Abstract
Objective
It is widely believed that the perinatal brain injuries seen in youth with cerebral palsy (CP) impact neuronal processing of sensory information and the production of leg motor actions during gait. However, very limited efforts have been made to evaluate the connection between neural activity within sensorimotor networks and the altered spatiotemportal gait biomechanics seen in youth with CP. The objective of this investigation was to use magnetoencephalographic (MEG) brain imaging and biomechanical analysis to probe this connection.
Methods
We examined the cortical beta oscillations serving motor control of the legs in a cohort of youth with CP (N = 20; Age = 15.5 ± 3 years; GMFCS levels I‐III) and healthy controls (N = 15; Age = 14.1 ± 3 years) using MEG brain imaging and a goal‐directed isometric knee target‐matching task. Outside the scanner, a digital mat was used to quantify the spatiotemporal gait biomechanics.
Results
Our MEG imaging results revealed that the participants with CP exhibited stronger sensorimotor beta oscillations during the motor planning and execution stages compared to the controls. Interestingly, we also found that those with the strongest sensorimotor beta oscillations during motor execution also tended to walk slower and have a reduced cadence.
Interpretation
These results fuel the impression that the beta sensorimotor cortical oscillations that underlie leg musculature control may play a central role in the altered mobility seen in youth with CP.
Introduction
Cerebral palsy (CP) is the most prevalent pediatric neurologic impairment diagnosed in the United States and often results in lifelong mobility challenges. 1 The breadth of the mobility deficiencies seen in youth with CP have largely fueled the impression that the altered spatiotemporal gait biomechanics seen in these youth primarily originates from the musculoskeletal machinery. 2 , 3 , 4 , 5 , 6 However, this impression has been challenged, as it is becoming widely recognized that the perinatal brain insults seen in these youth may impact activity within the key brain networks that are involved in processing sensory information and the production of the leg motor actions during gait. 7 Despite this revised direction, very limited efforts have been made to evaluate the connection between neural activity in the sensorimotor cortices and the gait performance of youth with CP. 8 , 9 , 10 , 11
Numerous electroencephalographic and magnetoencephalographic (MEG) brain imaging experiments have well established that the sensorimotor cortical oscillatory activity at the beta frequency is tightly yoked to the production of a motor action. 12 , 13 , 14 , 15 , 16 , 17 Specifically, such beta cortical oscillations are known to decrease prior to the onset of movement, and this decrease is sustained throughout the movement. The consensus is that the decrease seen prior to the onset of movement represents the neural computations involved in the planning of motor actions, because it occurs prior to the onset of any muscular activity, is influenced by the ease of the motor task, and is influenced by the certainty of the movement pattern to be performed. 18 , 19 , 20 , 21 , 22 , 23 , 24 Although there is substantial evidence that sensorimotor beta oscillations play a prominent role in the production of motor actions in healthy controls, we still have a limited understanding of the nature of these cortical oscillations in youth with CP. Previously, we conducted a series of foundational MEG studies, which revealed that beta sensorimotor cortical oscillations were notably stronger in youth with CP during the motor planning and execution stages of a knee extension motor task. 25 , 26 In the latter work, we identified that these aberrant beta oscillations were tightly coupled with the slower reaction times and motor production errors seen these youth. 26 From these studies, we have inferred that these abnormal beta cortical dynamics may play a role in the atypical gait seen in youth with CP. However, this premise has yet to be fully tested.
In this investigation, we used MEG and a knee isometric force‐matching task to quantify and map the beta sensorimotor cortical oscillations serving lower limb motor control in a cohort of youth with CP and a demographically matched group of healthy controls. In addition, we quantified the participant’s spatiotemporal gait dynamics and assessed if the deviations in the gait spatiotemporal biomechanics were linked with the strength of the beta cortical oscillations. Our primary hypothesis was that the youth with CP would display aberrant beta oscillations relative to controls during the planning and motor execution stages, and that these deviant beta oscillations would be associated with atypical spatiotemporal gait biomechanics.
Materials and methods
Subjects
Twenty youth with CP that had a spastic diplegic presentation (Age = 15.5 ± 3 years; 8 females; Gross Motor Function Classification Score (GMFCS) levels I‐III) and 15 healthy controls (Age = 14.1 ± 3 years; 5 females) with no neurological or musculoskeletal impairments participated in this investigation. The youth with CP were excluded if they had orthopedic surgery or anti‐spasticity treatments within the last 6‐months. The Institutional Review Board at the University of Nebraska Medical Center reviewed and approved the protocol for this investigation. All the parents provided written consent that their child could participate in the investigation and the youth assented.
MEG data acquisition and experimental paradigm
Neuromagnetic responses were sampled continuously at 1 kHz with an acquisition bandwidth of 0.1–330 Hz using an Elekta/MEGIN MEG system (Helsinki, Finland). All recordings were conducted in a one‐layer magnetically shielded room with active shielding engaged for advanced environmental noise compensation. A custom‐built head stabilization device that consisted of a series of inflatable airbags that surrounded the sides of the head and filled the void between the head and MEG helmet was worn for all data collections. This system stabilized the head and reduced the probability of any large head movements occurring during the data collections.
The children were seated upright in a magnetically silent chair during the experiment. A custom‐built magnetically silent force transducer was developed for this investigation to measure the isometric knee extension forces generated by the children. 26 This device consisted of a 20 × 10 cm airbladder that was inflated to 317 kPa, and fixed to the anterior portion of the lower leg just proximal to the malleoli. A thermoplastic shell encased the outer portion of the airbladder and was secured to the chair with ridged strappings. Changes in the pressure of the airbag as the child generated an isometric contraction were quantified by an air pressure sensor (Phidgets Inc., Calgary, Alberta, CA) and were subsequently converted into units of force.
The experimental paradigm involved the participant generating an isometric knee extension force with their right leg in order to match target forces that varied between 15 and 30% of the participant’s maximum isometric force (Figure 1), which was determined prior to beginning the experiment. The maximal isometric force was determined by having the participant generate a maximum sustained contraction for 2‐3 seconds on two separate trials and taking the average. The target force was visually displayed as a moth and the force generated by the participant was shown as a frog that was animated vertically, based on the isometric force generated. The participants were instructed to match the presented targets as fast and as accurately as possible. The target forces were presented in a random order, and a successful match occurred when the bug that represented the target force was inside of the frog’s mouth for 0.3 seconds. The stimuli were shown on a back‐projection screen that was approximately ~ 1 meter in front of the participant and at eye‐level. Each trial was 10 seconds in length. The participants started each trial at rest, fixated at the center of the screen for 5 seconds. After this rest period, the target moth would appear, prompting the participant to match the force value. The target was available to be matched for up to 5 seconds. Once the target was matched or 5 seconds elapsed, feedback was given to indicate the end of the trial, and the participant returned to rest and fixated on the center of the screen while waiting for the next target to appear. The participants completed 120 target matching trials.
Figure 1.
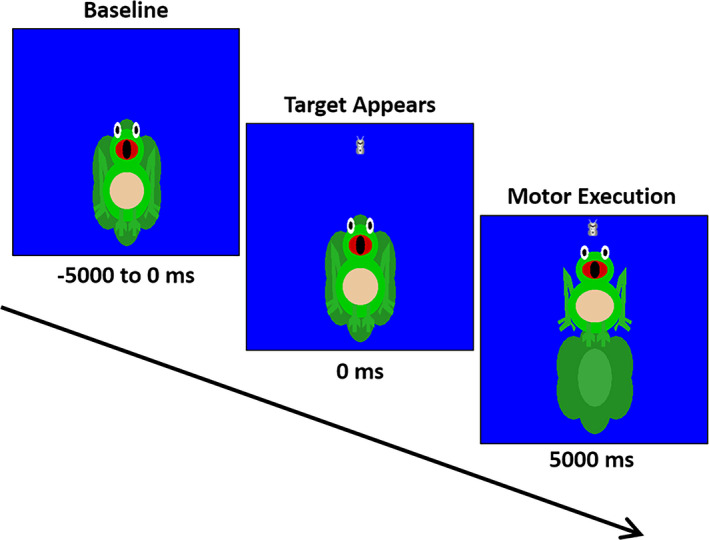
Depiction of the MEG experimental paradigm. The trial length was 10 s in length. The participants started each trial at rest for 5000 ms. Subsequently, a target moth would appear, prompting the participant to generate an isometric knee extension force that matched the force value. The target forces varied between 15 and 30% of the participant’s maximum isometric force and were available to be matched for up to 5000 ms. A successful match occurred when the bug that represented the target force was inside of the frog’s mouth for 300 ms. The participants completed 120 target matching trials.
MEG coregistration & structural MRI processing
Structural MRI data were acquired using a Siemens Prisma 3T scanner. MPRAGE High‐resolution T1‐weighted images were obtained with a 64‐channel head/neck coil using the following parameters: TR: 1720 ms; TE: 2.48 ms; flip angle = 8 deg; FOV: 230 mm; slice thickness: 1 mm slice; in‐plane resolution: 1.0 × 0.89 × 0.89 mm.
For the MEG experiment, four coils were affixed to the head of the participant and were used for continuous head localization throughout the experiment. The location of the coils, three fiducial points and the scalp surface were digitized to determine their three‐dimensional coordinates (Fastrak 3SF0002, Polhemus Navigator Sciences, Colchester, VT, USA). An electric current with a unique frequency label (e.g., 322 Hz) was fed to each of the four coils during the MEG experiment. This induced a measurable magnetic field, which allowed for each coil to be localized in reference to the sensors throughout the recording session. Since the coil locations were also known in head coordinates, all MEG measurements could be transformed into a common coordinate system. With this coordinate system (including the scalp surface points), each child’s MEG data were coregistered with native space neuroanatomical MRI data using the three external landmarks (i.e., fiducials) and the digitized scalp surface points prior to source space analyses. The neuroanatomical MRI data were aligned parallel to the anterior and posterior commissures and transformed into standardized space.
MEG preprocessing, time‐frequency transformation, and statistics
Using the MaxFilter software (Elekta), each MEG dataset was individually corrected for head motion, and were subjected to noise reduction using the signal space separation method with a temporal extension. 27 Artifact rejection was performed at the level of the entire trial and was based on a fixed threshold method, supplemented with visual inspection. There was no significant difference in the number of trials accepted for the respective groups (CP = 76.7 ± 20.4; Controls = 79.7 ± 10.2; P = 0.292). The continuous magnetic time series was divided into epochs of 4500 ms in length and centered on movement onset. For all time windows, 0.0 ms was defined as the onset of the isometric force and the baseline was defined as −2000 to −1500 ms. Artifact‐free epochs for each sensor were transformed into the time‐frequency domain using complex demodulation and averaged over trials. 28 In brevity, the complex demodulation approach involves applying a heterodyning procedure to the Fourier transformed MEG signal through a series of complex sinusoids that have increasing carrier frequencies. A 4 Hz low pass finite impulse response filter (frequency step of 2 Hz and a time‐step of 25 ms) was subsequently applied to the complex signals to prevent spectral leakage. These sensor‐level data were normalized per time‐frequency bin by using the respective bin’s mean power during the baseline. Essentially, the normalized sensor‐level data are the measured percent change in the neural oscillatory power relative to the baseline period. The specific time‐frequency windows used for imaging were determined by statistical analysis of the sensor‐level spectrograms across the entire array of gradiometers. Each data point in the spectrogram was initially evaluated using a mass univariate approach based on the general linear model. 29 , 30 To reduce the risk of false‐positive results while maintaining reasonable sensitivity, a two‐stage procedure was followed to control for Type 1 error. In the first stage, paired‐sample t‐tests against baseline were conducted on each data point and the output spectrogram of t‐values was thresholded at P < 0.05 to define time‐frequency bins containing potentially significant oscillatory deviations across all participants. In stage two, time‐frequency bins that survived the threshold were clustered with temporally and/or spectrally neighboring bins that were also above the (P < 0.05) threshold, and a cluster value was derived by summing all of the t‐values of all data points in the cluster. Nonparametric permutation testing was then used to derive a distribution of cluster‐values and the significance level of the observed clusters (from stage one) were tested directly using this distribution. For each comparison, 1,000 permutations were computed to build a distribution of cluster values. For a more detailed description of our methodology, see Wiesman and Wilson. 31
MEG source imaging & statistics
The Dynamic Imaging of Coherent Sources (DICS) beamformer was employed to calculate the source power across the entire brain volume. 32 The single images were derived from the cross‐spectral densities of all combinations of MEG sensors, and the solution of the forward problem for each location on a grid specified by input voxel space. Following convention, the source power in these images were normalized per subject and trial using an averaged prestimulus noise period of equal duration and bandwidth. 33 , 34 Thus, the normalized power per voxel was computed over the entire brain volume per participant at 4.0 × 4.0 × 4.0 mm resolution, and across trials this was used to compute pseudo‐t values per voxel. Each participant’s functional images, which were co‐registered to anatomical images prior to beamforming, were transformed into standardized space using the transform previously applied to the structural MRI volume and spatially resampled. The final images (pseudo‐t units) were used to quantify the relative change in the activity per voxel across the entire cortex. MEG preprocessing and imaging used the BESA software (BESA v6.0; Grafelfing, Germany).
Virtual sensors were extracted by applying the sensor weighting matrix derived through the forward computation to the preprocessed signal vector, which resulted in a time series with the same temporal resolution as the original MEG recording. 35 , 36 , 37 Once the virtual sensors were extracted, they were transformed into the time‐frequency domain and the two orientations for each peak voxel per individual were combined using a vector‐summing algorithm. The change in power within each neural region (relative to the baseline) was averaged across the time window of interest for each individual to assess the magnitude of the oscillatory responses seen during the motor planning and execution stages. For a more detailed description of our imaging methodology, see Wiesman and Wilson. 31 Separate repeated‐measures ANOVAs (Group × Time Period) performed to determine if the beta power changes were significantly different between the youth with CP and the controls during the motor planning and execution stages.
MEG behavioral data & gait spatiotemporal biomechanics
The output of the force transducer was simultaneously collected at 1 kHz along with the MEG data, and was used to quantify the participant’s motor performance during the isometric force‐matching task. The participant’s reaction time was calculated from the time when the target was presented to when force production was initiated. The amount of error in the feedforward execution of the motor plan was quantified based on the percent overshoot of the target. Finally, the time to match the target was used to quantify the online corrections that were made after the initial motor plan was executed. The online corrections were calculated based on the time difference between the reaction time and the time to reach the target.
Lastly, participants walked across a gaitRITE digital mat (CIR Systems, Franklin, NJ) that quantified their step length, cadence and walking speed. Each participant completed two representative trials at their preferred walking speed, and the average of the respective spatiotemporal variables were subsequently computed. The youth with CP were allowed to complete the walking trials with their prescribed ankle‐foot orthosis, forearm crutches or wheeled walker.
Separate independent‐sample t‐tests were used to determine if there were differences in the percent change in the strength of the beta ERD, maximum isometric force, MEG behavioral variables and spatiotemporal gait variables for the youth with CP and the controls. In addition, Spearman rho rank order correlations were calculated between the percent change of the beta ERD, the MEG behavioral variables, and the gait spatiotemporal kinematics. Nonparametric correlations were selected for the entire dataset because Kolmogorov–Smirnov statistical tests showed that some of the gait and MEG behavioral variables were not normally distributed.
Results
Sensor‐level and beamforming results
The spectrogram permutation tests revealed that there was a significant beta (18‐24 Hz) event‐related desynchronization (ERD; i.e., power decrease) across a large number of sensors near the sensorimotor cortex (P < 0.0001, corrected). Inspection of the time‐frequency components shown in Figure 2 indicated that the beta ERD began approximately 250 ms prior to the onset of the isometric force, and was sustained as the participants attempted to match the presented force targets (e.g., ~2500 ms). Similarly, there was a notable alpha (10‐14 Hz) ERD that emerged 250 ms before movement onset and was sustained up to 2500 ms.
Figure 2.
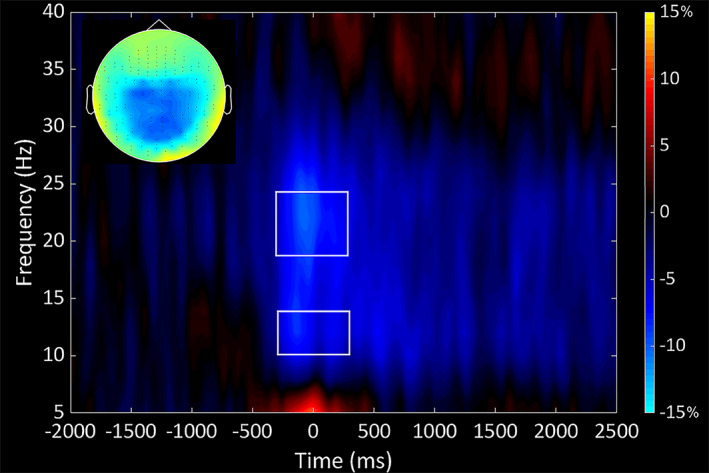
Time‐frequency spectrogram grand averaged across both groups. Frequency (Hz) is shown on the y‐axis and time (ms) is denoted on the x‐axis, with 0 ms defined as the onset of the isometric force. The event‐related spectral changes during the isometric knee task are expressed as the percent difference from baseline (−2000 to −1500 ms), with the scale shown to the far right. The inset shows a topographical map that depicts the location of the decrease in the beta power seen across the respective MEG sensors. The time‐frequency spectrograph of the MEG sensor with the greatest response amplitude is shown, which was located near the sensorimotor cortices and contralateral to the (right) leg generating the force. As can be discerned, there was a strong decrease in the alpha (10‐14 Hz) and beta (18‐24 Hz) band power (desynchronization) that started 250 ms prior to the initiation of the isometric force and was sustained through the duration of the contraction (2500 ms). The respective alpha and beta time windows that were beamformed are outlined on the spectrogram.
We imaged the beta ERD response from −250 to 250 ms in each participant using a baseline period of equal duration and bandwidth (18‐24 Hz; −2000 to −1500 ms) to identify the underlying cortical regions. The resulting images indicated that the beta ERD was centered on the leg region of the contralateral sensorimotor cortices (Figure 3A), with additional clusters seen in the occipital cortices. The local maxima of these responses in the grand‐averaged images were subsequently used as seeds for extracting virtual sensors in each participant. We then calculated the average activity seen during the motor planning (−250 to 0 ms) and execution (0 to 250 ms) time periods separately in each participant. Inspection of the neural time courses showed that the strength of the beta ERD (i.e., magnitude of the decrease in the sensorimotor beta oscillations) was greater in the youth with CP compared with the healthy controls (Figure 3A). Our statistical analysis indicated that there was no main effect for time (P = 0.99) indicating that the strength of the beta ERD was similar across the motor planning and execution stages. However, there was a significant group main effect (CP = −22.47 ± 3.1%; TD = −12.06 ± 2.2%; P = 0.018), confirming the observation that the beta ERD was stronger for the youth with CP across the motor planning and execution stages when compared with the controls (Figures 3B,C). The group x time interaction was not significantly different (P = 0.71).
Figure 3.
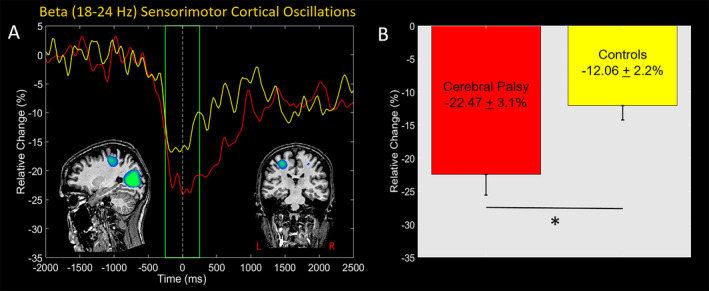
A, Grand‐averaged pseudo‐t maps across both groups for the beta (18‐24 Hz) event‐related desynchronization (ERD) across the −250 to 250 ms time window are shown in the inset. These images confirm that there was a prominent beta ERD in the contralateral leg region of the sensorimotor cortices. The displayed neural time courses were extracted from the peak voxel in the sensorimotor cortices identified in the grand average beamformer image. The yellow trace is the average neural time course for the controls, whereas the red time course is the average for the youth with CP. Time (ms) is denoted on the x‐axis, with 0 ms defined as the movement onset, and relative power (% of baseline) denoted on the y‐axis. The green box signifies the beamformed time window. Inspection of the neural time courses reveals that the youth with CP had a stronger beta ERD within the sensorimotor cortices across the motor planning and execution stages. B, These observations are supported by our statistical analysis that shows the group main effect was significant, indicating that the average strength of the beta ERD across the motor planning and the motor execution stages was stronger for the youth with CP. * denotesP ≤ 0.05.
Our beamforming results also indicated that there were additional clusters of beta oscillatory activity bilaterally in the occipital cortices. The local maxima of these responses were subsequently used as seeds for extracting virtual sensors, and the neural time courses from these seeds were averaged since the paradigm was not designed to interrogate hemispheric effects. Our statistical analysis indicated that there was no main effect for time (P = 0.597), group (P = 0.832) or a group x time interaction effect (P = 0.947). Hence, indicating that the strength of the occipital beta ERD was similar across the motor planning and execution stages for both groups (Motor Planning: CP = −20.3 ± 3.1%; Controls = −21.5 ± 3.3%; Execution: CP = −21.8 ± 4.2%; Controls = −20.6 ± 5.5%).
We also imaged the alpha ERD seen across the −250 to 250 ms time windows using a baseline period of −2000 to −1500 ms. The resulting images indicated that the alpha ERD responses were confined to the occipital cortices. Time series were then extracted from the local maxima and averaged across the respective hemispheres, as we had no hemispheric hypotheses. The average activity during the motor planning (−250 to 0 ms) and execution (0 to 250 ms) stages was then computed and evaluated separately. Our statistical analysis indicated that there was no main effect for time (P = 0.597), group (P = 0.832) or a group x time interaction effect (P = 0.947). Thus, the strength of the occipital beta ERD was similar during the motor planning and execution stages for both groups (Motor Planning: CP = −27.1 ± 4%; Controls = −17.8 ± 7%; Execution: CP = −29.7 ± 4%; Controls = −22.1 ± 9%).
MEG motor behavioral results
The youth with CP had lower isometric knee extension force values compared with the controls (CP = 103 ± 3 N; Controls = 206 ± 5 N; P = 0.001). The reaction time was similar between the respective groups (CP = 584 ± 4 ms; Controls = 537 ± 5 ms; P = 0.481), but the youth with CP had a larger initial overshoot of the presented targets (CP = 14.9 ± 0.6 %; Controls = 7.3 ± 0.3%; P = 0.001) and took longer to match the targets (CP = 3030 ± 200 ms; Controls = 2300 ± 100 ms; P = 0.0001). Furthermore, the youth with CP matched fewer targets than the controls (CP = 52.32 ± 6.48 targets, TD = 74.70 ± 4.49 targets, P = 0.026). For the entire group, there was a negative rank order correlation (rho = −0.645; P = 0.0001) between the percent overshoot and the number of targets matched. Similarly, there was a negative rank‐order correlation for the percent overshoot and number of targets matched within the youth with CP (rho = −0.507; P = 0.023) and the controls (rho = −0.781; P = 0.0001). Altogether this implies that participants that overshot the target by a greater amount also tended to match fewer targets.
For the entire group, there also was a rank order correlation (rho = −0.40; P = 0.01; Figure 4A) between the percent overshoot and the strength of the sensorimotor beta ERD during the motor planning stage (−250 to 0 ms). Indicating that participants with a stronger sensorimotor beta ERD (i.e., larger reduction in power) during the motor planning stage also tended to have a larger overshoot of the target’s position.
Figure 4.
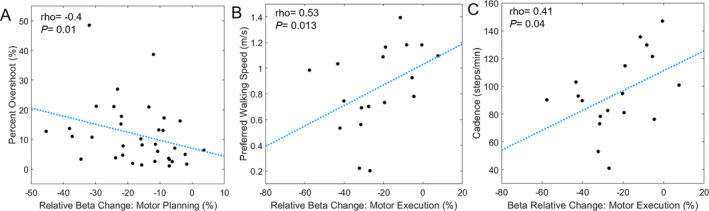
A, Scatter plot of the relationship between the relative change in the sensorimotor beta oscillations during the motor planning stage and the percent overshoot of the presented targets across all participants. As shown, participants with stronger sensorimotor beta event‐related desynchronizations (ERD) tended to have larger overshoots in their isometric force production when initially attempting to match the presented targets. B, Scatter plot of the relationship between the relative change in beta oscillations during the motor execution stage and the preferred walking speed of the youth with cerebral palsy. These data indicate that the youth with cerebral palsy who had stronger beta ERD responses during motor execution also tended to have slower preferred walking speeds. C, Scatter plot of the relative change in beta oscillations and walking cadence in the youth with cerebral palsy. The plot shows that stronger beta ERD response during the motor execution stage was associated with slower cadence in the youth with cerebral palsy.
Gait biomechanical results
We were unable to collect viable data from two participants with CP due to technical difficulties. These technical difficulties were due to the participants’ assistive walking device creating artifacts that interfered with the ability to distinguish between the participants’ footfalls and the device. Compared with the controls, the remaining youth with CP had a slower preferred walking speed (CP = 0.84 ± 0.08 m/s; Controls = 1.13 ± 0.05 m/s; P = 0.004). This slower walking speed was accompanied by a shorter step length (CP = 0.52 ± 0.03 m; Controls = 0.64 ± 0.03 m; P = 0.004), and a slower cadence when compared with the controls (CP = 94.7 ± 6.5 step/min; Controls = 103.4 ± 4.3 steps/min; P = 0.035). Altogether, these results further support the notion that youth with CP have abnormal gait biomechanics.
Across groups, there was a positive rank‐order correlation between the strength of the beta ERD during the motor planning stage and the participant’s walking speed (rho = 0.41; P = 0.008) and cadence (rho = 0.39; P = 0.01). This implied that participants with a slower walking speed were more likely to have a stronger beta ERD (e.g., more negative) during the motor planning stage. There was a positive rank‐order correlation between the beta ERD during the motor execution stage and the participants walking speed (rho = 0.42; P = 0.007) and cadence (rho = 0.47; P = 0.003). This suggested that participants with a slower preferred walking speed also tended to have a stronger beta ERD during the motor execution stage of the leg isometric task performed in the MEG.
For the youth with CP, there was a positive rank‐order correlation between the strength of the beta ERD during the motor execution stage and the preferred walking speed (rho = 0.53; P = 0.013; Figure 4B) and cadence (rho = 0.41; P = 0.04; Figure 4C). This suggests that youth with CP that had a slower walking speed and cadence tended to have a stronger beta ERD during the motor execution stage. Similar relationships were not found for the controls (Ps> 0.05).
Discussion
This investigation aimed to determine if there is a connection between the strength of the sensorimotor beta oscillations seen in youth with CP and their abnormal gait biomechanics. To this end, we utilized MEG and a goal‐directed isometric knee target‐matching task to quantify the beta oscillations serving motor control of the leg, and separately used a digital mat to quantify their spatiotemporal gait biomechanics. Our MEG imaging results revealed that the sensorimotor beta ERD was stronger during the motor planning and execution stages in youth with CP relative to their typically‐developing peers. In addition, we found that youth with CP who had stronger beta ERD responses in the motor cortex during the motor execution stage tended to walk slower and have a slower cadence. In sum, these results suggest that the abnormal beta sensorimotor cortical oscillations underlying control of the leg musculature may play a central role in the altered mobility seen in youth with CP. Further discussions of these results are provided in the following paragraphs.
One of our key findings was that the youth with CP exhibited a stronger beta ERD within the sensorimotor cortices during the motor planning and execution stages of the knee isometric target‐matching task. These results corroborate outcomes from our prior investigations, which have also shown that sensorimotor beta oscillations are stronger when youth with CP perform a lower extremity task. 25 , 26 The altered beta ERD seen prior to the onset of the isometric force further supports the notion that youth with CP have difficulty formulating an effective motor plan that accurately achieves the desired motor goal. 38 , 39 This premise is also supported by the MEG behavioral outcomes, which showed that the targets were initially overshot by about 14% in the youth with CP, and that the amount of overshoot was predicted by the strength of the beta ERD during the motor planning stage. The recurrent finding that youth with CP have a stronger beta ERD when executing their leg motor actions seems to align with the idea that youth with CP must generate a stronger cortical response to adequately excite the spinal motor neuron pools underlying the generation of a muscular contraction that is comparable to the controls. 40
Our biomechanical results were aligned with the current literature that has overwhelmingly showed that youth with CP have slower walking speeds, shorter step lengths and a reduced cadence compared with the controls. 9 , 41 , 42 , 43 However, our correlation results provide insight on the possible neurological mechanisms that may contribute to these spatiotemporal gait deficiencies by revealing that the youth with CP who had a stronger beta ERD during the motor execution stage also tended to walk slower and utilized a slower cadence. Nevertheless, this relationship did not appear to exist for the controls. We suggest that the deviant somatosensory processing often seen in youth with CP, but not controls, might partially contribute to the altered beta ERD and gait biomechanics. 9 , 44 This notion is based on prior research that has shown the influence of the Ib sensory afferents (i.e., golgi tendon organs) on the activity of the spinal cord alpha motor neurons is diminished in individuals with CP during gait. 45 Potentially, the spatiotemporal biomechanics seen in the youth with CP might be more dependent on activity within sensorimotor neural populations since the afferent spinal cord feedback loops are aberrant. In contrast, the intact afferent feedback loops seen in the controls may allow for spinal cord neural circuits to play a more prominent role in the spatiotemporal biomechanics while walking at a preferred speed. Nevertheless, this premise needs to be tested to better understand the unique contributions of both the spinal cord interneurons and cortical oscillations on the gait patterns seen in individuals with CP. Paradigms that combine H‐reflex and MEG measures of the sensorimotor cortical dynamics could significantly advance understanding in this area, and such experiments are direly needed.
Our results also indicated that the occipital alpha and beta cortical oscillations were similar between the youth with CP and the controls while performing the isometric knee target‐matching task. These results were somewhat unexpected, as visual processing impairments in youth with CP are becoming more prominent in the clinical literature. 46 , 47 , 48 Furthermore, our prior MEG experimental results have shown that the cortical oscillations seen in the visual middle temporal (MT) and occipital cortical areas are often abnormal in youth with CP. 26 , 49 It is possible that the participants in this investigation had more selective brain injuries that spared the occipital cortices, or that our specific task did not strongly burden these regions. Nevertheless, we cannot rule out the possibility that some of the participants did have visual impairments, as no ophthalmological assessments were performed in this study. Overall, we are cautious and recommend further work to clarify whether visual processing selectively affects motor actions in youth with CP.
The precise neurophysiological mechanism underlying the altered motor‐related beta ERD seen across our investigations remains unknown. 25 , 26 Pharmaco‐MEG studies with healthy controls have provided supporting evidence that an increased concentration of the inhibitory γ‐Aminobutyric acid (GABA) neurotransmitter within the sensorimotor cortices results in a stronger motor‐related beta ERD. 50 , 51 Given that prior PET studies have identified that individuals with CP tend to have increased GABAA receptor binding potential within the motor cortices, 52 , 53 a possible neurophysiological mechanism is heightened GABAergic activity. That being said, transcranial magnetic stimulation (TMS) studies have also shown that a higher stimulation intensity is necessary to elicit short‐latency intracortical inhibition in the motor cortices of youth with CP. 54 , 55 This suggests that the inhibitory cortical interneurons might actually have a lower excitability in persons with CP, implying that the stronger beta ERD might be related to hyper‐excitability of the sensorimotor cortical neurons. Both of these hypotheses are plausible and need to be thoroughly tested to better understand how the neurophysiology of youth with CP influences the beta ERD involved in the production of leg motor actions. These potential alternative explanations could be tested through multimodal neuroimaging approaches that combine TMS, GABA spectroscopy, and the MEG methods employed in this investigation, and such studies would significantly advance understanding in this area.
Overall, our results are somewhat consistent with what has been reported in the adult stroke literature. Briefly, it has been well established that adults who have incurred a stroke are likely to have difficulties matching prescribed target forces with the upper and lower extremities. 56 , 57 , 58 Furthermore, prior MEG studies have also identified that, when compared with controls, the sensorimotor beta cortical oscillations are also abnormal in individuals that have incurred a stroke, although the directionality of the differences in beta oscillatory strength have been mixed (i.e., stronger vs. weaker) across the respective studies. 59 , 60 , 61 , 62 Nevertheless, we suggest care should be taken in linking our results in CP to observations in the stroke literature, as the neural injuries in those who incurred a stroke generally occur in the less plastic adult brain, while those seen in CP generally occurred in the highly plastic perinatal de novo brain. The enormous capacity for neuroplasticity in the developing brain obviously raises challenges in making direct comparisons.
Conclusion
In this study, we demonstrated that motor‐related beta oscillations are aberrant in youth with CP during both the planning and execution of knee motor actions. Moreover, we identified that these altered beta oscillations are at least partially concomitant with the aberrant spatiotemporal gait biomechanics seen in CP. Together these results suggest that motor‐related beta oscillations have a notable influence on the degree of mobility impairments seen in youth with CP. While there is growing consensus that beta oscillations in the motor cortex are central to the motor impairments seen in youth with CP, whether the current therapeutic gait training trends are actually improving sensorimotor beta oscillations in individuals with CP remains an open question. Finally, prior research from our lab has shown that there is a connection between somatosensory cortical activity and the altered mobility seen in youth with CP. 9 It is probable that these sensory processing deficits may play a large role in the deviant gait patterns. Alternatively, it is possible that the altered motor‐related and somatosensory cortical oscillations are directly interconnected such that somatosensory processing might moderate the strength of the motor‐related beta ERD. These alternative options deserve consideration when interpreting the results presented here and should be used to drive the next generation of MEG‐mobility experiments.
Conflict of Interest
The authors have no conflicts of interest to report.
Acknowledgements
This work was partially supported by grants from the National Institutes of Health (R01‐HD086245; R01‐HD101833; P20‐GM130447).
Funding Information
No funding information provided.
Funding Statement
This work was funded by National Institutes of Health grants P20‐GM130447, R01‐HD086245, and R01‐HD101833.
References
- 1. Christensen D, Van Naarden BK, Doernberg NS, et al. Prevalence of cerebral palsy, co‐occurring autism spectrum disorders, and motor functioning ‐ Autism and Developmental Disabilities Monitoring Network, USA, 2008. Dev Med Child Neurol 2014;56(1):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Damiano DL, Dodd K, Taylor NF. Should we be testing and training muscle strength in cerebral palsy? Dev Med Child Neurol 2002;44(1):68–72. [DOI] [PubMed] [Google Scholar]
- 3. Arpin DJ, Stuberg W, Stergiou N, Kurz MJ. Motor control of the lower extremity musculature in children with cerebral palsy. Res Dev Disabil 2013;34(4):1134–1143. [DOI] [PubMed] [Google Scholar]
- 4. Hoffman RM, Corr BB, Stuberg WA, et al. Changes in lower extremity strength may be related to the walking speed improvements in children with cerebral palsy after gait training. Res Dev Disabil 2017;12(73):14–20. [DOI] [PubMed] [Google Scholar]
- 5. Moreau NG, Falvo MJ, Damiano DL. Rapid force generation is impaired in cerebral palsy and is related to decreased muscle size and functional mobility. Gait Posture 2012;35(1):154–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moreau NG, Simpson KN, Teefey SA, Damiano DL. Muscle architecture predicts maximum strength and is related to activity levels in cerebral palsy. Phys Ther 2010;90(11):1619–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Graham HK, Rosenbaum P, Paneth N, et al. Cerebral palsy. Nat Rev Dis Primers 2016;7(2):15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kurz MJ, Wilson TW, Corr B, Volkman KG. Neuromagnetic activity of the somatosensory cortices associated with body weight‐supported treadmill training in children with cerebral palsy. J Neurol Phys Ther 2012;36(4):166–172. [DOI] [PubMed] [Google Scholar]
- 9. Kurz MJ, Heinrichs‐Graham E, Becker KM, Wilson TW. The magnitude of the somatosensory cortical activity is related to the mobility and strength impairments seen in children with cerebral palsy. J Neurophysiol 2015;113(9):3143–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Short MR, Damiano DL, Kim Y, Bulea TC. Children with unilateral cerebral palsy utilize more cortical resources for similar motor output during treadmill gait. Front Hum Neurosci 2020;14:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kurz MJ, Wilson TW, Arpin DJ. An fNIRS exploratory investigation of the cortical activity during gait in children with spastic diplegic cerebral palsy. Brain Dev 2014;36(10):870–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gehringer JE, Arpin DJ, VerMaas JR, et al. The strength of the movement‐related somatosensory cortical oscillations differ between adolescents and adults. Sci Rep 2019;9(1):18520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Trevarrow MP, Kurz MJ, McDermott TJ, et al. The developmental trajectory of sensorimotor cortical oscillations. NeuroImage 2019;1(184):455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kurz MJ, Proskovec AL, Gehringer JE, et al. Developmental trajectory of beta cortical oscillatory activity during a knee motor task. Brain Topogr 2016;29(6):824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tzagarakis C, West S, Pellizzer G. Brain oscillatory activity during motor preparation: effect of directional uncertainty on beta, but not alpha, frequency band. Front Neurosci 2015;9:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grent‐'t‐Jong T, Oostenveld R, Jensen O, et al. Competitive interactions in sensorimotor cortex: oscillations express separation between alternative movement targets. J Neurophysiol 2014;112(2):224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heinrichs‐Graham E, McDermott TJ, Mills MS, et al. The lifespan trajectory of neural oscillatory activity in the motor system. Dev Cogn Neurosci 2018;30:159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaiser J, Birbaumer N, Lutzenberger W. Event‐related beta desynchronization indicates timing of response selection in a delayed‐response paradigm in humans. Neurosci Lett 2001;312(3):149–152. [DOI] [PubMed] [Google Scholar]
- 19. Jurkiewicz MT, Gaetz WC, Bostan AC, Cheyne D. Post‐movement beta rebound is generated in motor cortex: evidence from neuromagnetic recordings. NeuroImage 2006;32(3):1281–1289. [DOI] [PubMed] [Google Scholar]
- 20. Wilson TW, Slason E, Asherin R, et al. An extended motor network generates beta and gamma oscillatory perturbations during development. Brain Cogn 2010;73(2):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tzagarakis C, Ince NF, Leuthold AC, Pellizzer G. Beta‐band activity during motor planning reflects response uncertainty. J Neurosci 2010;30(34):11270–11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heinrichs‐Graham E, Wilson TW. Coding complexity in the human motor circuit. Hum Brain Mapp 2015;36(12):5155–5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gehringer JE, Arpin DJ, Heinrichs‐Graham E, et al. Neurophysiological changes in the visuomotor network after practicing a motor task. J Neurophysiol 2018;120(1):239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kilner J, Bott L, Posada A. Modulations in the degree of synchronization during ongoing oscillatory activity in the human brain. Eur J Neurosci 2005;21(9):2547–2554. [DOI] [PubMed] [Google Scholar]
- 25. Kurz MJ, Becker KM, Heinrichs‐Graham E, Wilson TW. Neurophysiological abnormalities in the sensorimotor cortices during the motor planning and movement execution stages of children with cerebral palsy. Dev Med Child Neurol 2014;56(11):1072–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kurz MJ, Proskovec AL, Gehringer JE, et al. Children with cerebral palsy have altered oscillatory activity in the motor and visual cortices during a knee motor task. Neuroimage Clin 2017;15:298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Taulu S, Simola J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol 2006;51(7):1759–1768. [DOI] [PubMed] [Google Scholar]
- 28. Kovach CK, Gander PE. The demodulated band transform. J Neurosci Methods 2016;1(261):135–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maris E, Oostenveld R. Nonparametric statistical testing of EEG‐ and MEG‐data. J Neurosci Methods 2007;164(1):177–190. [DOI] [PubMed] [Google Scholar]
- 30. Ernst MD. Permutation methods: a basis for exact inference. Stat Sci 2004;19(4):676–685. [Google Scholar]
- 31. Wiesman AI, Wilson TW. Attention modulates the gating of primary somatosensory oscillations. NeuroImage 2020;1(211):116610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gross J, Kujala J, Hamalainen M, et al. Dynamic imaging of coherent sources: studying neural interactions in the human brain. Proc Natl Acad Sci USA 2001;98(2):694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Van Veen BD, van Drongelen W, Yuchtman M, Suzuki A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans Biomed Eng 1997;44(9):867–880. [DOI] [PubMed] [Google Scholar]
- 34. Hillebrand A, Singh KD, Holliday IE, et al. A new approach to neuroimaging with magnetoencephalography. Hum Brain Mapp 2005;25(2):199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cheyne D, Bakhtazad L, Gaetz W. Spatiotemporal mapping of cortical activity accompanying voluntary movements using an event‐related beamforming approach. Hum Brain Mapp 2006;27(3):213–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heinrichs‐Graham E, Arpin DJ, Wilson TW. Cue‐related Temporal Factors Modulate Movement‐related Beta Oscillatory Activity in the Human Motor Circuit. J Cogn Neurosci 2016;28(7):1039–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Heinrichs‐Graham E, Wilson TW. Is an absolute level of cortical beta suppression required for proper movement? Magnetoencephalographic evidence from healthy aging. Neuroimage 2016;1(134):514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lust JM, Spruijt S, Wilson PH, Steenbergen B. Motor planning in children with cerebral palsy: a longitudinal perspective. J Clin Exp Neuropsychol 2018;40(6):559–566. [DOI] [PubMed] [Google Scholar]
- 39. Surkar SM, Hoffman RM, Davies B, et al. Impaired anticipatory vision and visuomotor coordination affects action planning and execution in children with hemiplegic cerebral palsy. Res Dev Disabil 2018;80:64–73. [DOI] [PubMed] [Google Scholar]
- 40. Condliffe EG, Jeffery DT, Emery DJ, et al. Full activation profiles and integrity of corticospinal pathways in adults with bilateral spastic cerebral palsy. Neurorehabil Neural Repair 2019;33(1):59–69. [DOI] [PubMed] [Google Scholar]
- 41. Kurz MJ, Arpin DJ, Corr B. Differences in the dynamic gait stability of children with cerebral palsy and typically developing children. Gait Posture 2012;36(3):600–604. [DOI] [PubMed] [Google Scholar]
- 42. Kim CJ, Son SM. Comparison of spatiotemporal gait parameters between children with normal development and children with diplegic cerebral palsy. J Phys Ther Sci 2014;26(9):1317–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Damiano DL, Abel MF. Relation of gait analysis to gross motor function in cerebral palsy. Dev Med Child Neurol 1996;38(5):389–396. [DOI] [PubMed] [Google Scholar]
- 44. Kurz MJ, Wiesman AI, Coolidge NM, Wilson TW. Children with cerebral palsy hyper‐gate somatosensory stimulations of the foot. Cereb Cortex 2018;28(7):2431–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Frisk RF, Jensen P, Kirk H, et al. Contribution of sensory feedback to plantar flexor muscle activation during push‐off in adults with cerebral palsy. J Neurophysiol 2017;118(6):3165–3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Costa MF, Pereira JC. Correlations between color perception and motor function impairment in children with spastic cerebral palsy. Behav Brain Funct 2014;25(10):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. da Costa MF, Salomao SR, Berezovsky A, et al. Relationship between vision and motor impairment in children with spastic cerebral palsy: new evidence from electrophysiology. Behav Brain Res 2004;149(2):145–150. [DOI] [PubMed] [Google Scholar]
- 48. Deramore Denver B, Froude E, Rosenbaum P, et al. Measurement of visual ability in children with cerebral palsy: a systematic review. Dev Med Child Neurol 2016;58(10):1016–1029. [DOI] [PubMed] [Google Scholar]
- 49. VerMaas JR, Gehringer JE, Wilson TW, Kurz MJ. Children with cerebral palsy display altered neural oscillations within the visual MT/V5 cortices. Neuroimage Clin 2019;23:101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Muthukumaraswamy SD, Myers JF, Wilson SJ, et al. The effects of elevated endogenous GABA levels on movement‐related network oscillations. NeuroImage 2013;1(66):36–41. [DOI] [PubMed] [Google Scholar]
- 51. Hall SD, Stanford IM, Yamawaki N, et al. The role of GABAergic modulation in motor function related neuronal network activity. NeuroImage 2011;56(3):1506–1510. [DOI] [PubMed] [Google Scholar]
- 52. Park HJ, Kim CH, Park ES, et al. Increased GABA‐A receptor binding and reduced connectivity at the motor cortex in children with hemiplegic cerebral palsy: a multimodal investigation using 18F‐fluoroflumazenil PET, immunohistochemistry, and MR imaging. J Nuclear Med 2013;54(8):1263–1269. [DOI] [PubMed] [Google Scholar]
- 53. Lee JD, Park HJ, Park ES, et al. Motor pathway injury in patients with periventricular leucomalacia and spastic diplegia. Brain 2011;134(Pt 4):1199–1210. [DOI] [PubMed] [Google Scholar]
- 54. Kuo HC, Zewdie E, Ciechanski P, et al. Intervention‐induced motor cortex plasticity in hemiparetic children with perinatal stroke. Neurorehabil Neural Repair 2018;32(11):941–952. [DOI] [PubMed] [Google Scholar]
- 55. Zewdie E, Damji O, Ciechanski P, et al. Contralesional corticomotor neurophysiology in hemiparetic children with perinatal stroke. Neurorehabil Neural Repair 2017;31(3):261–271. [DOI] [PubMed] [Google Scholar]
- 56. Chow JW, Stokic DS. Force control of quadriceps muscle is bilaterally impaired in subacute stroke. J Appl Physiol 2011;111(5):1290–1295. [DOI] [PubMed] [Google Scholar]
- 57. Lodha N, Naik SK, Coombes SA, Cauraugh JH. Force control and degree of motor impairments in chronic stroke. Clin Neurophysiol 2010;121(11):1952–1961. [DOI] [PubMed] [Google Scholar]
- 58. Lodha N, Patel P, Casamento‐Moran A, et al. Strength or motor control: what matters in high‐functioning stroke? Front Neurol 2018;9:1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rossiter HE, Borrelli MR, Borchert RJ, et al. Cortical mechanisms of mirror therapy after stroke. Neurorehabil Neural Repair 2015;29(5):444–452. [DOI] [PubMed] [Google Scholar]
- 60. Rossiter HE, Boudrias MH, Ward NS. Do movement‐related beta oscillations change after stroke? J Neurophysiol 2014;112(9):2053–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tang CW, Hsiao FJ, Lee PL, et al. beta‐oscillations reflect recovery of the paretic upper limb in subacute stroke. Neurorehabil Neural Repair 2020;34(5):450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wilson TW, Fleischer A, Archer D, et al. Oscillatory MEG motor activity reflects therapy‐related plasticity in stroke patients. Neurorehabil Neural Repair 2011;25(2):188–193. [DOI] [PubMed] [Google Scholar]


