Cyclin D1 is an oncogenic protein that drives G1-S cell cycle transition.1 Aberrant cyclin D1 expression due to CCND1 rearrangement has been identified in more than 90% of cases of mantle cell lymphoma (MCL) and is also seen in approximately 15% of plasma cell neoplasms.2 Aberrant expression of Cyclin D1 protein has been observed rarely in cases of diffuse large B-cell lymphoma (DLBCL), but usually in the absence of any gene alterations.3,4 Nevertheless, CCND1 rearrangement has been reported at relapse in some B-cell lymphomas including DLBCL,5 chronic lymphocytic leukemia/small lymphocytic lymphoma,6 and follicular lymphoma.7 These data suggest that CCND1 rearrangement can be a secondary event during lymphoma evolution and is not restricted to MCL.
Recently, Hu et al8 reported 17 cases that they proposed to represent MCL with secondary MYC gene rearrangement. Their cases were associated with aggressive pathological and clinical features and a poor prognosis. Similarly, Zanetto et al9 described cases classified as MCL that had rearrangements of both CCND1 and BCL6 and were positive for CD10. Closer inspection raises questions as to whether they are indeed all examples of MCL. Most were either not examined for SOX11 or were negative,8 and a number were negative for CD5 as well.9 These data indicate that in some instances, it can be challenging to determine if aberrations in CCND1 are primary, as occurs in MCL, or take place as a secondary event in another form of B-cell lymphoma.
We recently identified 2 cases of high-grade B-cell lymphoma, in which direct or indirect evidence implicated CCND1 rearrangement as a secondary event in tumor evolution, and not indicative of MCL. Both cases carried other oncogene rearrangements leading to a designation as “quadruple hit” [MYC, BCL2, BCL6, CCND1] or “triple hit” [MYC, BCL2, CCND1] lymphomas. We searched the literature for similar cases. The clinical history and pathological features are summarized in the Table 1.10–13
Table 1.
Pathological and Clinical Features of 7 Cases of High-Grade B-Cell Lymphoma With Triple or Quadruple Hits Involving CCND1 Gene Rearrangement.
| Case | Age/Gender | Clinical History | Diagnosis | Stage | FISH/Cytogenetics | Immunohistochemistry | Treatment | Outcome | Ref |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 37/M | Diffuse lymphadenopathy, breast mass, Bell’s palsy | High-grade B-cell lymphoma | IV-B | CCND1 RMYC RBCL2 R | Cyclin D1+ CD5- SOX11- CD10+ BCL2+ BCL6- MUM1+ Ki67 90% TDT focal + | EPOCH-R, HSCT | DOD (18 mo) | |
| 2 | 74/F | Mediastinal mass | High-grade B-cell lymphoma | II | CCND1 RMYC RBCL2 RBCL6 R | Cyclin D1+ CD5- SOX11-CD10- BCL2+ BCL6+ MUM1+ Ki67 90% | R-CHOP | DOD (8 mo) | |
| 3 | 51/F | Diffuse lymphadenopathy | DLBCL | III | CCND1 RMYC RBC2 RBCL6 R | CD10+ BCL2+ BCL6+ MUM1+ Ki67 80% | R-CHOP | A, NED (30 mo) | 10 |
| 4 | 81/F | Bone marrow involved by B-cell lymphoma | DLBCL | Unknown | BCL6 R/t(3;18)(q27;q21) MYC R/t(8;14)(q24;q32) CCND1 R/t(11;14)(q13;q32) BCL2 R/t(14;18)(q32;q21) | Unknown | Cyclophosphamide containing chemotherapy | Death due to unknown causes (9 d) | 11 |
| 5 | 68/M | Bone marrow involved by B-cell lymphoma | BL/B-ALL | Unknown | BCL6 R/t(2;3)(p12;q27) MYC R/t(8;14)(q24;q32) CCND1 R/t(11;14)(q13;q32) BCL2 R/t(14;18)(q32;q21) | Unknown | Unknown | DOD (6 d) | 11 |
| 6 | 79/M | Paranesthesia for 4 mo and diffuse lymphadenopathy | DLBCL | III/IV | CCND1 RMYC RBCL2 RBCL6 R | Cyclin D1+ CD5- SOX11- CD10- BCL2+ BCL6+Ki67 80% | RICE | DOD (4 mo) | 12 |
| 7 | Unknown | Meningeal lymphomatosis and diffuse lymphadenopathy | B-cell lymphoma | Unknown | t(3;14)(q27;q32) t(8;14)(q24;q32) t(11;22)(q13;q11) t(14;18)(q32;q21) | Cyclin D1+ CD5- CD10+ BCL2+ BCL6+ TDT-Ki67 60% | Doxorubicin containing chemo/rituximab | DOD | 13 |
ALL = acute lymphoblastic leukemia, BL = Burkitt Lymphoma, CR = complete response, DLBCL = diffuse large B-cell lymphoma, EPOCH-R = rituximab, cyclophosphamide, hydroxydaunomycin, prednisone, etoposide, FISH = fluorescence in situ hybridization, HSCT = hematopoietic stem cell transplant, LAD = lymphadenopathy, R = gene rearranged by FISH, R-CHOP = rituximab, cyclophosphamide, hydroxydaunomycin, vincristine sulfate, prednisone, RICE = rituximab, ifosfamide, carboplatin, etoposide.
Case 1 was a 37-year-old man without significant medical history, first seen in January 2013 with symptoms of Bell’s palsy. Clinical evaluation showed diffuse lymphadenopathy involving the axillary, cervical, celiac, mesenteric, and inguinal regions, as well as a mass in the right breast. Biopsy of the breast mass biopsy was performed showing an infiltrate of medium to large blastoid cells with finely clumped chromatin, small basophilic nuclei, and scant cytoplasm. There were abundant mitoses and apoptotic bodies, with admixed histiocytes imparting a starry-sky appearance. Neoplastic cells were positive for CD20, CD10, BCL2, Cyclin D1, MYC with high Ki67 proliferation rate (4+). They were negative for BCL6, CD5, CD34, and SOX11. Interestingly, a small percentage of atypical cells were positive for TdT (Figure 1). Break-apart fluorescence in situ hybridization (FISH) demonstrated the presence of gene rearrangements of MYC, BCL2, and CCND1 in 80% of nuclei. Next-generation sequencing (NGS) using the Illumina TruSight Oncology 500 (TSO500) panel, performed as previously described,14 identified additional pathogenic mutations in BCL2 (p.Ala131Val, VAF 45%) and MYC (p.Pro72Thr, VAF 79%). A subsequent bone marrow biopsy further implicated underlying follicular lymphoma, with paratrabecular lymphoid aggregates that were positive for CD20, PAX5, and BCL2, and negative for cyclin D1, CD10, BCL6, and CD5.
Figure 1.
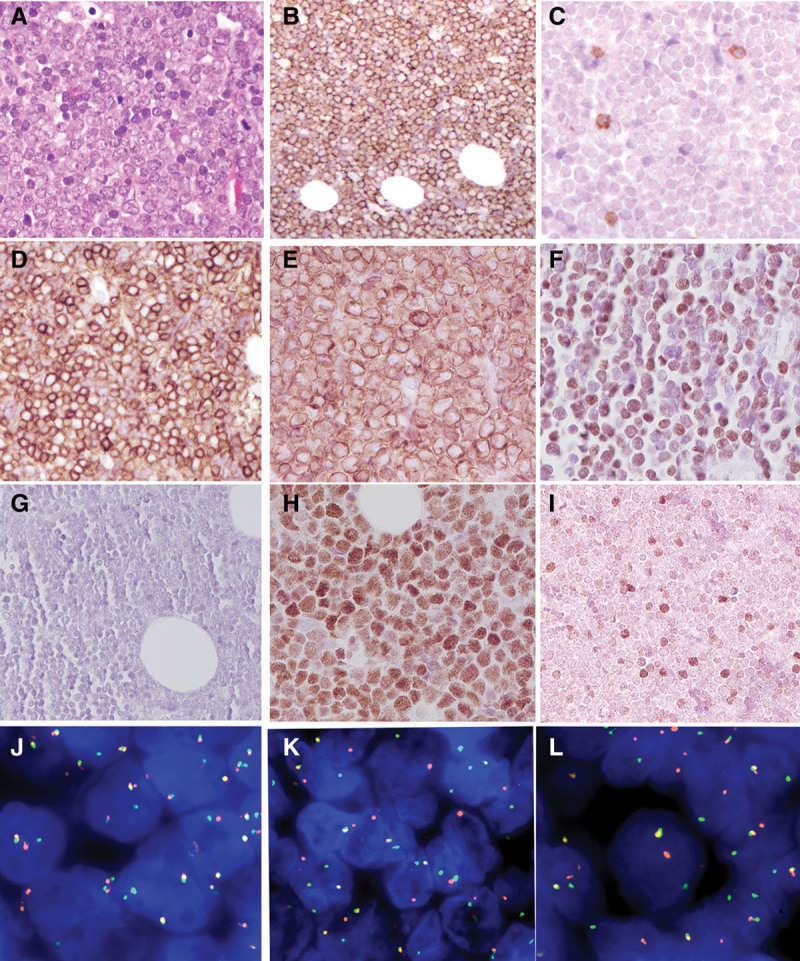
Breast mass, Case 1. (A), Biopsy shows an infiltrate of atypical lymphoid cells, medium to large in size with vesicular nuclei and basophilic nucleoli (400×). The tumor cells are CD20 positive (B), CD5 negative (C), CD10 positive (D), BCL2 positive (E), cyclin D1 positive (F), SOX11 negative (G), MYC positive (H), and focally positive for TDT (I). FISH studies of the breast mass revealed breaks in BCL2 (J), MYC (K), and CCND1 (L) in approximately 80% of nuclei. BCL6 gene rearrangement was negative. FISH = fluorescence in situ hybridization.
The patient was treated with 6 cycles of Dose adjusted etoposide, prednisone, oncovin (vincristine), cyclophosmamide, hydroxydaunomycin + rituximab (DA-EPOCH-R) and was thought to be in complete remission, as of June 2013. However, symptoms of headache led to magnetic resonance imaging, which showed diffuse leptomeningeal enhancement. A leptomeningeal biopsy confirmed involvement by lymphoma, leading to high-dose methotrexate and high-dose Ara-C followed by cranial radiation therapy. He subsequently received an unrelated (9/10) allogeneic stem cell transplant following a conditioning regimen with cyclophosmamide and total body irradiation in December 2013. However approximately day 100 after allograft, a computerized tomography scan showed a large perirenal mass (10 × 10 × 10 cm), and a biopsy confirmed a diagnosis high-grade B-cell lymphoma with confirmed “triple hit” by FISH, similar to the initial breast mass. Palliative care was initiated; the patient expired in June 2014, 18 months after initial presentation.
Case 2 was a 74-year-old female with medical history of obesity, diabetes, hypertension, postsurgical treatment for breast cancer followed by Arimidex (stage T1cN0). She presented with shortness of breath, hemoptysis, an 8.8 × 7.2 cm mediastinal mass on imaging scan. The biopsy showed a diffuse infiltrate of medium to large cells with oval nuclei, vesicular chromatin, prominent nucleoli, and variable cytoplasm. The atypical cells were positive for CD20, CD23 subset, PAX5, cyclin D1, BCL2, BCL6, CMYC, with high proliferation rate (4+/4), and negative for SOX11, MUM1, CD10, CD5, CD30, TDT, and EBER (Figure 2). Rearrangements of CCND1, BCL2, BCL6, and MYC were identified in approximately 70% of cells by FISH. Further evaluation by the Illumina TruSight Oncology 500 (TSO500) NGS panel identified additional somatic pathogenic mutations including CDKN2A p.R80* (VAF 39%), KRAS p.G13D (VAF 35%), TP53 p.F270S (VAF 33%), and TNFRSF14 p.T169fs*65 (VAF 32%).
Figure 2.
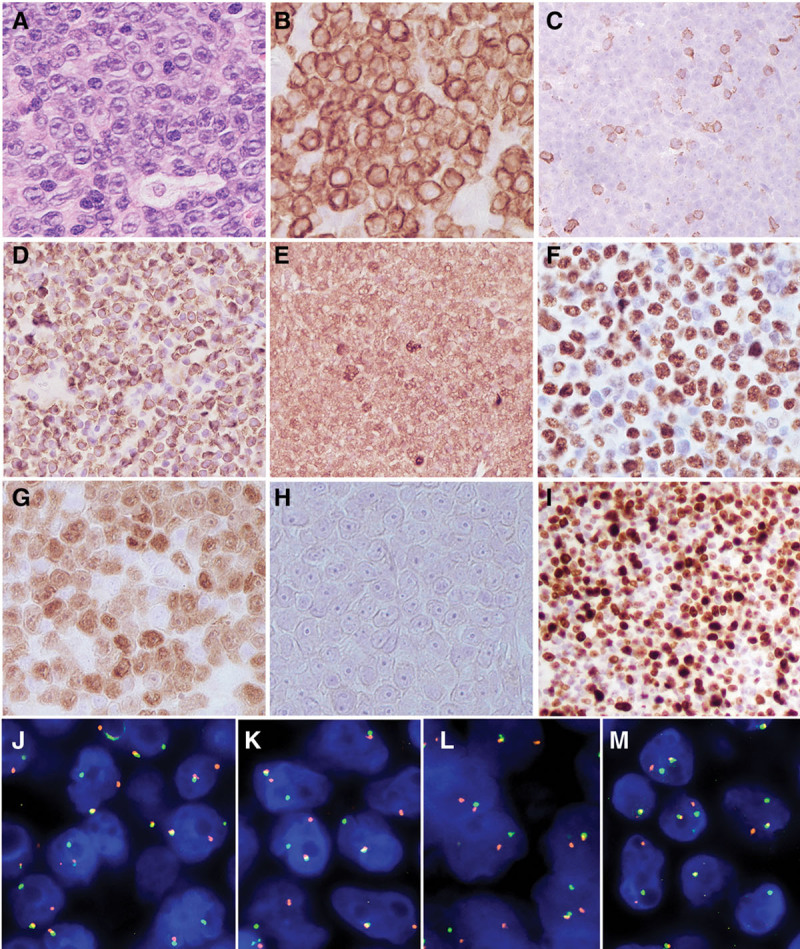
Mediastinal mass, Case 2. (A), Hematoxylin and eosin–stained sections show a high-grade lymphoma with a starry sky pattern and prominent central nucleoli (400×). The tumor cells are CD79 positive (B), CD5 negative (C), BCL2 positive (D), BCL6 positive (E), MYC positive (F), cyclin D1 positive (G), SOX11 negative (H), with high Ki67 proliferation rate (I). The tumor cells were negative for MUM1, TdT, and CD10 (not shown). FISH studies revealed breaks in BCL2 (J), BCL6 (K), CCND1 (L), and MYC (M) in approximately 80% of nuclei. FISH = fluorescence in situ hybridization.
A diagnosis of high-grade B-cell lymphoma with a quadruple hit genetic profile was made (clinical stage II). Her underlying medical issues precluded high-dose intensive chemotherapy or stem cell transplant. She was treated with rituximab, cyclophosphamide, hydroxydaunomycin, vincristine sulfate, prednisone for 6 cycles. Although partial response was noted, post-treatment scan showed refractory mediastinal disease and growth of new lesions, which was not controlled by additional radiation therapy. Clinical progress led to palliative care. The patient expired 8 months after initial diagnosis.
Morphologically, both cases had blastoid cytological features, raising consideration for the blastoid variant of MCL. However, CD5 and SOX11 were negative in both, arguing against a diagnosis of MCL.2,15 Additionally, both cases were positive for either BCL6 or CD10, further supporting a germinal center B-cell derivation. The mutational profiles by NGS also favored tumors of germinal center derivation rather than MCL, with mutations in BCL2 and MYC in one case. NGS studies identified mutations in KRAS and TNFRSF14, which have not been reported in MCL,16 but can be seen in tumors of follicle center derivation.16,17
Case 1 had a subpopulation of cells positive for TDT. TDT expression can be seen in the B-lymphoblastic transformation of follicular lymphoma, which is usually a late event in the evolution of the disease, and has been linked to secondary MYC rearrangement.18 As TDT was present only in a subset of the atypical cells, we favor a designation of high-grade B-cell lymphoma with MYC and BCL2 rearrangement, as defined in the World Health Organization classification. It is difficult to be confident of the molecular sequence of events in our cases. However, the bone marrow findings in case 1 showed evidence of low-grade follicular lymphoma, suggesting that a BCL2 rearrangement was the initiating event, with MYC and CCND1 translocations occurring secondarily.
Review of the literature reveals 5 more cases with quadruple translocations involving CCND1, BCL2, BCL6, and MYC.6,7,10–12 Most such cases were diagnosed as DLBCLs (3 of 5). They appeared to have an aggressive clinical course, with limited response to chemotherapy. Nevertheless, Yoshida et al10 reported 1 patient with complete remission to R-CHOP chemotherapy and the patient was still alive at 30 months after diagnosis.
In summary, we report 2 cases of high-grade B-cell lymphoma in which CCND1 rearrangement appears to be a secondary event, appearing in cells that exhibit rearrangements involving BCL2, MYC, and additionally BCL6 in 1 case. These cases need to be distinguished from MCL with secondary MYC rearrangement. Further evidence against a diagnosis of MCL was provided by the immunophenotype (negative for CD5 and SOX11) and the mutational profile observed by NGS, which differed from that seen in MCL.19 Knowledge of this rare lymphoma variant is important, to avoid misclassification as blastoid MCL. Moreover, these observations expand the spectrum of the World Health Organization–defined entity of high-grade B-cell lymphoma with “double or triple hit,” providing evidence for an additional variant.
Sources of funding
This research was supported by the Intramural Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Baldin V, Lukas J, Marcote MJ, et al. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993; 7:812–821 [DOI] [PubMed] [Google Scholar]
- 2.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016; 127:2375–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vela-Chávez T, Adam P, Kremer M, et al. Cyclin D1 positive diffuse large B-cell lymphoma is a post-germinal center-type lymphoma without alterations in the CCND1 gene locus. Leuk Lymphoma. 2011; 52:458–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ok CY, Xu-Monette ZY, Tzankov A, et al. Prevalence and clinical implications of cyclin D1 expression in diffuse large B-cell lymphoma (DLBCL) treated with immunochemotherapy: a report from the International DLBCL Rituximab-CHOP Consortium Program. Cancer. 2014; 120:1818–1829 [DOI] [PubMed] [Google Scholar]
- 5.Al-Kawaaz M, Mathew S, Liu Y, et al. Cyclin D1-positive diffuse large B-cell lymphoma with IGH-CCND1 translocation and BCL6 rearrangement: a report of two cases. Am J Clin Pathol. 2015; 143:288–299 [DOI] [PubMed] [Google Scholar]
- 6.Nishida Y, Takeuchi K, Tsuda K, et al. Acquisition of t(11;14) in a patient with chronic lymphocytic leukemia carrying both t(14;19)(q32;q13.1) and +12. Eur J Haematol. 2013; 91:179–182 [DOI] [PubMed] [Google Scholar]
- 7.Koduru PR, Chen W, Garcia R, et al. Acquisition of a t(11;14)(q13;q32) in clonal evolution in a follicular lymphoma with a t(14;18)(q32;q21) and t(3;22)(q27;q11.2). Cancer Genet. 2015; 208:303–309 [DOI] [PubMed] [Google Scholar]
- 8.Hu Z, Medeiros LJ, Chen Z, et al. Mantle cell lymphoma with MYC rearrangement: a report of 17 patients. Am J Surg Pathol. 2017; 41:216–224 [DOI] [PubMed] [Google Scholar]
- 9.Zanetto U, Dong H, Huang Y, et al. Mantle cell lymphoma with aberrant expression of CD10. Histopathology. 2008; 53:20–29 [DOI] [PubMed] [Google Scholar]
- 10.Yoshida M, Ichikawa A, Miyoshi H, et al. Clinicopathological features of double-hit B-cell lymphomas with MYC and BCL2, BCL6 or CCND1 rearrangements. Pathol Int. 2015; 65:519–527 [DOI] [PubMed] [Google Scholar]
- 11.Kawakami K, Miyanishi S, Sonoki T, et al. Case of B-cell lymphoma with rearrangement of the BCL1, BCL2, BCL6, and c-MYC genes. Int J Hematol. 2004; 79:474–479 [DOI] [PubMed] [Google Scholar]
- 12.Ittel A, Hélias C, Wissler MP, et al. Four genetic lymphoma-specific events (MYC, BCL2, BCL6 and CCND1) identified in a high grade B lymphoma case. Blood Cancer J. 2015; 5:e374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bacher U, Haferlach T, Alpermann T, et al. Several lymphoma-specific genetic events in parallel can be found in mature B-cell neoplasms. Genes Chromosomes Cancer. 2011; 50:43–50 [DOI] [PubMed] [Google Scholar]
- 14.Galera P, Flavin R, Savage NM, et al. Epstein-Barr virus-negative marginal zone lymphoma as an uncommon form of monomorphic posttransplant lymphoproliferative disorder. Am J Surg Pathol. 2020; 44:1340–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsiao SC, Cortada IR, Colomo L, et al. SOX11 is useful in differentiating cyclin D1-positive diffuse large B-cell lymphoma from mantle cell lymphoma. Histopathology. 2012; 61:685–693 [DOI] [PubMed] [Google Scholar]
- 16.Beà S, Valdés-Mas R, Navarro A, et al. Landscape of somatic mutations and clonal evolution in mantle cell lymphoma. Proc Natl Acad Sci U S A. 2013; 110:18250–18255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmitz R, Wright GW, Huang DW, et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med. 2018; 378:1396–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geyer JT, Subramaniyam S, Jiang Y, et al. Lymphoblastic transformation of follicular lymphoma: a clinicopathologic and molecular analysis of 7 patients. Hum Pathol. 2015; 46:260–271 [DOI] [PubMed] [Google Scholar]
- 19.Nadeu F, Martin-Garcia D, Clot G, et al. Genomic and epigenomic insights into the origin, pathogenesis, and clinical behavior of mantle cell lymphoma subtypes. Blood. 2020; 136:1419–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]


