Abstract
Objective
Lewy body (LB) synucleinopathies such as Parkinson’s disease (PD) entail profound cardiac norepinephrine deficiency. The status of sympathetic noradrenergic innervation at other extracranial sites has been unclear. Although in vivo neuroimaging studies have indicated a cardioselective noradrenergic lesion, no previous study has surveyed peripheral organs for norepinephrine contents in LB diseases. We reviewed 18F‐dopamine (18F‐DA) positron emission tomographic images and postmortem neurochemical data across several body organs of controls and patients with the LB synucleinopathies PD and pure autonomic failure (PAF) and the non‐LB synucleinopathy multiple system atrophy (MSA).
Methods
18F‐DA–derived radioactivity in the heart, liver, spleen, pancreas, stomach, kidneys, thyroid, and submandibular glands were analyzed from 145 patients with LB synucleinopathies (112 PD, 33 PAF), 74 controls, and 85 MSA patients. In largely separate cohorts, postmortem tissue norepinephrine data were reviewed for heart, liver, spleen, pancreas, kidney, thyroid, submandibular gland, and sympathetic ganglion tissue from 38 PD, 2 PAF, and 5 MSA patients and 35 controls.
Results
Interventricular septal 18F‐DA–derived radioactivity was decreased in the LB synucleinopathy group compared to the control and MSA groups (P < 0.0001 each). The LB and non‐LB groups did not differ in liver, spleen, pancreas, stomach, or kidney 18F‐DA–derived radioactivity. The LB synucleinopathy group had markedly decreased apical myocardial norepinephrine, but normal tissue norepinephrine in other organs. The MSA group had normal tissue norepinephrine in all examined organs.
Interpretation
By in vivo sympathetic neuroimaging and postmortem neurochemistry peripheral noradrenergic deficiency in LB synucleinopathies is cardioselective. MSA does not involve peripheral noradrenergic deficiency.
Introduction
Lewy body (LB) diseases such as sporadic Parkinson’s disease (PD) are characterized by cytoplasmic intraneuronal deposition of the protein alpha‐synuclein in the brain and autonomic nervous system and are considered to be in a family of disorders termed synucleinopathies. 1 LB synucleinopathies feature severe myocardial norepinephrine depletion, 2 implying that the disease process is not confined to the central nervous system and involves pathology in postganglionic sympathetic noradrenergic nerves. In contrast, the non‐LB synucleinopathy multiple system atrophy (MSA) is characterized by alpha‐synuclein deposits in glial cells in the central nervous system 3 and typically does not involve peripheral noradrenergic deficiency. 4
Studying peripheral noradrenergic innervation in LB forms of synucleinopathy is important scientifically and clinically because autonomic involvement seems to occur early in the pathogenetic sequence leading to relatively morbid “body‐first” PD. 5 , 6 , 7 , 8 Several studies have reported alpha‐synuclein aggregates in the enteric nervous system, which might then be transmitted in a prion‐like manner to the CNS. 9 , 10 , 11
Pathological studies in PD have yielded inconsistent findings about which plexuses and neurons in the periphery are most affected. 12 Whether patients with LB synucleinopathies have decreased norepinephrine contents in organs other than the heart has been unknown. Neuroimaging studies have provided evidence for decreased sympathetic innervation in the thyroid gland, submandibular gland, or renal cortex of patients with PD; however, postmortem data have so far not been reported. 13 , 14 Of particular interest is the pancreas, where autonomic neuronal, adrenomedullary hormonal, and autocrine–paracrine catecholaminergic systems converge, 15 , 16 , 17 yet to date no study has described neuroimaging or postmortem neurochemical data about the pancreas in synucleinopathies.
The purpose of this study therefore was to assess in vivo and postmortem cardiac and extracardiac sympathetic noradrenergic innervation in individuals with synucleinopathies and controls. 18F‐dopamine positron emission tomography (18F‐DA PET) scanning was used to visualize peripheral noradrenergic innervation in organs such as the heart, liver, spleen, stomach, kidneys, thyroid, submandibular glands, and pancreas. In largely separate cohorts, postmortem tissue NE contents in the heart, liver, spleen, kidneys, thyroid gland, submandibular glands, pancreas, and sympathetic ganglia were assayed.
We hypothesized that the results of in vivo neuroimaging and postmortem neurochemistry would agree in terms of the extent of peripheral noradrenergic deficiency in LB synucleinopathies. Based on the in vivo neuroimaging literature, we expected that LB synucleinopathy patients would have severely decreased norepinephrine content in the apical myocardium and less decreased or normal norepinephrine contents in other organs. We also expected that in most MSA patients there would be normal norepinephrine contents in all extracranial organs including the heart.
Methods
Subjects
All the living subjects in this study gave written informed consent to participate in protocols approved by the Institutional Review Board of the National Institute of Neurological Disorders and Stroke (NINDS). We reviewed the neuroimaging data from all 18F‐DA PET scans done under protocols of the Autonomic Medicine Section (formerly Clinical Neurocardiology Section) at the National Institutes of Health (NIH) between 1990 and 2020. Patients had been referred for evaluation of chronic autonomic failure or were healthy volunteers or subjects with chronic hypertension who were included in a protocol at the NIH that included 18F‐DA PET scanning.
When a subject underwent more than one 18F‐DA PET scan, we averaged the radioactivity concentrations over time for the same organ of interest. Autonomic function testing to identify neurogenic orthostatic hypotension included continuous blood pressure recording associated with the performance of the Valsalva maneuver and orthostatic plasma catechols. A diagnosis was assigned to each patient based on the medical and neurological history and the physical and neurological examinations, supported by the results of specialized tests described below.
Lewy body synucleinopathies
Patients with PD and pure autonomic failure (PAF) were included in the LB synucleinopathy group. The study did not include a patient cohort with LB dementia.
Parkinson’s disease
A diagnosis of PD was assigned based on at least three of the following four clinical criteria: bradykinesia, resting tremor, cogwheel rigidity, and good response of the movement disorders to levodopa treatment. 18 Supportive clinical laboratory findings included central dopaminergic deficiency as indicated by low putamen/occipital cortex ratios of 18F DOPA‐derived radioactivity. 19 The PD cohort was stratified into two groups: those with neurogenic orthostatic hypotension (PD + OH, N = 52) and those without neurogenic orthostatic hypotension (PD No OH, N = 60).
Pure autonomic failure
A diagnosis of PAF (N = 33) was assigned based on chronic, persistent neurogenic OH, 20 without a known cause (e.g., diabetic autonomic neuropathy, autoimmune autonomic ganglionopathy) and supported by clinical laboratory evidence of sympathetic noradrenergic deficiency. 4 , 21
Multiple system atrophy
The clinical diagnosis of probable MSA was made based on consensus criteria. 22 The MSA cohort was divided into two groups, that is, parkinsonian (MSA‐P, N = 68) and cerebellar (MSA‐C, N = 17).
Controls
A control group consisted of 67 healthy volunteers and seven subjects with chronic hypertension who were referred for evaluation prior to treatment with sympathectomy.
18F‐Dopamine positron emission tomographic scanning
PET scans were acquired on a GE Advance Tomograph (GE Healthcare) prior to January 2016 and on a Siemens PET/CT scanner after the GE Advance scanner was retired in January 2016. 18F‐DA PET scanning was done as described previously. 23 Briefly, 18F‐DA–derived radioactivity was recorded for the 5‐minute frame with a midpoint about 8 minutes after initiation of the 3‐minute infusion of 1 mCi of the tracer. Radioactivity concentrations in nCi/cc in regions of interest were adjusted for the radioactivity dose in mCi per kg body mass and expressed in units of nCi‐kg/cc‐mCi.
PET images were analyzed with Pixelwise modeling computer software (PMOD 2.61; PMOD Group). The organs of interest were visualized easily on 18F‐DA PET scans, and when the images were acquired on a PET/CT scanner the PMOD image registration/fusion tool was used to confirm the localization of each organ of interest on CT. The organs of interest were the cardiac interventricular septum, left ventricular (LV) chamber, liver, spleen, pancreas, stomach, renal cortex, renal pelvis, thyroid, and submandibular glands. We placed regions of interest within the borders of the organs. In dynamic emission scans of the thoraco‐abdominal region, regions of interest were drawn on transverse tomographic slices of the interventricular septum, LV chamber, liver, spleen, renal cortex, renal pelvis, and pancreas and coronal tomographic slices of the stomach. For the stomach, the inner region of interest was the lumen and the outer region of interest was the margin of the stomach. We subtracted the inner region of interest from the outer region of interest in order to assess radioactivity concentration in the stomach wall. For the renal cortex and renal pelvis, we exploited the fact that 18F‐DA–derived radioactivity always appears in the renal cortex before it appears in the renal pelvis. In static emission scans of the head and neck, circular regions of interest were drawn on transverse tomographic slices of the submandibular glands and thyroid.
The mean ± SEM concentrations of 18F‐DA–derived radioactivity in the various organs were compared among the subject groups. Differences between groups were assessed by Dunnett’s multiple comparisons with the controls defined as the control group. When only two groups were available for comparison, independent means t‐tests were used. A P‐value of < 0.05 defined statistical significance.
Postmortem observations
Postmortem neuropathologic diagnostic confirmation was obtained for a total of 80 individuals. Forty patients had a diagnosis of LB synucleinopathy (38 PD, 2 PAF), 5 had MSA, and 35 were controls. Postmortem neuropathologic descriptions were provided by the Banner Sun Health Research Institute (29 LB disease with neuropathologically demonstrated LBs in the brainstem, 27 controls) or the Laboratory of Pathology at the NIH Clinical Center (11 LB disease, 5 MSA, 8 controls).
Assays of norepinephrine in tissue samples were conducted by the Autonomic Medicine Section at the NIH, as described previously. 24 The assay personnel were blinded as to the individual diagnosis until the data were tabulated. Norepinephrine concentrations were expressed as fmol/mg wet weight. Mean norepinephrine concentrations were compared between the LB and non‐LB groups by independent‐means t‐tests. Postmortem data were not available for the stomach in any of the groups because of rapid postmortem autolysis. A P‐value < 0.05 defined statistical significance.
Results
18F‐DA–derived radioactivity
Patients with LB synucleinopathies had decreased 18F‐DA–derived radioactivity in the cardiac septum compared to controls (P < 0.0001, Figure 1). Septal 18F‐DA–derived radioactivity was increased in MSA patients compared to controls (P = 0.02, Figure 1). Patients with MSA also had higher 18F‐DA–derived radioactivity in the LV chamber (P = 0.01, Figure 1). Numerical data for 18F‐DA–derived radioactivity concentrations in body organs in synucleinopathies are summarized in a supplementary table (Supplementary data).
Figure 1.
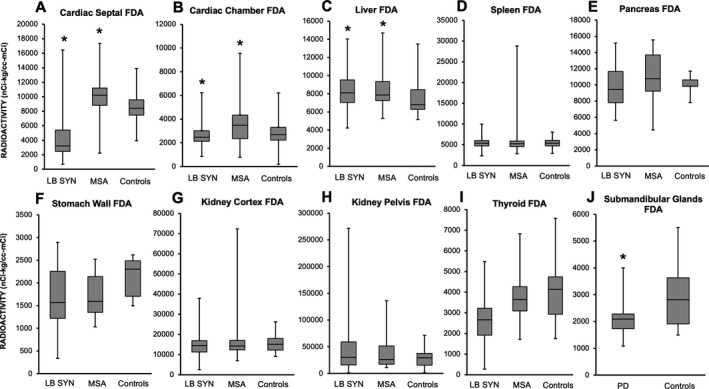
18F‐Dopamine (18F‐DA‐)–derived radioactivity in the cardiac septum (panel A), left ventricular (LV) chamber (panel B), liver (panel C), spleen (panel D), pancreas (panel E), stomach (panel F), kidney cortex (panel G), kidney pelvis (panel H), thyroid (panel I), and submandibular glands (panel J) in the patient groups and controls. In the box plots, the boundary of the box closest to zero indicates the 25th percentile, a black line within the box marks the median, and the boundary of the box farthest from zero indicates the 75th percentile. Whiskers above and below the box indicate the minimum and maximum values. Patients with LB synucleinopathies (LB SYN) had decreased septal18F‐DA–derived radioactivity compared to controls (P < 0.0001).Cardiac septal18F‐DA–derived radioactivity was increased in multiple system atrophy (MSA) compared to controls (P = 0.02).18F‐DA–derived radioactivity in the LV chamber was higher in MSA than in controls (P = 0.01).18F‐DA–derived radioactivity in the liver was increased in LB SYN (P = 0.01) and MSA (P = 0.03) compared to controls, whereas submandibular gland18F‐DA–derived radioactivity was decreased in Parkinson’s disease (PD) compared to controls (P = 0.04)
18F‐DA–derived radioactivity in the liver was higher in LB synucleinopathies (P = 0.01) and MSA (P = 0.03) than in controls (Figure 1). The groups did not differ in radioactivity in the spleen, pancreas, stomach, renal cortex, renal pelvis, or thyroid. 18F‐DA–derived radioactivity was decreased in the submandibular glands in LB synucleinopathies compared to the controls (P = 0.04, Figure 1).
Postmortem data
Myocardial norepinephrine was substantially decreased in the LB group (P < 0.0001; Figure 2) compared to the non‐LB (MSA and controls) group. The groups did not differ in mean norepinephrine concentrations in the liver, spleen, pancreas, thyroid, submandibular glands, or sympathetic ganglia (Figure 2). Mean ± SEM tissue norepinephrine concentrations in the different organs in the patients with LB versus non‐LB groups are summarized in a supplementary table (supplementary data).
Figure 2.
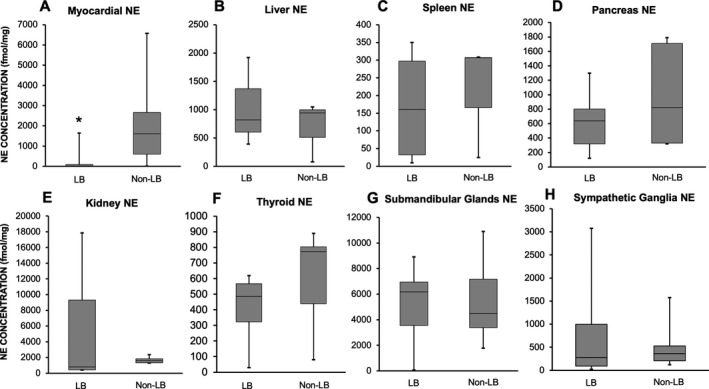
Postmortem tissue norepinephrine (NE) concentrations in apical left ventricular myocardium (panel A), liver (panel B), pancreas (panel C), spleen (panel D), kidney (panel E) thyroid (panel F), submandibular glands (panel G), and sympathetic ganglion (panel H) in the Lewy body (LB) and non‐LB groups. In the box plots, the boundary of the box closest to zero indicates the 25th percentile, a black line within the box marks the median, and the boundary of the box farthest from zero indicates the 75th percentile. Whiskers above and below the box indicate the minimum and maximum values. Myocardial NE was significantly lower in the LB group compared to the non‐LB group (P < 0.0001). There was no difference in tissue NE between the two groups for the liver, spleen, pancreas, thyroid, submandibular glands, and sympathetic ganglia
Validation of 18F‐DA PET scanning as a biomarker of myocardial noradrenergic innervation
A total of 11 patients (3 PD + OH, 3 PAF, 4 MSA, 1 with persistent neurogenic orthostatic hypotension without evidence of sympathetic noradrenergic deficiency) had both cardiac 18F‐DA PET scanning and postmortem data. Myocardial tissue was available from assayed 10 patients (Table 1). There was a strong positive correlation between 18F‐DA–derived radioactivity and myocardial tissue norepinephrine content (r = 0.8, Figure 3). All the patients with a diagnosis of LB synucleinopathy had both low cardiac 18F‐DA–derived radioactivity and low tissue norepinephrine compared to controls. One patient with pathologically confirmed MSA had low myocardial tissue norepinephrine and low 18F‐DA–derived radioactivity. The patient with neurogenic orthostatic hypotension and no neuroimaging evidence of sympathetic noradrenergic deficiency had low myocardial norepinephrine content and no LB pathology.
Table 1.
Demographics and pathology of subjects with both in vivo neuroimaging and postmortem data
| Subject | Clinical diagnosis | Sex |
Age at onset y |
Disease duration y |
Septum FDA nCi‐kg/cc‐mCi |
Myocardium NE fmol/mg |
Pathology | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
PMI h |
LBs | GCIs | AD | Other | |||||||
| 1 | PD + OH | F | 69 | 14.6 | 2293 | 36 | 20 | SN+ | No | Hirano bodies: H+ | No |
| 2 | PD + OH | F | 61 | 22.5 | 1765 | 48 | 30 | Intraneuronal α‐synuclein in brainstem and sympathetic ganglia | |||
| 3 | PD + OH | M | 75 | 2.8 | 3729 | 431 | 24 | SN ++ | No | No | No |
| 4 | PAF | F | 48 | 35.6 | 1733 | 7 | <24h |
SN+ LC+++ |
No | No | Marked cerebral atherosclerosis |
| 5 | PAF | M | 66 | 3.1 | 3101 | 18 | 23 |
Am+ CG+ SN++ Brainstem++ |
No |
NFT: H+, EC+ BA: no |
No |
| 6 | PAF | M | 53 | 15.9 | 3218 | 34 | 12 | SN+ | No |
NP: FC+, TC+, PC+ No NFT |
No |
| 7 | NOH | M | 50 | 7.7 | 7434 | 184 | 15 | No | No | No | No |
| 8 | MSA‐C | M | 46 | 11 | 8913 | NA | <24h | No |
Midbrain+++ Pons+++ Cereb.+++ SN+ |
No | No |
| 9 | MSA‐P | M | 60 | 6.2 | 11308 | 6569 | 8 | No |
SN+++ St.+++ NB+ |
Tau: Th+, NB+, Am+ BA: Am+, Th+, BG+ |
No |
| 10 | MSA‐P | M | 69 | 3.2 | 2219 | 4 | 8 | No | SN+, CG+, H+, EC+ | No | No SYN in heart, SG |
| 11 | MSA‐P | M | 71 | 6.7 | 5924 | 4428 | <24h | Final Pathology report requested – unavailable at the time of submission | |||
AD, Alzheimer’s disease pathology; Am, amygdala; BA, beta‐amyloid; Cereb., cerebellum; CG, cingular cortex; EC, entorhinal cortex; F, female; FC, frontal cortex; GCIs, glial cytoplasmic inclusions; H, hippocampus; LBs, Lewy bodies; M, male; M; MSA‐C, multiple system atrophy subtype cerebellar; MSA‐P, multiple system atrophy subtype parkinsonian; NB, nucleus basalis; NFT, neurofibrillary tangles; NP, neuritic plaques; NOH, neurogenic orthostatic hypotension; PAF, pure autonomic failure; PC, parietal cortex; PD + OH, Parkinson’s disease with orthostatic hypotension; PMI, postmortem interval; SG, sympathetic ganglia; St, striatum; SN, substantia nigra; TC, temporal cortex; Th, thalamus; SYN, synuclein immunoreactivity. +, mild or focal pathological burden; ++, moderate pathological burden; +++, severe pathological burden.
Figure 3.
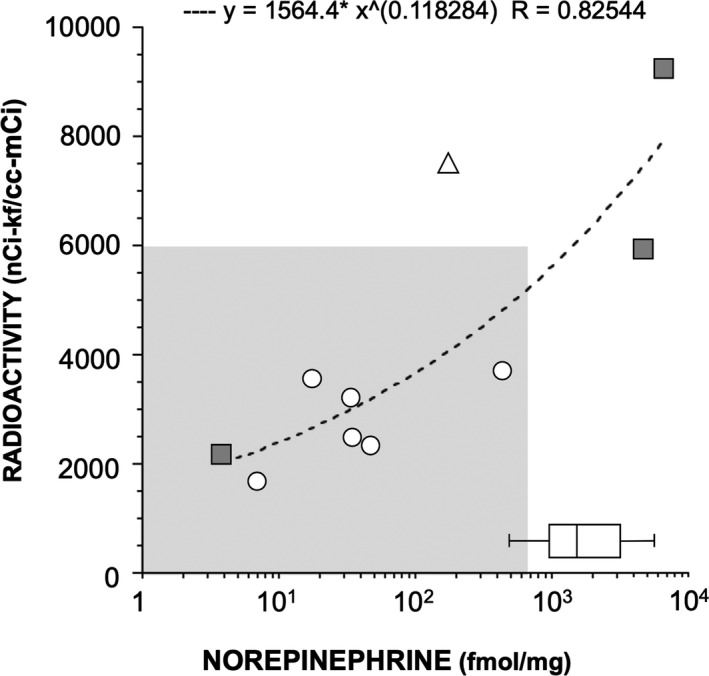
Scatter plot relating individual values for in vivo cardiac septal18F‐dopamine(18F‐DA)–derived radioactivity and postmortem myocardial norepinephrine (NE). Six subjects had a diagnosis of LB synucleinopathy (circle), three MSA (gray filled squares), and one subject with chronic neurogenic OH (triangle) (r = 0.8). The horizontal box plot represents postmortem myocardial NE concentration in all available controls. The boundary of the box plot closest to zero indicates the 25thpercentile, a black line within the box marks the median, and the boundary of the box farthest from zero indicates the 75thpercentile. All patients with LB synucleinopathies had low postmortem myocardial NE concentration and low septal18F‐DA–derived radioactivity (below 6000 nCi‐kg/cc‐mCi). One patient with MSA (empty square) had low postmortem myocardial NE concentration and low septal18F‐DA–derived radioactivity
Discussion
The results of this study show that LB forms of alpha‐synucleinopathy involve peripheral noradrenergic deficiency that is especially prominent in the heart, by both in vivo 18F‐DA PET scanning and postmortem neurochemistry. A major and unique strength of the study was the assays of tissue norepinephrine contents in tissues from a variety of extracranial organs.
Validation of 18F‐DA PET scanning as a biomarker of cardiac noradrenergic innervation
We validated low 18F‐DA–derived radioactivity as a biomarker of the cardiac noradrenergic deficiency by comparing in vivo radioactivity with postmortem myocardial norepinephrine content in the same patients. Analogously, heart/mediastinum ratios of 123I‐metaiodobenzylguanidine (123I‐MIBG)–derived radioactivity have been found to correlate positively with myocardial immunoreactive tyrosine hydroxylase. 25
It should be noted that low 18F‐DA–derived radioactivity does not imply sympathetic noradrenergic denervation. A functional abnormality in intact sympathetic nerves could produce the same abnormality. 26 , 27 For instance, a recent multi‐tracer imaging study found that PAF patients have a combination of a moderate amount of loss of cardiac noradrenergic innervation and a substantial vesicular storage defect in residual neurons. 28
Profound cardiac norepinephrine deficiency in LB synucleinopathies
Interventricular septal myocardial 18F‐DA–derived radioactivity was decreased in three forms of LB synucleinopathy – PD + OH, PD No OH, and PAF. These results fit with those from numerous previous reports based on cardiac sympathetic neuroimaging by 18F‐DA or 123I‐MIBG scanning in these conditions. 23 , 29 The current data provide postmortem confirmation of drastic myocardial noradrenergic deficiency in LB disease. Mean myocardial norepinephrine content was decreased by 97% from that in controls.
In contrast, septal 18F‐DA–derived radioactivity was significantly increased in the group with the non‐LB synucleinopathy MSA. The increased radioactivity might reflect decreased postganglionic sympathetic nerve traffic, which tends to increase tissue contents of the tracer. 27
Mean myocardial norepinephrine content was normal in a small group of MSA patients.
No previous study has reported on myocardial norepinephrine content in MSA, possibly because MSA is a rare disease, and autopsy studies rarely involve extracranial tissue harvesting. A patient with pathologically confirmed MSA had both PET and postmortem neurochemical data demonstrating severe cardiac noradrenergic deficiency. This patient had an increased “synuclein index” in arrector pili muscles during life, indicating a peripheral intraneuronal synucleinopathic process. 30 We speculate that the patient had an early peripheral LB process. Unfortunately, in this patient postmortem harvesting of sympathetic ganglion tissue was not done. Overall, the results are consistent with other studies where most MSA patients have biomarkers of intact cardiac sympathetic innervation, although rare cases of MSA with neuroimaging evidence of cardiac noradrenergic deficiency have been reported. 31 , 32 , 33
No evidence for decreased norepinephrine contents in extracardiac organs in LB synucleinopathies
In this study, tissue norepinephrine contents in extracardiac organs were similar in the LB and non‐LB groups. Alpha‐synuclein deposition in salivary glands has been proposed as a potential biomarker of early autonomic involvement in PD. 34 , 35 , 36 Despite neuroimaging evidence that suggests noradrenergic deficiency in the submandibular gland in PD, in this study tissue norepinephrine content was normal in the LB synucleinopathy group. Discordance between neuroimaging and tissue norepinephrine data might reflect a functional abnormality that decreases neuronal uptake of sympathetic imaging agents without actual denervation. No previous studies have assessed submandibular gland norepinephrine contents in synucleinopathies. A recent report noted lower 123I‐MIBG–derived radioactivity in the parotid and submandibular glands in PD than in control subjects. 37 The authors concluded that decreased 123I‐MIBG uptake could be explained by alpha‐synuclein pathology in the superior cervical ganglion. 37 This study did not include postmortem neuropathological or neurochemical data.
Our group and others reported previously that patients with PD and OH have decreased 18F‐DA–derived radioactivity in the thyroid. 13 , 38 , 39 Taki et al. did not find evidence of reduced 123I‐MIBG–derived radioactivity in PD patients without autonomic dysfunction. 40 The different results could be due to sample size and the fact that only PD patients without autonomic failure were included. 40 In the current study, postmortem thyroid norepinephrine concentrations did not differ between the LB and non‐LB groups. No previous reports have described thyroid norepinephrine contents in synucleinopathies.
The role of the sympathetic nervous system in the regulation of pancreatic functions is incompletely understood. Splanchnic nerve stimulation has been shown to decrease plasma insulin levels, possibly via direct actions of norepinephrine on pancreatic beta‐cells. 41 A link between disrupted brain insulin signaling and PD has been suggested by an increased rate of subsequent PD following type 2 diabetes mellitus in a large cohort study. 42 Furthermore, cytoplasmic phosphorylated alpha‐synuclein has been found in the pancreas of subjects with synucleinopathies or with diabetes melitus, 43 and there is evidence for alpha‐synuclein inhibiting insulin secretion and dopamine synthesis. 44 Our study is the first to report on in vivo imaging of noradrenergic innervation and postmortem tissue norepinephrine in the human pancreas. By both methodologies the current findings do not suggest pancreatic noradrenergic deficiency in synucleinopathies. Further studies should further characterize the role of α‐synuclein on pancreatic neurotransmitter, hormonal, and autocrine–paracrine functions.
Larger decrease in norepinephrine in myocardium than in sympathetic ganglion tissue in LB synucleinopathies
The large decrease in myocardial tissue norepinephrine in LB compared to non‐LB controls was not accompanied by similarly decreased tissue norepinephrine in sympathetic ganglion tissue. There are a variety of explanations for this difference. One for which there is relevant literature is that the pathogenetic process may proceed in a centripetal fashion. 45 It has been suggested that synucleinopathies may involve decreased axonal transport relatively early in the disease process, 46 , 47 and since vesicles and vesicle‐associated proteins are delivered by axonal transport, there could be decreased populations of functionally intact storage vesicles at terminals in the target tissue. 48 , 49
Why are cardiac sympathetic nerves susceptible in LB diseases?
The heart relies almost exclusively on aerobic oxidation for the generation of energy. Therefore, in comparison to other organs, sympathetic nerves in the heart might be particularly sensitive to alterations of mitochondrial functions and oxidative stress; both have been shown to play a role in the pathogenesis of PD. 50 The heart also stands out in the magnitude of oxidative deamination of cytoplasmic catecholamines. Thus, between the arterial and coronary sinus, the plasma concentration of 3,4‐dihydroxyphenylglycol, the main product of oxidative deamination of norepinephrine in sympathetic nerves, approximately doubles. 51 , 52 Moreover, unlike other organs, about 80% of norepinephrine in coronary arterial plasma is removed in the passage through the heart. 52 If there were a circulating sympathetic neurotoxin there might be greater damage to sympathetic nerves in the heart than in other organs.
Limitations
Except for 10 patients, the subject cohort that underwent in vivo neuroimaging was different from the cohort that underwent postmortem tissue neurochemistry. Therefore, there is a risk of imperfect concordance across subjects for the organs sampled. Comprehensive clinical data (e.g., clinical diagnosis, disease duration) or other pathologic findings (e.g., LB subtype, NIA‐AA Alzheimer type pathology, DLB criteria, or co‐morbid TDP‐43 or cerebrovascular pathologies) were unavailable for most of the cohort that underwent postmortem tissue harvesting.
We did not obtain gastrointestinal tissue samples for neurochemical assays because of rapid postmortem autolysis, which would obviate obtaining valid data about tissue NE contents. Alpha‐synuclein deposition and LBs have been reported in the gastrointestinal tract, 53 but whether PD patients have increased gastrointestinal alpha‐synuclein deposition has been questioned. No differences between PD and controls have been reported in numbers of tyrosine hydroxylase‐positive myenteric and submucosal neurons in gastrointestinal organs. 54 , 55 One should bear in mind that the occurrence of LB pathology does not imply catecholamine deficiency. The status of noradrenergic innervation in the enteric nervous system in LB diseases therefore remains unknown. This is an important matter for future research involving gastrointestinal tissue sampling with short postmortem intervals.
We did not assay alpha‐synuclein and norepinephrine in the same tissue samples. The focus of the present study was on noradrenergic innervation, not on the putative pathophysiologic role of intraneuronal alpha‐synuclein deposition. This is a matter of current research.
Conclusion and outlook
In conclusion, LB synucleinopathies entail cardioselective peripheral noradrenergic deficiency. It is possible that a better understanding of bases for this selectivity may apply also to the unusual susceptibility of nigrostriatal dopaminergic neurons in PD.
Authors’ Contributions
All authors made substantial contributions. Guillaume Lamotte contributed to the conception and design of the study, acquisition, analysis and interpretation of data, drafting the manuscript and preparing the figures, and reviewing the manuscript; Patricia Sullivan and Courtney Holmes contributed to the analysis and interpretation of data and reviewing the manuscript; Abhishek Lenka contributed to the interpretation of data, reviewing the manuscript; David S. Goldstein contributed to the conception and design of the study, acquisition, analysis and interpretation of data, preparing the figures, and reviewing the manuscript.
Conflicts of Interest
The Corresponding Author affirms that none of the authors has a conflict of interest.
Supporting information
Table S1. Reports numerical data for 18F‐Dopamine (18F‐DA‐)–derived radioactivity in body organs in synucleinopathies and controls. Cardiac septal 18F‐DA–derived radioactivity was decreased in Lewy body synucleinopathies (Parkinson’s disease with orthostatic hypotension (PD + OH), PD without OH (OH), and pure autonomic failure (PAF)) compared to controls. Cardiac septal and left ventricular chamber 18F‐DA–derived radioactivity concentrations were increased in multiple system atrophy (MSA) compared to controls. Liver 18F‐DA–derived radioactivity was increased in PD + OH, PAF, and MSA compared to controls. 18F‐DA–derived radioactivity concentration in submandibular glands was decreased in PD compared to controls. The groups did not differ in radioactivity in the spleen, pancreas, stomach, renal cortex, renal pelvis, or thyroid.
Table S2. Reports numerical data for postmortem tissue norepinephrine (NE) in the different organs of interest in the Lewy body (LB) group (Parkinson’s disease = 37, pure autonomic failure = 3) and the non‐Lewy body group (controls = 35, multiple system atrophy = 5). Myocardial NE was substantially decreased in the LB group (P < 0.0001) compared to the non‐LB (MSA and controls) group.
Acknowledgments
We thank the Banner Sun Health Research Institute and the Laboratory of Pathology at the NIH Clinical Center for providing postmortem tissue samples. The research reported here was supported by the Division of Intramural Research, NINDS.
Funding Information
This research was supported by the Division of Intramural Research of the National Institute of Neurological Disorders and Stroke (NINDS), NIH.
Funding Statement
This work was funded by National Institute of Neurological Disorders and Stroke grants 18N0140 and 03N0004; Health Research grant ; NIH Clinical Center grant .
References
- 1. Del Tredici K, Hawkes CH, Ghebremedhin E, Braak H. Lewy pathology in the submandibular gland of individuals with incidental Lewy body disease and sporadic Parkinson's disease. Acta Neuropathol 2010;119(6):703–713. [DOI] [PubMed] [Google Scholar]
- 2. Goldstein DS, Sharabi Y. The heart of PD: Lewy body diseases as neurocardiologic disorders. Brain Res 2019;1(1702):74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wakabayashi K, Yoshimoto M, Tsuji S, Takahashi H. Alpha‐synuclein immunoreactivity in glial cytoplasmic inclusions in multiple system atrophy. Neurosci Lett 1998;249(2–3):180–182. [DOI] [PubMed] [Google Scholar]
- 4. Goldstein DS, Holmes C, Sharabi Y, et al. Plasma levels of catechols and metanephrines in neurogenic orthostatic hypotension. Neurology 2003;60(8):1327–1332. [DOI] [PubMed] [Google Scholar]
- 5. Cersosimo MG, Benarroch EE. Autonomic involvement in Parkinson's disease: pathology, pathophysiology, clinical features and possible peripheral biomarkers. J Neurol Sci 2012;313(1–2):57–63. [DOI] [PubMed] [Google Scholar]
- 6. Gelpi E, Navarro‐Otano J, Tolosa E, et al. Multiple organ involvement by alpha‐synuclein pathology in Lewy body disorders. Mov Disord 2014;29(8):1010–1018. [DOI] [PubMed] [Google Scholar]
- 7. Fereshtehnejad SM, Romenets SR, Anang JB, et al. New clinical subtypes of Parkinson disease and their longitudinal progression: A prospective cohort comparison with other phenotypes. JAMA Neurol 2015;72(8):863–873. [DOI] [PubMed] [Google Scholar]
- 8. Horsager J, Andersen KB, Knudsen K, et al. Brain‐first versus body‐first Parkinson's disease: a multimodal imaging case‐control study. Brain 2020. [DOI] [PubMed] [Google Scholar]
- 9. Masuda‐Suzukake M, Nonaka T, Hosokawa M, et al. Pathological alpha‐synuclein propagates through neural networks. Acta Neuropathol Commun 2014;6(2):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prusiner SB, Woerman AL, Mordes DA, et al. Evidence for alpha‐synuclein prions causing multiple system atrophy in humans with parkinsonism. Proc Natl Acad Sci USA 2015;112(38):E5308–E5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Borghammer P. How does parkinson's disease begin? Perspectives on neuroanatomical pathways, prions, and histology. Mov Disord 2018;33(1):48–57. [DOI] [PubMed] [Google Scholar]
- 12. Chalazonitis A, Rao M. Enteric nervous system manifestations of neurodegenerative disease. Brain Res 2018;1693(Pt B):207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tipre DN, Goldstein DS. Cardiac and extra‐cardiac sympathetic denervation in Parkinson disease with orthostatic hypotension and in pure autonomic failure. J Nucl Med 2005;46:1775–1781. [PubMed] [Google Scholar]
- 14. Li ST, Dendi R, Holmes C, Goldstein DS. Progressive loss of cardiac sympathetic innervation in Parkinson's disease. Ann Neurol 2002;52(2):220–223. [DOI] [PubMed] [Google Scholar]
- 15. Oomori Y, Iuchi H, Ishikawa K, et al. Immunocytochemical study of tyrosine hydroxylase and dopamine beta‐hydroxylase immunoreactivities in the rat pancreas. Histochemistry 1994;101:313–323. [DOI] [PubMed] [Google Scholar]
- 16. Struthers AD, Brown DC, Brown MJ, et al. Selective alpha 2 receptor blockade facilitates the insulin response to adrenaline but not to glucose in man. Clin Endocrinol (Oxf) 1985;23(5):539–546. [DOI] [PubMed] [Google Scholar]
- 17. Eisenhofer G. The role of neuronal and extraneuronal plasma membrane transporters in the inactivation of peripheral catecholamines. Pharmacol Ther 2001;91(1):35–62. [DOI] [PubMed] [Google Scholar]
- 18. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico‐pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55(3):181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goldstein DS, Holmes C, Bentho O, et al. Biomarkers to detect central dopamine deficiency and distinguish Parkinson disease from multiple system atrophy. Parkinsonism Relat Disord 2008;14(8):600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goldstein DS, Sharabi Y. Neurogenic orthostatic hypotension: a pathophysiological approach. Circulation 2009;119(1):139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goldstein DS, Holmes C, Kopin IJ, Sharabi Y. Intra‐neuronal vesicular uptake of catecholamines is decreased in patients with Lewy body diseases. J Clin Invest 2011;121(8):3320–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008;71(9):670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goldstein DS, Holmes C, Cannon RO 3rd, et al. Sympathetic cardioneuropathy in dysautonomias. N Engl J Med 1997;336(10):696–702. [DOI] [PubMed] [Google Scholar]
- 24. Goldstein DS, Pekker MJ, Eisenhofer G, Sharabi Y. Computational modeling reveals multiple abnormalities of myocardial noradrenergic function in Lewy body diseases. JCI Insight 2019;23:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takahashi M, Ikemura M, Oka T, et al. Quantitative correlation between cardiac MIBG uptake and remaining axons in the cardiac sympathetic nerve in Lewy body disease. J Neurol Neurosurg Psychiatry 2015;86(9):939–944. [DOI] [PubMed] [Google Scholar]
- 26. Goldstein DS, Grossman E, Tamrat M, et al. Positron emission imaging of cardiac sympathetic innervation and function using 18F‐6‐fluorodopamine: effects of chemical sympathectomy by 6‐hydroxydopamine. J Hypertens 1991;9:417–423. [DOI] [PubMed] [Google Scholar]
- 27. Goldstein DS, Chang PC, Eisenhofer G, et al. Positron emission tomographic imaging of cardiac sympathetic innervation and function. Circulation 1990;81:1606–1621. [DOI] [PubMed] [Google Scholar]
- 28. Goldstein DS, Isonaka R, Holmes C, Ding Y‐S, Sharabi Y. Cardiac sympathetic innervation and vesicular storage in pure autonomic failure. Ann Clin Transl Neurology 2020;(in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Treglia G, Cason E, Stefanelli A, et al. MIBG scintigraphy in differential diagnosis of Parkinsonism: a meta‐analysis. Clin Auton Res 2012;22(1):43–55. [DOI] [PubMed] [Google Scholar]
- 30. Isonaka R, Rosenberg AZ, Sullivan P, et al. Alpha‐synuclein deposition within sympathetic noradrenergic neurons is associated with myocardial noradrenergic deficiency in neurogenic orthostatic hypotension. Hypertension 2019;73(4):910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cook GA, Sullivan P, Holmes C, Goldstein DS. Cardiac sympathetic denervation without Lewy bodies in a case of multiple system atrophy. Parkinsonism Relat Disord 2014;20(8):926–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brandl SJ, Braune S. Sensitivity and specificity of cardiac metaiodobenzylguanidine scintigraphy in the early diagnosis of Parkinson's disease. Clin Auton Res 2019;29(6):567–574. [DOI] [PubMed] [Google Scholar]
- 33. Goldstein DS, Holmes C, Sullivan P, et al. Autoimmunity‐associated autonomic failure with sympathetic denervation. Clin Auton Res 2017;27(1):57–62. [DOI] [PubMed] [Google Scholar]
- 34. Adler CH, Serrano GE, Zhang N, et al. Feasibility of repeat and bilateral submandibular gland needle biopsies in Parkinson's disease. Parkinsonism Relat Disord 2019;68:69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Beach TG, Adler CH, Serrano G, et al. Prevalence of submandibular gland synucleinopathy in Parkinson's disease, dementia with lewy bodies and other lewy body disorders. J Parkinsons Dis 2016;6(1):153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Campo F, Carletti R, Fusconi M, et al. Alpha‐synuclein in salivary gland as biomarker for Parkinson's disease. Rev Neurosci 2019;30(5):455–462. [DOI] [PubMed] [Google Scholar]
- 37. Haqparwar J, Pepe A, Fassbender K, et al. Reduced MIBG accumulation of the parotid and submandibular glands in idiopathic Parkinson's disease. Parkinsonism Relat Disord 2017;34:26–30. [DOI] [PubMed] [Google Scholar]
- 38. Matsui H, Udaka F, Oda M, et al. Metaiodobenzylguanidine (MIBG) scintigraphy at various parts of the body in Parkinson's disease and multiple system atrophy. Auton Neurosci 2005;119(1):56–60. [DOI] [PubMed] [Google Scholar]
- 39. Matsui H, Udaka F, Oda M, et al. Metaiodobenzylguanidine (MIBG) uptake in Parkinson's disease also decreases at thyroid. Ann Nucl Med 2005;19(3):225–229. [DOI] [PubMed] [Google Scholar]
- 40. Taki J, Nakajima K, Hwang EH, et al. Peripheral sympathetic dysfunction in patients with Parkinson's disease without autonomic failure is heart selective and disease specific. Eur J Nucl Med 2000;27:566–573. [DOI] [PubMed] [Google Scholar]
- 41. Dunning BE, Ahrén B, Veith RC, Taborsky GJ Jr. Nonadrenergic sympathetic neural influences on basal pancreatic hormone secretion. Am J Physiol 1988;255(6 Pt 1):E785–E792. [DOI] [PubMed] [Google Scholar]
- 42. De Pablo‐Fernandez E, Goldacre R, Pakpoor J, et al. Association between diabetes and subsequent Parkinson disease: a record‐linkage cohort study. Neurology 2018;91(2):e139–e142. [DOI] [PubMed] [Google Scholar]
- 43. Martinez‐Valbuena I, Amat‐Villegas I, Valenti‐Azcarate R, et al. Interaction of amyloidogenic proteins in pancreatic β cells from subjects with synucleinopathies. Acta Neuropathol 2018;135(6):877–886. [DOI] [PubMed] [Google Scholar]
- 44. Vidal‐Martinez G, Yang B, Vargas‐Medrano J, Perez RG. Could α‐synuclein modulation of insulin and dopamine identify a novel link between Parkinson's disease and diabetes as well as potential therapies? Front Mol Neurosci 2018;11:465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Orimo S, Uchihara T, Nakamura A, et al. Axonal alpha‐synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson's disease. Brain 2008;131(Pt 3):642–650. [DOI] [PubMed] [Google Scholar]
- 46. Chu Y, Morfini GA, Langhamer LB, et al. Alterations in axonal transport motor proteins in sporadic and experimental Parkinson's disease. Brain 2012;135(Pt 7):2058–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Volpicelli‐Daley LA. Effects of alpha‐synuclein on axonal transport. Neurobiol Dis 2017;105:321–327. [DOI] [PubMed] [Google Scholar]
- 48. Janezic S, Threlfell S, Dodson PD, et al. Deficits in dopaminergic transmission precede neuron loss and dysfunction in a new Parkinson model. Proc Natl Acad Sci USA 2013;110(42):E4016–E4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pifl C, Rajput A, Reither H, et al. Is Parkinson's disease a vesicular dopamine storage disorder? Evidence from a study in isolated synaptic vesicles of human and nonhuman primate striatum. J Neurosci 2014;34(24):8210–8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lotharius J, Brundin P. Pathogenesis of Parkinson's diease: Dopamine, vesicles, and [alpha]‐synuclein. Nat Rev Neurosci 2002;3:932–942. [DOI] [PubMed] [Google Scholar]
- 51. Eisenhofer G, Smolich JJ, Cox HS, Esler MD. Neuronal reuptake of norepinephrine and production of dihydroxyphenylglycol by cardiac sympathetic nerves in the anesthetized dog. Circulation 1991;84:1354–1363. [DOI] [PubMed] [Google Scholar]
- 52. Eisenhofer G, Esler MD, Meredith IT, et al. Sympathetic nervous function in human heart as assessed by cardiac spillovers of dihydroxyphenylglycol and norepinephrine. Circulation 1992;85:1775–1785. [DOI] [PubMed] [Google Scholar]
- 53. Wakabayashi K. Parkinson's disease: the distribution of Lewy bodies in the peripheral autonomic nervous system. No To Shinkei 1989;41:965–971. [PubMed] [Google Scholar]
- 54. Annerino DM, Arshad S, Taylor GM, et al. Parkinson's disease is not associated with gastrointestinal myenteric ganglion neuron loss. Acta Neuropathol 2012;124(5):665–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Corbille AG, Coron E, Neunlist M, et al. Appraisal of the dopaminergic and noradrenergic innervation of the submucosal plexus in PD. J Parkinsons Dis 2014;4(4):571–576. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Reports numerical data for 18F‐Dopamine (18F‐DA‐)–derived radioactivity in body organs in synucleinopathies and controls. Cardiac septal 18F‐DA–derived radioactivity was decreased in Lewy body synucleinopathies (Parkinson’s disease with orthostatic hypotension (PD + OH), PD without OH (OH), and pure autonomic failure (PAF)) compared to controls. Cardiac septal and left ventricular chamber 18F‐DA–derived radioactivity concentrations were increased in multiple system atrophy (MSA) compared to controls. Liver 18F‐DA–derived radioactivity was increased in PD + OH, PAF, and MSA compared to controls. 18F‐DA–derived radioactivity concentration in submandibular glands was decreased in PD compared to controls. The groups did not differ in radioactivity in the spleen, pancreas, stomach, renal cortex, renal pelvis, or thyroid.
Table S2. Reports numerical data for postmortem tissue norepinephrine (NE) in the different organs of interest in the Lewy body (LB) group (Parkinson’s disease = 37, pure autonomic failure = 3) and the non‐Lewy body group (controls = 35, multiple system atrophy = 5). Myocardial NE was substantially decreased in the LB group (P < 0.0001) compared to the non‐LB (MSA and controls) group.


