Abstract
Neuronal injury is a universal event that occurs in disease processes that affect both the central and peripheral nervous systems. A blood biomarker linked to neuronal injury would provide a critical measure to understand and treat neurologic diseases. Neurofilament light chain (NfL), a cytoskeletal protein expressed only in neurons, has emerged as such a biomarker. With the ability to quantify neuronal damage in blood, NfL is being applied to a wide range of neurologic conditions to investigate and monitor disease including assessment of treatment efficacy. Blood NfL is not specific for one disease and its release can also be induced by physiological processes. Longitudinal studies in multiple sclerosis, traumatic brain injury, and stroke show accumulation of NfL over days followed by elevated levels over months. Therefore, it may be hard to determine with a single measurement when the peak of NfL is reached and when the levels are normalized. Nonetheless, measurement of blood NfL provides a new blood biomarker for neurologic diseases overcoming the invasiveness of CSF sampling that restricted NfL clinical application. In this review, we examine the use of blood NfL as a biologic test for neurologic disease.
Introduction
The last few decades have seen important advances in the field of neuroscience and this improved knowledge of pathological mechanisms has led to the development of new drugs for neurological disorders. Sophisticated imaging techniques have allowed direct visualization of disease processes in the brain. A critical biologic component of neurologic disease is neuronal damage and a means to measure and quantify it would provide a much needed clinical and research tool. In this context, neurofilament light chain (NfL), a cytoskeletal protein specific for neurons has emerged as blood biomarker able to capture neuronal damage in a wide variety of neurologic conditions. NfL is released into the cerebrospinal fluid (CSF) and blood following damage to both central 1 and peripheral neurons. 2 , 3 , 4 The exact mechanism by which NfL is released from damaged neurons is not completely understood though it most likely is a direct consequence of the loss of cell membrane integrity. 5 The possibility that the increase in NfL in neurologic diseases is due to a compensatory overproduction is not supported by gene expression studies which revealed that NfL expression is not increased in amyotrophic lateral sclerosis (ALS) 6 and Alzheimer disease (AD) 7 patients. The clinical aspects of neurofilaments were reviewed by Khalil, et al in 2018. 1 Since then, over 200 papers have been published on the subject of blood NfL. More recently the biology of NfL was reviewed by Gafson et al. 8
The first report of the use of NfL as biomarker of neuronal damage was in 1989 when Karlsson, Rosengren, and colleagues purified NfL and used polyclonal antibodies that were used to detect NfL by immunoblot and ELISA methods. 5 The availability of monoclonal antibodies generated with the use of hybridoma technology enabled the spread of NfL quantification in CSF. 9 Nonetheless, the invasiveness of CSF sampling restricted its clinical application. Initially, the ability to measure NfL in the blood was hampered by the lack of a blocker for heterophilic antibodies 10 and detection levels did not have sufficient sensitivity to quantification of NfL in blood. 11 Improved sensitivity was reached using a electrochemiluminescence (ECL) platform which enabled quantification of NfL in blood samples. 12 The breakthrough that enabled widespread measurement of NfL in blood was developed by Blennow and Zetterberg who adapted single‐molecule array technology (SIMOA®, Quanterix, US). 13 This technology leads to a higher sensitivity by the use of microwells which were miniaturized a thousand‐fold from the 6.4 mm in diameter in ELISA/ECL assays to 4.25 µm in diameter in the SIMOA® assay. 14 Furthermore, NfL is then captured on paramagnetic microbeads and single antibody–antigen complexes are dispersed to 216,000 microwells thus requiring a much smaller number NfL molecules to emit a detectable signal than previous technologies. 14 The SIMOA® technology has created a sensitive and reproducible method to quantify NfL in the blood of both patients and healthy controls. 11 The reproducible preanalytical and analytical performances of NfL measurements has led to its use in the multiple centers worldwide.
The validation of blood NfL as a biomarker of neuronal damage took advantage of samples from a large number of biorepositories and it was found across multiple neurological disorders, that the neuronal damage quantified by NfL reflected clinical and imaging measures of disease. 1 NfL levels were also found to have prognostic value 15 , 16 , 17 and were able to detect treatment effects. 18 Importantly, blood NfL is not specific for one disease and its levels can also be influenced by physiological processes including body‐mass‐index, diabetes, and hypertension. Longitudinal studies on multiple sclerosis (MS), traumatic brain injury, and stroke show an increase in NfL levels over days which remained elevated over months. 19 , 20 , 21 , 22 The kinetics of NfL was explored in diseases with a known timepoint for the neuronal damage, whereas in chronic diseases such as Alzheimer’s Disease (AD) and Parkinson’s Disease (PD) it remains difficult to investigate acute changes over time. Therefore, it may be difficult to determine with a single measurement when the peak of NfL is reached and when the levels are normalized. Because blood NfL levels are measured as a concentration, a change in the blood volume can affect NfL levels. Other biomarkers such as neurofilament heavy chain (NfH) are also being investigated 23 , 24 ; because of the standardized measurement method of NfL and its growing recognition in various clinical research centers worldwide we have focused this review on blood NfL. Here we review the physiological and pathological conditions that are most relevant for the clinical implementation of NfL (Fig. 1).
Figure 1.
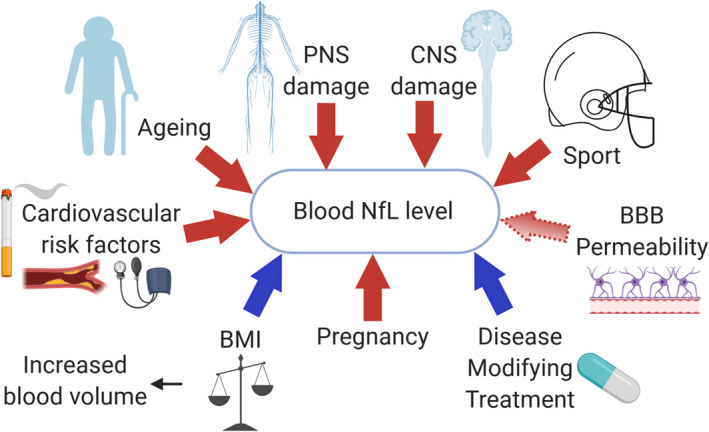
Physiological and pathological factors increasing or decreasing the blood levels of NfL. NfL is released as a consequence of neuronal damage. A rise in NfL (red arrows) is not specific for a specific disease factor and may be caused by both neurodegenerative diseases or a head impact during sports. Cardiovascular risk factors and aging may cause subclinical damage due to silent ischemic events. NfL is not specific for the central nervous system and occurs with injury to the peripheral nervous system. BBB permeability may influence blood NfL levels (light red arrow). Some factors may contribute to a decrease in NfL (blue arrows) including an increase in blood volume associated with BMI. Pregnancy is associated with a physiological increase in NfL. Treatment that decreases neuronal damage results in lower blood NfL levels. BBB, blood–brain barrier; BMI, body‐mass‐index; CNS, central nervous system; PNS, peripheral nervous system.
Preanalytical and Analytical Variability of Blood NfL Measurements
Preanalytical variability
refers to the variation that occurs between the time the sample is collected and when the sample is analyzed. Plasma or serum can be used for quantification of blood NfL and NfL levels between them are highly correlated 25 , 26 thus we refer to blood NfL levels in this review. Of note, EDTA plasma showed lower NfL concentrations than serum, 27 though it remains unclear whether this applies also to specimen collected by heparin or citrate treatment. Thus, direct comparisons of absolute values can only be done on the same source fluid, that is, plasma or serum. Several studies investigated the stability of NfL, both in serum and plasma, by exposing aliquots of the same sample to multiple freeze‐thawing steps or prolonged exposure at room temperature. 12 , 25 , 27 , 28 , 29 The consensus is that there is good stability of the analyte following multiple freeze‐thawing 12 , 28 , 29 , 30 as well as when left at room temperature. 12 , 25 , 27 , 28 , 29 (Table 1) The preanalytical stability of a biomarker is a basic property for assuring reduced inter‐center variability particularly when using different sampling protocols. 31 The stability of blood NfL at room temperature allows the collection of samples by normal post shipments, 25 thus enabling the recruitment of study participants from diverse geographical locations.
Table 1.
Factors influencing preanalytical and analytical variability of blood NfL.
| Factor | Type of test | Platform | Number of samples | Influence on NfL levels | References |
|---|---|---|---|---|---|
| Freeze‐thaw cycles | 5 freeze‐thaw cycles | ECL | 4 | ‐ | [12] |
| 4 freeze‐thaw cycles | 3 | ‐ | [28] | ||
| 4 freeze‐thaw cycles | SIMOA® HD‐1 | 12 | ‐ | [30] | |
| 3 freeze‐thaw cycles | 6 | ‐ | [27] | ||
| 1,2,3 freeze‐thaw cycles | 5 | Increase, P < 0.05 | [29] | ||
| Exposure to room temperature | 1 to 8 days at 4°C | ECL | 4 | ‐ | [12] |
| 1 to 8 days at RT | 4 | ‐ | [12] | ||
| 8 days at RT | 3 | ‐ | [28] | ||
| 1 to 7 days at RT | SIMOA® HD‐1 | 241 | ‐ | [25] | |
| 5 days at 4°C | 5 | Increase, P < 0.05 | [29] | ||
| 5 days at RT | 5 | Increase, P < 0.05 | [29] | ||
| 3 or 5 days at RT | 12 | ‐ | [27] | ||
| Position‐in plate | Sample processing order | SIMOA® HD‐1 | 24 | Later processed samples present higher NfL levels | [34] |
| Serum vs. plasma levels | Correlation | SIMOA® HD‐1 |
16 129 |
r: 0.684, P = 0.009 r: 0.96, P < 0.0001 |
[25] [26] |
| Comparison absolute values | 129 | 23.1% lower in plasma | [26] | ||
| 52 | 12.0% lower in EDTA plasma | [27] |
‐, no influence; ECL, electrochemiluminescence; SIMOA® HD‐1, single‐molecule array analyzer HD‐1 (Quanterix, Bellerica, US); RT, room temperature.
Analytical variability
The majority of published studies on blood NfL use assays based on two working protocols that primarily differ in the blocker used to control for nonspecific binding. One assay has been used by University Hospital in Basel 32 and the other is a commercial kit (NfL Advantage Kit®, Quanterix, US) that has now become the primary kit used by investigators studying blood NfL. Both protocols utilize the SIMOA® platform. Because of the differences of the blockers used in these assays, studies using the commercial kit obtain half the levels of blood NfL compared to the other assay. The reason could be a different efficacy of casein compared to bovine serum albumin in serum/plasma matrix or to the white color that casein gives to the assay diluent, concept known in fluorescence‐ and luminescence‐based assays where white‐colored plates are used to enhance the signal. The performance of these two assays primarily differ in the “spike recovery” in which protein is added to the sample and the measured concentration compared to the expected (“spiked”) concentration. 31 Disanto and colleagues reported a spike recovery of 107% for their assay that used casein‐based blocker, 32 whereas the commercial kit that uses bovine serum albumin reported 68% (see Advantage Kit® data sheet). This could explain the two‐fold difference between the two. 32 , 33 In addition, on the SIMOA® platform, depending on how the samples are loaded, there may be a time difference between the reading of the first and last sample. It has been shown that a position‐effect bias may occur in which the order of how the samples are processed can affect the NfL concentration. 34 The adoption of NfL in clinical care requires reproducible and stable measurements. The performance of SIMOA® NfL kit is under evaluation in a multicenter study. 35 Preliminary results showed an excellent performance in terms of intercenter variability. 35 The confirmation of these findings could lead to the worldwide implementation of NfL measurements in clinical chemistry laboratories. The biomarker field is rapidly evolving and other technologies like the proximity extension assay based on the Olink platform hold promise as they enable the parallel determination of several other biomarkers. 36 Established and new platforms will need to provide identical NfL levels to ensure a role for NfL in clinical practice.
Physiological Factors Influencing Blood NfL Concentration
In addition to neuronal damage secondary to nervous system damage, other factors may affect blood NfL levels (Table 2). These need to be taken into account when measuring blood NfL and when choosing control populations for studies.
Table 2.
Factors influencing blood NfL levels in health and disease.
| Type of factor | Factor | Diagnosis | Association with NfL/ increase per unit | Reference |
|---|---|---|---|---|
| Demographic | Age (y) | HC |
r: 0.7, P < 0.0001 +0.8 ‐ 3.7% (range) By age category: 40‐50y: 0.9%; 50‐60y: 2.7%; >60y: 4.7% |
[49] |
| MS | +1.5 ‐ 1.8% (range) | [16, 32] | ||
| AD | r: 0.65, P < 0.001 | [29, 90] | ||
| ALS | r: 0.32, P: 0.065 | [101] | ||
| FTD |
r: 0.770, P < 0.0001 r: 0.327, P: 0.002 |
[132] [131] |
||
| PD |
r: 0.25, P < 0.001 r: 0.78, P < 0.0001 |
[92] [46] |
||
| AN | P < 0.0001 | [59] | ||
| Stroke | +7.6% | [118] | ||
| Sex | ALS | Higher in females, P < 0.0001 | [47, 52] | |
| Race/ethnicity | HC (DM2) | Higher in non‐Caucasian patients, P < 0.01 | [55] | |
| Cardiovascular risk factors and scores | Diabetes mellitus type 2 | AF | +62.5% in patients with diabetes, P < 0.05 | [56] |
| HbA1C | HC (DM2) | Increased by higher Hb1AC, P < 0.01 | [55] | |
| BMI | HC | Decreased with higher BMI, P < 0.01 | [58, 59] | |
| AN | Decreased with higher BMI, P: 6.9x10‐12 | [59] | ||
|
Systolic blood pressure |
HC (DM2) AD |
Increased with higher blood pressure, P < 0.01 Increased in hypertensive patients |
[55] [93] |
|
| CHA2DS2VASc | AF | +22.3% per score point | [56] | |
| Smoke | MS | +20% in smokers | [136] | |
|
Sport related traumatic brain injury |
Hockey | HC | Increased 1h after head trauma and further increasing over the next days | [20, 126] |
| Football | Increased during sport season, P < 0.001 | [123] | ||
| Soccer | +26% 1h after head trauma; +311% 22 days later | [125] | ||
| Box | Increased after bout and in recovery phase, P < 0.0001 | [20] | ||
| Physiological | Pregnancy | HC |
Increased postpregnancy, P < 0.0001 Increased during pregnancy, P < 0.001 |
[137] [61] |
| MS | Decreased during pregnancy, P < 0.001 | [62] | ||
| Blood–CSF barrier | Mixed patients’ group | Higher with higher Qalb/CSF flow but not correlated | [40] | |
| MS | Not correlated | [42] | ||
| Increased blood NfL with higher Qalb permeability, P < 0.001 | [41] | |||
| SMA | Not correlated | [43, 138] | ||
| GFR < 60ml/min per 1.73 m2 | HC (DM2) | Increased in patients with low GFR, P < 0.01 | [55] |
AD: Alzheimer disease; AF: atrial fibrillation; BBB: blood–brain barrier; CIS: clinically isolated syndrome; DM2: diabetes mellitus type 2; GFR: glomerular filtration rate; HbA1C: glycated hemoglobin; HC: healthy controls; MS: multiple sclerosis; qAlb: albumin quotient; SMA: spinal muscular atrophy; y: years
CSF versus blood quantification of NfL
NfL has been identified in both the CSF and blood. 12 Because there are much higher levels of NfL in the CSF versus blood, initial studies which‐correlated NfL levels with neurologic diseases focused on the CSF. 5 The degree to which permeability of blood–brain barrier (BBB) and blood–CSF barrier (BCB) influences the levels of blood NfL is not clear. Acute inflammatory processes of the CNS such as that which occurs in multiple sclerosis can cause acute disruption of the BBB which can be envisioned by gadolinium‐enhancing areas on the MRI. 37 Nonetheless, it is not clear the degree to which increased blood NfL in MS is related to BBB disruption or to CNS damage as increased NfL is observed in the CSF which is independent of BBB breakdown. Explorative works used the ratio between CSF and serum albumin as an estimation of the BBB permeability although this ratio is a marker of blood CSF barrier (BCB) and should be interpreted as such. 38 , 39 Patients with the highest CSF/serum albumin ratio also had the highest CSF and blood NfL levels, 40 , 41 suggesting that an altered permeability of the BCB contributes to serum NfL, though this relationship was not observed in all studies. 40 , 41 , 42 , 43 Of note, there is increased disruption of the BBB with aging which may contribute to the increased levels of blood NfL with age‐related neurodegenerative diseases. 37
Investigation of the correlation between the NfL levels in CSF and blood was used to establish that blood NfL measurements accurately reflected what was happening in CNS (Table 3). Factors that affect blood NfL levels independent of the CSF such as BMI (discussed below) or peripheral neuropathies weaken the correlation. For example, in MS a 10% increase in CSF NfL corresponded to an increase of 5.9% in the blood 32 and the ability to predict and detect disease activity was stronger for CSF than blood NfL levels. 44 The CSF/blood NfL correlations show variability among healthy controls (range 0.35–0.77), underscoring the importance of obtaining comprehensive health data apart from neurologic diseases. Moreover, conditions with the lowest NfL levels are associated with a weaker correlation between the CSF and blood compartments; for example, Parkinson Disease (PD) 45 , 46 and healthy controls 15 , 27 , 44 , 46 , 47 present on average a moderate correlation of 0.52 with an average blood NfL of 11 pg/mL compared to ALS patients 47 , 48 which show a strong correlation of 0.79 with an average blood NfL of 135 pg/mL (Table 3).
Table 3.
Correlation between CSF and blood NfL levels.
| Diagnosis | Correlation strength | Blood NfL levels (pg/mL, range/IQR/±SD) | Fluid | Platform | CSF NfL levels (pg/mL, range/IQR/±SD) | Platform | Ref. |
|---|---|---|---|---|---|---|---|
| HC |
r: 0.350, P: 0.014 r: 0.574, P: 0.008 r: 0.59, P: 0.004 r: 0.772, P < 0.0001 r: 0.39, P: 0.029 |
‐ 22.0, (12.0 ‐ 36.5) 11, (8 ‐ 14) ‐, (2.8 ‐ 53.8) 11.5 (± 6.5) |
Serum Serum Serum Serum Serum |
Simoa HD‐1* ECL Simoa HD‐1 Simoa HD‐1 Simoa HD‐1 |
‐ 466.5, (338.7 ‐ 651.7) 212, (151 ‐ 289) ‐ 1.265 (±551) |
Simoa HD‐1* ECL ELISA Simoa HD‐1 NA |
[15] [47] [44] [27] [46] |
| AD |
r: 0.612, P < 0.0001 r: 0.580, P < 0.001 r: 0.568, P < 0.001 r:0.666, P = 0.003 |
‐ ‐, (31.0‐44.1) 46.8, (32.7‐70.7) 38.1, ‐ |
Serum Plasma Plasma Serum |
Simoa HD‐1 Simoa HD‐1 Simoa HD‐1 Simoa HD‐1 |
‐ ‐, 1070‐2778 608.3 (429.1, 817.7) 1595, ‐ |
Simoa HD‐1 ELISA ELISA ELISA |
[15] [88] [93] [134] |
| ALS |
r: 0.781, P < 0.001 r: 0.79, P < 0.0001 |
90, (54.5 ‐ 151.0) 179, ‐ |
Serum Serum |
ECL ECL |
7304, (4376 ‐ 11736) ‐ |
ECL ELISA |
[47] [48] |
| FTD | r: 0.706, P < 0.0001 | 56.9, ‐ | Serum | Simoa HD‐1 | 2948, ‐ | ELISA | [134] |
| HAD | r: 0.89, P < 0.0001 | 114 (46.0 ‐ 235) | Plasma | Simoa HD‐1 | 16185 (1513 ‐ 43010) | ELISA | [13] |
| HD | r:0.868, P < 0.0001 | 31.7 (24.9 ‐ 50.6) | Plasma | Simoa HD‐1 | 1871, (1312 ‐ 2461) | ELISA | [139] |
| MS |
r: 0.77, P < 0.001 r: 0.79, P < 0.0001 r: 0.72, P < 0.0001 r: 0.63, P < 0.001 |
35.9, (22.1 ‐ 61.7) 16.4 (±14.4) 25.0 (±43.9) 17, (12 ‐ 22) |
Serum Plasma Serum Serum |
Simoa HD‐1* Simoa HD‐1 Simoa HD‐1 Simoa HD‐1 |
1521, (814 ‐ 2888) 2368 (±1947) 2368 (±1947) 895, (300 ‐ 2060) |
Simoa HD‐1* Simoa HD‐1 Simoa HD‐1 ELISA |
[32] [26] [26] [44] |
| PD |
r: 0.589, P < 0.001 r: 0.34, P:0.012 |
9, (4 ‐ 19)** 10.4, (±4.9) |
Plasma Serum |
Simoa HD‐1 Simoa HD‐1 |
896, ‐ 1.249, (±666) |
ELISA NA |
[45] [46] |
| TBI ‐ Boxers | r: 0.86, P: 0.0003 | 30, (20‐70)** | Serum | Simoa HD‐1 | 845, ‐ | ELISA | 20 |
AD: Alzheimer’s Disease; ALS: amyotrophic lateral sclerosis; FTD: frontotemporal dementia; HAD: HIV‐associated dementia; HD: Huntington disease; IQR: Interquartile range; MS: multiple sclerosis; ‐: not available; PD: Parkinson’s disease; TBI: traumatic brain injury.
Basel protocol, the concentrations in blood are ~ 2 fold higher compared to the commercial Simoa NfL kit.
Concentrations derived from Figure.
Demographical characteristics
Age. Studies report an increase in NfL levels with increasing age both in patients and healthy individuals. The yearly increase in NfL was most pronounced in subjects above 60. 49 Furthermore, the variability of NfL levels among older individuals was higher than in younger groups. 49 The variability at older ages is important to consider when comparing NfL levels of elderly patients with age‐matched healthy controls. Considering the higher prevalence of comorbidities in the elderly population, 50 the increase in blood NfL levels with age may be driven by the emergence of co‐morbidities with age rather than by the aging process alone. The influence of comorbidities on blood NfL levels has not been broadly studied and is often not routinely collected even in observational studies. 51
Sex. Blood NfL does not appear to be influenced by sex either in healthy individuals 49 or those with neurologic disease apart from ALS in which blood NfL levels were reported to be higher in female versus male ALS patients. 47 , 52 This can be explained by the higher severity of the disease in female versus male patients, with a higher prevalence of the bulbar ALS form (38% in female vs. 27% in male) and shorter survival in female patients (13.6 months in females vs. 14.8 months in male) reported on a large study of >1000 ALS patients. 53 Of note, a recent meta‐analysis reported that CSF NfL was 26% higher in healthy males compared to females, 54 thus suggesting that CSF measurement could be more sensitive to NfL changes than blood measurements.
Race/ethnicity. Although not extensively investigated one study reported higher NfL in non‐Caucasian subjects. 55
Cardiovascular risk factors
Dietary habits, smoking, and blood pressure are important factors associated with the risk of cerebrovascular disease which is more prevalent in older populations 50 who are also at increased risk for Alzheimer’s and Parkinson’s disease. Individual risk factors 55 as well as combined factors (CHA2DS2‐VASc) 56 were associated with increased levels of blood NfL. Thus, increased levels of blood NfL in older subjects could in part be due to subclinical CNS ischemic damage and a clue to underlying hypoperfusion of neuronal tissue due to cardiovascular disease. 21 In addition, hypoperfusion that occurs in diabetic neuropathy and that affects peripheral nerves could be associated with increased blood NfL as was shown for NfH. 57 Blood NfL levels could thus be considered as a marker of peripheral neuropathy and cerebrovascular disease.
Body mass index (BMI)
Body fluid biomarkers are measured as the concentration that is a ratio between the quantity of analyte and the volume of diluent. Some studies reported that decreased blood NfL levels were associated with increased BMI, and therefore blood volume, in healthy adults. 58 , 59 Interestingly, it was shown that even if the blood volume was inversely correlated with the concentration of NfL in blood, it did not correlate with CSF NfL levels. 58 Thus, variations in blood volume/BMI were not reflected by a proportional change in CSF volume. Thus, two individuals with the same CSF NfL but different BMIs will have different concentrations of NfL in the blood. During pregnancy, there is an increase in blood volume (approximately 45%) though this may vary between individuals. 60 Paradoxically, in both women at risk that did not develop preeclampsia 61 and in healthy pregnant women 62 blood NfL levels progressively increased during pregnancy until delivery, perhaps due to the developing brain of the fetus. It may be appropriate to consider BMI and pregnancy as a confounder when measuring NfL.
Blood NfL in Neurological Diseases
Multiple sclerosis
MS is a chronic, inflammatory, and neurodegenerative disease of the CNS. 63 The approval of a large number of treatments for MS and the heterogenous clinical presentation raise the question of which drug is best used in which patient and for how long of a period of time (Table 4).
In initial studies of blood NfL using ECL technology showed an association between serum NfL and brain atrophy over 2 years and number of MRI lesions, 64 though it had a poor prognostic value for patients with clinically isolated syndrome later converting to MS. 65 These studies on a small group of patients were limited by the sensitivity of the assay. 11 The high sensitivity of the SIMOA® technology provided the reliable quantification of blood NfL in MS. 11 On the group level, blood NfL in MS was higher than in healthy controls 32 , 44 , 62 , 66 with progressive MS having higher levels of blood NfL than relapsing MS. 16 , 32 The increased levels of blood NfL in progressive versus relapsing MS was unexpected and contrasted with lower levels of NfL in the CSF in progressive versus relapsing MS. 67 This remains unexplained but could be related to a breakdown of the BBB in progressive MS linked to aging and/or a contribution from the peripheral nervous system. Ascherio’s group reported that in a military cohort, blood NfL was able to identify neuronal damage 6 years prior to the clinical disease onset, 68 thus neurodegeneration was already present at the prodromal stage of MS. A number of studies have found that disease activity as measured by clinical or MRI assessment is associated with an increase in blood NfL levels in both adult 16 , 32 , 66 , 69 , 70 , 71 , 72 , 73 and pediatric patients. 74 , 75 Of note, the association of blood NfL with disability was less in progressive MS, which may be related to the older age of this population that also have comorbidities. 32 Elevated levels of blood NfL following a relapse were detectable for as long as 60 days later. 18 , 19 , 32 Prolonged elevation of blood NfL after neurologic damage was also observed in traumatic brain injury and stroke (discussed below). This prolonged elevation may be related to slow degradation in the blood or continued release from the brain into the blood.
A decrease in blood NfL levels has also been observed with several disease‐modifying treatments 25 , 32 , 42 , 76 and blood NfL may be able to segregate treatments by their potency. 33 Observational studies suggested a prognostic value of NfL in being able to predict brain and spinal cord atrophy over 2, 5, and 10 years. 16 , 69 , 70 The known reduction in disease activity during pregnancy 77 was reflected by similar blood NfL levels among pregnant healthy women and MS patients. 62 It is not known whether this was related to the physiological increase in blood volume that occurs during pregnancy. 60
With a better understanding of the role of NfL and the development of sensitive and reproducible assays, a fertile ground has developed for the use of blood NfL in clinical studies. In the FREEDOMS trial, there was a decrease in blood NfL with fingolimod treatment versus placebo and a decrease in blood NfL with fingolimod versus interferon in the TRANSFORMS study. 18 Furthermore, blood NfL levels discriminated against the active from the control arm after only 6 months of treatment. 18 , 26 It was shown in clinical trials that there was an association between NfL, MRI, and clinical disease activity. 18 , 78 In the ASCLEPIOS trials of Ofatumumab versus teriflunomide, the pronounced effect of treatment on both MRI and disease activity was accompanied by a marked decrease in blood NfL levels. 79 In the EXPAND study of siponimod in progressive MS, there was a decrease in blood NfL and was more pronounced in those who had relapses. 80 Overall, high‐frequency determinations of blood NfL can be a cost‐effective tool to detect clinical and subclinical disease activity earlier than a routine 6‐/12‐monthly visit. Notably, high NfL can have other causes than MS and low NfL could be a more precise tool to exclude disease activity. 81 Thus, low NfL in clinically stable patients could indicate that costly assessments such as MRI are not needed at that time point. Of note, neuronal damage secondary to comorbidities can be an important confounder in the older population of progressive MS patients. In fact, the levels detected in the healthy population older than 60 years of age ranged from 7.0 pg/mL to 106.6 pg/mL 49 making the use of reference ranges in the elderly MS patients potentially misleading. Therefore, the NfL level may be compared with a previous NfL level from the same patient rather than with an age‐matched healthy control population. In clinical trials, the use of blood NfL as a biomarker could reduce the number of patients required to show a treatment effect, follow‐up time, and trial costs. 82 In particular, post hoc analysis of the FREEDOMS trial showed that NfL was more sensitive to treatment effect than MRI measures, therefore NfL was suggested as a potential surrogate endpoint that was noninferior to MRI. 82 Of note, the earliest decrease in NfL was observed at 3–6 months after treatment initiation. 18 , 26 , 79 However, most trials do not have a sampling timepoint earlier than 3 months from baseline. Thus, a treatment effect might be appreciable even earlier.
Alzheimer disease
AD is characterized by the accumulation of β‐amyloid (A), neurofibrillary tangles plaques (T), and neurodegeneration (N) in the brain parenchyma. 83 The core AD CSF biomarkers (Aβ42, phospho‐tau, total‐tau) enabled the quantification of these pathological processes supporting the diagnosis of AD. 83 , 84 However, the complexity of the blood matrix, peripheral sources, and protein degradation have hampered the translation of this biomarker panel in the blood, 84 , 85 although new assays for tau and phospho‐tau are being investigated. 85 , 86 The pathological process of AD begins years before symptoms are manifest. 83 Thus, a biomarker that facilitates early detection of the disease would enable early treatment and better prognosis for AD patients with familial AD. 87 Studies in both sporadic and familial AD demonstrated that blood NfL levels correlate with cortical thinning and cognitive decline and have prognostic value for future decline. 15 , 29 , 88 , 89 , 90 , 91 , 92 , 93 Asymptomatic individuals that carry mutations had increased NfL levels and a higher rate of increase 9–15 years before symptom onset than individuals without a mutation. 15 , 94 , 95 , 96
The incidence of sporadic AD increases with increasing age. The average age of onset is 80 years for the sporadic form (99% of patients) and 45 years for familial forms. 83 The older age of sporadic AD patients is associated with a higher prevalence of comorbidities, including cardiovascular conditions, which is associated with CNS ischemic damage and subsequent release of NfL. This is a confounding co‐variable that must be taken into account when interpreting blood NfL in patients suspected of having sporadic forms of AD (Fig. 2). This confounder would not be present in younger patients suspected of having familial AD. Of note, the change in blood NfL levels measured longitudinally over time in an individual had a better prognostic value than the absolute blood NfL level at a single time point. 15 Thus, the confounding effect of comorbidities was controlled for using longitudinal measurements. Blood NfL is a nonspecific measure of neurodegeneration without the diagnostic power of the AD core CSF biomarkers. 86 However, the simplicity of a blood NfL measurement enables the monitoring of neurodegeneration over time and provides a tool for detecting the effect of prospective treatments. 97
Figure 2.
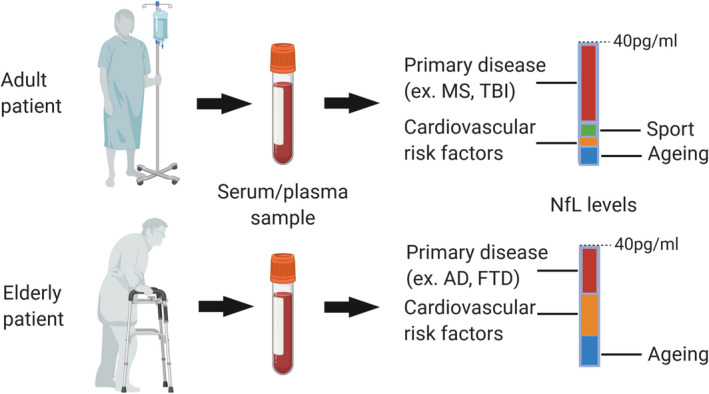
Interpretation of NfL levels in adult and elderly patients. The relative contribution of the primary disease to the overall NfL level is affected by other causes of neuronal damage. This is particularly relevant in the older populations, where aging and comorbidities lead to a substantial variability between individuals. The increase in neuronal damage caused by the primary disease, for example, AD, may be masked by silent damage due to comorbidities. AD, Alzheimer disease; FTD, frontotemporal dementia; MS, multiple sclerosis; NfL, neurofilament light chain; TBI, traumatic brain injury.
Amyotrophic lateral sclerosis
ALS is a neurodegenerative disease of the upper and lower motoneurons. 98 The diagnosis of ALS is based on the exclusion of alternative diagnoses. 98 NfL shows in this disease the strongest diagnostic value. In fact, NfL levels may have the ability to discriminate ALS from ALS‐mimics. 48 , 99 Although the majority of ALS cases are sporadic, a small proportion of patients are familial. 100 However, in those carrying a mutation associated with familial ALS, an increase in NfL prior to symptom onset could not be clearly identified, though the samples may have been drawn too soon before symptom onset. 100 A longitudinal study reported that patients that converted to the symptomatic stage had blood NfL levels higher than healthy controls up to 1 year prior to symptom onset, 101 findings confirmed by an additional study by the same group. 102 Blood NfL has also been investigated as a prognostic biomarker once the disease has been diagnosed. Several studies found that NfL levels at symptom onset were prognostic of the disease progression rate. 47 , 48 , 52 , 100 , 103 Thus NfL levels at symptom onset could be used to stratify patients into groups with a similar prognosis in clinical trials. Of note, the elevated levels of blood NfL remain relatively stable throughout the disease course. 47 , 48 , 101
As emerged from a recent meta‐analysis on CSF NfL, ALS presents particularly high NfL levels. 54 This is explained by the severity of the disease, but also by the fact that the degeneration affects large myelinated axons that have a high expression of NfL, which could be further amplified by the long length of the axons. 104
The phosphorylated form of NfH, the heavy subunit of neurofilaments, is being investigated in ALS as a blood biomarker. 105 Similar to NfL, increased pNfH levels have been observed prior to symptom onset, 102 discriminated ALS from ALS mimics 99 and was associated with the disease progression rate. 106 Studies that investigated both NfL and pNfH suggested that pNfH was better at discriminating ALS from other motor neuron diseases, 107 thought NfL was better at reflecting disease severity and progression. 108 , 109 Nevertheless, these studies were not powered or aimed at comparing the performance of the two biomarkers and confirmatory studies are needed. A phase 1‐2 clinical trial that explored the safety of Tofersen, a treatment based on antisense oligonucleotides targeting SOD1 messenger RNA, resulted in not only a decrease in CSF SOD1 levels but also a decrease in both NfL and pNfH levels whereas levels in the placebo arm remained stable. 110
Affected neurons in ALS have altered metabolism that results in a decreased production of NfH versus NfL. 111 This alters the normal stoichiometry of the three neurofilament proteins from 7:3:2 (NfL:Neurofilament Medium Chain (NfM):NfH, respectively) to 24:2.4:1.6 (NfL:NfM:NfH) 111 and can be found accumulated as aggregate in the motor neurons. 112 This is a further explanation for increased NfL levels. The added value of measuring both pNfH and NfL is yet to be determined. Nevertheless, the changes in neurofilaments stoichiometry suggest that their clinical value might depend on the disease stage. Thus, the determination of both NfL and pNfH might ensure better diagnostic and prognostic performances at earlier and later stages of the disease.
Parkinson disease
PD is associated with the accumulation of α‐synuclein in the CNS and the selective loss of dopaminergic neurons in the substantia nigra. 113 Studies of blood NfL levels in PD showed an elevation versus age‐matched healthy controls 45 , 92 , 114 though the differences were not as pronounced as with other CNS diseases. PD patients with elevated blood NfL levels had worse cognitive decline, 45 , 92 , 114 , 115 brain cortical atrophy, 115 and motor score. 45 , 92 Multiple system atrophy and progressive supranuclear palsy manifest similar symptoms as early stage of PD and are thus considered atypical Parkinsonian disorders. A correct diagnosis in the early stages may be difficult. 113 Blood NfL levels in these atypical forms of Parkinson’s disease are higher than in PD and thus may be used to help differentiate PD from atypical parkinsonian disorders. 45 , 46 As with Alzheimer’s disease and progressive MS the presence of comorbidities needs to be controlled for in interpreting blood NfL levels.
Stroke
Acute neuronal damage to the brain caused by a stroke may be related to hypoperfusion (ischemic stroke) or bleeding (hemorrhagic stroke). 116 In the first days after stroke, there is a continuous increase in blood NfL over time which remains increased over 3–6 months. 21 , 22 Thus, blood NfL levels reflect infarct size at 1 week but not 1 day after the stroke. 22 Furthermore, cerebrovascular events are accompanied by a rise in blood NfL proportional to the lesion burden 17 , 21 , 22 , 117 , 118 , 119 and clinical scores. 17 , 22 , 117 , 118 , 119 , 120 , 121 In addition blood NfL is prognostic for disability at 3 months 17 , 22 , 121 and at 3–7 years, 17 , 118 , 121 as well as survival at 17 years, 118 though not all studies have found a prognostic association. 119 , 120 Following an acute stroke, prophylaxis to prevent or reduce further events is initiated. 116 Thus, a blood biomarker that reflects subclinical events would help both in monitoring and in determining the best treatment for stroke prophylaxis. Indeed, blood NfL levels link to the risk of developing stroke in the years following the acute event 17 and low NfL may reflect a real‐time measure of effective prophylactic therapy. In addition, blood NfL may be helpful in those who have not had a stroke, but who are at risk for stroke, such as subjects with diabetes. 55 Blood NfL levels are increased by subclinical ischemic events 21 and the risk of these subclinical events is associated with cardiovascular risk factors. 116 Of note, the use of NfL in stroke should take into account the time between event and blood NfL measurement and the long half‐life of the biomarker.
Traumatic brain injury
Traumatic brain injury (TBI) can be a consequence of a penetrating or dull injury and can result in a mild, moderate or severe brain damage. 122 It has been repeatedly shown that brain trauma that occurs in sports such as football, 123 , 124 soccer, 125 hockey, 20 , 126 and boxing 20 , 127 result in increased NfL concentrations. Patients with different grades of TBI showed corresponding changes in NfL levels. 20 , 123 , 125 , 126 , 128 Interestingly, similarly to what has been described in stroke patients, NfL showed a progressive increase over the first 12 days. 126 , 128 Strikingly, neuronal damage quantified 24h postevent was associated with 1‐year disability outcomes. 128 NfL has been proposed as an objective biomarker of trauma severity which in turn may allow a more precise time‐window for recovery. 122 Because the majority of traumatic events are classified as mild, such subjects are only evaluated clinically when imaging with MRI might be appropriate. 122
Exposure to a second trauma before complete recovery can result in an augmented degree of brain injury 122 and chronic exposure to TBI is associated with the development of a chronic traumatic encephalopathy. 122 Notably, the levels of NfL may remain increased for months, 20 which could relate to slow degradation and long half‐life of NfL in the blood. In athletes, estimation of the brain damage is critical for avoiding premature exposure to further trauma. The use of blood NfL may provide an additional measure to assess brain damage and help guide in long‐term management and treatment of athletes that undergo repeated head injury. Blood NfL could also find usefulness in other type of head trauma that may occur in automobile accidents or in the military. Elevated blood NfL levels in subjects with other neurologic diseases such as MS should take into account whether the subject has other risk factors, such as recent head trauma.
Frontotemporal dementia
Frontotemporal dementia (FTD) is characterized by a range of symptoms from personality changes to aphasia. 129 The differential diagnosis includes other forms of dementia and primary psychiatric disorders. Blood NfL was able to discriminate patients with the behavioral form of FTD from patients with primary psychiatric disorders. 130 , 131 In 10% of cases FTD is caused by an autosomal‐dominant mutation in C9orf72 (a common mutation to ALS), or MAPT or GRN genes. 129 Studies on the presymptomatic stage showed that mutation carriers tend to have increased NfL when older than 48 years of age 132 thought this was not described in a previous study. 133 However, at symptom onset blood NfL discriminated FTD patients from healthy controls. 130 , 132 , 133 , 134 , 135 Clinical subtypes could not be discriminated by blood NfL, 135 but higher concentrations reflected worse clinical and MRI measures of disease activity 131 , 132 , 134 , 135 as well as shorter survival. 133 Thus, blood NfL could be used to support the diagnosis of the behavioral form of FTD, monitor disease progression, and prognosis of FTD. Of note, some FTD patients also have ALS 129 which itself causes an increase in blood NfL levels and should be considered when interpreting blood NfL.
Conclusion and Outlook
Neuronal injury is a universal event that occurs following damage to the nervous system and the measurement of blood NfL has emerged as an important biomarker for a wide variety of nervous system conditions. Nevertheless, NfL does not differentiate disease processes and other factors besides the primary neurologic disease including age, pregnancy, BMI, diabetes, cardiovascular risk factors, and unrecognized head trauma can be confounding factors that influence blood NfL and thus should be taken into account when assessing blood NfL in individual patients. The use of reference ranges to interpret NfL levels in young patients is appropriate but could be misleading in elderly patients because of the physiological variability observed with aging. The study of elderly patients could benefit from longitudinal measurements that allow the evaluation of the individual NfL profile without the need for reference ranges to avoid inter‐individual variability.
A critical point in applying blood NfL levels to clinical practice is the understanding of the dynamics of NfL. The time required to reach peak of NfL concentrations in the blood following neuronal injury as well as its half‐life in the blood is not precisely known. This further underscores the importance of obtaining longitudinal measurements in an individual patient.
In summary, the measurement of blood NfL in the clinical practice provides a relatively simple and quantitative way to measure neuronal damage. Future studies will define its best use in clinical practice including the establishment of reference levels and a deeper understanding of the biological process responsible for the release of NfL in disease.
Author Contributions
CB, CT, and HLW did literature search, reviewed the manuscript, drafted the manuscript, and prepared the figures and tables. All authors read and approved the final version of the manuscript.
Conflict of Interest
CB has nothing to disclose. CT received personal compensation for advisory board/consulting for Biogen‐Idec, Merck Serono, Novartis, Sanofi, Bayer, Celgene, Alexion, and received financial support for research activities from Merck Serono and Novartis Pharmaceuticals. HLW reports grants from National Institutes of Health, National Multiple Sclerosis Society, Verily Life Sciences, Google Life Sciences, EMD Serono, Inc., Biogen, Teva Pharmaceuticals, and Novartis; grants and consulting fees from Sanofi US Services, Inc. and Genentech, Inc.; consulting and advising fees from Tilos Therapeutics; consulting and advising fees from Tiziana Life Sciences; consulting and advising fees from IM Therapeutics; personal, consulting, and advising fees from vTv Therapeutics; personal, consulting, and advising fees from MedDay Pharmaceuticals.
Table 4.
Blood NfL in neurological diseases.
| Disease | Role as biomarker | Current evidence associated with NfL | Ref |
|---|---|---|---|
| Multiple sclerosis | Susceptibility risk | Increased 6 years prior to symptom onset | [68] |
| Diagnostic | NA | – | |
| Disease monitoring | Association with clinical and MRI measures of disease activity | [16, 18, 32, 44, 64, 69, 71, 72, 76, 78] | |
| Treatment response | Decreased by effective treatment | [18, 26, 79, 80] | |
| Prognostic | Associated with conversion from CIS to MS, future EDSS, annualized relapse rate, brain, and spinal cord atrophy | [16, 18, 32, 44, 65, 69, 70, 73, 78] | |
| Alzheimer’s disease | Susceptibility risk | Increased NfL levels and annual rate of NfL change years prior to symptoms onset | [15, 94, 95, 96] |
| Diagnostic | NA | – | |
| Disease monitoring | Associated with cortical thinning and cognitive decline | [15, 29, 88, 89, 90, 92, 94] | |
| Treatment response | NA | – | |
| Prognostic | Associated with magnitude of future cortical thinning and cognitive changes | [15, 89, 93] | |
| Amyotrophic lateral sclerosis | Susceptibility risk | Increased months before symptom onset | [101] |
| Diagnostic | Higher in ALS compared to ALS‐mimics | [48, 99, 103] | |
| Disease monitoring | Negative – levels remain stable over disease progression | [47, 48, 101] | |
| Treatment response | NA | – | |
| Prognostic | Higher levels associated with shorter survival | [47, 48, 52, 100, 103] | |
| Parkinson’s disease | Susceptibility risk | NA | – |
| Diagnostic | Discriminate PD from atypical parkinsonian disorders | [45, 46] | |
| Disease monitoring | Increased with higher cognitive and motor decline and cortical atrophy | [45, 92, 114, 115] | |
| Treatment response | NA | – | |
| Prognostic | NA | ||
| Stroke | Susceptibility risk | Increased in individuals at higher risk of developing stroke | [17, 55] |
| Diagnostic | NA | – | |
| Disease monitoring |
Associated with clinical/MRI measures of stroke severity Increased with subclinical ischemic events |
[17, 21, 22, 117, 118, 119, 120, 121] [21] |
|
| Treatment response | NA | – | |
| Prognostic | Associated with future disability and survival | [17, 22, 118, 121] | |
| Traumatic brain injury | Susceptibility risk | NA | – |
| Diagnostic | NA | – | |
| Disease monitoring | Associated with degree of neuronal damage | [20, 123, 125, 126, 128] | |
| Treatment response | NA | – | |
| Prognostic | Associated with future disability outcomes | [128] | |
| Frontotemporal dementia | Susceptibility risk | Increased in presymptomatic stage | [132] |
| Diagnostic | Discriminate bvFTD from primary psychiatric disorders | [130, 131] | |
| Disease monitoring | Associated clinical and/or brain atrophy assessments | [131, 132, 134, 135] | |
| Treatment response | NA | – | |
| Prognostic | Associated with worse disease course | [133] |
NfL, serum neurofilament light chain; bvFTD, behavioral frontotemporal dementia; NA, not explored/not applicable.
Acknowledgment
Figures were created with BioRender.com.
Funding InformationCB is supported by a postdoctoral fellowship from the Swiss National Science Foundation (P400PM_191077). This study was funded in part by the U.S. Department of Defense (W81XWH‐18‐1‐0648 to TC), the National MS Society SUMMIT Consortium, and the Nancy Davis Center Without Walls.
Funding Statement
This work was funded by Swiss National Science Foundation grant P400PM_191077; U.S. Department of Defense grant W81XWH‐18‐1‐0648; National MS Society SUMMIT Consortium grant ; Nancy Davis Center Without Walls grant .
Contributor Information
Christian Barro, Email: cbarro1@bwh.harvard.edu.
Howard L. Weiner, Email: hweiner@rics.bwh.harvard.edu.
References
- 1. Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol 2018;14:577–589. [DOI] [PubMed] [Google Scholar]
- 2. Bischof A, Manigold T, Barro C, et al. Serum neurofilament light chain: a biomarker of neuronal injury in vasculitic neuropathy. Ann Rheum Dis 2018;77;1093–1094. 10.1136/annrheumdis-2017-212045 [DOI] [PubMed] [Google Scholar]
- 3. van Lieverloo GGA, Wieske L, Verhamme C, et al. Serum neurofilament light chain in chronic inflammatory demyelinating polyneuropathy. J Peripher Nerv Syst 2019;24:187–194. [DOI] [PubMed] [Google Scholar]
- 4. Sandelius A, Zetterberg H, Blennow K, et al. Plasma neurofilament light chain concentration in the inherited peripheral neuropathies. Neurology 2018;90:e518–e524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rosengren LE, Karlsson JE, Karlsson JO, et al. Patients with amyotrophic lateral sclerosis and other neurodegenerative diseases have increased levels of neurofilament protein in CSF. J. Neurochem 1996;67:2013–2018. [DOI] [PubMed] [Google Scholar]
- 6. Wong NKY, He BP, Strong MJ. Characterization of neuronal intermediate filament protein expression in cervical spinal motor neurons in sporadic Amyotrophic Lateral Sclerosis (ALS). J Neuropathol Exp Neurol 2000;59:972–982. [DOI] [PubMed] [Google Scholar]
- 7. Robinson CA, Clark AW, Parhad IM, et al. Gene expression in Alzheimer neocortex as a function of age and pathologic severity. Neurobiol Aging 1994;15:681–690. [DOI] [PubMed] [Google Scholar]
- 8. Gafson AR, Barthélemy NR, Bomont P, et al. Neurofilaments: neurobiological foundations for biomarker applications. Brain 2020;143:1975–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Norgren N, Karlsson JE, Rosengren L, Stigbrand T. Monoclonal antibodies selective for low molecular weight neurofilaments. Hybrid Hybridomics 2002;21:53–59. [DOI] [PubMed] [Google Scholar]
- 10. Bolstad N, Warren DJ, Nustad K. Heterophilic antibody interference in immunometric assays. Best Pract Res Clin Endocrinol Metab 2013;27:647–661. [DOI] [PubMed] [Google Scholar]
- 11. Kuhle J, Barro C, Andreasson U, et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med 2016;54:1655–1661. [DOI] [PubMed] [Google Scholar]
- 12. Gaiottino J, Norgren N, Dobson R, et al. Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS One 2013;8:e75091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gisslén M, Price RW, Andreasson U, et al. Plasma concentration of the Neurofilament Light Protein (NFL) is a biomarker of CNS injury in HIV infection: a cross‐sectional study. EBioMedicine 2016;3:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wilson DH, Rissin DM, Kan CW, et al. The simoa HD‐1 analyzer: A novel fully automated digital immunoassay analyzer with single‐molecule sensitivity and multiplexing. J Lab Autom 2016;21:533–547. 10.1177/2211068215589580 [DOI] [PubMed] [Google Scholar]
- 15. Preische O, Schultz SA, Apel A, et al. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer’s disease. Nat Med 2019;25:277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barro C, Benkert P, Disanto G, et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain 2018;141:2382–2391. [DOI] [PubMed] [Google Scholar]
- 17. Uphaus T, Bittner S, Gröschel S, et al. NfL (Neurofilament Light Chain) levels as a predictive marker for long‐term outcome after ischemic stroke. Stroke 2019;50:3077–3084. [DOI] [PubMed] [Google Scholar]
- 18. Kuhle J, Kropshofer H, Haering DA, et al. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology 2019;92:e1007–e1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rosso M, Gonzalez CT, Healy BC, et al. Temporal association of sNfL and gad‐enhancing lesions in multiple sclerosis. Ann Clin Transl Neurol 2020;7:945–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shahim P, Zetterberg H, Tegner Y, Blennow K. Serum neurofilament light as a biomarker for mild traumatic brain injury in contact sports. Neurology 2017;88:1788–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gattringer T, Pinter D, Enzinger C, et al. Serum neurofilament light is sensitive to active cerebral small vessel disease. Neurology 2017;89:2108–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tiedt S, Duering M, Barro C, et al. Serum neurofilament light: a biomarker of neuroaxonal injury after ischemic stroke. Neurology 2018;91:e1338–e1347. [DOI] [PubMed] [Google Scholar]
- 23. Wilke C, Pujol‐Calderón F, Barro C, et al. Correlations between serum and CSF pNfH levels in ALS, FTD and controls: a comparison of three analytical approaches. Clin Chem Lab Med 2019;57:1556–1564. [DOI] [PubMed] [Google Scholar]
- 24. Gray E, Oeckl P, Amador MDM, et al. A multi‐center study of neurofilament assay reliability and inter‐laboratory variability. Amyotroph Lateral Scler Frontotemporal Degener 2020;2:452–458. 10.1080/21678421.2020.1779300 [DOI] [PubMed] [Google Scholar]
- 25. Piehl F, Kockum I, Khademi M, et al. Plasma neurofilament light chain levels in patients with MS switching from injectable therapies to fingolimod. Mult Scler 2018;24:1046–1054. [DOI] [PubMed] [Google Scholar]
- 26. Sejbaek T, Nielsen HH, Penner N, et al. Dimethyl fumarate decreases neurofilament light chain in CSF and blood of treatment naïve relapsing MS patients. J Neurol Neurosurg Psychiatry 2019;90:1324–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hviid CVB, Knudsen CS, Parkner T. Reference interval and preanalytical properties of serum neurofilament light chain in Scandinavian adults. Scand J Clin Lab Invest 2020;80:291–295. [DOI] [PubMed] [Google Scholar]
- 28. Kuhle J, Plattner K, Bestwick JP, et al. A comparative study of CSF neurofilament light and heavy chain protein in MS. Mult. Scler 2013;19:1597–1603. [DOI] [PubMed] [Google Scholar]
- 29. Lewczuk P, Ermann N, Andreasson U, et al. Plasma neurofilament light as a potential biomarker of neurodegeneration in Alzheimer’s disease. Alzheimer's Res Ther 2018;10:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Keshavan A, Heslegrave A, Zetterberg H, Schott JM. Stability of blood‐based biomarkers of Alzheimer’s disease over multiple freeze‐thaw cycles. Alzheimer’s Dement 2018;10:448–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Andreasson U, Perret‐Liaudet A. van Waalwijk van Doorn LJ, et al. A practical guide to immunoassay method validation. Front Neurol 2015;6:1664–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Disanto G, Barro C, Benkert P, et al. Serum Neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol 2017;81:857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Delcoigne B, Manouchehrinia A, Barro C, et al. Blood neurofilament light levels segregate treatment effects in multiple sclerosis. Neurology 2020;94:e1201–e1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sharma A, Petrillo M, Zhao G, et al. ECTRIMS 2018 ‐ poster session 3. Mult Scler 2018;24(2_suppl):530–737. [Google Scholar]
- 35. Kuhle J, Barro C, Hrusovsky K, et al. ECTRIMS 2018 ‐ poster session 1. Mult Scler 2018;24(2_suppl):121–327.29059003 [Google Scholar]
- 36. Chitnis T, Yano H, Saxena S, et al. ECTRIMS 2019 ‐ poster session 3. Mult Scler 2019;25(2_suppl):581–805. [Google Scholar]
- 37. Sweeney MD, Sagare AP, Zlokovic BV. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 2018;14:133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reiber H. Flow rate of cerebrospinal fluid (CSF)–a concept common to normal blood‐CSF barrier function and to dysfunction in neurological diseases. J Neurol Sci 1994;122:189–203. [DOI] [PubMed] [Google Scholar]
- 39. Freedman MS, Thompson EJ, Deisenhammer F, et al. Recommended standard of cerebrospinal fluid analysis in the diagnosis of multiple sclerosis: a consensus statement. Arch Neurol 2005;62:865–870. [DOI] [PubMed] [Google Scholar]
- 40. Kalm M, Boström M, Sandelius Å, et al. Serum concentrations of the axonal injury marker neurofilament light protein are not influenced by blood‐brain barrier permeability. Brain Res 2017;1668:12–19. [DOI] [PubMed] [Google Scholar]
- 41. Uher T, McComb M, Galkin S, et al. Neurofilament levels are associated with blood–brain barrier integrity, lymphocyte extravasation, and risk factors following the first demyelinating event in multiple sclerosis. Mult Scler 2020;135245852091237 Epub ahead of print. 10.1177/1352458520912379 [DOI] [PubMed] [Google Scholar]
- 42. Novakova L, Zetterberg H, Sundstrom P, et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology 2017;89:2230–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wurster CD, Steinacker P, Günther R, et al. Neurofilament light chain in serum of adolescent and adult SMA patients under treatment with nusinersen. J Neurol 2020;267:36–44. [DOI] [PubMed] [Google Scholar]
- 44. Håkansson I, Tisell A, Cassel P, et al. Neurofilament levels, disease activity and brain volume during follow‐up in multiple sclerosis. J Neuroinflammation 2018;15:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hansson O, Janelidze S, Hall S, et al. Blood‐based NfL: a biomarker for differential diagnosis of parkinsonian disorder. Neurology 2017;88:930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Marques TM, van Rumund A, Oeckl P, et al. Serum NFL discriminates Parkinson disease from atypical parkinsonisms. Neurology 2019;92:e1479–e1486. [DOI] [PubMed] [Google Scholar]
- 47. Lu CH, Macdonald‐Wallis C, Gray E, et al. Neurofilament light chain: a prognostic biomarker in amyotrophic lateral sclerosis. Neurology 2015;84:2247–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gille B, De Schaepdryver M, Goossens J, et al. Serum neurofilament light chain levels as a marker of upper motor neuron degeneration in patients with Amyotrophic Lateral Sclerosis. Neuropathol Appl Neurobiol 2019;45:291–304. [DOI] [PubMed] [Google Scholar]
- 49. Khalil M, Pirpamer L, Hofer E, et al. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat Commun 2020;11:812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Divo MJ, Martinez CH, Mannino DM. Ageing and the epidemiology of multimorbidity. Eur Respir J 2014;44:1055–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Marrie RA, Miller A, Sormani MP, et al. Recommendations for observational studies of comorbidity in multiple sclerosis. Neurology 2016;86:1446–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Thouvenot E, Demattei C, Lehmann S, et al. Serum neurofilament light chain at time of diagnosis is an independent prognostic factor of survival in amyotrophic lateral sclerosis. Eur J Neurol 2020;27:251–257. [DOI] [PubMed] [Google Scholar]
- 53. Traxinger K, Kelly C, Johnson BA, et al. Prognosis and epidemiology of amyotrophic lateral sclerosis: analysis of a clinic population, 1997–2011. Neurology 2013;3:313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bridel C, van Wieringen WN, Zetterberg H, et al. Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: a systematic review and meta‐analysis. JAMA Neurol 2019;76:1035–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Korley FK, Goldstick J, Mastali M, et al. Serum NfL (Neurofilament Light Chain) levels and incident stroke in adults with diabetes mellitus. Stroke 2019;50:1669–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Aeschbacher S, Kuhle J, Benkert P, et al. P2908Serum light‐chain neurofilament, a brain lesion marker, correlates with CHA2DS2‐VASc score among patients with atrial fibrillation: a cross‐sectional study. Eur Heart J 2018;39:614–615. 10.1093/eurheartj/ehy565.P2908AUTHOR [DOI] [Google Scholar]
- 57. Qiao X, Zhang S, Zhao W, et al. Serum phosphorylated neurofilament‐heavy chain, a potential biomarker, is associated with peripheral neuropathy in patients with type 2 diabetes. Medicine 2015;94:e1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Manouchehrinia A, Piehl F, Hillert J, et al. Confounding effect of blood volume and body mass index on blood neurofilament light chain levels. Ann Clin Transl Neurol 2020;7:139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nilsson IAK, Millischer V, Karrenbauer VD, et al. Plasma neurofilament light chain concentration is increased in anorexia nervosa. Transl Psychiatry 2019;9:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sanghavi M, Rutherford JD. Cardiovascular physiology of pregnancy. Circulation 2014;130:1003–1008. [DOI] [PubMed] [Google Scholar]
- 61. Evers KS, Atkinson A, Barro C, et al. Neurofilament as neuronal injury blood marker in preeclampsia. Hypertension 2018;71:1178–1184. [DOI] [PubMed] [Google Scholar]
- 62. Cuello JP, Martínez Ginés ML, Kuhle J, et al. Neurofilament light chain levels in pregnant multiple sclerosis patients: a prospective cohort study. Eur J Neurol 2019;26:1200–1204. [DOI] [PubMed] [Google Scholar]
- 63. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018;17:162–173. [DOI] [PubMed] [Google Scholar]
- 64. Kuhle J, Nourbakhsh B, Grant D, et al. Serum neurofilament is associated with progression of brain atrophy and disability in early MS. Neurology 2017;88:826–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dalla Costa G, Martinelli V, Sangalli F, et al. Prognostic value of serum neurofilaments in patients with clinically isolated syndromes. Neurology 2019;92:e733–e741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jakimovski D, Kuhle J, Ramanathan M, et al. Serum neurofilament light chain levels associations with gray matter pathology: a 5‐year longitudinal study. Ann Clin Transl Neurol 2019;6:1757–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Martin S‐J, McGlasson S, Hunt D, Overell J. Cerebrospinal fluid neurofilament light chain in multiple sclerosis and its subtypes: a meta‐analysis of case–control studies. J Neurol Neurosurg Psychiatry 2019;90:1059–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bjornevik K, Munger KL, Cortese M, et al. Serum neurofilament light chain levels in patients with presymptomatic multiple sclerosis. JAMA Neurol 2020;77:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cantó E, Barro C, Zhao C, et al. Association between serum neurofilament light chain levels and long‐term disease course among patients with multiple sclerosis followed up for 12 years. JAMA Neurol 2019;76:1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chitnis T, Gonzalez C, Healy BC, et al. Neurofilament light chain serum levels correlate with 10‐year MRI outcomes in multiple sclerosis. Ann Clin Transl Neurol 2018;5:1478–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bittner S, Steffen F, Uphaus T, et al. Clinical implications of serum neurofilament in newly diagnosed MS patients: a longitudinal multicentre cohort study. EBioMedicine 2020;56:102807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Siller N, Kuhle J, Muthuraman M, et al. Serum neurofilament light chain is a biomarker of acute and chronic neuronal damage in early multiple sclerosis. Mult Scler 2019;25:678–686. [DOI] [PubMed] [Google Scholar]
- 73. Manouchehrinia A, Stridh P, Khademi M, et al. Plasma neurofilament light levels are associated with the risk of disability in multiple sclerosis. Neurology 2020;94:e2457–e2467. 10.1212/WNL.0000000000009571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wong YYM, Bruijstens AL, Barro C, et al. Serum neurofilament light chain in pediatric MS and other acquired demyelinating syndromes. Neurology 2019;93:e968–e974. [DOI] [PubMed] [Google Scholar]
- 75. Reinert M‐C, Benkert P, Wuerfel J, et al. Serum neurofilament light chain is a useful biomarker in pediatric multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2020;7:e749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Varhaug KN, Barro C, Bjornevik K, et al. Neurofilament light chain predicts disease activity in relapsing‐remitting MS. Neurol Neuroimmunol Neuroinflamm 2018;5:e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Confavreux C, Hutchinson M, Hours MM, et al. Rate of pregnancy‐related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med 1998;339:285–291. [DOI] [PubMed] [Google Scholar]
- 78. Kuhle J, Plavina T, Barro C, et al. Neurofilament light levels are associated with long‐term outcomes in multiple sclerosis. Mult Scler 2019;26:1691–1699. 10.1177/1352458519885613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hauser S, Bar‐Or A, Cohen J, et al. ECTRIMS 2019 – late breaking news abstracts. Mult Scler J 2019;25(2_suppl):890–938. 10.1177/1352458519869496 [DOI] [Google Scholar]
- 80. Kuhle J, Kropshofer H, Barro C, et al. Siponimod reduces neurofilament light chain blood levels in secondary progressive multiple sclerosis patients (S8.006). Neurology 2018;90(15 Supplement):S8.006. [Google Scholar]
- 81. Uher T, Schaedelin S, Srpova B, et al. Monitoring of radiologic disease activity by serum neurofilaments in MS. Neurol Neuroimmunol Neuroinflamm 2020;7:e714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sormani MP, Haering DA, Kropshofer H, et al. Blood neurofilament light as a potential endpoint in Phase 2 studies in MS. Ann Clin Transl Neurol 2019;6:1081–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Masters CL, Bateman R, Blennow K, et al. Alzheimer's disease. Nat Rev Dis Primers 2015;1:15056. [DOI] [PubMed] [Google Scholar]
- 84. Blennow K, Zetterberg H. The past and the future of Alzheimer’s disease fluid biomarkers1. JAD 2018;62:1125–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mengel D, Janelidze S, Glynn RJ, et al. Plasma NT1 tau is a specific and early marker of Alzheimer’s disease. Ann Neurol 2020;88:878–892. 10.1002/ana.25885 [DOI] [PubMed] [Google Scholar]
- 86. Karikari TK, Pascoal TA, Ashton NJ, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol 2020;19:422–433. [DOI] [PubMed] [Google Scholar]
- 87. Sperling RA, Karlawish J, Johnson KA. Preclinical Alzheimer disease—the challenges ahead. Nat Rev Neurol 2013;9:54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Pereira JB, Westman E, Hansson O. Association between cerebrospinal fluid and plasma neurodegeneration biomarkers with brain atrophy in Alzheimer’s disease. Neurobiol Aging 2017;58:14–29. [DOI] [PubMed] [Google Scholar]
- 89. Mattsson N, Andreasson U, Zetterberg H, et al. Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol 2017;74:557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sánchez‐Valle R, Heslegrave A, Foiani MS, et al. Serum neurofilament light levels correlate with severity measures and neurodegeneration markers in autosomal dominant Alzheimer’s disease. Alzheimer's Res Ther 2018;10:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ashton NJ, Leuzy A, Lim YM, et al. Increased plasma neurofilament light chain concentration correlates with severity of post‐mortem neurofibrillary tangle pathology and neurodegeneration. Acta Neuropathol Commun 2019;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lin Y‐S, Lee W‐J, Wang S‐J, Fuh J‐L. Levels of plasma neurofilament light chain and cognitive function in patients with Alzheimer or Parkinson disease. Sci Rep 2018;8:17368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mielke MM, Syrjanen JA, Blennow K, et al. Plasma and CSF neurofilament light: Relation to longitudinal neuroimaging and cognitive measures. Neurology 2019;93:e252–e260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Weston PSJ, Poole T, Ryan NS, et al. Serum neurofilament light in familial Alzheimer disease: a marker of early neurodegeneration. Neurology 2017;89:2167–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Weston PSJ, Poole T, O’Connor A, et al. Longitudinal measurement of serum neurofilament light in presymptomatic familial Alzheimer’s disease. Alzheimer's Res Ther 2019;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Quiroz YT, Zetterberg H, Reiman EM, et al. Plasma neurofilament light chain in the presenilin 1 E280A autosomal dominant Alzheimer’s disease kindred: a cross‐sectional and longitudinal cohort study. Lancet Neurol 2020;19:513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Cullen NC, Zetterberg H, Insel PS, et al. Comparing progression biomarkers in clinical trials of early Alzheimer’s disease. Ann Clin Transl Neurol 2020;7:1661–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hardiman O, Al‐Chalabi A, Chio A, et al. Amyotrophic lateral sclerosis. Nat Rev Dis Primers 2017;3:17071. [DOI] [PubMed] [Google Scholar]
- 99. Feneberg E, Oeckl P, Steinacker P, et al. Multicenter evaluation of neurofilaments in early symptom onset amyotrophic lateral sclerosis. Neurology 2018;90:e22–e30. [DOI] [PubMed] [Google Scholar]
- 100. Weydt P, Oeckl P, Huss A, et al. Neurofilament levels as biomarkers in asymptomatic and symptomatic familial amyotrophic lateral sclerosis. Ann Neurol 2016;79:152–158. [DOI] [PubMed] [Google Scholar]
- 101. Benatar M, Wuu J, Andersen PM, et al. Neurofilament light: A candidate biomarker of presymptomatic amyotrophic lateral sclerosis and phenoconversion. Ann Neurol 2018;84:130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Benatar M, Wuu J, Lombardi V, et al. Neurofilaments in pre‐symptomatic ALS and the impact of genotype. Amyotroph Lateral Scler Frontotemporal Degener 2019;20(7–8):538–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Verde F, Steinacker P, Weishaupt JH, et al. Neurofilament light chain in serum for the diagnosis of amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2019;90:157–164. [DOI] [PubMed] [Google Scholar]
- 104. Yuan A, Rao MV, Veeranna NRA. Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb Perspect Biol 2017;9(4):a018309 http://www.ncbi.nlm.nih.gov/pubmed/28373358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Turner MR, Gray E. Are neurofilaments heading for the ALS clinic?. J Neurol Neurosurg Psychiatry 2016;87:3–4. [DOI] [PubMed] [Google Scholar]
- 106. De Schaepdryver M, Jeromin A, Gille B, et al. Comparison of elevated phosphorylated neurofilament heavy chains in serum and cerebrospinal fluid of patients with amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2018;89:367–373. [DOI] [PubMed] [Google Scholar]
- 107. Poesen K, De SM, Stubendorff B, et al. Neurofilament markers for ALS correlate with extent of upper and lower motor neuron disease. Neurology 2017;88:2302–2309. [DOI] [PubMed] [Google Scholar]
- 108. Benatar M, Zhang L, Wang L, et al. CReATe Consortium. Validation of serum neurofilaments as prognostic and potential pharmacodynamic biomarkers for ALS. Neurology 2020;95:e59–e69. 10.1212/WNL.0000000000009559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Vocht JD, Blommaert J, Devrome M, et al. Use of multimodal imaging and clinical biomarkers in presymptomatic carriers of C9orf72 repeat expansion. JAMA Neurol 2020;77:1008–1017. 10.1001/jamaneurol.2020.1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Miller T, Cudkowicz M, Shaw PJ, et al. Phase 1–2 trial of antisense oligonucleotide tofersen for SOD1 ALS. N Engl J Med 2020;383:109–119. [DOI] [PubMed] [Google Scholar]
- 111. Zucchi E, Lu C, Cho Y, et al. A motor neuron strategy to save time and energy in neurodegeneration: adaptive protein stoichiometry. J Neurochem 2018;146:631–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Figlewicz DA, Krizus A, Martinoli MG, et al. Variants of the heavy neurofilament subunit are associated with the development of amyotrophic lateral sclerosis. Hum Mol Genet 1994;3:1757–1761. [DOI] [PubMed] [Google Scholar]
- 113. Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nat Rev Dis Primers 2017;3:17013. [DOI] [PubMed] [Google Scholar]
- 114. Oosterveld LP, Verberk IMW, Majbour NK, et al. CSF or serum neurofilament light added to α‐Synuclein panel discriminates Parkinson’s from controls. Mov Disord 2020;35:288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Sampedro F, Pérez‐González R, Martínez‐Horta S, et al. Serum neurofilament light chain levels reflect cortical neurodegeneration in de novo Parkinson’s disease. Parkinsonism Relat Disord 2020;74:43–49. [DOI] [PubMed] [Google Scholar]
- 116. Campbell BCV, De Silva DA, Macleod MR, et al. Ischaemic stroke. Nat Rev Dis Primers 2019;5:70. [DOI] [PubMed] [Google Scholar]
- 117. Duering M, Konieczny MJ, Tiedt S, et al. Serum neurofilament light chain levels are related to small vessel disease burden. J Stroke 2018;20:228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Gravesteijn G, Rutten JW, Verberk IMW, et al. Serum Neurofilament light correlates with CADASIL disease severity and survival. Ann Clin Transl Neurol 2019;6:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Onatsu J, Vanninen R, Jäkälä P, et al. Serum neurofilament light chain concentration correlates with infarct volume but not prognosis in acute ischemic stroke. J Stroke Cerebrovasc Dis 2019;28:2242–2249. [DOI] [PubMed] [Google Scholar]
- 120. De Marchis GM, Katan M, Barro C, et al. Serum neurofilament light chain in patients with acute cerebrovascular events. Eur J Neurol 2018;25:562–568. [DOI] [PubMed] [Google Scholar]
- 121. Pedersen A, Stanne TM, Nilsson S, et al. Circulating neurofilament light in ischemic stroke: temporal profile and outcome prediction. J Neurol 2019;266:2796–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Blennow K, Brody DL, Kochanek PM, et al. Traumatic brain injuries. Nat Rev Dis Primers 2016;2:16084. [DOI] [PubMed] [Google Scholar]
- 123. Oliver JM, Jones MT, Kirk KM, et al. Serum neurofilament light in american football athletes over the course of a season. J Neurotrauma 2016;33:1784–1789. [DOI] [PubMed] [Google Scholar]
- 124. Rubin LH, Tierney R, Kawata K, et al. NFL blood levels are moderated by subconcussive impacts in a cohort of college football players. Brain Inj 2019;33:456–462. [DOI] [PubMed] [Google Scholar]
- 125. Wallace C, Smirl JD, Zetterberg H, et al. Heading in soccer increases serum neurofilament light protein and SCAT3 symptom metrics. BMJ Open Sport Exerc Med 2018;4:e000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Shahim P, Tegner Y, Marklund N, et al. Neurofilament light and tau as blood biomarkers for sports‐related concussion. Neurology 2018;90:e1780–e1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Bernick C, Zetterberg H, Shan G, et al. Longitudinal performance of plasma neurofilament light and tau in professional fighters: the professional fighters brain health study. J Neurotrauma 2018;35:2351–2356. [DOI] [PubMed] [Google Scholar]
- 128. Shahim P, Gren M, Liman V, et al. Serum neurofilament light protein predicts clinical outcome in traumatic brain injury. Sci Rep 2016;6:2045–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Bang J, Spina S, Miller BL. Frontotemporal dementia. The Lancet 2015;386:1672–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Al Shweiki MR, Steinacker P, Oeckl P, et al. Neurofilament light chain as a blood biomarker to differentiate psychiatric disorders from behavioural variant frontotemporal dementia. J Psychiatr Res 2019;113:137–140. [DOI] [PubMed] [Google Scholar]
- 131. Katisko K, Cajanus A, Jääskeläinen O, et al. Serum neurofilament light chain is a discriminative biomarker between frontotemporal lobar degeneration and primary psychiatric disorders. J Neurol 2020;267:162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. van der Ende EL, Meeter LH, Poos JM, et al. Serum neurofilament light chain in genetic frontotemporal dementia: a longitudinal, multicentre cohort study. Lancet Neurol 2019;18:1103–1111. [DOI] [PubMed] [Google Scholar]
- 133. Meeter LH, Dopper EG, Jiskoot LC, et al. Neurofilament light chain: a biomarker for genetic frontotemporal dementia. Ann Clin Transl Neurol 2016;3:623–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Steinacker P, Anderl‐Straub S, Diehl‐Schmid J, et al. Serum neurofilament light chain in behavioral variant frontotemporal dementia. Neurology 2018;91:e1390–e1401. [DOI] [PubMed] [Google Scholar]
- 135. Rohrer JD, Woollacott IO, Dick KM, et al. Serum neurofilament light chain protein is a measure of disease intensity in frontotemporal dementia. Neurology 2016;87:1329–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Cortese M, Munger KL, Martínez‐Lapiscina EH, et al. Vitamin D, smoking, EBV, and long‐term cognitive performance in MS: 11‐year follow‐up of BENEFIT. Neurology 2020;94(18):e1950–e1960. 10.1212/WNL.0000000000009371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Evers KS, Huhn EA, Fouzas S, et al. Impact of parturition on maternal cardiovascular and neuronal integrity in a high risk cohort – a prospective cohort study. BMC Pregnancy Childbirth 2019;19:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Wurster CD, Günther R, Steinacker P, et al. Neurochemical markers in CSF of adolescent and adult SMA patients undergoing nusinersen treatment. Ther Adv Neurol Disord 2019;12:175628641984605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Byrne LM, Rodrigues FB, Blennow K, et al. Neurofilament light protein in blood as a potential biomarker of neurodegeneration in Huntington’s disease: a retrospective cohort analysis. Lancet Neurol 2017;16:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]


