Abstract
Matrix metalloproteinase-8 (MMP-8) is a gene associated with inflammation and prognosis in colorectal cancer (CRC). Here, we studied the link between the rs11225395 polymorphism of MMP-8 gene and CRC risk. We recruited 551 CRC cases and 623 controls from among a subpopulation of Han Chinese patients. Data found that this variant was connected to an increased risk of CRC (TT versus CC: OR, 1.76; 95%CI, 1.09–2.84; P = 0.021; T versus C: OR, 1.29; 95%CI, 1.07–1.56; P = 0.007). Stratified analyses indicated a positive association among smokers (TT versus CC: OR, 2.31; 95%CI, 1.12–4.79; P = 0.024), males, and patients ≥ 60 years old. Crossover analysis showed that the potential interaction between smoking or drinking and the MMP-8 rs11225395 polymorphism was related to elevated risk for CRC. The rs11225395 polymorphism was also connected with lymph node metastasis and TNM stage. Moreover, the CRC cases carrying a TT genotype of MMP-8 rs11225395 presented had poorer overall survival than the CC genotype carriers. These findings show that MMP-8 rs11225395 correlates with an elevated risk of CRC and poor patient prognosis in a subpopulation of the Han Chinese subpopulation. Thus, the MMP-8 rs11225395 polymorphism could potentially function as a biomarker predictive of CRC susceptibility.
Keywords: colorectal cancer, MMP-8, polymorphism, case-control study, survival, susceptibility
INTRODUCTION
Colorectal cancer (CRC) is one of the most deadly cancers, causing about 900,000 cancer related deaths every year [1, 2]. The number of CRC patients is projected to increase to more than 2.2 million by 2030 [3]. CRC ranks as the fifth and fourth most prevalent cancer among men and women in China, respectively [4]. The incidence and mortality among women are ~25% lower than those in men [1]. Nationwide screening programs, improved lifestyle and diet, and increased colonoscopy screenings have contributed to the decreasing trend in the incidence of this disease [5]. CRC patients show classical signs and symptoms including altered bowel habits, occult or overt rectal bleeding, anemia, or abdominal pain [6]. Unfortunately, CRC pathogenesis is poorly understood and only becomes symptomatic at advanced stages, with surgery currently being the most effective treatment. Genetic predisposition, unhealthy lifestyles, obesity, and other environmental factors such as smoking and drinking corelate with the development of CRC [1, 6, 7]. Genome wide association studies (GWASs) revealed a host of novel risk loci for CRC patients [8–11].
Matrix metalloproteinases (MMPs), zinc and calcium-dependent proteolytic enzymes could degrade extracellular matrix proteins and components. Some MMPs are related with cancer progression, metastasis and invasion [12, 13]. MMP-8 is an enzyme in the connective tissue that is primarily produced by neutrophils and that cleaves collagens, growth factors, cell adhesion proteins, and cytokines [14]. Increased MMP-8 levels in CRC correlated with disease progression and inflammation [15]. Böckelman et al. showed that MMP-8 levels served as prognostic biomarker for CRC [16]. In addition, Sirniö et al. showed that high MMP-8 serum levels corelated with decreased survival in CRC [17]. Elevated MMP-8 levels were also observed in CRC patients who developed anastomotic leakage after surgery [18].
The MMP-8 gene is shown to locate on chromosome 11q22.2. Rs11225395 polymorphism is in the promoter region of MMP-8 gene. The T allele of the rs11225395 polymorphism resulted in increased protein expression of MMP-8 [19]. Several studies addressed the link between the rs11225395 polymorphism of MMP-8 gene and different cancer risk, but with conflicting findings [20–31]. Here, we aimed to explore the relationships between both CRC risk and survival prognosis and the MMP-8 rs11225395 polymorphism.
RESULTS
Population characteristics
Table 1 summarizes the clinicopathological characteristics and data of all included patients. The frequencies of gender, age, smoking and drinking showed no obvious differences between these two groups. In this study, 551 CRC patients and 623 controls were recruited. In terms of tumor site, CRC patients were divided into two groups: the rectum (66.9%) and colon (33.1%). Adenocarcinoma accounted for the most common pathology subtype (95.1%). Other relevant data are presented in Table 1.
Table 1. Patient demographics and risk factors in colorectal cancer.
| Characteristics | Case (N=551) | Control (N=623) | P |
| Age | 61.86±12.62 | 60.40±13.33 | 0.055 |
| Sex | 0.249 | ||
| Male | 341(61.9%) | 365(58.6%) | |
| Female | 210(38.1%) | 258(41.4%) | |
| Smoking | 0.149 | ||
| Yes | 315(57.2%) | 330(53.0%) | |
| No | 236(42.8%) | 293(47.0%) | |
| Alcohol | 0.333 | ||
| Yes | 303(54.9%) | 325(52.2%) | |
| N0 | 248(45.1%) | 298(47.8%) | |
| BMI | 22.24±2.79 | 22.54±2.88 | 0.066 |
| CRP, mg/dL | |||
| <0.4 | 315(57.2%) | 593(95.2%) | |
| ≥0.4 | 236(42.8%) | 30(4.8%) | |
| ESR, mm/hr | |||
| <15 | 303(55.0%) | 597(95.8%) | |
| ≥15 | 248(45.0%) | 26(4.2%) | |
| Family History | |||
| Yes | 117(21.2%) | ||
| No | 434(78.8%) | ||
| Histological grade | |||
| well differentiated | 77(13.9%) | ||
| Moderate differentiated | 406(73.7%) | ||
| Poor differentiated | 68(12.4%) | ||
| TNM stage | |||
| I+II | 234(42.5%) | ||
| III+IV | 317(57.5%) | ||
| Tumor size | |||
| >5 cm | 321(58.3%) | ||
| ≤5 cm | 230(41.7%) | ||
| Lymph node metastasis | |||
| No | 318(57.7%) | ||
| Yes | 233(42.3%) | ||
| Histology | |||
| Adenocarcinoma | 524(95.1%) | ||
| Squamous cell carcinoma | 22(4.0%) | ||
| Others | 5(0.9%) | ||
| Location of colorectal cancer | |||
| colon cancer | 182(33.1%) | ||
| rectal cancer | 369(66.9%) |
TNM, tumor node metastasis; BMI, body mass index; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate.
Association of MMP-8 gene rs11225395 polymorphism with the risk of CRC
The relationship between this locus and CRC risk is presented in Table 2. The genotype distributions in control groups were in accordance with HWE test (P > 0.05). The minor allele frequency (MAF) of the rs11225395 polymorphism for CRC cases was 0.27. In addition, we summarized the MAF for different populations around the world in Figure 1, which was obtained from the 1000 Genomes repository [33]. The TT or CT+TT genotypes were shown to increase the susceptibility to CRC (TT vs. CC: OR, 1.76; 95%CI, 1.09–2.84; P = 0.021). The associations remained statistically significant even after adjusting for gender and age. Additionally, the T allele was shown to increase the risk of CRC.
Table 2. Logistic regression analysis of associations between MMP-8 rs11225395 polymorphism and risk of colorectal cancer.
| Genotype | Casesa(n=551) | Controlsa(n=623) | OR (95% CI) | P | *OR (95% CI) | *P | ||
| n | % | n | % | |||||
| rs11225395C/T | ||||||||
| CC | 287 | 52.2% | 367 | 59.0% | 1.00 | - | - | - |
| CT | 219 | 39.8% | 223 | 35.9% | 1.26(0.99-1.60) | 0.065 | 1.25(0.98-1.59) | 0.073 |
| TT | 44 | 8% | 32 | 5.1% | 1.76(1.09-2.84) | 0.021 | 1.76(1.09-2.85) | 0.022 |
| CT+TT | 263 | 47.8% | 255 | 41% | 1.32(1.05-1.66) | 0.019 | 1.31(1.04-1.66) | 0.021 |
| CC+CT | 506 | 92% | 590 | 94.9% | 1.00 | |||
| TT | 44 | 8% | 32 | 5.1% | 1.60(1.00-2.57) | 0.049 | 1.61(1.00-2.58) | 0.048 |
| C allele | 793 | 72.1% | 957 | 76.9% | 1.00 | - | - | - |
| T allele | 307 | 27.9% | 287 | 23.1% | 1.29(1.07-1.56) | 0.007 | - | - |
aThe genotyping was successful in 550 cases and 622 controls for rs11225395 polymorphism;
Bold values are statistically significant (P < 0.05).
*Adjustment for sex and age.
Figure 1.
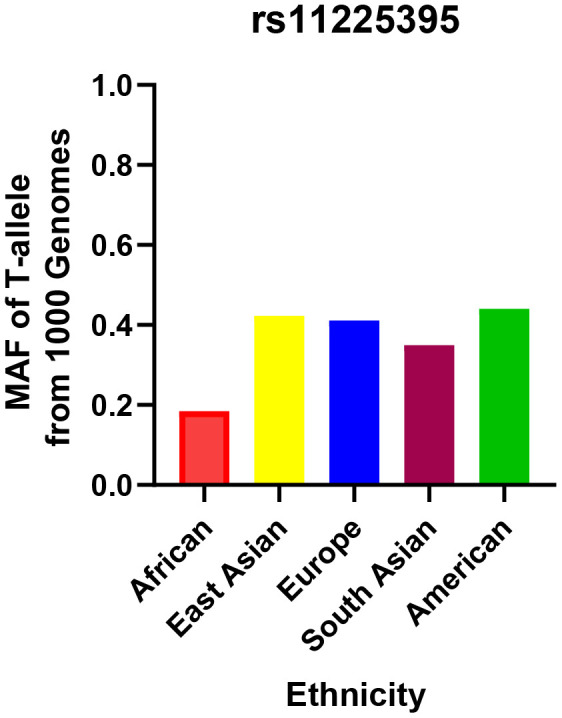
Minor allele frequencies for MMP-8 rs11225395 polymorphism in controls, stratified by ethnicity.
Stratified analysis of the rs11225395 polymorphism and CRC risk
Stratified analyses of gender, age, smoking, BMI, ESR, CRP, and drinking were conducted to measure the connection between the rs11225395 polymorphism and the risk of CRC (Table 3). We observed an increased risk for CRC patients among smokers, males, and those individuals aged ≥ 60 years. However, no positive findings were reported in the stratified analysis of drinking, BMI, ESR, and CRP.
Table 3. Stratified analyses between MMP-8 rs11225395 polymorphism and the risk of colorectal cancer.
| Variable | (case/control) | CT vs. CC | TT vs.CC | TT vs. CT+CC | TT+CT vs.CC | ||
| CC | CT | TT | |||||
| Sex | |||||||
| Male | 173/212 | 143/138 | 25/14 | 1.27(0.93-1.73); 0.129 | 2.19(1.10-4.34); 0.025 | 1.98(1.01-3.87); 0.047 | 1.35(1.01-1.82); 0.046 |
| Female | 114/155 | 76/85 | 19/18 | 1.22(0.82-1.80); 0.330 | 1.44(0.72-2.86); 0.304 | 1.33(0.68-2.61); 0.402 | 1.25(0.87-1.81); 0.229 |
| Smoking | |||||||
| Yes | 161/194 | 130/123 | 23/12 | 1.14(0.83-1.58); 0.426 | 2.31(1.12-4.79); 0.024 | 2.09(1.02-4.27); 0.040 | 1.37(1.00-1.87); 0.050 |
| No | 126/173 | 89/100 | 21/20 | 1.11(0.77-1.60); 0.569 | 1.44(0.75-2.77); 0.273 | 1.38(0.73-2.62); 0.318 | 1.26(0.89-1.78); 0.192 |
| Alcohol | |||||||
| Yes | 163/199 | 115/108 | 25/18 | 1.30(0.93-1.81); 0.124 | 1.70(0.89-3.22); 0.106 | 1.53(0.82-2.87); 0.181 | 1.36(0.99-1.86); 0.060 |
| No | 124/168 | 104/115 | 19/14 | 1.23(0.86-1.74); 0.259 | 1.84(0.89-3.81); 0.101 | 1.69(0.83-3.43); 0.151 | 1.29(0.92-1.81); 0.139 |
| Age (years) | |||||||
| <60 | 122/161 | 105/113 | 18/20 | 1.22(0.85-1.74); 0.275 | 1.18(0.60-2.33); 0.632 | 1.08(0.56-2.10); 0.814 | 1.23(0.86-1.70); 0.266 |
| ≥60 | 165/206 | 114/110 | 26/12 | 1.30(0.93-1.81); 0.122 | 2.72(1.33-5.55); 0.006 | 2.46(1.22-4.97); 0.012 | 1.44(1.05-1.98); 0.025 |
| BMI | |||||||
| <25 | 231/271 | 181/176 | 33/24 | 1.21(0.92-1.58);0.176 | 1.61(0.93-2.81);0.089 | 1.49(0.87-2.57);0.146 | 1.26(0.97-1.63);0.087 |
| ≥25 | 56/96 | 38/47 | 11/8 | 1.39(0.81-2.38);0.235 | 2.36(0.90-6.21);0.076 | 2.09(0.81-5.39);0.120 | 1.53(0.92-2.54);0.101 |
| CRP, mg/dL | |||||||
| <0.4 | 168/350 | 131/215 | 15/27 | 1.27(0.96-1.69);0.100 | 1.16(0.60-2.23);0.663 | 1.05(0.55-2.00);0.883 | 1.26(0.95-1.66);0.104 |
| ≥0.4 | 119/17 | 88/8 | 29/5 | 1.57(0.65-3.81);0.313 | 0.83(0.28-2.43);0.732 | 0.70(0.25-1.97);0.499 | 1.29(0.60-2.77);0.519 |
| ESR, mm/hr | |||||||
| <15 | 160/350 | 125/219 | 17/28 | 1.25(0.94-1.67);0.131 | 1.33(0.71-2.50);0.377 | 1.21(0.65-2.25);0.542 | 1.26(0.95-1.66);0.107 |
| ≥15 | 127/17 | 94/5 | 27/4 | 2.54(0.91-7.14);0.068 | 0.90(0.28-2.90);1.000 | 0.67(0.22-2.10);0.491 | 1.80(0.77-4.19);0.168 |
BMI: body mass index; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; Bold values are statistically significant (P < 0.05).
Combined and interactive effects of the rs11225395 polymorphism and drinking or smoking on CRC risk
Due to the association of the rs11225395 polymorphism with environmental factors observed in Table 3, we next used cross-over analysis to further evaluate the impact of the interactions between the MMP-8 gene rs11225395 polymorphism and environmental factors on CRC susceptibility. Smokers with CT or TT genotype increased the risk of CRC when compared with non-smokers with CC genotype (Table 4). Similarly, CRC patients who carried the CT genotype and drank alcohol presented a higher risk of developing CRC than individuals who did not carry the CC genotype and drink alcohol (drinking + CT vs. non-drinking +CC: OR, 1.44, 95% CI, 1.02-2.05; P = 0.040). In summary, the data showed potential associations between the rs11225395 polymorphism and contributions from smoking or drinking to an increased risk of developing CRC.
Table 4. Genetic (G) and environmental (E) factors 2*4 fork analysis.
| Ga | Eb | Case | Control | OR (95%CI); P value | Reflecting information |
| rs11225395 | |||||
| TT vs. CC | Smoking | ||||
| + | + | 23 | 12 | 2.63(1.26,5.49); 0.008 | G, E combined effect |
| + | - | 21 | 20 | 1.44(0.75,2.77); 0.271 | G alone effect |
| - | + | 161 | 194 | 1.14(0.84,1.55); 0.410 | E alone effect |
| - | - | 126 | 173 | 1.00 (reference) | Common control |
| CT vs. CC | Smoking | ||||
| + | + | 130 | 123 | 1.45(1.04,2.03); 0.030 | G, E combined effect |
| + | - | 89 | 100 | 1.22(0.85,1.76); 0.283 | G alone effect |
| - | + | 161 | 194 | 1.14(0.84,1.55); 0.410 | E alone effect |
| - | - | 126 | 173 | 1.00 (reference) | Common control |
| TT vs. CC | Drinking | ||||
| + | + | 25 | 18 | 1.88(0.98,3.60); 0.053 | G, E combined effect |
| + | - | 19 | 14 | 1.84(0.89,3.81); 0.097 | G alone effect |
| - | + | 163 | 199 | 1.11(0.81,1.52); 0.512 | E alone effect |
| - | - | 124 | 168 | 1.00 (reference) | Common control |
| CT vs. CC | Drinking | ||||
| + | + | 115 | 108 | 1.44(1.02,2.05); 0.040 | G, E combined effect |
| + | - | 104 | 115 | 1.23(0.86,1.74); 0.258 | G alone effect |
| - | + | 163 | 199 | 1.11(0.81,1.52); 0.512 | E alone effect |
| - | - | 124 | 168 | 1.00 (reference) | Common control |
aG (+): MMP8 gene rs11225395 variants (Heterozygous or homozygous); G (-): wild type; bE(+): smoking/non-smoking; E(-): non-smoking/non-drinking; Bold values are statistically significant (P < 0.05).
MMP-8 gene rs11225395 polymorphism and clinicopathological characteristics of CRC patients
Next, we assessed whether the MMP-8 gene rs11225395 polymorphism was connected to CRC clinicopathologic features (Table 5). The CRC patients with TT genotype were linked with TNM III+IV stage and lymph node metastasis. No positive association was observed for histological grade, tumor size, family history of CRC, histology, and location of CRC.
Table 5. The associations between MMP-8 rs11225395 polymorphism and clinical characteristics of colorectal cancer.
| Characteristics | Genotype distributions | |||
| CC | CT | TT | CT+TT | |
| Histological grade | ||||
| MD/WD | 205/42 | 170/31 | 30/4 | 200/35 |
| OR (95%CI); P-value | 1.0 (reference) | 1.12(0.68-1.87); 0.652 | 1.54(0.51-4.60); 0.439 | 1.17(0.72-1.91); 0.527 |
| Histological grade | ||||
| PD/WD | 40/42 | 18/31 | 10/4 | 28/35 |
| OR (95%CI); P-value | 1.0 (reference) | 0.61(0.30-1.26); 0.179 | 2.63(0.76-9.05); 0.117 | 0.84(0.44-1.62); 0.604 |
| Family History | ||||
| Yes/No | 59/228 | 50/169 | 7/37 | 57/206 |
| OR (95%CI); P-value | 1.0 (reference) | 1.14(0.75-1.75);0.538 | 0.73(0.31-1.72);0.472 | 1.07(0.71-1.61);0.749 |
| TNM stage | ||||
| III+IV/I+II | 164/123 | 118/101 | 34/10 | 152/111 |
| OR (95%CI); P-value | 1.0 (reference) | 0.85(0.60-1.21); 0.369 | 2.55(1.21-5.36); 0.011 | 1.03(0.73-1.44); 0.877 |
| Tumor size | ||||
| >5 cm/ ≤5 cm | 174/113 | 120/99 | 26/18 | 146/117 |
| OR (95%CI); P-value | 1.0 (reference) | 0.79(0.55-1.12); 0.188 | 0.94(0.49-1.79); 0.846 | 0.81(0.57-1.14); 0.225 |
| Lymph node metastasis | ||||
| Yes/No | 107/180 | 100/119 | 26/18 | 126/137 |
| OR (95%CI); P-value | 1.0 (reference) | 1.41(0.99-2.02); 0.057 | 2.43(1.27-4.64); 0.006 | 1.55(1.10-2.17); 0.012 |
| Histology | ||||
| Adenocarcinoma/others | 269/18 | 212/7 | 42/2 | 254/9 |
| OR (95%CI); P-value | 1.0 (reference) | 2.03(0.83-4.94); 0.114 | 1.41(0.32-6.28); 0.655 | 1.89(0.83-4.28); 0.122 |
| Location of colorectal cancer | ||||
| colon cancer/ rectal cancer | 99/188 | 73/146 | 9/35 | 82/181 |
| OR (95%CI); P-value | 1.0 (reference) | 0.95(0.66-1.38); 0.785 | 0.49(0.23-1.06); 0.064 | 0.86(0.60-1.23); 0.408 |
Bold values are statistically significant (P <0.05). PD = Poorly differentiation, MD= Moderately differentiation, WD= Well differentiation.
Genotype-based MMP-8 gene expression analysis and potential gene-gene interactions
Data from the GTEx portal uncovered that the rs11225395 polymorphism was shown to alter the expression of MMP-8 gene in whole-blood samples (P = 3.5e-17) (Figure 2). In addition, several genes including TIMP2, ELANE, MMP-9, DEFA4, TCN1, PLG, ARG1, LTF, LCN2, CXCL1 were involved in the interaction of MMP-8 (Figure 3), which was discovered by using the String online tool (http://string-db.org/).
Figure 2.
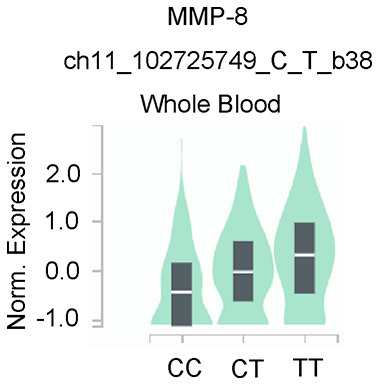
Genotype-based mRNA expression alteration in whole blood for MMP-8 rs11225395 polymorphism based on data from the GTEx portal database (https://www.gtexportal.org/home).
Figure 3.
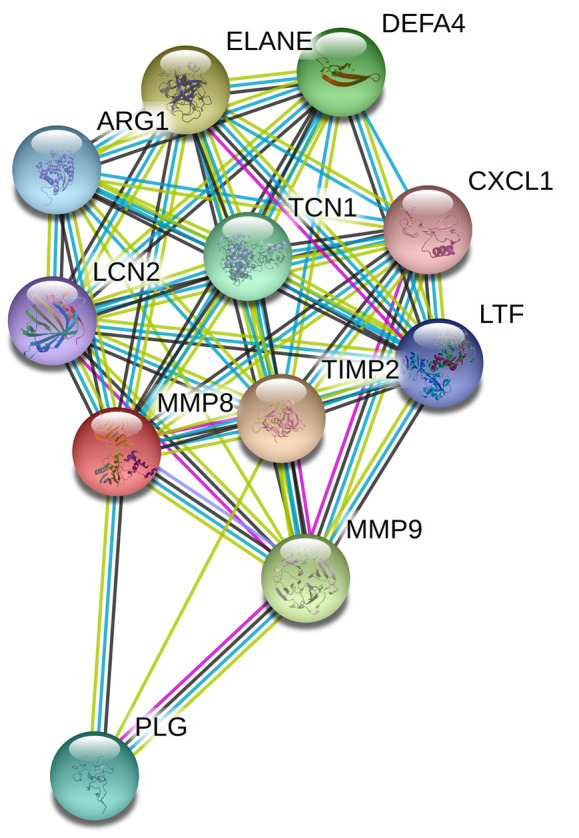
Human MMP-8 interactions with other genes obtained from the String server. The following genes participate in gene-gene interactions: TIMP2, Metalloproteinase inhibitor 2; ELANE, Neutrophil elastase; MMP9, Matrix metalloproteinase-9; DEFA4, Neutrophil defensin 4; TCN1, Transcobalamin-1; PLG, Plasminogen; ARG1, Arginase-1; LTF, Lactotransferrin; LCN2, Neutrophil gelatinase-associated lipocalin; CXCL1, Growth-regulated alpha protein.
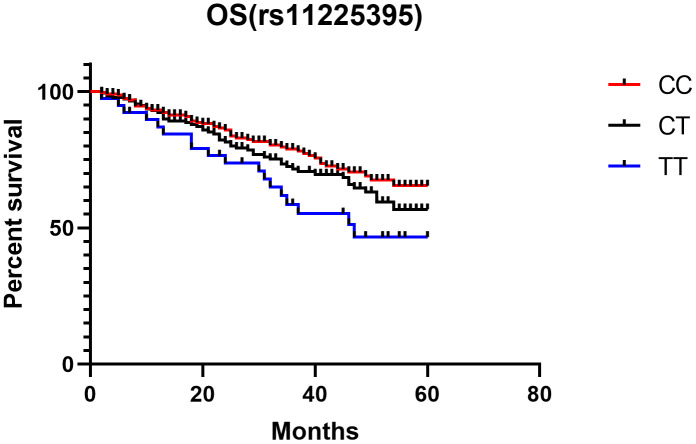
Survival analysis of CRC patients with the MMP-8 gene rs11225395 polymorphism
Last, Kaplan-Meier single factor analysis showed that CRC patients with TT genotype exhibited worse overall survival than those with a CC genotype (HR, 2.30, 95%CI, 1.19-4.43; log-rank P = 0.013, Figure 4).
Figure 4.
Kaplan-Meier analysis of the association between MMP-8 rs11225395 polymorphism and overall survival of colorectal cancer patients.
DISCUSSION
This study showed that the MMP-8 gene rs11225395 polymorphism was related with an elevated risk of CRC in a subpopulation of Chinese patients, especially among smokers, males, and those individuals aged ≥ 60 years. In addition, this polymorphism correlated with the occurrence of lymph node metastasis and TNM III+IV stage in CRC patients. Furthermore, TT genotype carriers showed worse overall survival with CC genotype carriers.
Recently, studies investigating the connection between the risk of cancers and rs11225395 polymorphism are increasingly emerging. Kubben et al. showed that the rs11225395 polymorphism was not related with gastric cancer risk and survival [23]. A subsequent study replicated the negative results obtained by Kubben et al., which revealed that the MMP-8 gene rs11225395 polymorphism was not related to hepatocellular carcinoma risk in a subpopulation of Han Chinese including 434 cases and 480 controls [26]. Nevertheless, an India study indicated that this SNP decreased the risk of bladder cancer in a population of 200 cases and 200 age-matched controls [28]. Unexpectedly, Debniak et al. from Poland uncovered that the rs11225395 polymorphism elevated the risk of malignant melanoma, while no association was detected between this SNP and breast cancer risk [20]. As for breast cancer, Hsiao et al. from Taiwan revealed that this SNP was not linked with breast cancer risk [21], but Wang et al. showed that rs11225395 polymorphism elevated the risk of breast cancer in a subpopulation of Han Chinese [30]. Other Taiwanese studies demonstrated no associations between the MMP-8 gene rs11225395 polymorphism and childhood leukemia [25], lung cancer [27], oral cancer [22], and bladder cancer risk [29]. Hashim et al. suggested that MMP-8 gene rs11225395 polymorphism was a protective factor associated with nasopharyngeal carcinoma susceptibility in a Malaysian population [24], and Arechavaleta-Velasco et al. detected that this locus increased the risk of ovarian cancer in Mexican women [31]. All such studies yielded conflicting findings regarding the association with this variant and cancer risk. There may be many reasons that may explain such contradictory findings: One, diverse exposure factors and dietary habits; two, different populations have genetic heterogeneity; three, the sample size among the aforementioned studies differed; four, the studies exhibited clinical heterogeneity among different cancers. To address these inconsistent results, a meta-analysis by Feng et al. to explore the connection between this SNP and cancer susceptibility, and found no association of the rs11225395 polymorphism and overall cancer risk [36]. However, elevated cancer risk was observed in non-Asian populations, and no relationship was detected in Asian populations [36]. Up to date, no studies investigated the link between CRC risk and the MMP-8 rs11225395 polymorphism. Therefore, we conducted this study to explore such association. Our study revealed that the MMP-8 rs11225395 polymorphism elevated the risk of CRC.
In addition, we uncovered an increased risk for developing CRC in males, smokers, and those subjects aged ≥ 60 years. However, Qiu et al. did not find any associations between hepatocellular carcinoma susceptibility and rs11225395 polymorphism when the analyses were stratified by age, gender, and drinking and smoking statuses [26]. Cross-over analysis showed that the integrated effects of the MMP-8 rs11225395 polymorphism and smoking or drinking were related to an increased risk of CRC. Our data also showed that the MMP-8 rs11225395 polymorphism related to TNM III+IV stage and lymph node metastasis among CRC patients.
Last, we explored the association between CRC prognosis and the MMP-8 rs11225395 polymorphism. We found that patients with TT genotype showed worse overall survival compared with patients with CC genotype. However, Velasco et al. indicated that the TT genetic carriers showed a poorer overall survival when compared with the CC + CT genotype carriers among ovarian cancer patients [31]. In addition, Kubben et al. observed that the MMP-8 gene rs11225395 polymorphism was not related to gastric cancer survival [23]. Decock et al. found that the rs11225395 polymorphism predicted a better overall survival in patients with breast cancer [19]. Different cancer tissues, disease stage, and clinical heterogeneity may contribute to these inconsistent findings. Further meta-analyses studying this issue are needed to clarify these contradictory results.
This study had some limitations. First, the sample size was relatively small, which may decrease the reliability of our results. Second, this case-control study failed to demonstrate a cause-effect relationship. Third, the current analysis only considered one polymorphism of the MMP8 gene. Fourth, other potential gene-gene interactions should be studied in future studies. Fifth, although the String online tool revealed an interaction between the MMP-8 gene and several other genes, we could not validate the endogenous expression of each candidate genes and whether their expression levels were linked to the survival of CRC patients. Sixth, we could not directly detect the MMP8 rs11225395 polymorphism site in clinical specimens. Last, why MMP-8 gene rs11225395 polymorphism was associated with the prognosis of CRC should be investigated. Nonetheless, our study here shows that the MMP-8 gene rs11225395 polymorphism correlates with the CRC risk and prognosis. Further studies in Chinese and other populations are warranted.
METHODS
Subjects
We enrolled 551 CRC cases and 623 controls from the First Hospital of Jilin University in this study. The diagnosis of CRC depended on pathological manifestations. The patients with digestive diseases, or CRC patients receiving chemotherapy or radiotherapy were excluded. Healthy controls were chosen from the same hospital. Written informed consent was got from each participant. Clinicopathological characteristics of all CRC patients were obtained from their medical records. All controls finished a standardized questionnaire including detailed clinical characteristics such age, sex, smoking and drinking. The Ethics Committees of the First Hospital of Jilin University approved this study; and it was in line with the standards of the Declaration of Helsinki.
Genotyping
We collected 2 ml of peripheral blood from all CRC patients and matched controls, which were stored at -80° C until used. We used the TIANamp Blood DNA kit to obtain DNA (Tiangen Biotech, Beijing, China). Genotyping of the studied SNP was analyzed by RFLP-PCR. The primers used for this polymorphism were as following: GCCAGAGACTCAAGTGGGAGACTACCATGCAGATC (forward) and reverse primer TTATGATTGCCCAGACATTTG (reverse). Approximately 10% of all enrolled individuals were re-genotyped, and the concordance was 100% [32, 33].
Genotype and gene expression correlation analysis
The data from the GTEx Portal database (https://www.gtexportal.org/home/) were utilized to evaluate the link between genotypes of the MMP-8 rs11225395 polymorphism and mRNA expression-level alteration [34].
Statistical analysis
We used student’s t-test and chi-square (χ2) test to evaluate continuous and categorical variables, respectively. The Hardy-Weinberg equilibrium (HWE) test among controls was calculated by a goodness-of-fit χ2 test. Logistic regression analysis was utilized to estimate the odds ratios (ORs) and their 95% confidence intervals (CIs). The Kaplan-Meier method was used to calculate overall survival [35]. P value < 0.05 indicated a statistical difference. All statistical analyses were addressed by SPSS 22.0 (SPSS Inc., Chicago, USA).
Availability of data and materials
The data can be made available by the corresponding author upon reasonable request.
Footnotes
AUTHOR CONTRIBUTIONS: Zhenhua Kang conceived of the study and participated in its design. Di Sun and Xu Wang conducted a systematic literature review. Zhenhua Kang performed data analyses. Jiandong Tai drafted the manuscript. All authors gave final approval and agreed to be accountable for all aspects of the work, ensuring integrity and accuracy.
CONFLICTS OF INTEREST: The authors declare that they have no conflicts of interest.
FUNDING: No funding was provided for this study.
REFERENCES
- 1.Dekker E, Tanis PJ, Vleugels JL, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019; 394:1467–80. 10.1016/S0140-6736(19)32319-0 [DOI] [PubMed] [Google Scholar]
- 2.Hofseth LJ, Hebert JR, Chanda A, Chen H, Love BL, Pena MM, Murphy EA, Sajish M, Sheth A, Buckhaults PJ, Berger FG. Early-onset colorectal cancer: initial clues and current views. Nat Rev Gastroenterol Hepatol. 2020; 17:352–64. 10.1038/s41575-019-0253-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016; 66:115–32. 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 5.Ait Ouakrim D, Pizot C, Boniol M, Malvezzi M, Boniol M, Negri E, Bota M, Jenkins MA, Bleiberg H, Autier P. Trends in colorectal cancer mortality in Europe: retrospective analysis of the WHO mortality database. BMJ. 2015; 351:h4970. 10.1136/bmj.h4970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019; 16:713–32. 10.1038/s41575-019-0189-8 [DOI] [PubMed] [Google Scholar]
- 7.Kaminski MF, Robertson DJ, Senore C, Rex DK. Optimizing the quality of colorectal cancer screening worldwide. Gastroenterology. 2020; 158:404–17. 10.1053/j.gastro.2019.11.026 [DOI] [PubMed] [Google Scholar]
- 8.Archambault AN, Su YR, Jeon J, Thomas M, Lin Y, Conti DV, Win AK, Sakoda LC, Lansdorp-Vogelaar I, Peterse EF, Zauber AG, Duggan D, Holowatyj AN, et al. Cumulative burden of colorectal cancer-associated genetic variants is more strongly associated with early-onset vs late-onset cancer. Gastroenterology. 2020; 158:1274–86.e12. 10.1053/j.gastro.2019.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huyghe JR, Bien SA, Harrison TA, Kang HM, Chen S, Schmit SL, Conti DV, Qu C, Jeon J, Edlund CK, Greenside P, Wainberg M, Schumacher FR, et al. Discovery of common and rare genetic risk variants for colorectal cancer. Nat Genet. 2019; 51:76–87. 10.1038/s41588-018-0286-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Law PJ, Timofeeva M, Fernandez-Rozadilla C, Broderick P, Studd J, Fernandez-Tajes J, Farrington S, Svinti V, Palles C, Orlando G, Sud A, Holroyd A, Penegar S, et al. , and PRACTICAL consortium. Association analyses identify 31 new risk loci for colorectal cancer susceptibility. Nat Commun. 2019; 10:2154. 10.1038/s41467-019-09775-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian J, Chang J, Gong J, Lou J, Fu M, Li J, Ke J, Zhu Y, Gong Y, Yang Y, Zou D, Peng X, Yang N, et al. Systematic functional interrogation of genes in GWAS loci identified ATF1 as a key driver in colorectal cancer modulated by a promoter-enhancer interaction. Am J Hum Genet. 2019; 105:29–47. 10.1016/j.ajhg.2019.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst. 1997; 89:1260–70. 10.1093/jnci/89.17.1260 [DOI] [PubMed] [Google Scholar]
- 13.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001; 17:463–516. 10.1146/annurev.cellbio.17.1.463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Lint P, Libert C. Matrix metalloproteinase-8: cleavage can be decisive. Cytokine Growth Factor Rev. 2006; 17:217–23. 10.1016/j.cytogfr.2006.04.001 [DOI] [PubMed] [Google Scholar]
- 15.Väyrynen JP, Vornanen J, Tervahartiala T, Sorsa T, Bloigu R, Salo T, Tuomisto A, Mäkinen MJ. Serum MMP-8 levels increase in colorectal cancer and correlate with disease course and inflammatory properties of primary tumors. Int J Cancer. 2012; 131:E463–74. 10.1002/ijc.26435 [DOI] [PubMed] [Google Scholar]
- 16.Böckelman C, Beilmann-Lehtonen I, Kaprio T, Koskensalo S, Tervahartiala T, Mustonen H, Stenman UH, Sorsa T, Haglund C. Serum MMP-8 and TIMP-1 predict prognosis in colorectal cancer. BMC Cancer. 2018; 18:679. 10.1186/s12885-018-4589-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sirniö P, Tuomisto A, Tervahartiala T, Sorsa T, Klintrup K, Karhu T, Herzig KH, Mäkelä J, Karttunen TJ, Salo T, Mäkinen MJ, Väyrynen JP. High-serum MMP-8 levels are associated with decreased survival and systemic inflammation in colorectal cancer. Br J Cancer. 2018; 119:213–19. 10.1038/s41416-018-0136-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasternak B, Matthiessen P, Jansson K, Andersson M, Aspenberg P. Elevated intraperitoneal matrix metalloproteinases-8 and -9 in patients who develop anastomotic leakage after rectal cancer surgery: a pilot study. Colorectal Dis. 2010; 12:e93–98. 10.1111/j.1463-1318.2009.01908.x [DOI] [PubMed] [Google Scholar]
- 19.Decock J, Long JR, Laxton RC, Shu XO, Hodgkinson C, Hendrickx W, Pearce EG, Gao YT, Pereira AC, Paridaens R, Zheng W, Ye S. Association of matrix metalloproteinase-8 gene variation with breast cancer prognosis. Cancer Res. 2007; 67:10214–21. 10.1158/0008-5472.CAN-07-1683 [DOI] [PubMed] [Google Scholar]
- 20.Dębniak T, Jakubowska A, Serrano-Fernández P, Kurzawski G, Cybulski C, Chauhan SR, Laxton RC, Maleszka R, Lubinski J, Ye S. Association of MMP8 gene variation with an increased risk of Malignant melanoma. Melanoma Res. 2011; 21:464–68. 10.1097/CMR.0b013e3283485fdd [DOI] [PubMed] [Google Scholar]
- 21.Hsiao CL, Liu LC, Shih TC, Chuang CL, Chen GL, Wang HC, Pan SY, Shen TC, Tsai CW, Chang WS, Way TD, Chung JG, Bau DT. The association of matrix metalloproteinase-8 promoter genotypes in breast cancer. Anticancer Res. 2018; 38:2181–85. 10.21873/anticanres.12459 [DOI] [PubMed] [Google Scholar]
- 22.Hung YW, Tsai CW, Wu CN, Shih LC, Chen YY, Liu YF, Hung HS, Shen MY, Chang WS, Bau DT. The contribution of matrix metalloproteinase-8 promoter polymorphism to oral cancer susceptibility. In Vivo. 2017; 31:585–90. 10.21873/invivo.11098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kubben FJ, Sier CF, Meijer MJ, van den Berg M, van der Reijden JJ, Griffioen G, van de Velde CJ, Lamers CB, Verspaget HW. Clinical impact of MMP and TIMP gene polymorphisms in gastric cancer. Br J Cancer. 2006; 95:744–51. 10.1038/sj.bjc.6603307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nor Hashim NA, Ramzi NH, Velapasamy S, Alex L, Chahil JK, Lye SH, Munretnam K, Haron MR, Ler LW. Identification of genetic and non-genetic risk factors for nasopharyngeal carcinoma in a Southeast Asian population. Asian Pac J Cancer Prev. 2012; 13:6005–10. 10.7314/apjcp.2012.13.12.6005 [DOI] [PubMed] [Google Scholar]
- 25.Pei JS, Chang WS, Hsu PC, Hung YW, Cheng SP, Tsai CW, Bau DT, Gong CL. The contribution of MMP-8 promoter genotypes to childhood leukemia. In Vivo. 2017; 31:1059–64. 10.21873/invivo.11170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiu W, Zhou G, Zhai Y, Zhang X, Xie W, Zhang H, Yang H, Zhi L, Yuan X, Zhang X, He F. No association of MMP-7, MMP-8, and MMP-21 polymorphisms with the risk of hepatocellular carcinoma in a Chinese population. Cancer Epidemiol Biomarkers Prev. 2008; 17:2514–18. 10.1158/1055-9965.EPI-08-0557 [DOI] [PubMed] [Google Scholar]
- 27.Shen TC, Hsia TC, Chao CY, Chen WC, Chen CY, Chen WC, Lin YT, Hsiao CL, Chang WS, Tsai CW, Bau DT. The contribution of MMP-8 promoter polymorphisms in lung cancer. Anticancer Res. 2017; 37:3563–67. 10.21873/anticanres.11726 [DOI] [PubMed] [Google Scholar]
- 28.Srivastava P, Kapoor R, Mittal RD. Association of single nucleotide polymorphisms in promoter of matrix metalloproteinase-2, 8 genes with bladder cancer risk in northern India. Urol Oncol. 2013; 31:247–54. 10.1016/j.urolonc.2011.01.001 [DOI] [PubMed] [Google Scholar]
- 29.Tsai TH, Wang YM, Chang WS, Tsai CW, Wu HC, Hsu HM, Wang YC, Li HT, Gong CL, Bau DT, Li CY. Association of matrix metalloproteinase-8 genotypes with the risk of bladder cancer. Anticancer Res. 2018; 38:5159–64. 10.21873/anticanres.12838 [DOI] [PubMed] [Google Scholar]
- 30.Wang K, Zhou Y, Li G, Wen X, Kou Y, Yu J, He H, Zhao Q, Xue F, Wang J, Zhao X. MMP8 and MMP9 gene polymorphisms were associated with breast cancer risk in a Chinese Han population. Sci Rep. 2018; 8:13422. 10.1038/s41598-018-31664-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arechavaleta-Velasco F, Cuevas-Antonio R, Dominguez-Lopez P, Estrada-Moscoso I, Imani-Razavi FS, Zeferino-Toquero M, Diaz-Cueto L. Matrix metalloproteinase-8 promoter gene polymorphisms in Mexican women with ovarian cancer. Med Oncol. 2014; 31:132. 10.1007/s12032-014-0132-3 [DOI] [PubMed] [Google Scholar]
- 32.Fu W, Zhuo Z, Hua RX, Fu K, Jia W, Zhu J, Zhang J, Cheng J, Zhou H, Xia H, He J, Liu G. Association of KRAS and NRAS gene polymorphisms with Wilms tumor risk: a four-center case-control study. Aging (Albany NY). 2019; 11:1551–63. 10.18632/aging.101855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J, Zhuo Z, Li W, Zhu J, He J, Su J. XRCC1 gene polymorphisms and risk of neuroblastoma in Chinese children. Aging (Albany NY). 2018; 10:2944–53. 10.18632/aging.101601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wan Q, Zhang D, Zhou Q, Li M, Wang Y, Song Y, Xu T. Association of CD44 gene rs187115 polymorphism with colorectal cancer risk and prognosis in Chinese Han population: a case-control study. Aging (Albany NY). 2019; 11:9616–25. 10.18632/aging.102408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qian H, Zhang D, Bao C. Two variants of interleukin-1B gene are associated with the decreased risk, clinical features, and better overall survival of colorectal cancer: a two-center case-control study. Aging (Albany NY). 2018; 10:4084–92. 10.18632/aging.101695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng J, Chen Y, Hua W, Sun X, Chen Y, Liu Y, Fan J, Zhao Y, Zhao L, Xu X, Yang X. The MMP -8 rs11225395 promoter polymorphism increases cancer risk of non-asian populations: evidence from a meta-analysis. Biomolecules. 2019; 9:570. 10.3390/biom9100570 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data can be made available by the corresponding author upon reasonable request.