involves almost all of the lungs. (cii) A 77-year-old woman with similar CT features.
Patients ≤39 years had a significantly lower number of pulmonary lobes affected by GGO and consolidation (p<0.001), while the two older groups showed no relevant difference between them. Moreover, the youngest group was more likely to have monolateral disease (p<0.001) and less likely to show the “crazy-paving” pattern (p=0.001) in comparison with the two older classes, which again showed no relevant offsets between them. On the other hand, pleural effusion was rare and more common in patients ≥61 years (p=0.019; Table 3).
The total CT score, utilised to quantify overall disease involvement of the parenchyma, was higher in older patients, with mean values of 6.2 ± 5.9 in patients ≤39 years old, 9.2 ± 4.6 in patients 40–60 years old and 12.7 ± 5.2 in patients ≥61 years old. The statistical significance, measured with Bonferroni test, was <0.005 in all groups.
The mean CT score for all the 331 patients was 10.7 ± 5.6. The mean CT score in men was 10.9 ± 5.5, while in women the value was 10.3 ± 5.6 with no statistically significant difference (p=0.31) not even after a score regression test was performed including both age and sex.
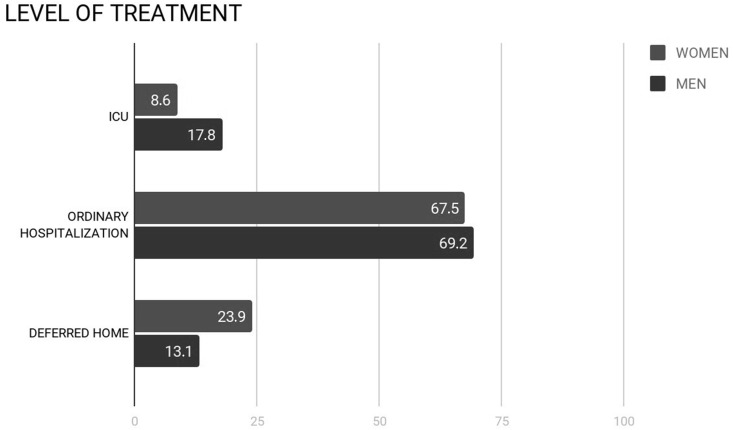
Regarding the required level of treatment, there was a significant difference between the age groups: older patients were more likely to be hospitalised in the ordinary regimen and in ICU and less likely to be deferred for home treatment. Considering also the two general sex groups, 28 (13.1%) males and 28 (23.9%) females were discharged, 148 (69.2%) males and 79 (67.5%) females were admitted on a general ward, 38 (17.8%) males and 10 (8.6%) females underwent treatment in the ICU (Fig 3 ). In the present population, females had a statistically significant higher probability to be discharged for home treatment and a statistically significant lower probability to be admitted to the ICU (p=0.008; Table 5 ). The male:female ratio of those admitted to the ICU was 3.8.
Figure 3.
Different hospitalisation rates in men and women. ICU, intensive care unit.
Table 5.
Analysis of treatment level between the sexes.
| General population | Treatment level | p-Valuea | ||
|---|---|---|---|---|
| Sex | Home treatment | Ordinary hospitalisation | ICU | |
| Males | 28 (13.1) | 148 (69.2) | 38 (17.8) | 0.008 |
| Females | 28 (23.9) | 79 (67.5) | 10 (8.6) | |
| ≤39 | ||||
| Sex | ||||
| Males | 13 (44.8) | 13 (44.8) | 3 (10.3) | 0.753 |
| Females | 5 (50) | 5 (50) | 0 (0) | |
| 40–60 | ||||
| Sex | ||||
| Males | 12 (17.4) | 44 (63.8) | 13 (18.8) | 0.010 |
| Females | 19 (43.2) | 21 (47.7) | 4 (9.1) | |
| ≥61 | ||||
| Sex | ||||
| Males | 3 (2.6) | 91 (78.5) | 22 (19) | 0.162 |
| Females | 4 (6.4) | 53 (84.1) | 6 (9.5) | |
Data are patients with percentages in parentheses.
ICU, intensive care unit.
Fisher's exact test.
A sub-analysis of the different age groups divided by sex was performed. A statistically significant difference (p=0.009) in the level of treatment between males and females was found in the patients 40–60 years. In this category, 12 (17.4%) males and 19 (43.2%) females were discharged, 44 (63.8%) males and 21 (47.7%) females were admitted to a general ward, 13 (18.8%) males and four (9.1%) females underwent treatment in the ICU (Table 5). No statistically significant difference was found in the other two age groups.
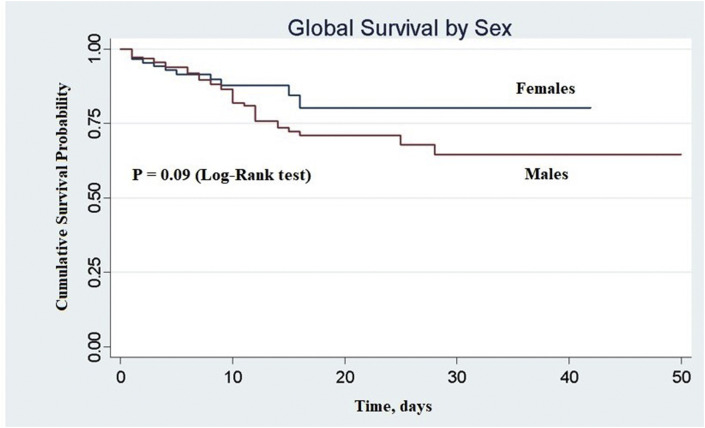
In the period of time considered, 53/275 patients (19.3%) died during the hospitalisation in the institution; 41 (77.4%) were men and 12 (22.6%) were women. The percentage of females admitted who died during their hospital stay was 13.5%, while the percentage of admitted males who died was 22%. A Kaplan–Meier analysis of in-hospital mortality is shown in Fig 4 . The difference between men and women is noteworthy but not statistically relevant (p=0.09).
Figure 4.
Cumulative survival probability in men and women followed for 50 days after emergency department access. Sex = 0, females; sex = 1, males.
Lastly, a univariate logistic analysis was performed to correlate each variable with the binary outcome. Those that proved to be statistically significant were age (p=0.004), sex (p=0.053), the presence of comorbidities (p=0.005), temperature (p=0.026), and the CT score (p<0.001). The other variables (cough, diarrhoea), were not determinant.
A multivariate analysis showed that the CT score was the only statistically significant independent value for the outcome prediction (p<0.001), while the others, i.e., sex, comorbidities, and age were not statistically significant (p=0.0788, p=0.1245 and p=0.9903, respectively). Receiver operating characteristic (ROC) curve analysis was performed of CT score accuracy in the binary outcome prediction (Fig 5 ), which showed an area under the ROC curve (AUC) of 0.78 (95% confidence interval [CI] 0.73–0.84).
Figure 5.
ROC curve. Logistic model: binary outcome (dependent variable); CT score (predictor).
Discussion
The percentage of females diagnosed with COVID-19 pneumonia during the study period was 35.3%, a result substantially in line with what reported by Mehra et al. 12 who reported a percentage of approximately 40%, and with the meta-analysis conducted by Fu et al.,16 which reported a median proportion of male patients equal to 56.5%, based on 42 studies conducted in the Hubei province.
Of the included patients 11.8% were <39 years, 34.1% were 40–60 years, and 54.1 were >60, a distribution consistent with the epidemiological data from other European countries, such as Netherlands and Spain, which at the beginning of April 2020 had a reported rate of infected patients in the same age groups equal to 15.3%, 26.6%, and 58.1%, and 16%, 34% and 50%, respectively.17 The mean age of the study participants is moderately higher in comparison to a similar study performed in Italy.18
Women were more likely than men to suffer from diarrhoea, a result that confirms the findings already highlighted by Han et al. 19 in a recent study. On the other hand, an elevated body temperature was more frequent in the male sex (p=0.001), which is partly discordant with the study of Jin et al.,20 who in a smaller number of patients reported an equal incidence of fever between the two sexes.
In line with the purposes of the study, the CT findings of the cohort were investigated analytically to demonstrate potential differences between the sexes, but there were no statistically significant differences in the CT features considered. Overall, the two sexes had a similar CT score and number of lobes involved by GGO and consolidation, and no relevant offset existed in the dichotomic CT characteristics such as bilaterality, crazy paving, reversed halo sign, or pleural effusion.
The present study does not concur with the results obtained by Dangis et al. and by Shang et al.,21 , 22 who found a CT score that was significantly greater in men using a similar method. Noteworthy, those authors performed a serial analysis on the same patients, considering a number of consecutive CT examinations, while the present study examined only the first tomography performed in the ED. This methodological difference may partially explain the offset between the results. Additionally, the visual CT score is not a precise and reproducible method, and there is interobserver variability.
The present CT findings appear to indicate that lung involvement is similar in the two sexes, as the CT features examined and the CT score evaluated were unable to demonstrate significant differences in the aggressiveness of Sars-CoV-2 towards the pulmonary tissue.
The multivariate analysis showed that the CT score was the only statistically significant independent predictor of the binary evaluated outcome. This seems to suggest that the CT score is a good predictor of “extreme” events, such as ICU admission and death, while stratifying the outcome in three ways other factors, such as sex and comorbidities, are more useful in predicting hospitalisation or home deferral. Additionally, although sex did not reach statistical significance in the univariate and multivariate analyses with the binary outcome, the p-value was borderline and may have reached significance with a larger cohort.
Chest CT as expected showed an overall milder lung involvement in patients <39 years, who showed a lower number of pulmonary lobes hit by GGO and consolidation and a lower CT score in comparison with the two older groups, with a higher chance of monolateral disease. Those results are consistent with the fact that COVID-19 is more severe in older patients.22 , 23 No relevant difference in the CT imaging findings existed between the two older groups, but for the higher prevalence of pleural effusion in patients ≥61 years, most likely because of the presence of concomitant illnesses, such as heart failure and bacterial superinfection.
In the present population, women affected by COVID-19 surprisingly had a higher number of comorbidities in comparison to men (p=0.024). In particular, a higher percentage of the women was affected by two or more comorbidities (17.1% versus 7.5%). These results seem to suggest that women affected by the illness may be generally more frail than men, while the latter have on average fewer comorbidities at the moment of the infection; however, additional research is needed to confirm these results, as there were no supplementary references in the current literature. Despite having a higher number of comorbidities, females required a lower rate of admission to the ICU, and were more likely to be immediately deferred home after ED access. Women had also an inferior intra-hospital mortality rate, but statistical significance was not achieved (p=0.09), again probably because of small cohort. These data are confirmatory of the existing literature, as hospitalisation rates were higher in males in all the countries which described them,24, 25, 26 with a range from 55% to 62%. Moreover, 73% of all ICU admissions in Europe were for men27 and the percentage of deaths was higher in males in an overwhelming majority of countries all over the world, ranging from 59% to 69%.24 , 26 The results of the present investigation suggest that this remarkable divergence in mortality and ICU admission rate was not due to a lower extension or to different types of lung parenchyma alterations as demonstrated by chest CT. Moreover, they suggest that sex differences in mortality and ICU admission persists among an infected population with the same characteristics and degree of lung involvement, thus excluding that a different rate of infection can be the cause. Women therefore seem to be overall more resistant to the disease despite having a superimposable lung disease severity and being affected by an equivalent or superior number of comorbidities. The difference may consequently be due to biological factors, i.e., the different immune response in the two sexes, as stated by Conti et al. 28 Specifically, women may be less susceptible to viral infections because of different natural immunity and different activity of steroid hormones because of sex-chromosome-related factors. In an experiment conducted in mice in 2017,29 males proved to be more susceptible to Sars-CoV infection and that the enhanced susceptibility was associated with elevated virus titres, increased vascular leakage, and alveolar oedema.
The present study suffers from several limitations. Firstly, the cohort of included patients, although quite numerous, is still not sufficient for some of the parameters that were assessed. A second limitation is the retrospective nature of the study, as patients presented autonomously to the ED and the number of days from symptoms onset was not recorded. A third limitation is the absence of stratification of comorbidities based on their severity. Finally, the patients' outcomes were measured only via the level of hospitalisation and mortality, without accounting for other clinical and laboratory findings.
In conclusion, in the authors' experience, as in previous reports, women were less affected than men by COVID-19 and they required an inferior level of hospitalisation and suffered a minor level of in-hospital mortality. Nevertheless, the present investigation seems to demonstrate that the difference was not explained by a lower extension of the lung parenchyma alterations as demonstrated by chest CT, which were substantially superimposable between the two sexes, and not even by comorbidities, which were more numerous in infected women in comparison to men. Biological factors, such as the different immune responses between the two sexes, can therefore be hypothesised.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
The authors thank Prof. Silvano Andorno, who provided invaluable help with the statistical analysis, and Dr. Fabio Falaschi for his help in editing and counselling. The authors would also like to acknowledge the healthcare professionals who took care of the patient participants in this study.
References
- 1.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vermeiren C., Marchand-Senécal X., Sheldrake E. Comparison of Copan Eswab and FLOQswab for COVID-19 PCR diagnosis: working around a supply shortage. J Clin Microbiol. 2020 doi: 10.1128/JCM.00669-20. JCM.00669-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutiérrez-Ocampo E. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li L.Q., Huang T., Wang Y.Q. COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garg S., Kim L., Whitaker M. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 — COVID-NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yi Y., Lagniton P.N.P., Ye S. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int J Biol Sci. 2020;16(10):1753-1766. doi: 10.7150/ijbs.45134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020 doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan W., Ni Z., Liang W. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ai T., Yang Z., Hou H. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020:200642. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J.J., Dong X., Cao Y. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 11.Istituto Superiore di Sanità (ISS) Epidemia COVID-19. Aggiornamento nazionale. https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_28-aprile-2020.pdf 28 aprile 2020 – ore 16:00. 30 APRILE 2020. Available at: (accessed 28 April 2020)
- 12.Mehra M.R., Desai S.S., Kuy S. Retraction: Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19. N Engl J Med. DOI: 10.1056/NEJMoa2007621. N Engl J Med. 2020 Jun 25;382(26):2582. doi: 10.1056/NEJMc2021225. Epub 2020 Jun 4. PMID: 32501665; PMCID: PMC7274164. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Simpson S., Kay F.U., Abbara S. Radiological society of North America expert consensus statement on reporting chest CT findings related to COVID-19. Endorsed by the society of thoracic radiology, the American College of Radiology, and RSNA. J Thorac Imag. 2020 doi: 10.1097/RTI.0000000000000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung M., Bernheim A., Mei X. CT Imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295(1):202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moradi B., Ghanaati H., Kazemi M. Implications of sex difference in CT scan findings and outcome of patients with COVID-19 pneumonia. Radiol Cardiothorac Imag. 2020;2 doi: 10.1148/ryct.2020200248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu L., Wang B., Yuan T. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J Infect. 2020;80(6):656–665. doi: 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bulut C., Kato Y. Epidemiology of COVID-19. Turk J Med Sci. 2020;50(SI-1):563–570. doi: 10.3906/sag-2004-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caruso D., Zerunian M., Polici M. Chest CT features of COVID-19 in Rome, Italy. Radiology. 2020:201237. doi: 10.1148/radiol.2020201237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han C., Duan C., Zhang S. Digestive symptoms in COVID-19 patients with mild disease severity: clinical presentation, stool viral RNA testing, and outcomes. Am J Gastroenterol. 2020 doi: 10.14309/ajg.0000000000000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin J.M., Bai P., He W. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dangis A., De Brucker N., Heremans A. Impact of gender on extent of lung injury in COVID-19. Clin Radiol. 2020 Jul;75(7):554–556. doi: 10.1016/j.crad.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shang Y., Xu C., Jiang F. Clinical characteristics and changes of chest CT features in 307 patients with common COVID-19 pneumonia infected SARS-CoV-2: a multicenter study in Jiangsu, China. Int J Infect Dis. 2020 Jul;96:157–162. doi: 10.1016/j.ijid.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li K., Wu J., Wu F. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol. 2020;55(6):327-331. doi: 10.1097/RLI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang X., Wei F., Hu L. Epidemiology and clinical characteristics of COVID-19. Arch Iran Med. 2020;23(4):268-271. doi: 10.34172/aim.2020.09. [DOI] [PubMed] [Google Scholar]
- 25.Serge R., Vandromme J., Charlotte M. Are we equal in adversity? Does Covid-19 affect women and men differently? Maturitas. 2020 doi: 10.1016/j.maturitas.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johns Hopkins Coronavirus Resource Center COVID-19 United States cases by county. https://coronavirus.jhu.edu/us-map Available at: (accessed 19 May 2020)
- 27.WHO Weekly surveillance report: coronavirus COVID-19. https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/weekly-surveillance-report Available at: (accessed 19 May 2020)
- 28.Conti P., Younes A. Coronavirus COV-19/SARS-CoV-2 affects women less than men: clinical response to viral infection. J Biol Regul Homeost Agents. 2020;34(2) doi: 10.23812/Editorial-Conti-3. [DOI] [PubMed] [Google Scholar]
- 29.Channappanavar R., Fett C., Mack M. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J Immunol. 2017;198(10):4046-4053. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]