Abstract
Metabolic syndrome is a complex and heterogeneous pathology characterized by a cluster of biochemical, clinical, and metabolic factors that came together in raising the risk of cardiovascular diseases, type 2 diabetes mellitus, and all-cause mortality. Some of these features are well defined in this syndrome like: obesity, inflammation, hypertension, insulin resistance, atherosclerotic dyslipidemias, endothelial dysfunction, and inflammation. This circuit is intermediated by a complex network of hormones, cytokines, transcription factors, and adipokines, among others. Some like leptin, adiponectin, Plasminogen activator inhibitor-1, interleukin-6, Tumor necrosis factor, and their influence on the metabolic syndrome are well described in the literature and new players are described continuously. One novel player was described in 2016 by Romere et al as a fasting-induced glycogenic protein hormone named asprosin.
In order to perform a state-of-the-art, nonsystematic review of asprosin, a study of the available literature was carried out in the main database (Pubmed) and the results were studied and correlated to better understand the mechanism of action of this hormone.
Asprosin is not only associated with the metabolic syndrome features like glucose and lipid metabolism, insulin resistance, obesity and inflammation but also in other pathologies metabolic syndrome related like diabetic retinopathy, polycystic ovary syndrome and anorexia nervosa. A limited number of pathways were already unveiled although much more research is needed to better understand the therapeutical potential of asprosin in the metabolic syndrome.
Keywords: asprosin, diabetes, inflammation, metabolic syndrome, metabolism, obesity
Introduction to metabolic syndrome
The Metabolic Syndrome (MetS) was first described by the Swedish physician Kylin in 1923 as a concept rather than a diagnosis. It stood for the association between hypertension, hyperglycemia, and gout.1 The evolution of MetS integrated new features over time and was identified with different designations. The combination of obesity in the MetS2 was termed as “insulin-resistance syndrome,” “Syndrome X” assembled insulin-stimulated glucose uptake, hypertension, type 2 diabetes mellitus (T2DM) and cardiovascular diseases (CVD),3 and “deadly quartet” brought together obesity, glucose intolerance, hypertriglyceridemia, and hypertension.4
As an extremely complex and heterogeneous pathology (Fig. 1), there are many controversies regarding the most accurate definition for MetS by different academic societies (Table 1). Regardless of cutoffs of criteria, most agree that MetS is a cluster of biochemical, physiologic metabolic features which lead to higher levels of mortality due to risk factors like diabetes and impaired glucose metabolism, insulin resistance, abdominal obesity, impaired lipid metabolism, and high blood pressure.5
Figure 1.
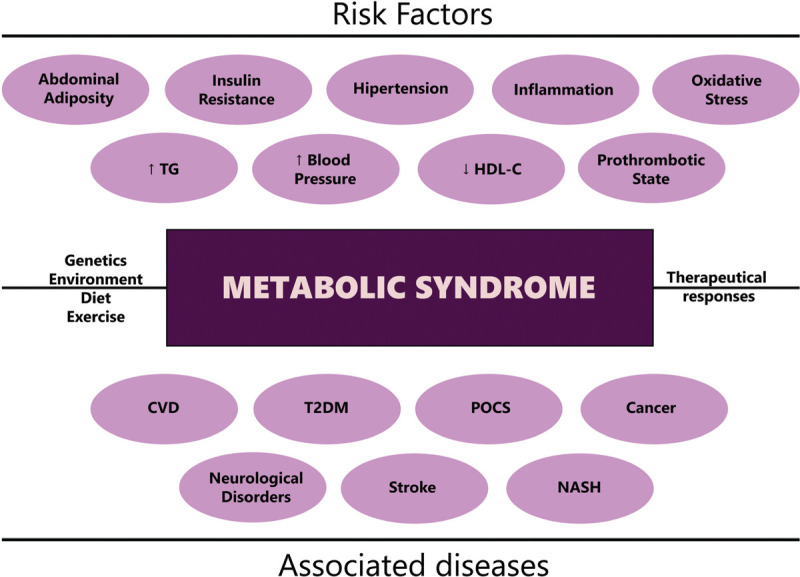
Risk factors and associated diseases of metabolic syndrome. CVD = cardiovascular disease; HDL-C = high-density lipoprotein cholesterol; NASH = nonalcoholic steatohepatitis; POCS = Polycystic Ovary Syndrome; T2DM = type 2 diabetes mellitus; TG = triglycerides.
Table 1.
Different cutoffs of the MetS criteria from scientific societies, based on Corneir et al, 2008.5
| Scientific society | WHO | IDF | EGIR | AACE | NCEP:ATPIII | |
|---|---|---|---|---|---|---|
| Main criteria | Insulin levels increasedIFG or IGT | Central obesity as defined by ethnic/racial, specific WC | Top 25% of the fasting insulin values among nondiabetic individuals | IGT | Three or more of the following: | |
| And two or more of the following: | Body fat features | WHR > 0.9BMI ≥ 30 kg/m2WC > 37 inches | N/D | WC in men: ≥ 94 cmWC in women ≥80 cm | N/D | WC in men: >40 inchesWC in women: >35 inches |
| Lipid profile | Triglycerides > 150 mg/dLHDL-C <35 mg/dL | Triglycerides ≥150 mg/dLHDL-C in men: < 40 mg/dLHDL-C in women: <50 mg/dL | Triglycerides ≥ 2.0 mmol/LHDL-C <1.0 mg/dL | Triglycerides ≥150 mg/dLHDL-C in men: <40 mg/dLHDL-C in women: <50 mg/dL | Triglycerides ≥150 mg/dLHDL-C in men: <40 mg/dLHDL-C in women: <50 mg/dL | |
| Blood pressure | BP >140/90 mm Hg | BP ≥130/85 mm Hg | BP ≥140/90 mm Hg or antihypertensive medication | BP ≥130/85 mm Hg | BP ≥130/85 mm Hg | |
| Glucose profile | N/D | FPG ≥ 100 mg/dL | Fasting glucose ≥6.1 mmol/L | N/D | FPG ≥100 mg/dL | |
AACE = American Association of Clinical Endocrinologists; BMI = body mass index; BP = blood pressure; EGIR = European Group for the Study of Insulin Resistance; FPG = fasting plasma glucose; HDL-C = high-density lipoprotein cholesterol; IDF = International Diabetes Federation; IFG = impaired fasting glucose; IGT = impaired glucose tolerance; N/D = not defined; NCEP:ATPIII = National Cholesterol Education Program, Adult Treatment Panel III; WC = waist circumference; WHO = World Health Organization; WHR = waist-to-hip ratio.
Obesity (abdominal obesity in particular) and insulin resistance are the core of MetS, as adipose tissue releases a panoply of local and systemic bioactive molecules like adipokines, cytokines, and transcription factors that results on increased insulin resistance, lipolysis of triglycerides, and increased free fatty acids (FFA)6 (Fig. 2).
Figure 2.
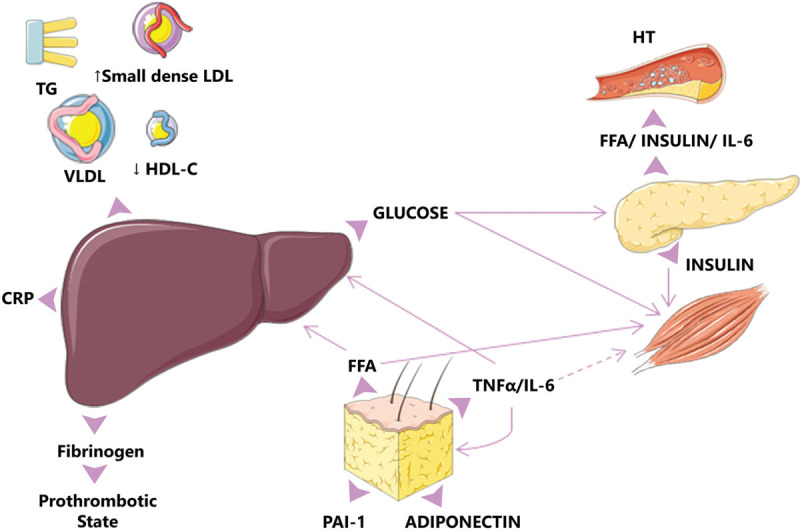
The marked produced FFA by the adipose tissue has an important contribution to the increased production of cytokines such as IL-6 and TNFα among others which results in increase insulin resistance. Cytokines may enhance hepatic glucose production, VLDL production by the liver, and insulin resistance in muscle. Based on the diagram from Cornier et al, 2008.5 CRP = C-reactive protein; FFA = free fatty acids; HDL = high-density lipoproteins; HT = hypertension; IL-6 = interleukin 6; LDL = low-density lipoproteins; PAI1 = plasminogen activator inhibitor-1; TG = triglycerides; TNFα = tumor necrosis factor α; VLDL = very low-density lipoproteins.
Moreover, the infiltration of immune cells in the adipose tissue promotes the state of chronic low-grade inflammation mediated by proinflammatory molecules (TNF-α and IL-6) and acute-phase proteins (CRP and PAI-1) increasing inflammatory signaling via the downstream activation of pathways like NFκB and JNK, resulting in an inhibition of the insulin signaling.7 In addition, adipokines (adiponectin and leptin) are insulin modulators by the maintenance of the energy balance and several peripheral metabolic mechanisms responsible for food intake, energy expenditure, and other neuroendocrine functions.8
Asprosin, a novel mediator
One recent player in the metabolic game is asprosin. Asprosin is a fasting-induced protein hormone secreted by the adipose tissue and recruited by the liver.9 Protein hormones are a subclass of hormones that usually use a cell-surface receptor to bind to the target cell, using a secondary messaging system they stimulate rapid signal transduction. Most of protein hormones result from the cleavage of larger proteins; asprosin is the result of the C-terminal cleavage product of profibrillin (encoded by FBN1) and exemplifies a glucogenic protein hormone. It was first described in Neonatal Progeroid Syndrome (NPS), which is associated with FBN1 truncating mutations which results in the ablation of asprosin. Ablation of asprosin is accountable for a distinctive symptom of NPS: subcutaneous lipoatrophy.9
Asprosin circulating levels are increased by fasting as a response to the low plasma glucose concentrations. In the liver, it bounds to a G-protein-coupled receptor—later known to be OLFR73410 and activates the G protein-cAMP-PKA pathway resulting in the secretion of hepatic glucose9 (Fig. 3).
Figure 3.
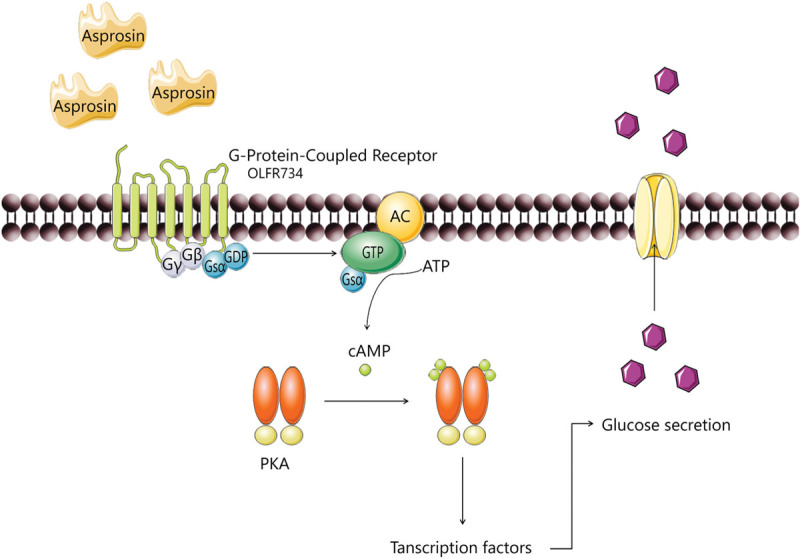
Asprosin pathway in the hepatocyte. G-protein-coupled receptors are activated and adenylyl cyclase (AC) synthesizes cAMP from ATP that binds to subunits of PKA, catalytic subunits will be released, will translocate to the nucleus, phosphorylating and subsequently activating the transcription factors. This downstream pathway will enhance the hepatic glucose release. Adapted from Romere et al, 2016.9
Observational studies of asprosin in glucose and lipid metabolism
Romere et al9 unveil the mechanism behind the glucose and lipid metabolic dysfunction but no studies succeed to fully understand if asprosin is the result of a protective feedback mechanism or the result of metabolic disorders. Nonetheless, evidence between glucose and lipid metabolism was shown in several observational studies.
The research by Wang et al recruited 143 Chinese subjects and divided them into three groups: normal glucose regulation (NGR, n = 52), impaired glucose regulation (IGR, n = 40), and newly diagnosed type 2 diabetes (nT2DM group, n = 51). Results show that plasma asprosin levels were significantly higher in IGR and nT2DM individuals and correlated with various clinical parameters of glucose and lipid metabolic disorders. The authors found a positive correlation with waist circumference (WC), fasting plasma glucose (FPG), post challenge plasma glucose (2hPG), glycated hemoglobin (HbA1c), triglyceride (TG), and homeostasis model assessment for insulin resistance (HOMA-IR). In addition, a negative correlation was found with homeostasis model assessment for β-pancreatic cell function (HOMA-β), area under the curve of the first-phase (0–10 min) insulin secretion (AUC), acute insulin response (AIR), and glucose disposition index (GDI).11 Combination of all parameters analyzed delineated a typical diabetic profile, where asprosin seems to play an important role and be a potential biomarker in T2DM and consequently in MetS.
Matching results were obtained by Zhang et al regarding individuals with NGR and nT2DM, serum asprosin concentrations were significantly increased in the nT2DM group12 and positively correlated with adiposity-related parameters like body mass index (BMI), WC and waist-hip ration (WHR) and also with TG.13
Asprosin and obesity management
Obesity is one of the main features in MetS, and already considered a growing epidemic and a public health problem. One target organ in the management of adiposity is the central nervous system: the response of hypothalamus to neurotransmitters released by the gastrointestinal tract plays a key role in energy homeostasis and appetite control.14
Appetite control is a complex neurometabolic network of physiology pathways in response to fasting hormones. Duerrschmid et al demonstrated that asprosin is able to cross the blood–brain barrier (BBB) and activate the hypothalamic feeding system and provoke appetite stimulation.15 The hypothalamus system responsible for appetite control consists on the regulation of NPY/AgRP+ (orexigenic) and POMC/CART (Anorexigenc) neurons. The experiments carry by Duerrschmid et al in mice model observed that asprosin, not only activated AgRP+ neurons, (only ∼50% were asprosin responsive by cells which contain the components necessary for transducing the asprosin-dependent signal) via a G-protein, adenylate cyclase, cAMP, and PKA pathway, but also inhibited ∼85% of the POMC+ neurons.15 The balance resulted in appetite stimulation16 (Fig. 4).
Figure 4.
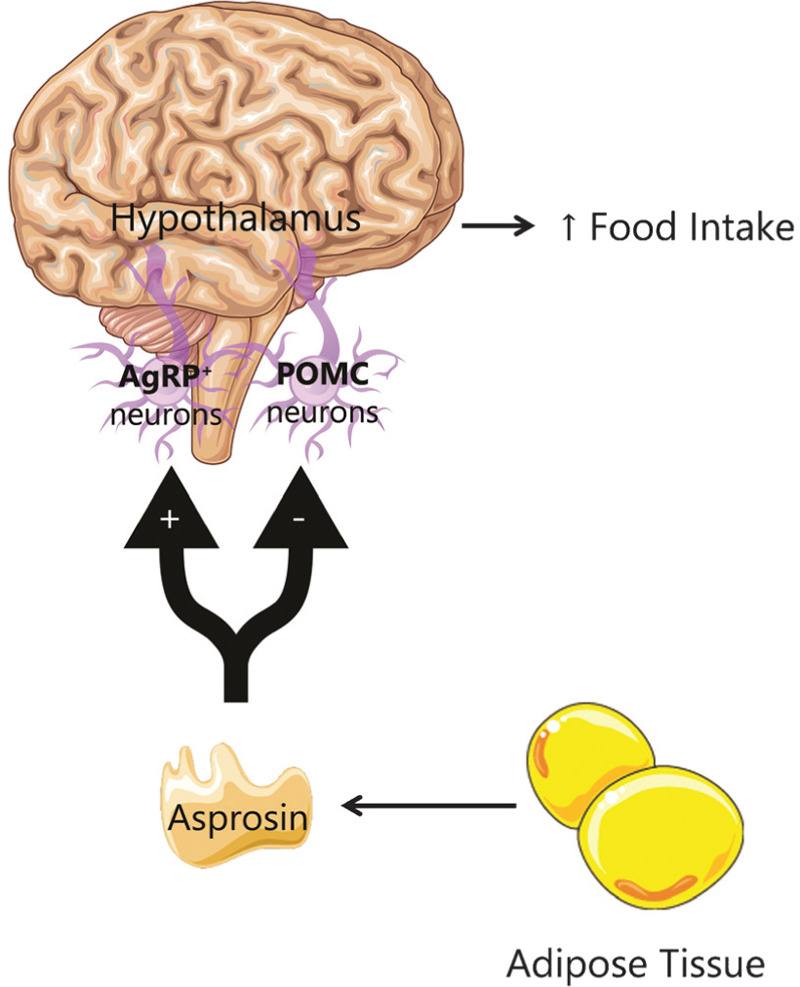
In the hypothalamus, asprosin released from the adipose tissue activates AgRP+ neurons and inhibits the POMC neurons, leading to increased appetite. Adapted from Yuan et al, 2020.16
This correlation is also supported by other studies. Ugur et al found a clear evidence that asprosin concentration increases in accordance with BMI, the method was performed both in blood and saliva and disclose that salivary glands also synthesize asprosin.17 This study revealed an advantage of using asprosin as a biomarker for it will be possible to obtain a non-invasive sample. Moreover, asprosin levels decreased significantly after bariatric surgery associated with the decrease of BMI.18
Research performed in children reveal controversial results. While Silistre et al as well as Wang et al obtained higher levels of asprosin in children with obesity19,20 as expected, Long et al observed a decrease in asprosin levels in children with obesity when compared with the normal weight control group, but no elucidation was given for these results.21
Interestingly enough, in anorexia nervosa (AN), a psychiatric disorder characterized by self-induced weight loss and appetite deregulation, the levels of asprosin were also increased and correlated with interoceptive awareness. The authors of this work postulate that the abnormal interaction between asprosin and insula is the responsible link; insula plays an important role in some of the symptoms of AN.22
Asprosin and inflammation
As it was described previously, inflammation is also a key regulation in MetS due to the inflammatory cytokines and acute phase proteins produced in the adipocytes. Lee et al reported that asprosin also contributes to inflammation, cellular dysfunction, and apoptosis in pancreatic β-cells and human primary islets.23 In this study, it was performed cellular assays with recombinant asprosin in MIN6 cells and human primary islets. The results reveal increased inflammation state, impaired glucose-stimulated insulin secretion, and promotion of apoptosis. These results suggests that not only adipocytes but also pancreatic β-cells can produce asprosin, furthermore, asprosin presented to be a novel ligand of toll-like receptor 4 (TLR4) that will promote inflammation and β-cell dysfunction by activation of JNK pathway23 (Fig. 5).
Figure 5.
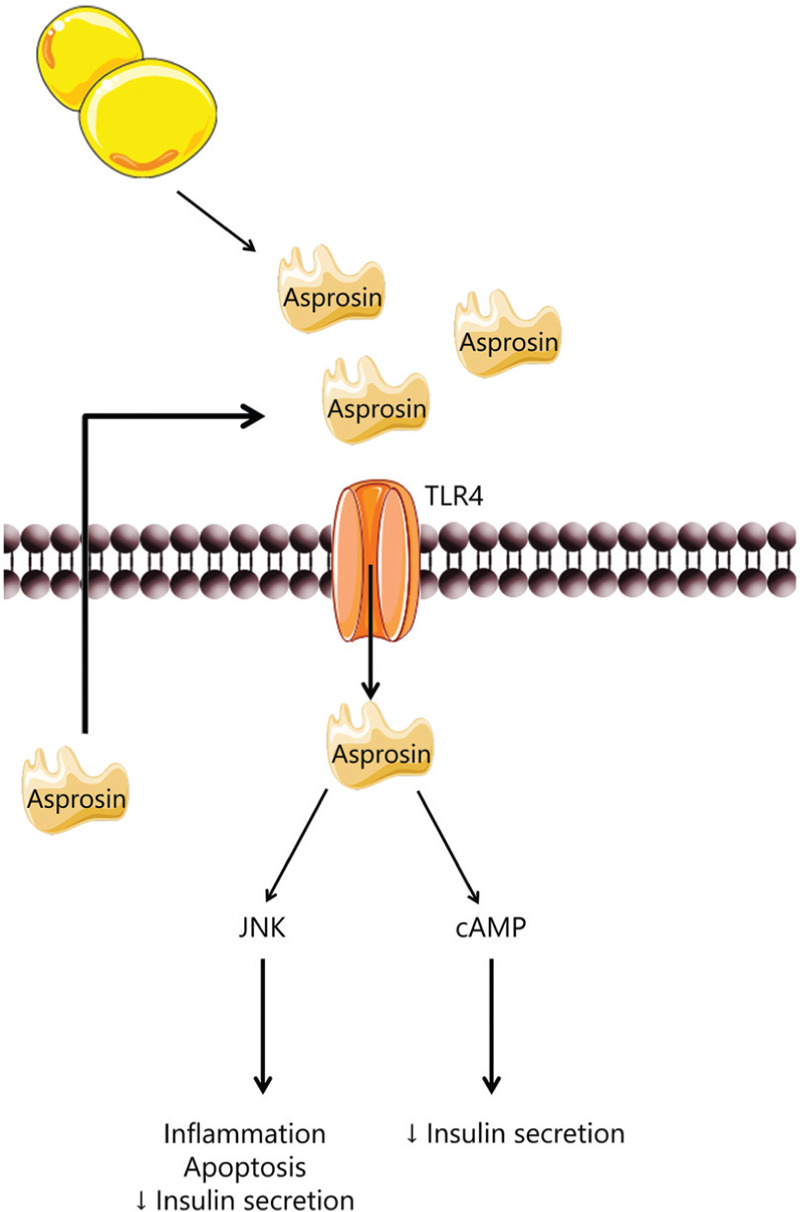
In β pancreatic cells, asprosin binds to TLR4 receptor promoting inflammation, apoptosis and decreasing insulin secretion. Adapted from Lee et al, 2019.22
In addition, FFA can promote endoplasmic reticulum (ER) stress which, by various inflammatory signals, will lead to augmented insulin signaling therefore to insulin resistance.24 Jung et al evaluated the effect of asprosin treatment in muscle cells: differentiated C2C12 myocytes and isolated mouse soleus skeletal muscle.25 They demonstrated that asprosin treatment resulted on elevated ER stress biomarkers (IRE-1—inositol-requiring enzyme 1; eIF2α—eukaryotic initiation factor 2α; CHOP—C/EBP homologous protein) that led to PKCδ activation and nuclear translocation, decreased SERCA activity and concluded on the impairment of insulin sensitivity in skeletal muscle by an unknown receptor25 (Fig. 6).
Figure 6.
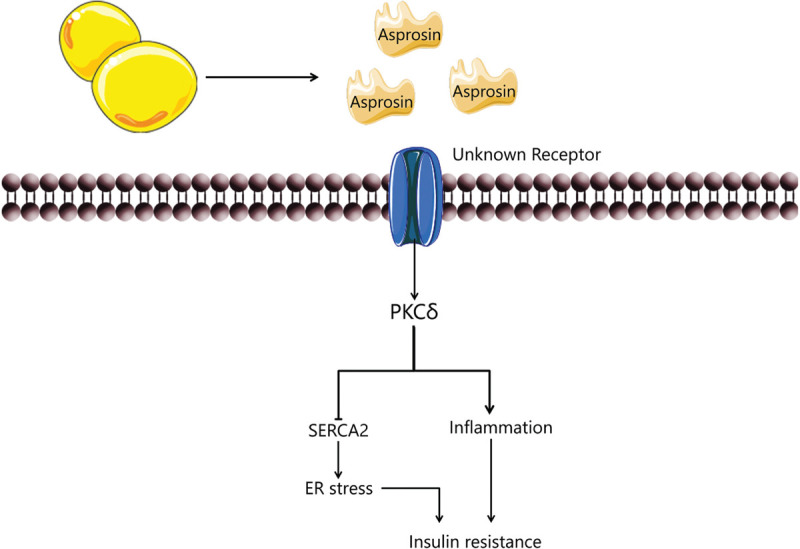
Asprosin in skeletal muscle induces insulin resistance by activating PKCd/SERCA2-mediated ER stress/inflammation pathways. Adapted from Yuan et al, 2020.16
Asprosin in pathologies—metabolic syndrome related
The correlation between asprosin and pathologies directly associated with MetS such as obesity, insulin impairment, T2DM, and inflammation has been subject of several studies in the scientific community. Other pathologies were also contemplated and asprosin correlated that are somewhat MetS associated like polycystic ovary syndrome (PCOS).
A research performed by Ko et al aim to study the influence of aerobic exercise on asprosin levels in type 1 diabetes mellitus (T1DM). Their results show that in streptozotocin-induced T1DM mouse model, the levels of asprosin was increased when compared to the non-diabetic control and that aerobic exercise decreased asprosin levels via PKA/TGF-β to control levels approximately.26
Other studies also associated increased plasma levels of asprosin in gestational diabetes mellitus (GDM). In a prospective study named early diagnosis of gestational diabetes mellitus (EDoGDM), a case-control of 40 pregnant women with GDM and 40 control cases with normal glucose tolerance (NGT) was performed to evaluate the levels of asprosin. Asprosin was higher in plasma of GDM as early as 18–20 gestational weeks and present in their offspring. The authors also observed asprosin expression in placenta from both groups.27 Moreover, a study performed by Baykus et al observed similar results, although asprosin levels were increased not only in GDM, but also in preeclampsia, severe preeclampsia and macrosomic fetuses, other interesting and unsolved result obtained was in intrauterine growth retardation where the levels of asprosin were significantly decrease.28
Regarding diabetic complications, diabetic retinopathy (DRP) is characterized by an increased edema and angiogenesis in the retina due to hyperglycemia. A quantification of blood and aqueous humor asprosin was performed in 30 patients with DRP and cataract, 30 patients with diabetes mellitus and cataract without DRP and 30 healthy controls. The results observed a significant increase in patients with DRP. The authors suggest that these findings could be a clinical tool to establish risk of DRP development.29
Also, an association was already observed between diabetes and chronic viral hepatitis as an additional risk factor,30 but no evidence has been shown in asprosin levels.31
On the subject of cardiovascular diseases, a study was performed by Chen et al reveal that asprosin can improve microvascular endothelium damage in the diabetic heart of mice through upregulation of the spartin/ROS signaling pathway.32 Another work from Zhang et al demonstrated that asprosin pretreatment of mesenchymal stromal cells (MSCs) and transplantation to the heart of mice that suffered myocardial infarction results in an improvement of cardiac health. Asprosin-pretreated MSCs are more resistant to apoptosis induced by both oxidative stress in vitro and myocardial ischemia in vivo, by activation of the ERK1/2 signaling pathway which upregulates antioxidant proteins (SOD2). SOD 2 will promote the decrease of Reactive Oxygen Species (ROS) and inhibit apoptosis.33 Moreover, asprosin levels can predict the severity of acute coronary syndrome in unstable angina pectoris.34
Polycystic ovary syndrome (PCOS) is a metabolic disorder in which insulin resistance plays a vital role. It is characterized by hyperandrogenism and/or hirsutism. Two studies demonstrated that asprosin levels are elevated in women with POCD. One demonstrated a positive correlation with insulin resistance, BMI, and free androgen index (FAI).35 The other a positive correlation with fasting blood glucose (FBG), hemoglobin A1c (HbA1c), LDL-c, APOB, APOE, testosterone and HOMA.36
Discussion and conclusions
The majority of the studies included in this review reveal increase levels of asprosin in pathologies associated with the metabolic syndrome. Still, few researchers had found decreased levels especially in obesity-related syndromes in children. A common consensus is needed to improve the knowledge of asprosin underlying mechanisms in order to uncover its potential as a diagnostic and/or therapeutical target. Research already recognized that anti-asprosin monoclonal antibodies can reduce blood glucose, decrease appetite therefore body weight and could be considered as a diabetic and obesogenic therapy.37 One promising therapeutical drug is a peripheral cannabinoid receptor 1 (CB1R) antagonism AM6545 which was able to lower the asprosin levels. In a monosodium glutamate (MSG)-induced obesity mice model characterized by abdominal obesity, dyslipidemia and insulin resistance, the treatment with AM6545 reduce not only asprosin levels but also others circulating adipokines including leptin with a positive metabolic improvement.38
Obesity and diabetes are the fundamental core of MetS and there's still no efficient therapy to either of these conditions. Asprosin disclosures not only an association with these pathologies but also with other diseases involved in MetS. Asprosin appears as a promising therapeutical target in MetS but there is still a hard road ahead. A better understanding of asprosin could bring new insights and reveal asprosin as the fundamental link between MetS risk factors.
Conflicts of interest
The author declares that there are no conflicts of interest.
References
- [1].E K. Studien ueber das Hypertonie-Hyperglyca “mie- Hyperurika” miesyndrom. Zentralblatt fuer Inn Medizin. 1923;1:105–127. [Google Scholar]
- [2].Despres JP. Abdominal obesity as important component of insulin-resistance syndrome. Nutrition. 1993;9:452–459. [PubMed] [Google Scholar]
- [3].Reaven GM. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. [DOI] [PubMed] [Google Scholar]
- [4].Kaplan NM. The deadly quartet. Upper-body obesity, glucose intolerance, hypertriglyceridemia, and hypertension. Arch Intern Med. 1989;149:1514–1520. [DOI] [PubMed] [Google Scholar]
- [5].Cornier M-A, Dabelea D, Hernandez TL, et al. The metabolic syndrome. Endocr Rev. 2008;29:777–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. [DOI] [PubMed] [Google Scholar]
- [7].Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–355. [DOI] [PubMed] [Google Scholar]
- [8].Nawrocki AR, Scherer PE. The delicate balance between fat and muscle: Adipokines in metabolic disease and musculoskeletal inflammation. Curr Opin Pharmacol. 2004;4:281–289. [DOI] [PubMed] [Google Scholar]
- [9].Romere C, Duerrschmid C, Bournat J, et al. Asprosin, a fasting-induced glucogenic protein hormone. Cell. 2016;165:566–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Li E, Shan H, Chen L, et al. OLFR734 Mediates Glucose Metabolism as a Receptor of Asprosin. Cell Metab. 2019;30:319–328.e8. [DOI] [PubMed] [Google Scholar]
- [11].Wang Y, Qu H, Xiong X, et al. Plasma asprosin concentrations are increased in individuals with glucose dysregulation and correlated with insulin resistance and first-phase insulin secretion. Mediators Inflamm. 2018;2018:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang X, Jiang H, Ma X, Wu H. Increased serum level and impaired response to glucose fluctuation of asprosin is associated with type 2 diabetes mellitus. J Diabetes Investig. 2020;11:349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhang L, Chen C, Zhou N, Fu Y, Cheng X. Circulating asprosin concentrations are increased in type 2 diabetes mellitus and independently associated with fasting glucose and triglyceride. Clin Chim Acta. 2019;489:183–188. [DOI] [PubMed] [Google Scholar]
- [14].Makaronidis JM, Batterham RL. The role of gut hormones in the pathogenesis and management of obesity. Current Opinion in Physiology. 2019;12:1–11. [Google Scholar]
- [15].Duerrschmid C, He Y, Wang C, et al. Asprosin is a centrally acting orexigenic hormone. Nat Med. 2017;23:1444–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yuan M, Li W, Zhu Y, Yu B, Wu J. Asprosin: a novel player in metabolic diseases. Front Endocrinol. 2020;11:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ugur K, Aydin S. Saliva and blood asprosin hormone concentration associated with obesity. Int J Endocrinol. 2019;2019:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang CY, Lin TA, Liu KH, et al. Serum asprosin levels and bariatric surgery outcomes in obese adults. Int J Obes. 2019;43:1019–1025. [DOI] [PubMed] [Google Scholar]
- [19].Wang M, Yin C, Wang L, et al. Serum asprosin concentrations are increased and associated with insulin resistance in children with obesity. Ann Nutr Metab. 2020;75:205–212. [DOI] [PubMed] [Google Scholar]
- [20].Sünnetçi Silistre E, Hatipoğlu HU. Increased serum circulating asprosin levels in children with obesity. Pediatr Int. 2020;62:467–476. [DOI] [PubMed] [Google Scholar]
- [21].Long W, Xie X, Du C, et al. Decreased circulating levels of asprosin in obese children. Horm Res Paediatr. 2019;91:271–277. [DOI] [PubMed] [Google Scholar]
- [22].Hu Y, Xu Y, Zheng Y, et al. Increased plasma asprosin levels in patients with drug-naive anorexia nervosa. Eat Weight Disord. 2020;Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [23].Lee T, Yun S, Jeong JH, Jung TW. Asprosin impairs insulin secretion in response to glucose and viability through TLR4/JNK-mediated inflammation. Mol Cell Endocrinol. 2019;486:96–104. [DOI] [PubMed] [Google Scholar]
- [24].Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jung TW, Kim HC, Kim HU, et al. Asprosin attenuates insulin signaling pathway through PKC(-activated ER stress and inflammation in skeletal muscle. J Cell Physiol. 2019;234:20888–20899. [DOI] [PubMed] [Google Scholar]
- [26].Ko JR, Seo DY, Kim TN, et al. Aerobic exercise training decreases hepatic asprosin in diabetic rats. J Clin Med. 2019;8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhong L, Long Y, Wang S, et al. Continuous elevation of plasma asprosin in pregnant women complicated with gestational diabetes mellitus: a nested case-control study. Placenta. 2020;93:17–22. [DOI] [PubMed] [Google Scholar]
- [28].Baykus Y, Yavuzkir S, Ustebay S, Ugur K, Deniz R, Aydin S. Asprosin in umbilical cord of newborns and maternal blood of gestational diabetes, preeclampsia, severe preeclampsia, intrauterine growth retardation and macrosemic fetus. Peptides. 2019;120:1–5. [DOI] [PubMed] [Google Scholar]
- [29].Oruc Y, Celik F, Ozgur G, et al. Altered blood and aqueous humor levels of asprosin, 4-hydroxynonenal, and 8-hydroxy-deoxyguanosine in patients with diabetes mellitus and cataract with and without diabetic retinopathy. Retina. 2020;Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [30].Mason AL, Lau JYN, Hoang N, et al. Association of diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;29:328–333. [DOI] [PubMed] [Google Scholar]
- [31].Eser Karlidag G, Arslan Solmaz O. Are adropin, apelin, elabela, asprosin and betatrophin biomarkers for chronic hepatitis and staging of fibrosis? Biotech Histochem. 2020;95:152–159. [DOI] [PubMed] [Google Scholar]
- [32].Chen S, Wang X, Qiu CM, et al. Study of the role and mechanism of asprosin/spartin pathway in cardiac microvascular endothelial injury induced by diabete mellitus. Sichuan da xue xue bao Yi xue ban=J Sichuan Univ Med Sci Ed. 2019;50:827–834. [PubMed] [Google Scholar]
- [33].Zhang Z, Tan Y, Zhu L, et al. Asprosin improves the survival of mesenchymal stromal cells in myocardial infarction by inhibiting apoptosis via the activated ERK1/2-SOD2 pathway. Life Sci. 2019;231:1–12. [DOI] [PubMed] [Google Scholar]
- [34].Acara AC, Bolatkale M, Kızıloğlu İ, İbişoğlu E, Can Ç. A novel biochemical marker for predicting the severity of ACS with unstable angina pectoris: asprosin. Am J Emerg Med. 2018;36:1504–1505. [DOI] [PubMed] [Google Scholar]
- [35].Alan M, Gurlek B, Yilmaz A, et al. Asprosin: a novel peptide hormone related to insulin resistance in women with polycystic ovary syndrome. Gynecol Endocrinol. 2019;35:220–223. [DOI] [PubMed] [Google Scholar]
- [36].Li X, Liao M, Shen R, et al. Plasma asprosin levels are associated with glucose metabolism, lipid, and sex hormone profiles in females with metabolic-related diseases. Mediators Inflamm. 2018;9:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hoffmann JG, Xie W, Chopra AR. Energy regulation mechanism and therapeutic potential of asprosin. Diabetes. 2020;69:559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ma H, Zhang G, Mou C, Fu X, Chen Y. Peripheral CB1 receptor neutral antagonist, AM6545, ameliorates hypometabolic obesity and improves adipokine secretion in monosodium glutamate induced obese mice. Front Pharmacol. 2018;9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]


