Abstract
INTRODUCTION:
Prostaglandin E-major urinary metabolite (PGE-MUM) is a novel biomarker reflecting endoscopic activity in ulcerative colitis (UC). However, there are no studies investigating the efficacy of PGE-MUM as a biomarker for predicting relapse. We investigated whether PGE-MUM can predict clinical relapse of UC.
METHODS:
The measurement of PGE-MUM and endoscopic evaluation were performed in 70 patients with UC in clinical remission. The optimal cutoff values predicting relapse and relapse-free rate were analyzed.
RESULTS:
Sixteen patients (22.9%) relapsed during the 12-month follow-up. The median PGE-MUM value of relapsed patients at entry was significantly higher than that of patients in clinical remission (P = 0.008). The cutoff value of PGE-MUM predicting future relapse was 25.2 μg/g Cr by receiver-operating characteristic (ROC) analysis, and the area under the ROC curve was 0.721 (95% confidence interval: 0.556–0.886). The relapse-free rate of patients with PGE-MUM ≥25.2 μg/g Cr was significantly lower than that in patients with PGE-MUM <25.2 μg/g Cr (log-rank test: P < 0.001). The ROC analysis of UC patients with disease duration more than 1–8 years showed that duration of more than 5 years had the largest area under the ROC curve 0.821 (95% confidence interval: 0.583–1.000) and that the optimal cutoff value was 26.3 μg/g Cr.
DISCUSSION:
PGE-MUM is a reliable biomarker for predicting future relapse, particularly in UC patients with long-disease duration.
INTRODUCTION
Ulcerative colitis (UC) is an idiopathic chronic inflammatory bowel disease (IBD) with repeated symptoms of remission and relapse (1). Although the initial goal of UC treatment was to achieve clinical remission, advances in methods of assessing UC activity and treatment have changed the goals of UC. In particular, recent studies have shown that the goal of IBD treatment is the achievement of mucosal healing, which reduces the rate of hospitalization and intestinal resection, leading to improved prognosis for patients with UC (2). As an evaluation method for achievement of mucosal healing in patients with UC, measurement of several biomarkers such as fecal calprotectin (FCP), fecal immunochemical occult blood test (FIT), and serum leucine-rich alpha-2 glycoprotein have been reported to be useful, instead of evaluation by endoscopy (3–18). By measuring these biomarkers, it is possible to avoid frequent endoscopic examination, leading to reduction in psychological and physical burden on patients, reduction of risk, and reduction of costs related to endoscopy.
In recent years, the “treat-to-target” strategy has been adopted for UC therapy. Prediction of clinical relapse is important for treatment according to this therapeutic strategy. Among the biomarkers mentioned above, FCP and FIT not only reflect inflammation of the colonic mucosa but also have been reported to be useful for predicting relapse in the clinical course (19–24). The usefulness of urinary prostaglandin E-major urinary metabolite (PGE-MUM) as a biomarker for UC was reported by Arai et al. (25). We also previously reported that PGE-MUM is useful as a biomarker that reflects endoscopic activity, comparable with FIT, and that it is a highly useful test, especially for UC patients with a long-disease duration (26). In addition, urinalysis can reduce the labor of collecting stool specimens and the hesitation of bringing them to the hospital. We believed that if PGE-MUM, a urine test that is simpler than the fecal test, could predict the relapse of UC, it would be even more useful. There has been no report examining the efficacy of PGE-MUM as a biomarker for predicting relapse.
Therefore, in this study, we investigated the efficacy of PGE-MUM as a biomarker for predicting relapse of patients with UC.
MATERIALS AND METHODS
Patients and study design
We enrolled patients with UC who were treated at the Hamamatsu University School of Medicine between April 2015 and June 2020 and had met the following criteria for at least 3 months: clinical remission (Lichtiger clinical activity index ≤3) and mucosal healing (Mayo endoscopic subscore [MES] ≤2) (27). The diagnosis of UC was made based on typical history and clinical features and endoscopic and histological evaluation in accordance with recent guidelines. Patients who were not diagnosed with UC, such as indeterminate colitis or IBD-unclassified, were excluded. Since smoking and chronic pulmonary fibrosis are reported to increase PGE-MUM levels, smokers and patients with chronic fibrosing interstitial pneumonia were excluded (28,29). In addition, patients who underwent colectomy were excluded because PGE-MUM has been suggested to reflect inflammation of the whole colon. We enrolled 70 patients with UC in clinical remission who met the above conditions. Endoscopic examination and the measurement of PEG-MUM levels were performed at entry, and the patients were observed during a 12-month follow-up.
This was a prospective observational study. The primary endpoint was the relationship between the occurrence of clinical relapse during follow-up and the PGE-MUM level at enrolment. The secondary endpoint was the association of clinical relapse prediction using PGE-MUM with disease duration.
Disease assessment
We evaluated clinical disease activity using the Lichtiger clinical activity index (CAI). The CAI is based on the following criteria: diarrhea (number of daily stools), nocturnal diarrhea, visible blood in stool (percentage of movements), fecal incontinence, abdominal pain or cramping, general well-being, abdominal tenderness, and need for antidiarrheal drugs (27). Clinical remission was defined as CAI ≤3. At entry, the values of C-reactive protein, albumin, hemoglobin, and erythrocyte sedimentation rate were measured in our facility.
Endoscopic assessment
Colonoscopy was performed with bowel preparation consisting of a polyethylene glycol–based electrolyte solution. UC mucosal status was assessed using the following criteria based on the MES: 0, normal or inactive disease; 1, mild disease with erythema, decreased vascular pattern, mild friability; 2, moderate disease with marked erythema, absence of vascular patterns, friability, erosions; and 3, severe disease with spontaneous bleeding, ulceration. Mucosal healing was defined as MES 0 or 1.
PGE-MUM analysis
Urine samples for measurement of PGE-MUM were obtained on the morning of endoscopic examination. These samples were sent to the SRL Hachioji Laboratory (Tokyo, Japan). The urinary PGE-MUM analysis was performed with a ɤ-counter (Hitachi, Tokyo, Japan) using a Bicyclic PGE-MUM RIA Kit (Fuji Rebio, Tokyo, Japan). We evaluated the PGE-MUM level corrected by urinary creatinine.
Patient follow-up
All enrolled patients visited our hospital every 1–3 months and were observed for symptoms of UC relapse. The patients were instructed to keep a daily record of their clinical symptoms based on the Lichtiger clinical activity index and present these clinical symptoms to their attending physician at the time of examination. Clinical relapse was defined as treatment modification or change due to exacerbation of defecation frequency and/or bloody stool and the enrolled patients whose CAI worsened to ≥4. The value of PEG-MUM at entry was not known by the attending physician, and correction or change of treatment was left to the discretion of each attending physician.
Statistical analysis
Statistical analyses of the data were performed using IBM SPSS Statistics for Windows, version 24 (IBM, Armonk, NY) and SAS version 9.4 (SAS Institute, Cary, NC). P < 0.05 was considered statistically significant. Differences between median values were compared by the Mann-Whitney U test. The receiver-operating characteristic (ROC) analysis was conducted to find the optimal cutoff value of PGE-MUM for predicting clinical relapse in the future. The accuracy of the predicted relapse value was evaluated by the area under the ROC curve (AUC). The relapse-free rate was analyzed using the Kaplan-Meier analysis using the log-rank test and the Cox proportional hazards model.
Ethical considerations
Written informed consent was obtained from all enrolled patients after explanation of the purpose of the study and the nature of the procedures involved. The study protocol was reviewed and approved by the Ethics Committee of Hamamatsu University School of Medicine (number 18-228). This study was conducted in accordance with Good Clinical Practice principles in adherence to the Declaration of Helsinki.
RESULTS
Patient characteristics
The characteristics of 70 patients with UC in clinical remission at entry are shown in Table 1. The mean patient age was 47.5 years, and the mean disease duration was 9.5 years (range, 0.3–37). Based on colonoscopy, 44 (62.9%) and 26 patients had an MES of 0 and 1, respectively. The mean PGE-MUM levels were 22.0 μg/g Cr. Regarding the medication at entry, 46 (65.7%) patients were taking oral 5-aminosalicylic acid, 4 (5.7%) were taking suppository 5-aminosalicylic acid, 9 (12.9%) were taking systematic steroids, 28 (40.0%) were taking immunomodulators, and 14 (20.0%) were taking biologics.
Table 1.
Demographic characteristics of 70 patients with ulcerative colitis in clinical remission
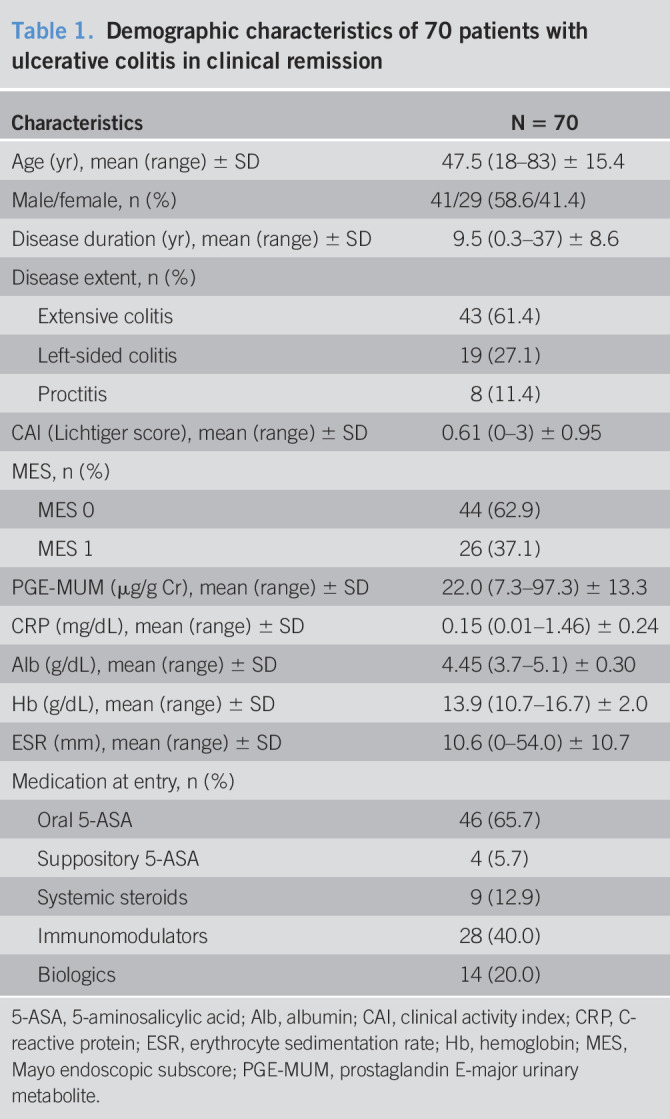
| Characteristics | N = 70 |
| Age (yr), mean (range) ± SD | 47.5 (18–83) ± 15.4 |
| Male/female, n (%) | 41/29 (58.6/41.4) |
| Disease duration (yr), mean (range) ± SD | 9.5 (0.3–37) ± 8.6 |
| Disease extent, n (%) | |
| Extensive colitis | 43 (61.4) |
| Left-sided colitis | 19 (27.1) |
| Proctitis | 8 (11.4) |
| CAI (Lichtiger score), mean (range) ± SD | 0.61 (0–3) ± 0.95 |
| MES, n (%) | |
| MES 0 | 44 (62.9) |
| MES 1 | 26 (37.1) |
| PGE-MUM (µg/g Cr), mean (range) ± SD | 22.0 (7.3–97.3) ± 13.3 |
| CRP (mg/dL), mean (range) ± SD | 0.15 (0.01–1.46) ± 0.24 |
| Alb (g/dL), mean (range) ± SD | 4.45 (3.7–5.1) ± 0.30 |
| Hb (g/dL), mean (range) ± SD | 13.9 (10.7–16.7) ± 2.0 |
| ESR (mm), mean (range) ± SD | 10.6 (0–54.0) ± 10.7 |
| Medication at entry, n (%) | |
| Oral 5-ASA | 46 (65.7) |
| Suppository 5-ASA | 4 (5.7) |
| Systemic steroids | 9 (12.9) |
| Immunomodulators | 28 (40.0) |
| Biologics | 14 (20.0) |
5-ASA, 5-aminosalicylic acid; Alb, albumin; CAI, clinical activity index; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; Hb, hemoglobin; MES, Mayo endoscopic subscore; PGE-MUM, prostaglandin E-major urinary metabolite.
Comparison of relapsed and patients in clinical remission
Sixteen patients (22.9%) with UC relapsed during the 12-month follow-up. The median PGE-MUM value of relapse patients at entry (26.5 μg/g Cr) was significantly higher than that of patients in clinical remission (19.4 μg/g Cr) (P = 0.008) (Figure 1). The ROC analysis of the subsequent clinical course of relapsed patients and those in remission showed that the cutoff value of PEG-MUM predicting clinical relapse was 25.2 μg/g Cr, and the AUC was 0.721 (95% confidence interval [CI]: 0.556–0.886) (Figure 2, Table 2).
Figure 1.
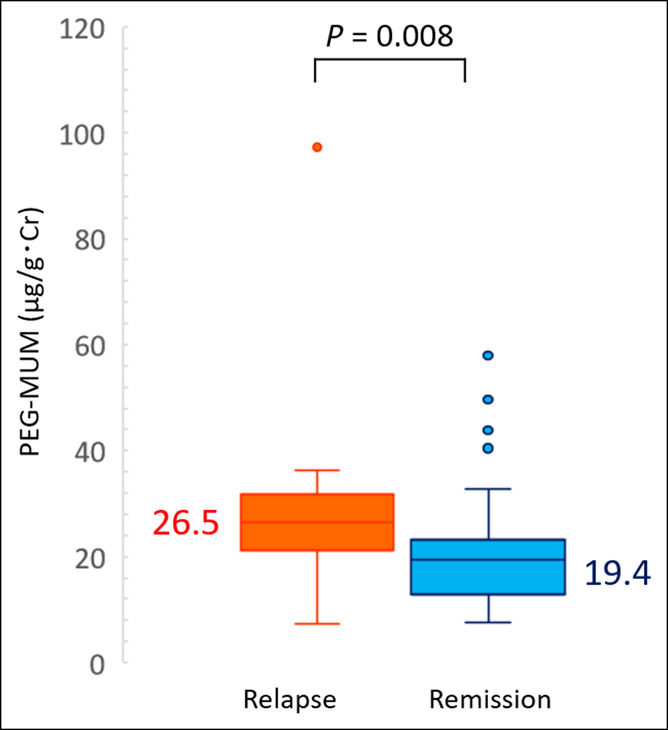
Differences in the prostaglandin E-major urinary metabolite (PGE-MUM) values at entry between patients with ulcerative colitis at relapse and clinical remission.
Figure 2.
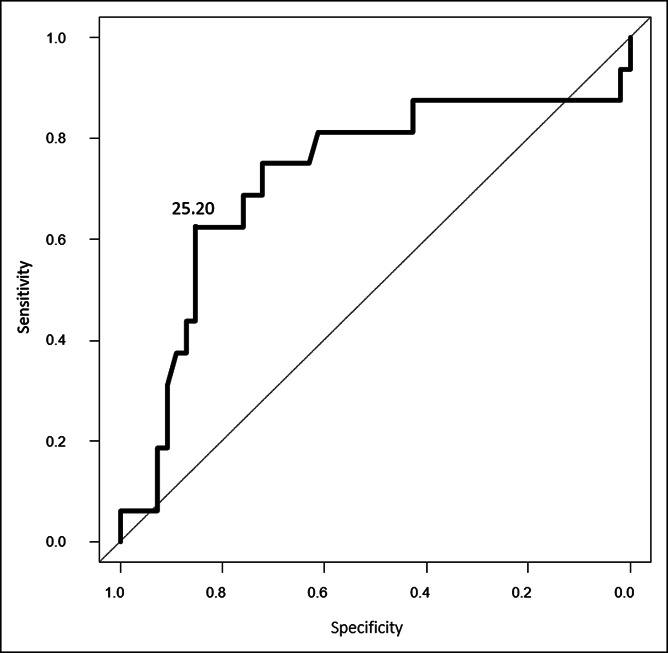
Receiver-operating characteristic analysis for prediction of future clinical relapse of patients with ulcerative colitis in clinical remission at entry.
Table 2.
Receiver-operating characteristic (ROC) analysis of PGE-MUM for predicting relapse of patients with ulcerative colitis in clinical remission
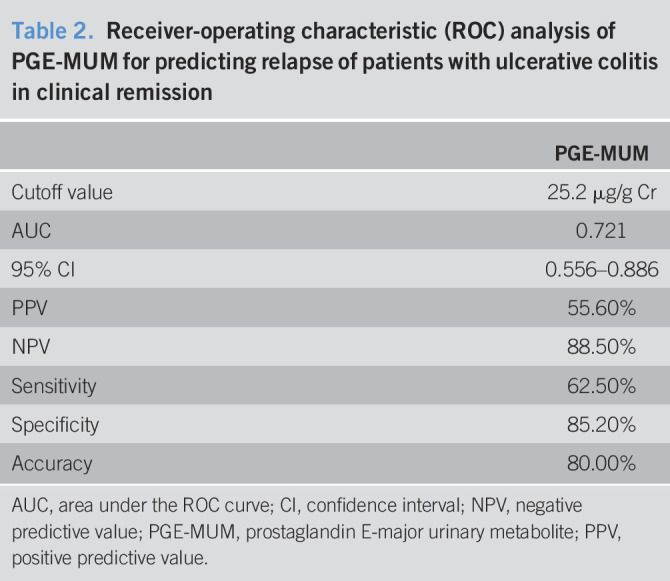
| PGE-MUM | |
| Cutoff value | 25.2 μg/g Cr |
| AUC | 0.721 |
| 95% CI | 0.556–0.886 |
| PPV | 55.60% |
| NPV | 88.50% |
| Sensitivity | 62.50% |
| Specificity | 85.20% |
| Accuracy | 80.00% |
AUC, area under the ROC curve; CI, confidence interval; NPV, negative predictive value; PGE-MUM, prostaglandin E-major urinary metabolite; PPV, positive predictive value.
Comparison of PGE-MUM and MES for the prediction of subsequent relapse
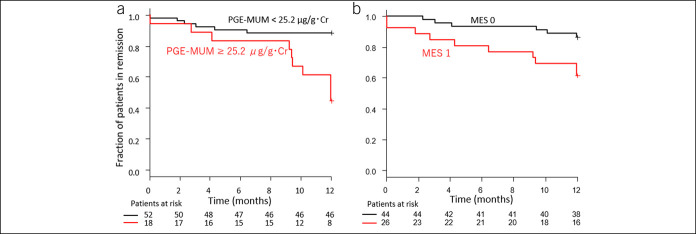
Remission-free survival was compared between the group of patients with PGE-MUM <25.2 μg/g Cr and that with PGE-MUM ≥25.2 μg/g Cr using Kaplan-Meier analysis (Figure 3a). There were 52 and 38 cases of PGE-MUM <25.2 μg/g Cr and PGE-MUM ≥25.2 μg/g Cr, respectively, and 6 cases (11.5%) and 10 cases (26.3%) of relapse, respectively. The relapse rate of the PGE-MUM ≥25.2 μg/g Cr group was significantly higher than that of the PGE-MUM <25.2 μg/g Cr group (log-rank test: P < 0.001) at 12 months of follow-up.
Figure 3.
Kaplan-Meier time-to-relapse curve of patients with ulcerative colitis in relation to the prostaglandin E-major urinary metabolite (PGE-MUM) value (a) and endoscopic score (b). MES, Mayo endoscopic subscore.
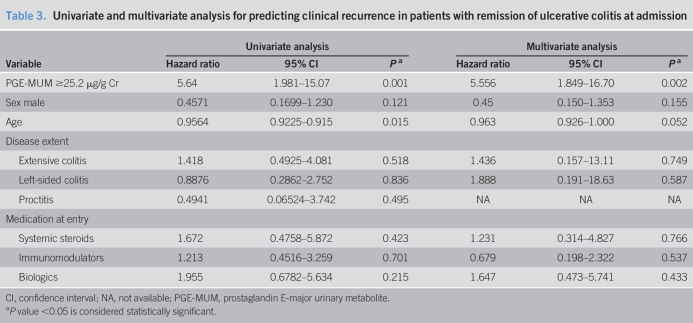
A Kaplan-Meier curve analysis using the endoscopic score at entry was also performed and compared. We analyzed relapse prediction by comparing the MES 0 group with the MES 1 group (Figure 3b). At entry, there were 44 and 26 patients in the MES 0 and MES 1 groups, respectively. The clinical symptoms of 6 (13.6%) and 10 (38.5%) patients in the MES 0 and MES 1 groups, respectively, showed relapse during the 12-month follow-up. The MES 1 group had a significantly higher clinical relapse rate in the Kaplan-Meier analysis than the MES 0 group (log-rank test: P = 0.013). However, the difference in relapse rate was greater in the PGE-MUM cutoff value comparison (PGE-MUM ≥25.2 μg/g Cr vs PGE-MUM <25.2 μg/g Cr) than in the endoscopic score comparison (MES 0 vs MES 1) (Figure 3a,b). Furthermore, multivariate Cox proportional hazards regression analysis was performed using PGE-MUM with UC relapse and a cutoff value of 25.2 μg/g Cr. In the univariate analysis, age was an independent predictive variable, and in the multivariate analysis, age was not an independent predictive factor (P = 0.015 and P = 0.052, respectively). Interestingly, it was shown that PGE-MUM was an independent predictive factor (hazard ratio 5.556; 95% CI 1.849–16.70; P = 0.002) (Table 3).
Table 3.
Univariate and multivariate analysis for predicting clinical recurrence in patients with remission of ulcerative colitis at admission
| Variable | Univariate analysis | Multivariate analysis | ||||
| Hazard ratio | 95% CI | Pa | Hazard ratio | 95% CI | Pa | |
| PGE-MUM ≥25.2 μg/g Cr | 5.64 | 1.981–15.07 | 0.001 | 5.556 | 1.849–16.70 | 0.002 |
| Sex male | 0.4571 | 0.1699–1.230 | 0.121 | 0.45 | 0.150–1.353 | 0.155 |
| Age | 0.9564 | 0.9225–0.915 | 0.015 | 0.963 | 0.926–1.000 | 0.052 |
| Disease extent | ||||||
| Extensive colitis | 1.418 | 0.4925–4.081 | 0.518 | 1.436 | 0.157–13.11 | 0.749 |
| Left-sided colitis | 0.8876 | 0.2862–2.752 | 0.836 | 1.888 | 0.191–18.63 | 0.587 |
| Proctitis | 0.4941 | 0.06524–3.742 | 0.495 | NA | NA | NA |
| Medication at entry | ||||||
| Systemic steroids | 1.672 | 0.4758–5.872 | 0.423 | 1.231 | 0.314–4.827 | 0.766 |
| Immunomodulators | 1.213 | 0.4516–3.259 | 0.701 | 0.679 | 0.198–2.322 | 0.537 |
| Biologics | 1.955 | 0.6782–5.634 | 0.215 | 1.647 | 0.473–5.741 | 0.433 |
CI, confidence interval; NA, not available; PGE-MUM, prostaglandin E-major urinary metabolite.
P value <0.05 is considered statistically significant.
ROC analysis of PGE-MUM for predicting relapse in long-disease duration group
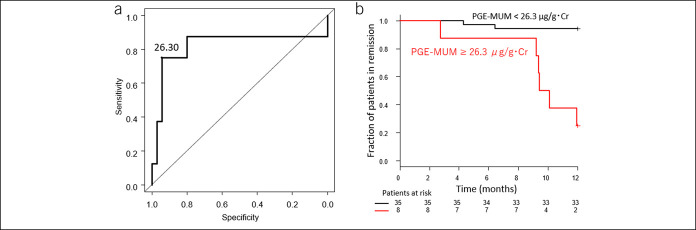
We then examined the relationship between UC disease duration and the accuracy of clinical relapse prediction. We analyzed the clinical relapse by dividing it into various disease periods (Table 4). The ROC analysis for predicting future clinical relapse during 12 months of follow-up in cases with a disease duration of more than 1–8 years showed the highest AUC in the group with a disease duration of more than 5 years (AUC 0.821, 95% CI: 0.583–1.000) (Figure 4a). The cutoff value of PGE-MUM that predicts relapse in this ROC analysis of the cases with disease duration more than 5 years was 26.3 μg/g Cr. Kaplan-Meier analysis for 12 months was performed in the groups with PGE-MUM of more and less than 26.3 μg/g Cr in the cases with a disease duration of 5 years or more. There were 35 and 8 patients in the groups with PGE-MUM <26.3 μg/g Cr and PGE-MUM ≥26.3 μg/g Cr at the time of entry, respectively. Clinical relapse occurred in 2 (5.7%) and 6 (7.5%) patients, respectively, during the 12-month follow-up. In this analysis, the group of patients with PGE-MUM ≥26.3 μg/g Cr had a significantly higher relapse rate than those with PGE-MUM <26.3 μg/g Cr (log-rank test: P < 0.001) (Figure 4b).
Table 4.
Receiver-operating characteristic analysis of PGE-MUM for predicting relapse in the long-disease duration group
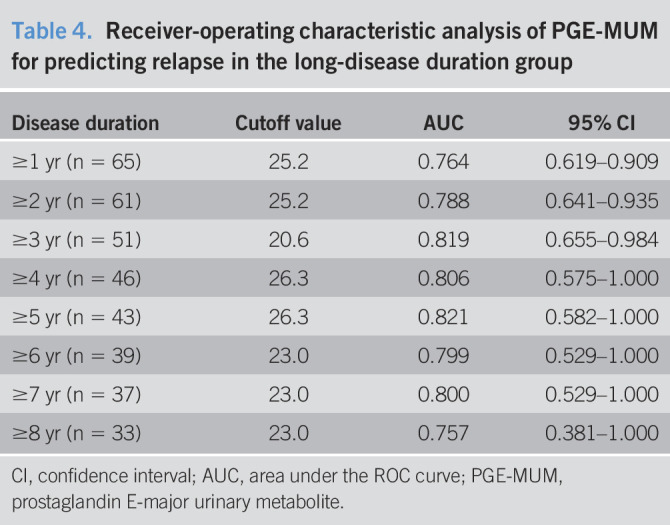
| Disease duration | Cutoff value | AUC | 95% CI |
| ≥1 yr (n = 65) | 25.2 | 0.764 | 0.619–0.909 |
| ≥2 yr (n = 61) | 25.2 | 0.788 | 0.641–0.935 |
| ≥3 yr (n = 51) | 20.6 | 0.819 | 0.655–0.984 |
| ≥4 yr (n = 46) | 26.3 | 0.806 | 0.575–1.000 |
| ≥5 yr (n = 43) | 26.3 | 0.821 | 0.582–1.000 |
| ≥6 yr (n = 39) | 23.0 | 0.799 | 0.529–1.000 |
| ≥7 yr (n = 37) | 23.0 | 0.800 | 0.529–1.000 |
| ≥8 yr (n = 33) | 23.0 | 0.757 | 0.381–1.000 |
CI, confidence interval; AUC, area under the ROC curve; PGE-MUM, prostaglandin E-major urinary metabolite.
Figure 4.
Receiver-operating characteristic analysis for predicting the future relapse (a) and Kaplan-Meier curve of ulcerative colitis patients with disease duration of more than 5 years (b). PGE-MUM, prostaglandin E-major urinary metabolite.
DISCUSSION
This is a study investigating whether PGE-MUM predicts clinical relapse of patients with UC in clinical remission. An important reason for using biomarkers in IBD is to reflect intestinal inflammatory and histological findings and to predict future clinical relapse. FCP and FIT in UC have been reported to correlate with endoscopic and histological scores and to predict future relapse. Urinary PEG-MUM is a stable product of urinary metabolism of prostaglandin E2 produced by inflammation in the colon, and its association with endoscopic scores and histological findings in UC was reported by Arai et al. (25). In addition, PGE-MUM was also reported to reflect endoscopic scores in pediatric UC (30). PGE-MUM is a minimally invasive marker that is measured using a urine sample. Although the association between such endoscopic scores of UC and histological findings has been demonstrated in PGE-MUM, to the best of our knowledge, the prediction of clinical relapse by PGE-MUM in patients with UC was not investigated previously. Therefore, we examined the association between PGE-MUM values and predictive clinical relapse during a 12-month follow-up in UC. In this study, the cutoff value of PGE-MUM was 25.2 μg/g Cr, which was predicted to represent clinical relapse by ROC analysis during the 12-month follow-up. Furthermore, the AUC was 0.721 (95% CI: 0.556–0.886). Although there are differences in conditions such as definition of clinical remission, definition of relapse, and follow-up period, the AUC of FIT and FCP that predict relapse was previously reported to be 0.69–0.72 and 0.65–0.81, respectively (19–23). The AUC of clinical relapse using PGE-MUM in this study was almost comparable with these previous reports (19–23).
Moreover, in this study, the clinical relapse rate evaluated by Kaplan-Meier analysis in the MES 0 group vs the MES 1 group showed that the MES 1 group had a significantly higher relapse rate than the MES 0 group. This result indicates that complete mucosal healing suppresses clinical relapse of UC as previously reported (31). A multicenter study investigating UC biomarkers conducted with the largest sample size to date was recently reported (24). In this study, cutoff values of FCP and FIT showing complete mucosal healing (MES 0 only) were calculated. The rate of patients who maintained clinical remission was then evaluated using Kaplan-Meier analysis by dividing them into 2 groups according to the level of the cutoff values. The results showed that the maintenance of the remission rate was significantly lower in the group with higher FCP or FIT than the cutoff value. This indicated that maintenance of mucosal healing was important for preventing clinical relapse.
Furthermore, in this study, it was shown that the relapse rate predicted using the PEG-MUM value was more accurate than the endoscopic score (MES 0 vs MES 1). Similar to this study, Yamamoto et al. (22) analyzed the clinical relapse prediction with endoscopic scores (MES 0 vs MES 1) and the relapse prediction using FCP, FIT, and lactoferrin. The cumulative relapse-free rate analyzed by the endoscopic score (MES 0 vs 1) did not show a significant difference between the MES 0 and MES 1 groups. However, in the analysis of clinical relapse prediction using the 3 biomarkers (FCP, FIT, and lactoferrin), the cumulative relapse-free rate using any of the biomarkers showed a significant difference between the groups with higher and lower values than the cutoff values, which proved their usefulness as biomarkers for predicting clinical relapse. Since biomarkers are only tests that predict the state of intestinal inflammation, it is necessary to regularly evaluate the intestinal mucosa evaluated by endoscopy. However, it is considered important to use biomarkers in combination with endoscopy in clinical practice from the perspective of relapse prediction.
We have previously reported that PEG-MUM is useful as a biomarker for reflecting endoscopic activity in UC patients with long-term disease (26). In this study as well, analysis of relapse prediction was conducted only for patients with long-term disease duration. The ROC analysis was performed to predict clinical relapse in patients with a disease duration of more than 1–8 years, and AUC of 0.821 (95% CI: 0.582–1.000) was the largest. The cutoff value of PGE-MUM calculated from this analysis was 26.3 μg/g Cr, and the relapse rate of the group with PGE-MUM ≥26.3 μg/g Cr was significantly higher than that of the group with PGE-MUM <26.3 μg/g Cr in the cases with disease duration of 5 years or more. Regarding the accuracy of biomarkers in the difference in disease duration, we have previously reported that the correlation between FIT and endoscopic score in patients with UC weakened when the disease duration was more than 5 years, but the correlation was strong when disease duration was within 4 years (32). It was reported that the usefulness of FCP in Crohn's disease was analyzed for long-term and short-term disease durations, showing that disease duration did not affect the reliability of FCP (33). Since this study did not include analysis of FIT, the relationship between disease duration and FIT as a biomarker for predicting UC relapse compared with PGE-MUM is not known at present and should be evaluated in a further study.
There are some limitations to this study. First, this is a single-center study with a few enrolled patients. Second, PGE-MUM has not been compared with other biomarkers. Although some analyses of relapse prediction with biomarkers in patients with UC have been reported, comparison with other reports, even with the same biomarker, is difficult owing to differences in observation periods and definitions of relapse. Third, there is no comparison with histological findings. Apart from biomarkers, endoscopic and histological findings are the key to predicting relapse of UC. In this study, a comparison of the MES 0 and MES 1 groups demonstrated that the MES 1 group had a higher relapse rate. However, the histological findings have not been investigated and warrant future studies.
In conclusion, urinary PEG-MUM was shown to be a reliable biomarker for predicting clinical relapse in patients with UC in clinical remission, even in long-term UC.
CONFLICTS OF INTEREST
Guarantor of the article: Ken Sugimoto, MD, PhD.
Specific author contributions: N.I.: contributed to this work. N.I. and K.S.: designed the study. K.S., T.M., S. Tamura, S.S., and S. Tani: collected the data. M.Y. M.I., and Y.H.: analyzed the data. N.I. and K.S.: wrote the paper. S.O. and T.F.: provided critical insight regarding paper preparation.
Financial support: This work was supported by a Grant-in-Aid for Scientific Research (C) (no. 18K07908) from the Japanese Ministry of Education, Culture, Sports, Science, and Technology.
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ Prostaglandin E-major urinary metabolite is a novel biomarker reflecting endoscopic activity in ulcerative colitis.
WHAT IS NEW HERE
✓ Prostaglandin E-major urinary metabolite reliably predicts ulcerative colitis relapse, particularly in patients with longer disease durations.
TRANSLATIONAL IMPACT
✓ Prostaglandin E-major urinary metabolite measurement could be useful for preventing clinical relapse of ulcerative colitis.
REFERENCES
- 1.Podolsky DK. Inflammatory bowel disease. N Engl J Med 2002;347:417–29. [DOI] [PubMed] [Google Scholar]
- 2.Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology 2011;141:1194–201. [DOI] [PubMed] [Google Scholar]
- 3.Schoepfer AM, Beglinger C, Straumann A, et al. Ulcerative colitis: Correlation of the Rachmilewitz endoscopic activity index with fecal calprotectin, clinical activity, C-reactive protein, and blood leukocytes. Inflamm Bowel Dis 2009;15:1851–8. [DOI] [PubMed] [Google Scholar]
- 4.D'Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis 2012;18:2218–24. [DOI] [PubMed] [Google Scholar]
- 5.Schoepfer AM, Beglinger C, Straumann A, et al. Fecal calprotectin more accurately reflects endoscopic activity of ulcerative colitis than the Lichtiger Index, C-reactive protein, platelets, hemoglobin, and blood leukocytes. Inflamm Bowel Dis 2013;19:332–41. [DOI] [PubMed] [Google Scholar]
- 6.Mooiweer E, Fidder HH, Siersema PD, et al. Fecal hemoglobin and calprotectin are equally effective in identifying patients with inflammatory bowel disease with active endoscopic inflammation. Inflamm Bowel Dis 2014;20:307–14. [DOI] [PubMed] [Google Scholar]
- 7.Patel A, Panchal H, Dubinsky MC. Fecal calprotectin levels predict histological healing in ulcerative colitis. Inflamm Bowel Dis 2017;23:1600–4. [DOI] [PubMed] [Google Scholar]
- 8.Mak WY, Buisson A, Andersen MJ, Jr, et al. Fecal calprotectin in assessing endoscopic and histological remission in patients with ulcerative colitis. Dig Dis Sci 2018;63:1294–301. [DOI] [PubMed] [Google Scholar]
- 9.Kim DJ, Jeoun YM, Lee DW, et al. Usefulness of fecal immunochemical test and fecal calprotectin for detection of active ulcerative colitis. Intest Res 2018;16:563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reinisch W, Bressler B, Curtis R, et al. Fecal calprotectin responses following induction therapy with vedolizumab in moderate to severe ulcerative colitis: A post hoc analysis of GEMINI 1. Inflamm Bowel Dis 2019;25:803–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mak LY, Tong TSM, Cheung KS, et al. Combined use of common fecal and blood markers for detection of endoscopically active inflammatory bowel disease. Clin Transl Gastroenterol 2020;11:e00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakarai A, Kato J, Hiraoka S, et al. Evaluation of mucosal healing of ulcerative colitis by a quantitative fecal immunochemical test. Am J Gastroenterol 2013;108:83–9. [DOI] [PubMed] [Google Scholar]
- 13.Takashima S, Kato J, Hiraoka S, et al. Evaluation of mucosal healing in ulcerative colitis by fecal calprotectin vs. fecal immunochemical test. Am J Gastroenterol 2015;110:873–80. [DOI] [PubMed] [Google Scholar]
- 14.Ryu DG, Kim HW, Park SB, et al. Assessment of disease activity by fecal immunochemical test in ulcerative colitis. World J Gastroenterol 2016;22:10617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi HY, Chan FKL, Chan AWH, et al. Accuracy of faecal immunochemical test to predict endoscopic and histological healing in ulcerative colitis: A prospective study based on validated histological scores. J Crohns Colitis 2017;11:1071–7. [DOI] [PubMed] [Google Scholar]
- 16.Hiraoka S, Inokuchi T, Nakarai A, et al. Fecal immunochemical test and fecal calprotectin results show different profiles in disease monitoring for ulcerative colitis. Gut Liver 2018;12:142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiraoka S, Takashima S, Inokuchi T, et al. The novel latex agglutination turbidimetric immunoassay system for simultaneous measurements of calprotectin and hemoglobin in feces. Intest Res 2019;17:202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shinzaki S, Matsuoka K, Iijima H, et al. Leucine-rich alpha-2 glycoprotein is a serum biomarker of mucosal healing in ulcerative colitis. J Crohns Colitis 2017;11:84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theede K, Holck S, Ibsen P, et al. Fecal calprotectin predicts relapse and histological mucosal healing in ulcerative colitis. Inflamm Bowel Dis 2016;22:1042–8. [DOI] [PubMed] [Google Scholar]
- 20.Hiraoka S, Kato J, Nakarai A, et al. Consecutive measurements by faecal immunochemical test in quiescent ulcerative colitis patients can detect clinical relapse. J Crohns Colitis 2016;10:687–94. [DOI] [PubMed] [Google Scholar]
- 21.Nakarai A, Hiraoka S, Takahashi S, et al. Simultaneous measurements of faecal calprotectin and the faecal immunochemical test in quiescent ulcerative colitis patients can stratify risk of relapse. J Crohns Colitis 2018;12:71–6. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto T, Shimoyama T, Umegae S, et al. Endoscopic score vs. fecal biomarkers for predicting relapse in patients with ulcerative colitis after clinical remission and mucosal healing. Clin Transl Gastroenterol 2018;9:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urushikubo J, Yanai S, Nakamura S, et al. Practical fecal calprotectin cut-off value for Japanese patients with ulcerative colitis. World J Gastroenterol 2018;24:4384–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naganuma M, Kobayashi T, Nasuno M, et al. Significance of conducting 2 types of fecal tests in patients with ulcerative colitis. Clin Gastroenterol Hepatol 2020;18:1102–11.e5. [DOI] [PubMed] [Google Scholar]
- 25.Arai Y, Arihiro S, Matsuura T, et al. Prostaglandin E-major urinary metabolite as a reliable surrogate marker for mucosal inflammation in ulcerative colitis. Inflamm Bowel Dis 2014;20:1208–16. [DOI] [PubMed] [Google Scholar]
- 26.Ishida N, Miyazu T, Takano R, et al. Prostaglandin E-major urinary metabolite versus fecal immunochemical occult blood test as a biomarker for patient with ulcerative colitis. BMC Gastroenterol 2020;20:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lichtiger S, Present DH. Preliminary report: Cyclosporin in treatment of severe active ulcerative colitis. Lancet 1990;336:16–9. [DOI] [PubMed] [Google Scholar]
- 28.Okayasu I, Ohnishi H, Sarandi I, et al. Significant increase of prostaglandin E major urinary metabolite in male smokers a screening study of age and gender differences using a simple radioimmunoassay. J Clin Lab Anal 2014;28:32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horikiri T, Hara H, Saito N, et al. Increased levels of prostaglandin E major urinary metabolite (PGE MUM) in chronic fibrosing interstitial pneumonia. Respir Med 2017;122:43–50. [DOI] [PubMed] [Google Scholar]
- 30.Hagiwara SI, Okayasu I, Fujiwara M, et al. Prostaglandin E-major urinary metabolite as a biomarker for pediatric ulcerative colitis activity. J Pediatr Gastroenterol Nutr 2017;64:955–61. [DOI] [PubMed] [Google Scholar]
- 31.Frøslie KF, Jahnsen J, Moum BA, et al. Mucosal healing in inflammatory bowel disease: Results from a Norwegian population-based cohort. Gastroenterology 2007;133:412–22. [DOI] [PubMed] [Google Scholar]
- 32.Ishida N, Miyazu T, Matsuura T, et al. Effect of ulcerative colitis duration on the usefulness of immunochemical fecal occult blood test result as a disease activity biomarker. Int J Colorectal Dis 2020;35:1729–39. [DOI] [PubMed] [Google Scholar]
- 33.Eder P, Stawczyk-Eder K, Łykowska-Szuber L, et al. Association between disease duration and usefulness of fecal calprotectin measurement in patients with Crohn's disease. Pol Arch Med Wewn 2014;124:51–7. [PubMed] [Google Scholar]