Abstract
Pathogen-associated molecular patterns (PAMPs) are some nonspecific and highly conserved molecular structures of exogenous specific microbial pathogens, whose products can be recognized by pattern recognition receptor (PRR) on innate immune cells and induce an inflammatory response. Under physiological stress, activated or damaged cells might release some endogenous proteins that can also bind to PRR and cause a harmful aseptic inflammatory response. These endogenous proteins were named damage-associated molecular patterns (DAMPs) or alarmins. Indeed, alarmins can also play a beneficial role in the tissue repair in certain environments. Besides, some alarmin cytokines have been reported to have both nuclear and extracellular effects. This group of proteins includes high-mobility group box-1 protein (HMGB1), interleukin (IL)-33, IL-1α, IL-1F7b, and IL-16. In this article, we review the involvement of nuclear alarmins such as HMGB1, IL-33, and IL-1α under physiological state or stress state and suggest a novel activity of these molecules as central initiators in the development of sterile inflammation.
1. Introduction
The mechanism of the immune system sensing exogenous pathogens and internal tissue damage has attracted increasing attention. There are two main modes to activate the body's immune defense system when confronted with damage caused by various factors: one is ectogenic Pathogen-associated molecular patterns (PAMPs), and the other one is endogenic damage-associated molecular patterns (DAMPs) or alarmins. Pathogen-associated molecular patterns (PAMPs) are some nonspecific and highly conserved molecular structures that are necessary for the survival and pathogenicity of a class or a group of specific microbial pathogens [1]. Pattern recognition receptors (PRRs) are germline-encoded receptors that can recognize PAMP, thus triggers innate and adaptive immunity through activating a series of signaling pathways. One of the most important responses is to induce the synthesis of proinflammatory cytokines and the activation of inflammasomes downstream [2]. DAMPs or alarmins are endogenous proteins or peptides released by leukocytes and epithelial cells when stimulated by danger signals. They strengthen the innate and adaptive immunity by recruiting and activating the antigen-presenting cells (APCs) [3]. These DAMPs include high-mobility group box-1 (HMGB1), defensins, antimicrobial peptides, eosinophilic neurotoxins, heat shock proteins, and some cytokines like IL-1α and IL-33. It was thought that the biological effects of cytokines were only to transmit signals through specific receptors on the cell membrane, but increasing studies suggest that certain cytokines also play a role in the nucleus, such as IL-33, HMGB1, and IL-1α [4–6]. Here, we review the involvement of three representative nuclear alarmins, HMGB1, IL-33, and IL-1α, in the development of inflammation.
2. Members of Nuclear Alarmins Involved in Inflammation
2.1. HMGB1
HMGB1 was named due to its low molecular weight and fast swimming in electrophoresis and was first recognized as an intranuclear protein [7]. It is present in almost all eukaryotic cells and is highly conserved between species [8]). Structurally, it is divided into three regions: Box A, Box B, and C-terminal domain. Both Box A and Box B are capable of binding to DNA; C-terminal is a residual terminal with a negative charge (Figure 1) [9]. Structure and function analysis showed that Box B had the biological activity of HMGB1, while Box A is an antagonist of HMGB1 and Box B, which can block the inflammatory effect of HMGB1 [10]. HMGB1 is a widely expressed nuclear protein and affects transcription regulation. It binds to the DNA grooves and loosens the DNA wrapped in the nucleosome, thus promoting chromatin remodeling [11]. HMGB1 can also bend the DNA significantly and promote the combination of DNA and relevant transcription factors, such as p53, NF-κB, and steroid receptor [12, 13]. HMGB1-deficient mice die soon after birth suggesting the key role of HMGB1 in the nucleus in maintaining life [14]. HMGB1 stays very short at specific DNA binding sites and moves quickly in the nucleus. The stimulation of inflammation can lead to the acetylation of lysine residues in HMGB1 and prevent it from moving into the nucleus [15].
Figure 1.
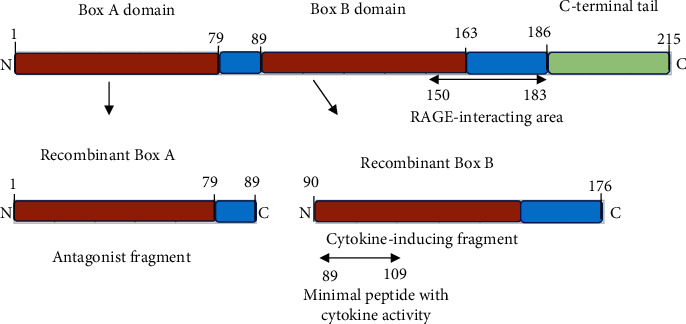
Molecular structure of HMGB1.
2.2. IL-33
Interleukin-33 (IL-33), also known as NF-HEV (nuclear factor from high endothelial venules), IL-1F11, is a new member of the IL-1 family originally reported by Schmitz et al. in 2005 [16]. It is widely expressed in the whole body, especially in the central nervous system and gastrointestinal [16]. It is composed of 270 amino acids, with an IL-1-like cytokine folding region at the C-terminal and a nuclear localization signal peptide and chromatin binding region at the N-terminal (Figure 2) [17]. IL-33 is synthesized at 30 KD in cellular and then cut into 18 KD by hydrolase as a mature form while secreted to extracellular [18]. Recent studies indicate that human IL-33 is processed at Asp178, not Asp112 as previously claimed [19, 20], and IL-33 is processed into bioactive forms and secreted to extracellular by neutrophil elastase and cathepsin G [21]. Recently, it has been reported that IL-33 is expressed in the nucleus, such as the human endothelial cells [22, 23]. The function of IL-33 in the nucleus is associated with the attachment to heterochromatin [24, 25].
Figure 2.
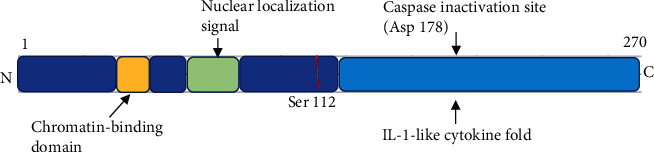
Molecular structure of IL-33.
IL-33 is derived from a wide range of tissues, but there are relatively few researches on which cell secreted IL-33 and its role in the disease. It has been reported that vascular endothelial cells (VECs) are the main source of IL-33. IL-33 are released from the nucleus when VECs are stimulated by inflammatory cytokines [26]. IL-33 is also expressed in the epithelial cells of the mucosa and the keratinocytes of skin [27–29], as well as some immune cells such as macrophages [30]. The secretory pathway of IL-33 is still unclear. It has been reported that it may be affected by the proteolytic enzyme, similar to that of IL-1β [31]. Researches also showed that cardiac fibroblasts stimulated by PMA and monocytes stimulated by LPS can secrete mature IL-33 [32, 33]. Recent studies suggested that the precursor IL-33 has biological activity, and its biological activity is reduced after proteolytic enzyme cleavage [19, 20, 30, 34].
2.3. IL-1α
IL-1α is also an important member of the IL-1 family. IL-1α lacks secretory protein as a signal peptide, so it can only be transformed from its precursor molecule. When the cell is stimulated, proteases (calpain, Granzyme B, etc.) cut pro-IL-1α into the 17 kDa mature form of IL-1α, both of which have biological activities [35, 36]. Pro-IL-1α is a 31 KD protein, which can be expressed in most dormant nonhematopoietic cells of humans, such as the epithelial cells of the gastrointestinal tract, liver, kidney, and skin [37, 38]. It consists of the N-terminal domain (NTD), nuclear localization signal (NLS), and C-terminal domain (CTD) (Figure 3) [39]. NLS induced pro-IL-1α to migrate to the nucleus as an intranuclear transcription factor and participates in gene regulation [40, 41]. Mature IL-1α plays a biological role by binding to IL-1R [42].
Figure 3.

Molecular structure of IL-1α.
3. Alarmin Receptors
Studies have shown that alarmins play a role in chemotaxis and activation of immune cells through G protein-coupled receptors (GPCRs) and non-G protein-coupled receptors (non-G PCR s) (Table 1).
Table 1.
Alarmins and relative receptors.
| Alarmin | Relative receptors |
|---|---|
| HMGB1 | RAGE, TLR2, TLR4 |
| IL-33 | ST2L |
| IL-1α | IL-1R |
3.1. Receptors for HMGB1
The Receptor for Advanced Glycation End Products (RAGE) is considered to be the receptor of HMGB1 [43]. RAGE is expressed on antigen-presenting cells (APC) [44–46], as well as endothelial cells and smooth muscle cells (SMCs) [47–51]. RAGE deficiency can significantly prolong the survival time of endotoxin mice. However, the deletion of RAGE does not completely prevent HMGB1 from stimulating macrophages to secrete inflammatory factors [52]. Other studies suggested that TLR2 and TLR4 are HMGB1's receptors as well [53]. However, there is no difference in the response of macrophages to HMGB1, whether the macrophages comes from TLR2-deficient mice or wild-type mice [54]. This suggests that TLR2, TLR4, and RAGE can bind to HMGB1, but RAGE may play a more important role for HMGB1.
Recent studies suggested that HMGB1 combined with other immune-stimulators, such as LPS, IL-1β, and DNA, can enhance its biological effect. This suggests that HMGB1 can simultaneously promote the activation of two receptors and produce biological effects. For example, HMGB1/DNA complex is easier to bind to RAGE than HMGB1, because the anchoring of DNA and TLR9 strengthens the combination of HMGB1 and RAGE [55, 56].
3.2. Receptors for IL-33
As the only specific receptor of ILl-33, ST2L is mainly expressed in Th2 lymphocytes [57], mast cells, and NKT, but not in Th1 lymphocytes [58]. IL-1 receptor accessory protein (IL-1RAcP) is essential for IL-33/ST2L to activate downstream signal pathways; IL-1RAcP-deficient mast cells cannot be stimulated to secrete IL-6 by IL-33 [59, 60]. IL-33 activates downstream signal pathways through ERK1/2, p38MAPK, and JNKs [16]; the TRAF6 pathway plays a key role in activating NF-κB and inducing Th2 cytokines by IL-33 [61]. However, the relationship between ST2L and NF-κB activation is controversial. It has been reported that the activation of ST2L has an anti-NF-κB effect, as in cardiomyocytes; IL-33-activated NF-κB inhibits angiotension II-induced NF-κB activation, thus alleviating the cardiac hypertrophy [62–64]. Soluble ST2 (sST2) is the extracellular segment of ST2L, which acts as a decoy receptor and binds to IL-33 competitively, thus blocking the effect of IL-33 [65]. In animal experiments, injection of sST2 or ST2 blocking antibody can alleviate asthma mediated by IL-33 and block the proinflammatory effect of IL-33 on rheumatoid arthritis [66–68].
4. Alarmins in Inflammation
4.1. HMGB1 in Inflammation
HMGB1 shows a strong proinflammatory effect when released into the extracellular environment, mainly through the following two mechanisms. First, necrotic cells release HMGB1 and activate the immune system [69, 70]. Recent studies indicated that apoptotic cells can also release HMGB1, but the reactive oxygen species produced by the activation of intracellular hydrolase can inactivate HGMB1 and block its proinflammatory activity [71]. Second, monocytes or macrophages can secret HMGB1 when activated by LPS, proinflammatory factors, or NO [72]. In endotoxemia, HMGB1 is considered a lethal factor in the late stage of endotoxic shock [73–75]. Increasing inflammatory factors, such as LPS, TNF-α, and IL-1, induce macrophages or DCs secrete HMGB1, which further stimulates macrophages or DCs to secrete inflammatory factors, thus forming a vicious circle [76].
4.2. IL-33 in Inflammation
Recent studies suggested that IL-33 is involved in the occurrence and progress of various diseases, and its mechanism is complex. It can promote the pathophysiological progress of asthma [77, 78], rheumatoid arthritis [79], and systemic lupus erythematosus [80], while in atherosclerosis, allogeneic transplantation, endotoxic shock, and parasitic infection, it inhibits the occurrence and development of diseases [81].
The dual function of IL-33 is mainly due to the different types of immune responses on different cells. IL-33 induce Th2 cells [82], mast cells [83], and basophils to secrete large amounts of IL-4, IL-5, IL-13, IgE, and IgA [83], which induce the pathological changes related to Th2 immune response. In vivo administration of recombinant IL-33 can cause histological changes in the lung and gastrointestinal tract, such as increased mucus secretion, epithelial hyperplasia, and overgrowth, which were considered to be related to Th2 immune response induced by IL-33 [16]. Previous studies also reported that IL-33 can induce the tolerance of allografts, which may be related to the differentiation of Th2 cells, MDSCs, and Treg cells induced by IL-33 [84–86]. The specific role of IL-33 in the cell nucleus is still not very clear, but studies have suggested that it can regulate gene expression. First, IL-33 would be lost when stimulated by inflammation in the resting vascular endothelial cell (VEC) nucleus [22]; second, when binding to NF-κB, IL-33 can block the related gene transcription induced by it [87]; and third, a short sequence of IL-33 precursor is involved in the formation of histone dimer, which is the components of higher-order chromatin structure [24].
4.3. IL-1α in Inflammation
IL-1α is an important alarmin that mediates aseptic inflammation. Studies have shown that the IL-1α expression can be upregulated in cells in the hypoxic environment, which activates aseptic inflammation. This is mainly due to the fact that hypoxia-inducible factor (HIF) induced by hypoxia can regulate the IL-1α transcription, thus affects the IL-1α-related inflammation by regulating the expression of IL-1α [88]. The expression and nuclear localization of IL-1α depend on the redox reaction. Overexpression of manganese superoxide dismutase leads to a corresponding increase of H2O2; meanwhile, a significant elevation of IL-1α is observed, in both mRNA and protein levels, as well as an increased localization of IL-1α in the nucleus [89].
5. Alarmins and Inflammatory Diseases
5.1. HMGB1 and Inflammatory Diseases
As a natural alarmin, HMGB1 is involved in the inflammatory response of acute local organ injury, as well as Th17-mediated autoimmune diseases, such as rheumatoid arthritis (RA), multiple sclerosis (MS), and its animal model-experimental autoimmune encephalomyelitis (EAE). HMGB1 is highly expressed in lesions of MS patients and EAE, and its three receptors RAGE, TLR2, and TLR4 are upregulated in macrophages or microglia. Besides, there is a positive feedback effect between HMGB1 and microglia, which promotes disease progression [90–92]. In the allograft rejection model, the expression of HMGB1 gradually increased over time. Notably, there was an ischemia-reperfusion injury in the process of obtaining the graft from the donor and during the surgery [93–96], which leads to the HMGB1 release from necrotic cells. These HMGB1 may be immediately involved in early and late graft rejection. The overexpression of HMGB1 is observed in colon cancer, breast cancer, and prostate cancer. With RAGE or HMGB1 blocked, tumor growth and metastasis are inhibited in animal models [97–99].
5.2. IL-33 and Inflammatory Diseases
In TNBS-induced enteritis, IL-33 upregulates CD103+IDO+ DCs through intestinal epithelial cells (IECs) and produces inhibitory Tregs to alleviate pathological changes mediated by Th1/Th17 [100]. IL-33 inhibits cardiac hypertrophy caused by AngII through activating NF-κB, and as a decoy receptor of IL-33, the serum expression of sST2 increases in patients with myocardial hypertrophy and heart failure caused by it [62], and the expression was correlated with the grade of heart failure.
5.3. IL-1α and Inflammatory Diseases
IL-1α is an important dual inflammatory factor, mainly involved in a variety of autoimmune diseases, as well as in anti-infection, anti-tumor, and other processes [101]. By inducing the release of TNF-α, G-CSF, and other inflammatory factors and recruiting concentrated granulocytes [102, 103], IL-1α can promote the progress of acute lung injury [104, 105], DSS-induced intestinal inflammation, and psoriasis. In addition, IL-1α can also be used as a prognostic indicator for distant metastasis of head and neck squamous cell carcinoma and promote the growth of melanoma, pancreatic ductal adenocarcinoma, and other tumors [105, 106].
6. Conclusions
DAMP or alarmin is actively released by cells or directly released by necrotic tissues when the tissue is stimulated or damaged, then produce certain biological effects by binding to relative receptors. Alarmins may play different roles in different locations of cells or in the microenvironment of different diseases. Researchers hope to achieve the goal of curing diseases by regulating alarmins and their relative signal pathways. However, before achieving this goal, the mechanism of these cytokines still needs further research. Does DAMP affect each other? How is DAMP released from intracellular to extracellular? Is there any difference in the function between DAMP that is actively released or passively released? Are there any differences between DAMP that is released by apoptotic cells or necrotic cells? All in all, there is still a long way to go to clarify the biological effects and related mechanisms of DAMP.
Acknowledgments
This work was supported by the Interdisciplinary Innovation Team, Frontier Science Key Research Project of Jiangxi Provincial People's Hospital (19-008), and Jiangxi Provincial Natural Science Foundation (20192ACB21006).
Contributor Information
Changsheng Ouyang, Email: oycs20011023@163.com.
Xiaohua Wang, Email: jxlnyxzx@163.com.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Authors' Contributions
LJ, YS, and YT reviewed the literature and wrote the first draft. LJ and XW reviewed the literature and finalized the manuscript. CO, XW, and LJ revised the manuscript. All authors have read and approved the final manuscript.
References
- 1.Kawai T., Akira S. Pathogen recognition with Toll-like receptors. Current Opinion in Immunology. 2005;17(4):338–344. doi: 10.1016/j.coi.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Cao X. Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nature Reviews. Immunology. 2016;16(1):35–50. doi: 10.1038/nri.2015.8. [DOI] [PubMed] [Google Scholar]
- 3.Nie Y., Yang D., Oppenheim J. J. Alarmins and antitumor immunity. Clinical Therapeutics. 2016;38(5):1042–1053. doi: 10.1016/j.clinthera.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferhat M., Robin A., Giraud S., et al. Endogenous IL-33 contributes to kidney ischemia-reperfusion injury as an alarmin. J Am Soc Nephrol. 2018;29(4):1272–1288. doi: 10.1681/ASN.2017060650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang H., Wang H., Andersson U. Targeting inflammation driven by HMGB1. Frontiers in Immunology. 2020;11:p. 484. doi: 10.3389/fimmu.2020.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malik A., Kanneganti T. D. Function and regulation of IL-1α in inflammatory diseases and cancer. Immunological Reviews. 2018;281(1):124–137. doi: 10.1111/imr.12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Einck L., Bustin M. The intracellular distribution and function of the high mobility group chromosomal proteins. Experimental Cell Research. 1985;156(2):295–310. doi: 10.1016/0014-4827(85)90539-7. [DOI] [PubMed] [Google Scholar]
- 8.Zlatanova J., Holde K. Binding to four‐way junction DNA: a common property of architectural proteins? The FASEB Journal. 1998;12(6):421–431. doi: 10.1096/fasebj.12.6.421. [DOI] [PubMed] [Google Scholar]
- 9.Bianchi M. E., Falciola L., Ferrari S., Lilley D. M. The DNA binding site of HMG1 protein is composed of two similar segments (HMG boxes), both of which have counterparts in other eukaryotic regulatory proteins. The EMBO Journal. 1992;11(3):1055–1063. doi: 10.1002/j.1460-2075.1992.tb05144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baxevanis A. D., Landsman D. The HMG-1 box protein family: classification and functional relationships. Nucleic Acids Research. 1995;23(9):1604–1613. doi: 10.1093/nar/23.9.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ritt C., Grimm R., Fernandez S., Alonso J. C., Grasser K. D. Four differently chromatin-associated maize HMG domain proteins modulate DNA structure and act as architectural elements in nucleoprotein complexes. The Plant Journal. 1998;14(5):623–631. doi: 10.1046/j.1365-313X.1998.00154.x. [DOI] [PubMed] [Google Scholar]
- 12.Livesey K. M., Kang R., Vernon P., et al. p53/HMGB1 complexes regulate autophagy and apoptosis. Cancer Research. 2012;72(8):1996–2005. doi: 10.1158/0008-5472.CAN-11-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melvin V. S., Roemer S. C., Churchill M. E. A., Edwards D. P. The C-terminal extension (CTE) of the nuclear hormone receptor DNA binding domain determines interactions and functional response to the HMGB-1/-2 co-regulatory proteins. The Journal of Biological Chemistry. 2002;277(28):25115–25124. doi: 10.1074/jbc.M110400200. [DOI] [PubMed] [Google Scholar]
- 14.Calogero S., Grassi F., Aguzzi A., et al. The lack of chromosomal protein Hmg1 does not disrupt cell growth but causes lethal hypoglycaemia in newborn mice. Nature Genetics. 1999;22(3):276–280. doi: 10.1038/10338. [DOI] [PubMed] [Google Scholar]
- 15.Assenberg R., Webb M., Connolly E., et al. A critical role in structure-specific DNA binding for the acetylatable lysine residues in HMGB1. The Biochemical Journal. 2008;411(3):553–561. doi: 10.1042/BJ20071613. [DOI] [PubMed] [Google Scholar]
- 16.Schmitz J., Owyang A., Oldham E., et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23(5):479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Smith D. E. IL-33: a tissue derived cytokine pathway involved in allergic inflammation and asthma. Clinical and Experimental Allergy. 2010;40(2):200–208. doi: 10.1111/j.1365-2222.2009.03384.x. [DOI] [PubMed] [Google Scholar]
- 18.Dinarello C. A. An IL-1 family member requires caspase-1 processing and signals through the ST2 receptor. Immunity. 2005;23(5):461–462. doi: 10.1016/j.immuni.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Lüthi A. U., Cullen S. P., McNeela E. A., et al. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity. 2009;31(1):84–98. doi: 10.1016/j.immuni.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Talabot-Ayer D., Lamacchia C., Gabay C., Palmer G. Interleukin-33 is biologically active independently of caspase-1 cleavage. The Journal of Biological Chemistry. 2009;284(29):19420–19426. doi: 10.1074/jbc.M901744200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lefrancais E., Roga S., Gautier V., et al. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proceedings of the National Academy of Sciences; 2012; pp. 1673–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Küchler A. M., Pollheimer J., Balogh J., et al. Nuclear interleukin-33 is generally expressed in resting endothelium but rapidly lost upon angiogenic or proinflammatory activation. The American Journal of Pathology. 2008;173(4):1229–1242. doi: 10.2353/ajpath.2008.080014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moussion C., Ortega N., Girard J. P. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel 'alarmin'? PLoS One. 2008;3(10, article e3331) doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roussel L., Erard M., Cayrol C., Girard J. P. Molecular mimicry between IL-33 and KSHV for attachment to chromatin through the H2A-H2B acidic pocket. EMBO Reports. 2008;9(10):1006–1012. doi: 10.1038/embor.2008.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carriere V., Roussel L., Ortega N., et al. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(1):282–287. doi: 10.1073/pnas.0606854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao X., Wang X., Yang Q., et al. Tumoral expression of IL-33 inhibits tumor growth and modifies the tumor microenvironment through CD8+ T and NK cells. Journal of Immunology. 2014;194(1):438–445. doi: 10.4049/jimmunol.1401344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verri W. A., Guerrero A. T. G., Fukada S. Y., et al. IL-33 mediates antigen-induced cutaneous and articular hypernociception in mice. Proceedings of the National Academy of Sciences; 2008; pp. 2723–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L., Lu R., Zhao G., Pflugfelder S. C., Li D. Q. TLR-mediated induction of pro-allergic cytokine IL-33 in ocular mucosal epithelium. The International Journal of Biochemistry & Cell Biology. 2011;43(9):1383–1391. doi: 10.1016/j.biocel.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cevikbas F., Steinhoff M. IL-33: a novel danger signal system in atopic dermatitis. The Journal of Investigative Dermatology. 2012;132(5):1326–1329. doi: 10.1038/jid.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohno T., Oboki K., Kajiwara N., et al. Caspase-1, caspase-8, and calpain are dispensable for IL-33 release by macrophages. Journal of Immunology. 2009;183(12):7890–7897. doi: 10.4049/jimmunol.0802449. [DOI] [PubMed] [Google Scholar]
- 31.Keller M., Rüegg A., Werner S., Beer H. D. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132(5):818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 32.Nile C. J., Barksby E., Jitprasertwong P., Preshaw P. M., Taylor J. J. Expression and regulation of interleukin-33 in human monocytes. Immunology. 2010;130(2):172–180. doi: 10.1111/j.1365-2567.2009.03221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu J., Carver W. Effects of interleukin-33 on cardiac fibroblast gene expression and activity. Cytokine. 2012;58(3):368–379. doi: 10.1016/j.cyto.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cayrol C., Girard J. P. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(22):9021–9026. doi: 10.1073/pnas.0812690106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Groß O., Yazdi A. S., Thomas C. J., et al. Inflammasome activators induce interleukin-1α secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity. 2012;36(3):388–400. doi: 10.1016/j.immuni.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 36.Garlanda C., Dinarello C. A., Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39(6):1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osei E. T., Brandsma C.-A., Timens W., Heijink I. H., Hackett T.-L. Current perspectives on the role of interleukin-1 signalling in the pathogenesis of asthma and COPD. European Respiratory Journal. 2020;55(2):p. 1900563. doi: 10.1183/13993003.00563-2019. [DOI] [PubMed] [Google Scholar]
- 38.Hernandez-Santana Y. E., Giannoudaki E., Leon G., Lucitt M. B., Walsh P. T. Current perspectives on the interleukin-1 family as targets for inflammatory disease. European Journal of Immunology. 2019;49(9):1306–1320. doi: 10.1002/eji.201848056. [DOI] [PubMed] [Google Scholar]
- 39.Wessendorf J. H., Garfinkel S., Zhan X., Brown S., Maciag T. Identification of a nuclear localization sequence within the structure of the human interleukin-1 alpha precursor. The Journal of Biological Chemistry. 1993;268(29):22100–22104. [PubMed] [Google Scholar]
- 40.Buryskova M., Pospisek M., Grothey A., Simmet T., Burysek L. Intracellular interleukin-1alpha functionally interacts with histone acetyltransferase complexes. The Journal of Biological Chemistry. 2004;279(6):4017–4026. doi: 10.1074/jbc.M306342200. [DOI] [PubMed] [Google Scholar]
- 41.Zamostna B., Novak J., Vopalensky V., Masek T., Burysek L., Pospisek M. N-terminal domain of nuclear IL-1α shows structural similarity to the C-terminal domain of Snf1 and binds to the HAT/core module of the SAGA complex. PLoS One. 2012;7(8, article e41801) doi: 10.1371/journal.pone.0041801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arend W. P., Palmer G., Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunological Reviews. 2008;223(1):20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- 43.van Beijnum J. R., Buurman W. A., Griffioen A. W. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1) Angiogenesis. 2008;11(1):91–99. doi: 10.1007/s10456-008-9093-5. [DOI] [PubMed] [Google Scholar]
- 44.Kokkola R., Andersson A., Mullins G., et al. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scandinavian Journal of Immunology. 2005;61(1):1–9. doi: 10.1111/j.0300-9475.2005.01534.x. [DOI] [PubMed] [Google Scholar]
- 45.Zhang F., Su X., Huang G., et al. sRAGE alleviates neutrophilic asthma by blocking HMGB1/RAGE signalling in airway dendritic cells. Scientific Reports. 2017;7(1):p. 14268. doi: 10.1038/s41598-017-14667-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.LeBlanc P. M., Doggett T. A., Choi J., et al. An immunogenic peptide in the A-box of HMGB1 protein reverses apoptosis-induced tolerance through RAGE receptor. The Journal of Biological Chemistry. 2014;289(11):7777–7786. doi: 10.1074/jbc.M113.541474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramasamy R., Yan S. F., Schmidt A. M. The RAGE axis and endothelial dysfunction: maladaptive roles in the diabetic vasculature and beyond. Trends in Cardiovascular Medicine. 2005;15(7):237–243. doi: 10.1016/j.tcm.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 48.Huang W., Liu Y., Li L., et al. HMGB1 increases permeability of the endothelial cell monolayer via RAGE and Src family tyrosine kinase pathways. Inflammation. 2012;35(1):350–362. doi: 10.1007/s10753-011-9325-5. [DOI] [PubMed] [Google Scholar]
- 49.Yang M., Yang X., Wang S., et al. HMGB1-induced endothelial cell pyroptosis is involved in systemic inflammatory response syndrome following radiofrequency ablation of hepatic hemangiomas. American Journal of Translational Research. 2019;11(12):7555–7567. [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Z., Zhang J., Chen L., Li J., Zhang H., Guo X. Glycine Suppresses AGE/RAGE Signaling Pathway and Subsequent Oxidative Stress by Restoring Glo1 Function in the Aorta of Diabetic Rats and in HUVECs. Oxidative Medicine and Cellular Longevity. 2019;2019:14. doi: 10.1155/2019/4628962.4628962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin Q., Fan C., Gomez-Arroyo J., et al. HIMF (hypoxia-induced mitogenic factor) signaling mediates the HMGB1 (high mobility group box 1)-dependent endothelial and smooth muscle cell crosstalk in pulmonary hypertension. Arteriosclerosis, Thrombosis, and Vascular Biology. 2019;39(12):2505–2519. doi: 10.1161/ATVBAHA.119.312907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liliensiek B., Weigand M. A., Bierhaus A., et al. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. The Journal of Clinical Investigation. 2004;113(11):1641–1650. doi: 10.1172/JCI200418704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu M., Wang H., Ding A., et al. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26(2):174–179. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- 54.Park J. S., Svetkauskaite D., He Q., et al. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. The Journal of Biological Chemistry. 2004;279(9):7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 55.Tian J., Avalos A. M., Mao S. Y., et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nature Immunology. 2007;8(5):487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 56.Avalos A. M., Kiefer K., Tian J., et al. RAGE-independent autoreactive B cell activation in response to chromatin and HMGB1/DNA immune complexes. Autoimmunity. 2009;43(1):103–110. doi: 10.3109/08916930903384591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li A., Herbst R. H., Canner D., et al. IL-33 Signaling Alters Regulatory T Cell Diversity in Support of Tumor Development. Cell Reports. 2019;29(10):2998–3008.e8. doi: 10.1016/j.celrep.2019.10.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Junttila I. S., Watson C., Kummola L., et al. Efficient cytokine-induced IL-13 production by mast cells requires both IL-33 and IL-3. Journal of Allergy and Clinical Immunology. 2013;132(3):704–712.e10. doi: 10.1016/j.jaci.2013.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lingel A., Weiss T. M., Niebuhr M., et al. Structure of IL-33 and its interaction with the ST2 and IL-1RAcP receptors--insight into heterotrimeric IL-1 signaling complexes. Structure. 2009;17(10):1398–1410. doi: 10.1016/j.str.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chackerian A. A., Oldham E. R., Murphy E. E., Schmitz J., Pflanz S., Kastelein R. A. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. Journal of Immunology. 2007;179(4):2551–2555. doi: 10.4049/jimmunol.179.4.2551. [DOI] [PubMed] [Google Scholar]
- 61.Funakoshi-Tago M., Tago K., Hayakawa M., et al. TRAF6 is a critical signal transducer in IL-33 signaling pathway. Cellular Signalling. 2008;20(9):1679–1686. doi: 10.1016/j.cellsig.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 62.Sanada S., Hakuno D., Higgins L. J., Schreiter E. R., McKenzie A. N. J., Lee R. T. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. The Journal of Clinical Investigation. 2007;117(6):1538–1549. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bhardwaj A., Januzzi J. L., Jr. ST2: a novel biomarker for heart failure. Expert Review of Molecular Diagnostics. 2014;10(4):459–464. doi: 10.1586/erm.10.25. [DOI] [PubMed] [Google Scholar]
- 64.Kunes P., Holubcova Z., Kolackova M., Krejsek J. Interleukin-33, a novel member of the IL-1/IL-18 cytokine family, in cardiology and cardiac surgery. The Thoracic and Cardiovascular Surgeon. 2010;58(8):443–449. doi: 10.1055/s-0030-1250436. [DOI] [PubMed] [Google Scholar]
- 65.Bandara G., Beaven M. A., Olivera A., Gilfillan A. M., Metcalfe D. D. Activated mast cells synthesize and release soluble ST2-a decoy receptor for IL-33. European Journal of Immunology. 2015;45(11):3034–3044. doi: 10.1002/eji.201545501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hayakawa H., Hayakawa M., Kume A., Tominaga S. I. Soluble ST2 blocks interleukin-33 signaling in allergic airway inflammation. The Journal of Biological Chemistry. 2007;282(36):26369–26380. doi: 10.1074/jbc.M704916200. [DOI] [PubMed] [Google Scholar]
- 67.Palmer G., Talabot-Ayer D., Lamacchia C., et al. Inhibition of interleukin-33 signaling attenuates the severity of experimental arthritis. Arthritis and Rheumatism. 2009;60(3):738–749. doi: 10.1002/art.24305. [DOI] [PubMed] [Google Scholar]
- 68.Kamradt T., Drube S. A complicated liaison: IL-33 and IL-33R in arthritis pathogenesis. Arthritis Research & Therapy. 2013;15(3):p. 115. doi: 10.1186/ar4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scaffidi P., Misteli T., Bianchi M. E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418(6894):191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 70.Dorostkar R., Bamdad T., Parsania M., Pouriayevali H. An endogenous immune adjuvant released by necrotic cells for enhancement of DNA vaccine potency. Iranian Journal of Immunology. 2012;9(4):215–225. [PubMed] [Google Scholar]
- 71.Kazama H., Ricci J. E., Herndon J. M., Hoppe G., Green D. R., Ferguson T. A. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity. 2008;29(1):21–32. doi: 10.1016/j.immuni.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pisetsky D. S., Jiang W. Role of Toll-like receptors in HMGB1 release from macrophages. Annals of the New York Academy of Sciences. 2007;1109(1):58–65. doi: 10.1196/annals.1398.008. [DOI] [PubMed] [Google Scholar]
- 73.Wang H., Bloom O., Zhang M., et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285(5425):248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 74.Deng M., Tang Y., Li W., et al. The Endotoxin Delivery Protein HMGB1 Mediates Caspase-11-Dependent Lethality in Sepsis. Immunity. 2018;49(4):740–753.e7. doi: 10.1016/j.immuni.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou H., Li Y., Gui H., et al. Antagonism of Integrin CD11b Affords Protection against Endotoxin Shock and Polymicrobial Sepsis via Attenuation of HMGB1 Nucleocytoplasmic Translocation and Extracellular Release. The Journal of Immunology. 2018;200(5):ji1701285–ji1701780. doi: 10.4049/jimmunol.1701285. [DOI] [PubMed] [Google Scholar]
- 76.Lotze M. T., Tracey K. J. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nature Reviews. Immunology. 2005;5(4):331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 77.Fux M., Pecaric-Petkovic T., Odermatt A., et al. IL-33 is a mediator rather than a trigger of the acute allergic response in humans. Allergy. 2014;69(2):216–222. doi: 10.1111/all.12309. [DOI] [PubMed] [Google Scholar]
- 78.Guo Z., Wu J., Zhao J., et al. IL-33 promotes airway remodeling and is a marker of asthma disease severity. The Journal of Asthma. 2014;51(8):863–869. doi: 10.3109/02770903.2014.921196. [DOI] [PubMed] [Google Scholar]
- 79.Chen S., Chen B., Wen Z., Huang Z., Ye L. IL-33/ST2-mediated inflammation in macrophages is directly abrogated by IL-10 during rheumatoid arthritis. Oncotarget. 2017;8(20):32407–32418. doi: 10.18632/oncotarget.16299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li P., Lin W., Zheng X. IL-33 neutralization suppresses lupus disease in lupus-prone mice. Inflammation. 2014;37(3):824–832. doi: 10.1007/s10753-013-9802-0. [DOI] [PubMed] [Google Scholar]
- 81.Kakkar R., Lee R. T. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nature Reviews. Drug Discovery. 2008;7(10):827–840. doi: 10.1038/nrd2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Murakami-Satsutani N., Ito T., Nakanishi T., et al. IL-33 promotes the induction and maintenance of Th2 immune responses by enhancing the function of OX40 ligand. Allergology International. 2014;63(3):443–455. doi: 10.2332/allergolint.13-OA-0672. [DOI] [PubMed] [Google Scholar]
- 83.Joulia R., L'Faqihi F.-E., Valitutti S., Espinosa E. IL-33 fine tunes mast cell degranulation and chemokine production at the single-cell level. Journal of Allergy and Clinical Immunology. 2017;140(2):497–509.e10. doi: 10.1016/j.jaci.2016.09.049. [DOI] [PubMed] [Google Scholar]
- 84.Yin H., Li X. Y., Jin X. B., et al. IL-33 prolongs murine cardiac allograft survival through induction of TH2-type immune deviation. Transplantation. 2010;89(10):1189–1197. doi: 10.1097/TP.0b013e3181d720af. [DOI] [PubMed] [Google Scholar]
- 85.Turnquist H. R., Zhao Z., Rosborough B. R., et al. IL-33 expands suppressive CD11b+ Gr-1(int) and regulatory T cells, including ST2L+ Foxp3+ cells, and mediates regulatory T cell-dependent promotion of cardiac allograft survival. Journal of Immunology. 2011;187(9):4598–4610. doi: 10.4049/jimmunol.1100519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brunner S. M., Schiechl G., Falk W., Schlitt H. J., Geissler E. K., Fichtner-Feigl S. Interleukin-33 prolongs allograft survival during chronic cardiac rejection. Transplant International. 2011;24(10):1027–1039. doi: 10.1111/j.1432-2277.2011.01306.x. [DOI] [PubMed] [Google Scholar]
- 87.Ali S., Mohs A., Thomas M., et al. The dual function cytokine IL-33 interacts with the transcription factor NF-κB to dampen NF-κB-stimulated gene transcription. Journal of Immunology. 2011;187(4):1609–1616. doi: 10.4049/jimmunol.1003080. [DOI] [PubMed] [Google Scholar]
- 88.Rider P., Kaplanov I., Romzova M., et al. The transcription of the alarmin cytokine interleukin-1 alpha is controlled by hypoxia inducible factors 1 and 2 alpha in hypoxic cells. Frontiers in Immunology. 2012;3 doi: 10.3389/fimmu.2012.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McCarthy D. A., Ranganathan A., Subbaram S., et al. Redox-control of the alarmin, interleukin-1α. Redox Biology. 2013;1(1):218–225. doi: 10.1016/j.redox.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sun Y., Chen H., Dai J., et al. Glycyrrhizin protects mice against experimental autoimmune encephalomyelitis by inhibiting high-mobility group box 1 (HMGB1) expression and neuronal HMGB1 release. Frontiers in Immunology. 2018;9:p. 1518. doi: 10.3389/fimmu.2018.01518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sun Y., Chen H., Dai J., et al. HMGB1 expression patterns during the progression of experimental autoimmune encephalomyelitis. Journal of Neuroimmunology. 2015;280:29–35. doi: 10.1016/j.jneuroim.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 92.Robinson A. P., Caldis M. W., Harp C. T., Goings G. E., Miller S. D. High-mobility group box 1 protein (HMGB1) neutralization ameliorates experimental autoimmune encephalomyelitis. Journal of Autoimmunity. 2013;43:32–43. doi: 10.1016/j.jaut.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang W., Wu L., Li J., et al. Alleviation of hepatic ischemia reperfusion injury by oleanolic acid pretreating via reducing HMGB1 release and inhibiting apoptosis and autophagy. Mediators of Inflammation. 2019;2019:10. doi: 10.1155/2019/3240713.3240713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chung H., Hong S. J., Choi S. W., et al. High mobility group box 1 secretion blockade results in the reduction of early pancreatic islet graft loss. Biochemical and Biophysical Research Communications. 2019;514(4):1081–1086. doi: 10.1016/j.bbrc.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 95.Braza F., Brouard S., Chadban S., Goldstein D. R. Role of TLRs and DAMPs in allograft inflammation and transplant outcomes. Nature Reviews. Nephrology. 2016;12(5):281–290. doi: 10.1038/nrneph.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cornide-Petronio M. E., Negrete-Sánchez E., Mendes-Braz M., et al. The effect of high-mobility group box 1 in rat steatotic and nonsteatotic liver transplantation from donors after brain death. American Journal of Transplantation. 2016;16(4):1148–1159. doi: 10.1111/ajt.13560. [DOI] [PubMed] [Google Scholar]
- 97.Wang M., Gauthier A., Daley L. A., et al. The role of HMGB1, a nuclear damage-associated molecular pattern molecule, in the pathogenesis of lung diseases. Antioxidants & Redox Signaling. 2019;31(13):954–993. doi: 10.1089/ars.2019.7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang K., Shan S., Wang S., Gu X., Zhou X., Ren T. HMGB1-containing nucleosome mediates chemotherapy-induced metastasis of human lung cancer. Biochemical and Biophysical Research Communications. 2018;500(3):758–764. doi: 10.1016/j.bbrc.2018.04.150. [DOI] [PubMed] [Google Scholar]
- 99.Gdynia G., Sauer S. W., Kopitz J., et al. The HMGB1 protein induces a metabolic type of tumour cell death by blocking aerobic respiration. Nature Communications. 2016;7(1) doi: 10.1038/ncomms10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang Z., Shi L., Hua S., Qi C., Fang M. IL-33 ameliorates experimental colitis involving regulation of autophagy of macrophages in mice. Cell & Bioscience. 2019;9(1) doi: 10.1186/s13578-019-0271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Di Paolo N. C., Shayakhmetov D. M. Interleukin 1α and the inflammatory process. Nature Immunology. 2016;17(8):906–913. doi: 10.1038/ni.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Menghini P., Corridoni D., Buttó L. F., et al. Neutralization of IL-1α ameliorates Crohn’s disease-like ileitis by functional alterations of the gut microbiome. Proceedings of the National Academy of Sciences of the United States of America; 2019; pp. 26717–26726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martin P., Palmer G., Rodriguez E., et al. Intracellular IL-1 receptor antagonist isoform 1 released from keratinocytes upon cell death acts as an inhibitor for the alarmin IL-1α. Journal of Immunology. 2020;204(4):967–979. doi: 10.4049/jimmunol.1901074. [DOI] [PubMed] [Google Scholar]
- 104.Michaudel C., Maillet I., Fauconnier L., et al. Interleukin-1α mediates ozone-induced myeloid differentiation factor-88-dependent epithelial tissue injury and inflammation. Frontiers in Immunology. 2018;9:p. 916. doi: 10.3389/fimmu.2018.00916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rabolli V., Badissi A. A., Devosse R., et al. The alarmin IL-1α is a master cytokine in acute lung inflammation induced by silica micro- and nanoparticles. Particle and Fibre Toxicology. 2014;11(1):p. 69. doi: 10.1186/s12989-014-0069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang W., Borcherding N., Kolb R. IL-1 signaling in tumor microenvironment. Advances in Experimental Medicine and Biology. 2020;1240:1–23. doi: 10.1007/978-3-030-38315-2_1. [DOI] [PubMed] [Google Scholar]


