Over the last decades, several surface antigens have been identified and validated for treatment of B-cell malignancies. CD20-targeting antibodies have emerged as an effective therapy for patients with B-cell malignancies and are now broadly used in clinical practice. Nonetheless, many patients develop resistance against CD20-targeting therapies, with little or no further treatment options.1 Tetraspanin CD37 is expressed almost exclusively on hematopoietic cells with high expression on mature B-cells, including their malignant counterparts,2,3 and is a well described and validated target for B-cell malignancies. A number of CD37 targeting agents are in (pre)clinical development, including antibody-drug conjugates (IMGN529 and AGS67E), an Fc-engineered antibody (BI836826), a homodimeric therapeutic protein (otlertuzumab/TRU-016), a radioimmunoconjugate (177Lu-lilotomab), and chimeric antigen receptor T-cells.4-6 We recently reported on the development of DuoHexaBody-CD37, a novel biparatopic CD37-bispecific immunoglobulin G1 (IgG1) antibody with an E430G hexamerization-enhancing single point mutation in the Fc-domain.7 In contrast to all other newly developed CD37 targeting agents, DuoHexaBody-CD37 mediates potent complement-dependent cytotoxicity (CDC), a powerful anti-tumor effector mechanism,8,9 in addition to its Fc gamma receptor (FcγR)-mediated tumor cell kill mechanisms including antibody-dependent cellular cytotoxicity and cellular phagocytosis. DuoHexaBody-CD37 was designed based on recent discoveries that (1) initiation of CDC is dependent on Fc-mediated hexamer-formation of IgG1 antibodies after target engagement on the cell surface,10 (2) antibody Fc–Fc interactions and hexamer formation can significantly be improved by introducing an E430G single point mutation in the Fc domain,10,11 and (3) dual-epitope targeting of CD37 can potentiate or further enhance CDC.7
Here, we investigated the ex vivo therapeutic potential of DuoHexaBody-CD37 by evaluating its unique CDC-inducing capacity in primary tumor cell samples from a large cohort of newly diagnosed (ND) and relapsed/refractory (RR) patients with a broad range of B-cell malignancies, including chronic lymphocytic leukemia (CLL) and B-cell non–Hodgkin lymphoma, including diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), mantle cell lymphoma, and marginal zone lymphoma (Supplementary Material and Methods, http://links.lww.com/HS/A121). DuoHexaBody-CD37 induced potent, dose-dependent CDC of malignant primary B-cells in 45-minute complement assays with a median maximal lysis of 86% (n = 51, range: 0%-99%) and a median half maximal effective concentration (EC50) of 0.10 μg/mL (range, 0.004-15.21 μg/mL) (Figure 1A). Strikingly, sensitivity of the patient samples to DuoHexaBody-CD37-induced CDC was highly homogeneous. Only in 8 of 51 patient samples maximal lysis was lower than 60%, of which 3 samples demonstrated responses below 20% lysis. The CDC activity of DuoHexaBody-CD37 was comparable in samples from ND patients (n = 33; median maximal lysis of 85%; range CDC 0%-98%) and RR patients (n = 18; median maximal lysis of 89%; range CDC 5%-99%). Furthermore, DuoHexaBody-CD37 was significantly more potent than the CD20-targeting antibody rituximab in samples from ND patients, that is, CD20 antibody treatment naive patients (Figure 1B), even though CD37 expression was generally lower than expression of CD20 (Supplementary Figure 1, http://links.lww.com/HS/A121).
Figure 1.
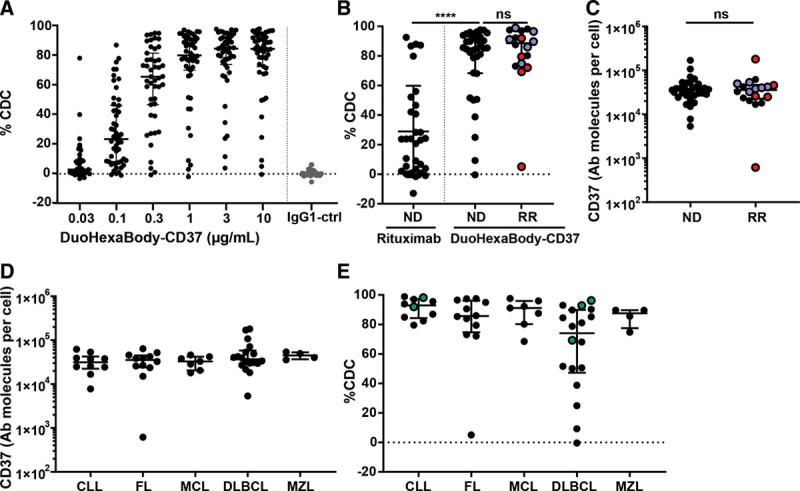
DuoHexaBody-CD37 induced potent CDC in samples obtained from ND and RR patients with various B-cell malignancies. A: Dose-dependent CDC induced by DuoHexaBody-CD37 in tumor B-cells (n = 51) derived from patients with various B-cell malignancy subtypes grouped together, in the presence of 20% NHS and in comparison to IgG1-ctrl (10 μg/mL). Levels of CDC-mediated tumor cell kill (% CDC) were determined by 7AAD-positive tumor cell staining, relative to a no antibody control sample. B: Comparison of CDC activity of 10 μg/mL DuoHexaBody-CD37 in samples from ND (n = 33) and RR (n = 18) patients (ns; Mann-Whitney U test), and comparison of rituximab (10 μg/mL) and DuoHexaBody-CD37 in ND patients (****P < 0.0001; Wilcoxon matched-pairs signed rank test). RR patients include CD20 therapy-refractory patients (n = 5;•), defined by progression of the disease within 6 mo post therapy, and CD20 therapy-relapse patients (n = 6;•). C: CD37 expression levels (defined as the number of CD37 molecules on the cell surface, assessed by quantitative flow cytometry), on tumor B-cells in ND and RR patient samples (ns; Mann-Whitney U test). D: Quantified CD37 expression levels (antibody molecules per cell) on tumor B-cells stratified according to B-cell malignancy subtype (ns, nonparametric Kruskal-Wallis test). All data are shown as the median and interquartile range. E: CDC activity of DuoHexaBody-CD37 (10 μg/mL) stratified according to B-cell malignancy subtype, including CLL (n = 10), FL (n = 12), MCL (n = 7), DLBCL (n = 18), and MZL (n = 4) (*P = 0.0321, nonparametric Kruskal-Wallis test with Dunn’s multiple comparisons [n])). Green symbols (•) indicatepatient samples with poor prognosis: ibrutinib-refractory CLL (n = 2) and double-hit DLBCL (n =3). AAD = xxx, CDC = complement-dependent cytotoxicity, CLL = chronic lymphocytic leukemia, DLBCL = diffuse large B-cell lymphoma, FL = follicular lymphoma, IgG1 = xxx, MCL = mantle cell lymphoma, MZL = marginal zone lymphoma, ND = newly diagnosed, NHS = normal human serum, NS = not significant, RR = relapsed/refractory.
Potent cytotoxicity was also observed in samples from patients who relapsed from (Figure 1B, blue symbols; n = 7) or were refractory to treatment regimens containing CD20-targeted antibodies (defined by progression of the disease within 6 months post treatment; Figure 1B, red symbols; n = 5), with the exception of 1 CD20-refractory FL patient. CD37 expression analysis (Figure 1C) revealed that this patient lacked CD37 expression on tumor B-cells. Since it has been reported that anti-CD20 therapy may induce CD20 antigen loss,12 and CD20 colocalizes with CD37 on the cell surface,13 we evaluated the possibility that CD37 expression levels were reduced on tumor B-cells derived from patients who had been exposed to anti-CD20. However, all anti-CD20–treated patient samples in our cohort showed high CD37 expression levels and no significant differences were observed in CD37 expression levels between samples from ND and RR patients (Figure 1C).
In addition, CD37 was expressed homogeneously in all B-cell malignancy subtypes (Figure 1D), which is in alignment with several other reports2,6 except for a recent study that suggested variable CD37 expression in DLBCL.14 This study-related discrepancy could be due to differences in assay-dependent detection limits in our study (flow cytometry) versus the other (immunohistochemistry) study. DuoHexaBody-CD37 induced an effective and homogeneous CDC response in all samples from CLL (n = 10; median max lysis 93%; range, 80%-99%), mantle cell lymphoma (n = 7; 91%; 69%-98%) and marginal zone lymphoma (n=4; 88%; 75%-90%), and 11 of 12 FL patient samples (n=12; 86%; 5%-98%), while a more heterogeneous response was observed in DLBCL patient samples (n = 18; 74%; 0%-96%) (Figure 1E) (Kruskal-Wallis one-way analysis of variance (ANOVA), *P = 0.0321). There were no differences in sensitivity between activated B-cell and germinal center B-cell subtypes of DLBCL (Supplementary Figure 2, http://links.lww.com/HS/A121). Of note, also samples from ibrutinib-refractory CLL (n = 2) and double-hit DLBCL (n = 3) patients with poor prognosis were highly susceptible to DuoHexaBody-CD37–induced CDC (maximum lysis >60%) (Figure 1E).
Complement activation and CDC-mediated tumor cell lysis is not only dependent on antigen density but also on expression levels of complement regulatory proteins (CRPs) CD46 and CD55 that inhibit complement convertases, and CD59 that inhibits the formation of the membrane attack complex.15 Expression of CRPs was not associated with B-cell malignancy subtype (Figure 2A) or treatment status (Supplementary Figure 3, http://links.lww.com/HS/A121).
Figure 2.
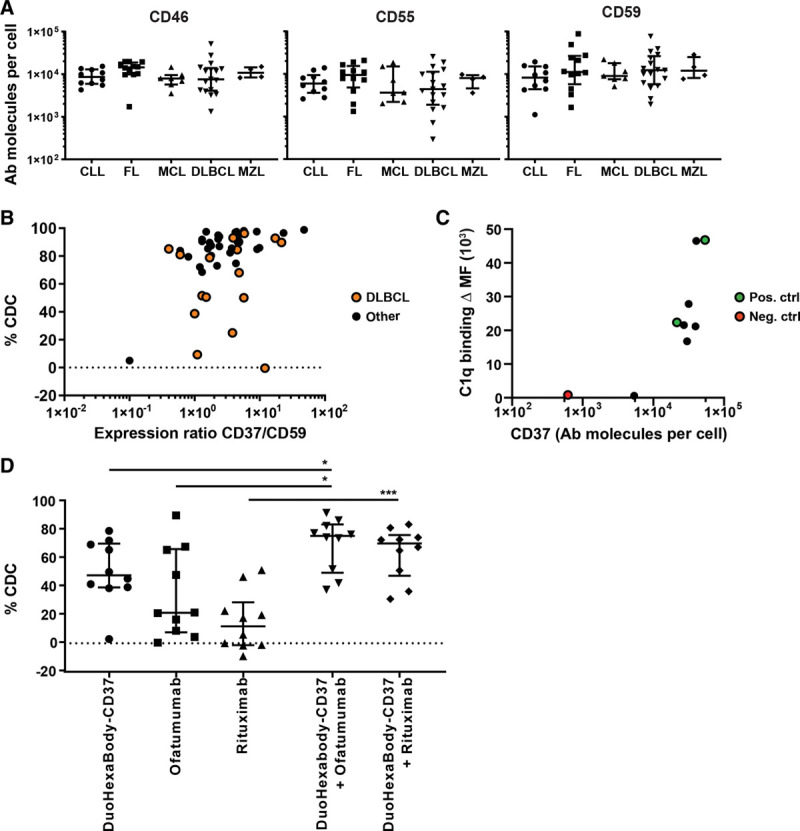
Low sensitivity to DuoHexaBody-CD37–mediated CDC is not associated with expression of CD37 and CRPs and can be improved by combination with CD20 mAbs. A, Quantified expression levels (antibody molecules per cell) of complement regulatory proteins CD46, CD55, and CD59 on tumor B-cells in specific B-cell malignancy subtypes; CLL (n = 10), FL (n = 12), MCL (n = 7), DLBCL (n = 18), and MZL (n = 4) (ns; nonparametric Kruskal-Wallis test). Data shown are CDC in individual patient samples, in addition to the median and interquartile range. B, CDC activity of DuoHexaBody-CD37 (10 μg/mL) correlated with the ratio of CD37/CD59 expression levels for all B-cell malignancy subtypes grouped together, for DLBCL patient samples specifically (•) and for B-cell malignancy subtypes other than DLBCL (Spearman’s correlation r = 0.4423, **P = 0.0013; r = 0.2843, P = 0.2678; and r = 0.5158, **P = 0.0025, respectively). Data are shown relative to a no antibody control sample. C, Expression levels of CD37 (antibody molecules per cell) correlated with C1q binding (ΔMFI) for 6 samples with low CDC response (CDC < 60%) (•), 1 CD37-negative sample as negative control (•) and 2 high responding samples as positive control (•) (Spearman’s correlation r = 0.7833, *P = 0.0172). D, CDC induced by a combination of DuoHexaBody-CD37 and rituximab or ofatumumab (10 μg/mL + 10 μg/mL) vs single antibody (10 μg/mL) (n = 10) (*P < 0.05, ***P < 0.001; Friedman test with Dunn multiple comparison test) relative to a no antibody control sample. All data are shown as the median and interquartile range. CDC = complement-dependent cytotoxicity, CLL = chronic lymphocytic leukemia, CRP = complement regulatory protein, DLBCL = diffuse large B-cell lymphoma, FL = follicular lymphoma, mAbs = monoclonal antibodies, MCL = mantle cell lymphoma, MFI = median fluorescence intensity, MZL = marginal zone lymphoma.
Furthermore, no correlation was observed between DuoHexaBody-CD37–induced CDC and expression of CD37 or CRPs (Supplementary Figure 4, http://links.lww.com/HS/A121). A weak, but statistically significant correlation was observed between sensitivity to DuoHexaBody-CD37 and the ratio of CD37 and CD59 expression levels (Figure 2B). However, in DLBCL patient samples, differences in (the ratio of) CD37 and CD59 expression could not explain the heterogeneous response to DuoHexaBody-CD37 ex vivo (Figure 2B, orange symbols). We therefore conclude that there is a limited impact of CRP expression on DuoHexaBody-CD37–mediated CDC. This was in contrast to rituximab-induced CDC, which already showed a strong correlation with CD20 expression but an even stronger correlation with the ratio of CD20 and CD55 (Supplementary Figure 5, http://links.lww.com/HS/A121).
The first step of complement activation is the binding of C1q to membrane-bound antibodies, that together with C1r and C1s forms the C1 complex, the first component of the classical complement pathway. Toward understanding the heterogeneity in CDC responses observed in DLBCL patient samples, we investigated the C1q binding capacity of membrane-bound DuoHexaBody-CD37 in DLBCL patient samples for which DuoHexaBody-CD37–mediated CDC levels were lower than 60% (N = 6). The C1q binding capacity in low CDC responders was comparable to that of 2 patient samples highly susceptible to DuoHexaBody-CD37–induced CDC (Figure 2C), indicating that the first step of complement activation is not impaired. Whether other steps in the complement activation pathway, such as membrane attack complex assembly and stability, or cell intrinsic mechanisms, play a role in resistance to CDC induction remains to be elucidated.
Finally, we also evaluated whether combination with rituximab and/or ofatumumab could further enhance the CDC activity of DuoHexaBody-CD37, as we have previously reported enhanced CDC in CLL and B-cell non–Hodgkin lymphoma primary patient cells with combinations of CD20 and CD37 antibodies.13 Indeed, in 10 patient samples including samples with intermediate to low sensitivity to DuoHexabody-CD37–mediated CDC (<80% CDC), the combination of DuoHexaBody-CD37 with ofatumumab significantly enhanced the tumor cell kill in an additive manner (Figure 2D). In contrast to ofatumumab, rituximab as single antibody could generally not induce CDC in these samples and the CDC effects of the combination with DuoHexaBody-CD37 are less striking.
In conclusion, this preclinical study indicates high therapeutic potential for DuoHexaBody-CD37 in a broad spectrum of B-cell malignancies either as single agent or in combination with CD20-targeting antibodies, and supports the recently initiated first-in-human clinical trial of DuoHexaBody-CD37 for patients with relapsed or refractory B-cell NHL (NCT04358458).
Disclosures
SCO, MBO, and ECWB are Genmab employees and own Genmab warrants and/or stock. HJvdH, SCO, MBO, MEDC, ECWB, and TM are inventors on Genmab patent applications. MEDC has received research support from Gilead, Genmab, and Celgene. SZ has received research support from Celgene, Janssen Pharmaceuticals and Takeda; and serves in advisory boards for Celgene, Janssen Pharmaceuticals, Takeda, Amgen, and Sanofi. TM has received research support from Janssen Pharmaceuticals, Genmab, Takeda, Onkimmune, and Gadeta. All the other authors have no conflicts of interest to disclose.
Acknowledgments
The authors thank Henk Lokhorst for his contribution in the early stage of the study.
Supplementary Material
Footnotes
This study was supported by research funding from Genmab (Utrecht, the Netherlands).
HJH and SCO contributed equally to this study.
Supplemental digital content is available for this article.
References
- 1.Salles G, Barrett M, Foà R, et al. Rituximab in B-cell hematologic malignancies: a review of 20 years of clinical experience. Adv Ther. 2017; 34:2232–2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deckert J, Park PU, Chicklas S, et al. A novel anti-CD37 antibody-drug conjugate with multiple anti-tumor mechanisms for the treatment of B-cell malignancies. Blood. 2013; 122:3500–3510 [DOI] [PubMed] [Google Scholar]
- 3.Schwartz-Albiez R, Dörken B, Hofmann W, et al. The B cell-associated CD37 antigen (gp40-52). Structure and subcellular expression of an extensively glycosylated glycoprotein. J Immunol. 1988; 140:905–914 [PubMed] [Google Scholar]
- 4.Witkowska M, Smolewski P, Robak T. Investigational therapies targeting CD37 for the treatment of B-cell lymphoid malignancies. Expert Opin Investig Drugs. 2018; 27:171–177 [DOI] [PubMed] [Google Scholar]
- 5.Scarfò I, Ormhøj M, Frigault MJ, et al. Anti-CD37 chimeric antigen receptor T cells are active against B- and T-cell lymphomas. Blood. 2018; 132:1495–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Köksal H, Dillard P, Josefsson SE, et al. Preclinical development of CD37CAR T-cell therapy for treatment of B-cell lymphoma. Blood Adv. 2019; 3:1230–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oostindie SC, van der Horst HJ, Kil LP, et al. DuoHexaBody-CD37®, a novel biparatopic CD37 antibody with enhanced Fc-mediated hexamerization as a potential therapy for B-cell malignancies. Blood Cancer J. 2020; 10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Gaetano N, Cittera E, Nota R, et al. Complement activation determines the therapeutic activity of rituximab in vivo. J Immunol. 2003; 171:1581–1587 [DOI] [PubMed] [Google Scholar]
- 9.de Weers M, Tai YT, van der Veer MS, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011; 186:1840–1848 [DOI] [PubMed] [Google Scholar]
- 10.Diebolder CA, Beurskens FJ, de Jong RN, et al. Complement is activated by IgG hexamers assembled at the cell surface. Science. 2014; 343:1260–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Jong RN, Beurskens FJ, Verploegen S, et al. A novel platform for the potentiation of therapeutic antibodies based on antigen-dependent formation of IgG hexamers at the cell surface. PLoS Biol. 2016; 14:e1002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy AD, Beum PV, Solga MD, et al. Rituximab infusion promotes rapid complement depletion and acute CD20 loss in chronic lymphocytic leukemia. J Immunol. 2004; 172:3280–3288 [DOI] [PubMed] [Google Scholar]
- 13.Oostindie SC, van der Horst HJ, Lindorfer MA, et al. CD20 and CD37 antibodies synergize to activate complement by Fc-mediated clustering. Haematologica. 2019; 104:1841–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu-Monette ZY, Li L, Byrd JC, et al. Assessment of CD37 B-cell antigen and cell of origin significantly improves risk prediction in diffuse large B-cell lymphoma. Blood. 2016; 128:3083–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorter A, Meri S. Immune evasion of tumor cells using membrane-bound complement regulatory proteins. Immunol Today. 1999; 20:576–582 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


