Abstract
Background
Clinical studies have suggested that prostate health index (phi) outperforms prostate-specific antigen (PSA) tests in prostate cancer detection. The cost-effectiveness of phi with different cutoffs is poorly understood in the context of decision making for prostate biopsy.
Methods
In a multicenter cohort, 3,348 men with elevated total PSA (tPSA) underwent initial prostate biopsy from August 2013 to May 2019. We constructed a decision model to evaluate the incremental cost-effectiveness ratios of different phi cutoffs. Total costs and reimbursement payments were based on the fee schedule of Shanghai Basic Medical Insurance and converted into United States dollars ($). Two willingness-to-pay thresholds were estimated as one or three times the average gross domestic product per capita of China ($7,760 or $23,279, respectively).
Results
The total costs of prostate biopsy and PSA tests were estimated at $315 and $19, respectively. The cost of phi test varied between $72 to $130 in different medical centers. Under different phi cutoffs (from 23 to 35), phi test predicted reductions of 420 (21.7%) to 972 (50.2%) in unnecessary biopsies, with a total gain of 23.77–57.58 quality adjusted life-years compared to PSA tests. All the cutoffs would be cost-effective for patients with tPSA levels of 2–10 ng/ml. Applying 27 as the cutoff was cost-effective for each tPSA range, with missing positive cases ranging from 11 (3.4%) to 33 (11.5%).
Conclusions
Using phi test was cost-effective in the decision-making process for initial prostate biopsy, especially for patients with tPSA values between 2–10 ng/ml. The phi cutoff of 27 was cost-effective regardless of tPSA ranges and should be recommended from a health-economic perspective.
Keywords: prostate health index, prostate biopsy, cost-effectiveness, cutoff, China
Introduction
Prostate-specific antigen (PSA) screening has been widely implemented in decision making for prostate biopsy in clinical practice. However, its low specificity causes large numbers of negative, unnecessary biopsies (1, 2), resulting in physiological and psychological burdens on patients (3). It also results in millions of dollars of unnecessary medical expenditures. To solve these clinical predicaments, novel indicators, such as the prostate health index (phi), derived from total PSA (tPSA), free PSA (fPSA), and [-2]proPSA (p2PSA), have been introduced. Phi has shown significantly better accuracy in predicting prostate cancer (PCa) than tPSA or %fPSA (fPSA/tPSA). It is now recommended by clinical guidelines for patients with elevated tPSA, especially for patients with tPSA between 2.0 and 10.0 ng/ml (4–7). Synthesizing several published results, using phi can reduce the number of unnecessary biopsies with good sensitivity and detect clinically significant PCa; thus, phi might play an important role reducing overdiagnosis and overtreatment in urologic practice (8, 9).
The cost of phi testing varies among different countries and regions from $35 to $370 (United States dollars) (9–14). Several cost-effectiveness analyses of phi have been performed in the United States, Europe, and Hong Kong, which are all based on the population-based screening models using a specific phi cutoff (11, 14–16). However, phi is mainly applied in men scheduled to undergo prostate biopsy (also known as the prostate biopsy cohort). The cost-effectiveness of phi application has never been reported in a biopsy cohort. In addition, differences in subsequent treatment options and medical insurance systems among countries make it difficult to apply the cost-effectiveness evaluation from previous studies to healthcare policy in mainland China. Therefore, we conducted the present study to evaluate the comparative cost-effectiveness using different cutoffs of phi in a multicenter biopsy cohort in mainland China.
Materials and Methods
Study Population
In this prospective observational multicenter study, we consecutively recruited 3,504 patients from August 2013 to May 2019 in seven tertiary hospitals in Shanghai, China. All the patients underwent initial prostate biopsies. The indications for prostate biopsy were the same across different tertiary hospitals: (1) a tPSA level >10.0 ng/ml; (2) a tPSA level >4.0 ng/ml with confirmation after 2–3 months; (3) %fPSA <0.16 when patients had a tPSA level >4.0 ng/ml; and (4) the presence of suspicious lesions detected by digital rectal examination (DRE), ultrasound or magnetic resonance imaging (MRI) at any level of tPSA. The phi calculation was not used in clinical decision making. MRI was not used for biopsy decision making in patients with a tPSA level >4.0 ng/ml.
Blood samples were collected prior to biopsies on the same day and were measured in a central certified lab. Transrectal ultrasound (TRUS)-guided biopsies were performed using a 10- to 14-core scheme. All biopsy specimens were independently examined by 2 experienced pathologists in the department of pathology at each hospital. Clinically significant PCa (csPCa) was defined as PCa with Grade Group (GG) ≥2. The study was approved by the institutional review board of each hospital, and written informed consent was obtained from each participant.
Patients were excluded from the present study if (1) information in the pathological report was missing, (2) serum antigen levels (tPSA, fPSA, or p2PSA) were unable to be tested due to poor serum sample quality, or (3) the tPSA level was <2.0 ng/ml.
Cost
Currently, application of phi (including tPSA, fPSA and p2PSA) has not been approved by the local Health Commission (but has been approved by the China Food and Drug Administration). Thus, the test fee was not fixed among medical centers. The price of phi test differed based on mutual agreements between each medical center and the certified lab. Therefore, in the present study, the cost of applying phi was assumed based on the information from the certified lab, with a range from $72 to $130 ( Table 1 ). Additional costs associated with the prostate biopsy procedure were estimated based on the fee schedule of Shanghai Basic Medical Insurance (SBMI) for employees and residents. We calculated the average reimbursement among the patients and entered it into our cost-effectiveness model ( Table 1 ). As described above, phi test had not been included in the reimbursement lists of SBMI. We assumed that phi test would have the same reimbursement percentage as prostate biopsy in our analysis.
Table 1.
Estimated total costs and reimbursement used in cost-effectiveness model.
| Charge list | Costs (CNY) | Costs (USD) |
|---|---|---|
| PSA tests, range* | 128 | 19 |
| phi test, range | 500–900 | 72–130 |
| Biopsy procedure | ||
| Ultrasonography | 385 | 56 |
| Prostate Biopsy | 779 | 113 |
| Medicine** | 111 | 16 |
| Pathological tests | 900 | 130 |
| Total | 2,175 | 315 |
| Reimbursement, mean (SD) | 1,781 (305) | 258 (44) |
PSA, prostate-specific antigen; phi, prostate health index; CNY, Chinese yuan; USD, United States dollar.
*PSA tests included both tPSA and fPSA test.
**Medicine included both anesthetic and antibiotic drugs.
All the costs noted above were reported in Chinese currency (CNY) and converted to United States dollar using the average exchange rate ($1 = 6.90 CNY) in 2019 (17). We evaluated the cost-effectiveness when making decisions at a single time point; therefore, the impacts of inflation and the annual discount rate were not considered in the analyses.
Utilities
We multiplied the utility estimates (ranging between 0 and 1, representing death and perfect health, respectively) and duration of health states to calculate the loss of QoL. We assigned a utility estimate of prostate biopsy of 0.90, and its duration of 3 weeks according to published studies (18, 19). Both life-years and quality-adjusted life-years (QALYs) were gained due to the reduction in unnecessary biopsies.
Outcomes
The primary outcome (necessary biopsy) was pathologically confirmed PCa. In contrast, unnecessary biopsies were defined as negative biopsy results. When considering different phi cutoffs, the number of unnecessary biopsies avoided was defined as a “true negative” number (the phi value was below the threshold and no cancer was indicated on biopsy). Similarly, a missing positive case was defined as a “false negative” result (the phi value was below the threshold but PCa was confirmed). The percentage of unnecessary biopsies avoided and missing positive cases was calculated as the number of unnecessary biopsies avoided and missing positive cases divided by the number of total negative biopsies and PCa, respectively. The secondary outcome was csPCa, and in this case, both indolent PCa (GG = 1) and negative results were classified as unnecessary biopsies.
Statistical Analysis
The derivative variable phi was calculated as follows: (p2PSA/fPSA) ×√tPSA. Since all patients with tPSA levels <2.0 ng/ml were excluded, the remaining patients were recommended to receive prostate biopsies mainly because of elevated tPSA levels. By assuming that men would receive or not receive prostate biopsies under a certain phi cutoff in the cohort (and make hypothetical biopsy decisions thereafter), we were able to compute the incremental costs and QALYs.
The incremental costs were calculated as follows: the cost of the [phi test] × N—the costs of [prostate biopsy] × n (N: the number of total biopsy times, n: the number of unnecessary biopsies under a certain phi cutoff). The incremental effectiveness (QALYs gained) was calculated as follows: the number of biopsies avoided × utility estimate × duration.
Eventually we calculated the incremental cost-effectiveness ratios (ICERs) of different phi cutoffs by dividing the incremental costs by the incremental QALYs. With each phi cutoff, both the incremental cost and ICER had a range depending on the unit price of phi test and the reimbursement percentage. Two willingness-to-pay (WTP) thresholds were estimated as one or 3 times the average gross domestic product (GDP) per capita of China from 2013 to 2018 (20) ($7,760 and $23,279 per QALY, respectively) on the basis of the World Health Organization (WHO) recommendation (21). Thus, it would be considered very cost-effective if the ICER was less than $7,760/QALY; cost-effective if the ICER was between $7,760 and $23,279/QALY gained; and not cost-effective for the remainder.
We evaluated four phi cutoffs from 23 to 35 according to the results from two previous studies (based on a Chinese population and a multi-institutional cohort) (7, 22). The cutoff threshold was defined as the number above which all phi cutoffs were cost-effective regardless of the unit price of phi test. The price threshold indicated that the unit price of phi test should be lower than that number to meet the WTP thresholds with each phi cutoff.
Sensitivity analyses were also performed by varying the range of tPSA levels. Continuous variables were tested by Student’s t-test for normally distributed variables, and Mann-Whitney U test for non‐normally distributed variables. All statistical analyses were performed using Stata 15.1 Special Edition (StataCorp, 4905 Lakeway Drive, College Station, Texas 77845 USA, 2017). A two-tailed P value < 0.05 was considered statistically significant.
Results
The characteristics and biopsy outcomes of the entire cohort and subgroups are shown in Table 2 and Table S1 . Based on our exclusion criteria, 156 patients were excluded, and 3,348 patients were included for further analysis. In the entire cohort, 1,411 (42.1%) patients had PCa documented on biopsy, and 1,145 (34.2%) had csPCa. As shown in Table 3 , phi tests reduced the number of unnecessary biopsies, with reductions ranging from 420 (21.7%) to 972 (50.2%) under different phi cutoffs (from 23 to 35). The number of missing positive cases ranged from 39 (2.8%) to 140 (9.9%). In patients with a tPSA level of 2–10 ng/ml, the number of unnecessary biopsies avoided ranged from 276 (28.5%) to 613 (63.4%), with the missing positive cases ranging from 19 (6.6%) and 74 (25.7%).
Table 2.
Characteristics and biopsy outcomes of entire cohort and subsets grouped by total prostate-specific antigen.
| Variables | Entire cohort | tPSA 2–10ng/ml | tPSA 10.1–20 ng/ml | tPSA 20.1–50 ng/ml | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCa | Non-PCa | P | PCa | Non-PCa | P | PCa | Non-PCa | P | ||||
| Patients, n (%) | 3,348 (100) | 288 (23.0) | 967 (77.1) | / | 373 (36.9) | 639 (63.1) | / | 319 (55.8) | 253 (44.2) | / | ||
| Median (IQR) | Median (IQR) | Median (IQR) | ||||||||||
| Age, year | 68 (62–74) | 70 (64–75) | 65 (60–70) | <0.001 | 71 (64–76) | 66 (61–71) | <0.001 | 71 (66–77) | 68 (63–73) | <0.001 | ||
| tPSA, ng/ml | 12.9 (8.1–25.8) | 7.5 (6.0–8.7) | 7.0 (5.3–8.5) | <0.001 | 13.9 (11.9–16.6) | 13.3 (11.7–16.0) | 0.031 | 29.6 (24.2–37.5) | 25.2 (22.1–30.8) | <0.001 | ||
| fPSA, ng/ml | 1.8 (1.1–3.4) | 0.9 (0.7–1.2) | 1.1 (0.7–1.5) | <0.001 | 1.6 (1.1–2.2) | 1.9 (1.3–2.7) | <0.001 | 2.8 (1.9–4.0) | 3.4 (2.2–5.0) | <0.001 | ||
| p2PSA, pg/ml | 22.1 (13.0–52.1) | 16.4 (11.3–23.6) | 12.2 (8.3–18.1) | <0.001 | 28.4 (18.3–45.7) | 19.6 (13.4–27.2) | <0.001 | 55.5 (32.7–105.7) | 30.6 (21.3–48.9) | <0.001 | ||
| f/tPSA | 0.13 (0.09–0.19) | 0.13 (0.09–0.18) | 0.17 (0.12–0.22) | <0.001 | 0.11 (0.08–0.15) | 0.14 (0.10–0.19) | <0.001 | 0.09 (0.07–0.13) | 0.13 (0.08–0.18) | <0.001 | ||
| p2/fPSA | 14.4 (9.5–23.1) | 18.5 (13.3–24.4) | 11.7 (8.5–16.3) | <0.001 | 18.4 (13.2–27.5) | 10.3 (7.5–14.9) | <0.001 | 21.3 (15.1–31.0) | 9.8 (6.8–14.5) | <0.001 | ||
| phi | 48.5 (29.8–106.4) | 49.1 (34.5–68.1) | 29.5 (21.7–41.0) | <0.001 | 69.2 (48.9–102.5) | 38.6 (27.7–54.6) | <0.001 | 114.6 (78.6–174.0) | 49.6 (35.0–74.1) | <0.001 | ||
PCa, prostate cancer; IQR, interquartile range; tPSA, total prostate-specific antigen; fPSA, free prostate-specific antigen; p2PSA, [-2]proPSA; phi, prostate health index.
Table 3.
Clinical endpoints, incremental effectiveness and ICER in entire cohort and patients with tPSA values between 2 and 10 ng/ml.
| Cutoff values | # Missing positive cases (%) | # Unnecessary biopsies avoided (%) | QALY gained (life-years) | ICER of total cost (USD per QALY) | ICER of reimbursement part (USD per QALY) |
|---|---|---|---|---|---|
| Entire cohort (n = 3,348, PCa = 1,411, non-PCa = 1,937) | |||||
| phi = 23 | 39 (2.8) | 420 (21.7) | 23.77 | 8,203 | 7,647 |
| phi = 27 | 63 (4.5) | 609 (31.4) | 34.80 | 3,674 | 3,643 |
| phi = 31 | 100 (7.1) | 802 (41.4) | 46.71 | 1,185 | 1,443 |
| phi = 35 | 140 (9.9) | 972 (50.2) | 57.58 | -189 | 229 |
| tPSA 2–10 ng/ml (n = 1,255, PCa = 288, non-PCa = 967) | |||||
| phi = 23 | 19 (6.6) | 276 (28.5) | 15.28 | 2,247 | 2,383 |
| phi = 27 | 33 (11.5) | 400 (41.4) | 22.42 | -409 | 35 |
| phi = 31 | 53 (18.4) | 528 (54.6) | 30.08 | -1,856 | -1,244 |
| phi = 35 | 74 (25.7) | 613 (63.4) | 35.57 | -2,508 | -1,821 |
ICER, incremental cost-effectiveness ratio; tPSA, total prostate-specific antigen; phi, prostate health index; QALY, quality-adjusted life-year; USD, United States dollar; PCa, prostate cancer.
The total costs of prostate biopsy were estimated at $315, with details shown in Table 1 (slight differences may exist due to the different antibiotic prescriptions, so the number was the average cost from 100 randomly selected patients). The fixed cost of PSA tests (including tPSA and fPSA) was $19. The cost range of phi tests for the three components, as described, was $72–$130. Compared to PSA tests, phi test led to a total gain in QALYs that ranged from 23.77 to 57.58 life-years due to the reduction in unnecessary biopsies, and 15.28–35.57 life-years in the subgroup with tPSA levels of 2–10 ng/ml ( Table 3 ).
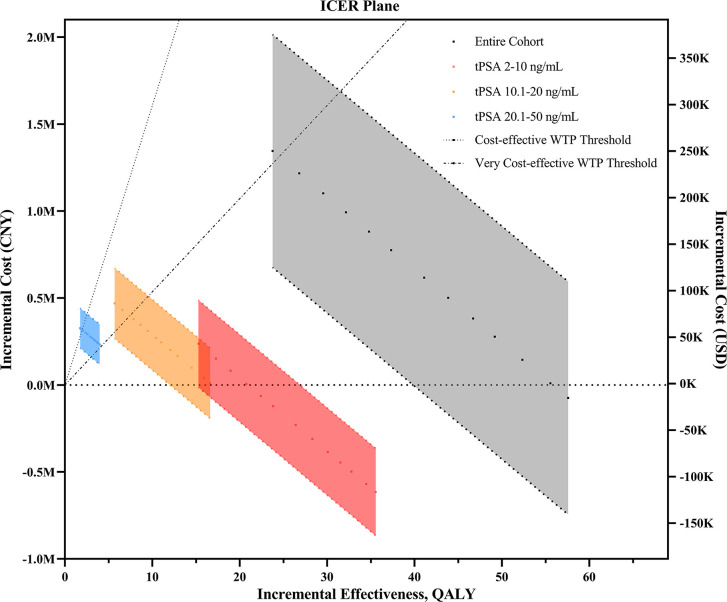
The comparative ICERs between different phi cutoffs were evaluated. Higher cutoffs were associated with lower incremental costs and ICERs in all subsets grouped by tPSA values ( Figure 1 ). In the entire cohort, the cost-effectiveness analysis showed that all phi cutoffs were cost-effective, and phi cutoffs over 27 were considered very cost-effective. Notably, when using 27 (or above) as the cutoff, phi test would be cost-effective at tPSA range, with limited missing positive cases varying from 11 (3.4%) to 33 (11.5%, Table 3 and Table S2 ).
Figure 1.
The ICER plane for the comparison of phi- and PSA-based prostate biopsy models upon changes in different phi cutoff values. ICER, incremental cost-effectiveness ratio; CNY, Chinese yuan; USD, United States dollar; QALY, quality-adjusted life years; tPSA, total prostate-specific antigen; WTP, willingness-to-pay.
In patients with tPSA levels of 2–10 ng/ml, phi test would be cost-effective regardless of the cutoffs and unit price. It would be very cost-effective if phi cutoffs of 23 or above were applied. Using cutoffs over 27 would even save the total costs (the incremental cost was less than zero). Similar results were found when evaluating the incremental costs of reimbursement parts ( Table 3 ). Therefore, under the most accepted phi cutoff (as 28) in China, as reported in a previous study (7), phi test would be very cost-effective for biopsy decision making. In addition, phi test with a cutoff over 27 strictly dominated PSA tests due to negative ICERs (indicating higher effectiveness but less cost). In patients with tPSA levels of 10.1–20 ng/ml, however, phi test with a cutoff of 23 or higher were cost-effective regardless of the unit price ( Table S2 and Table S3 ). In patients with tPSA levels of 20.1–50 ng/ml, the lowest cutoffs of 23 was extended dominance (more effective and more costly but higher than our WTP threshold). Similarly, the price thresholds varied from $90 to $122 with cutoffs ranging from 23 to 26 ( Table S3 ), and phi tests with a cutoff of 27 or higher were cost-effective regardless of the unit price.
Assuming that the cost of phi tests would be reimbursed proportionally at the same percentage as prostate biopsy, similar results were found from an insurance perspective ( Table 3 and Table S2 ). All the cutoffs would be very cost-effective in patients with tPSA levels of 2–10 ng/ml. Cutoffs over 27 were cost-effective at each tPSA range regardless of the unit price. Repeated analysis showed similar results after using csPCa as the biopsy outcome ( Table S4 ).
The expected incremental costs, also known as out-of-pocket payments, were also compared between different groups, as shown in Figure S1A (when the total cost of phi test was paid by the patients themselves) and Figure S1B (when the cost of phi test was reimbursed by SBMI). The expected incremental costs decreased with increased cutoffs in all subsets grouped by tPSA range. Furthermore, the out-of-pocket payment for phi test was lower in patients with tPSA values between 2–10 ng/ml than in the other two subgroups (all P values < 0.001).
Discussion
The objective of the present study was to evaluate whether phi test was cost-effective in decision making for initial prostate biopsy. In this multicenter study, we found that phi test was not only more accurate for PCa detection, but also more cost-effective than PSA tests, especially for patients with tPSA levels of 2–10 ng/ml in the Chinese population. In addition, applying 27 as phi cutoff would be very cost-effective in different tPSA subgroups, with very limited missing positive cases. The results from the present study suggested that phi test had health-economic advantages and could even save costs for national medical insurance in China.
Four published studies have demonstrated that phi test is cost-effective in screening models of developed regions (11, 14–16). However, all the previous studies inputted single cutoffs for phi test (25 and 35 for Western and Chinese populations, respectively) into their models and analyzed data in several screening cycles. Furthermore, the WTP thresholds ($50,000/QALY) in previous studies were much higher than those in our study ($7,760 or $23,279/QALY) because of socioeconomic differences. Regarding the costs of phi testing, a nonfixed price from $72 to $130 was allocated in our study, which was close to the price used in European and US models (10, 11, 15, 16), but much less than that in a Hong Kong model ($369.54) (14). Therefore, the results of previous studies may not be generalizable to our population. In the present study, we evaluated the ICERs of different phi cutoffs and performed subgroup analyses according to tPSA values. We found that applying 27 as phi cutoff was cost-effective regardless of tPSA range and unit price, with limited missing positive cases. In mainland China, basic medical insurance is provided nationwide and is nonprofitable. As a result, the high incidence of PCa and high reimbursement percentage of prostate biopsy increase the economic burden in the whole country. Hence, the present results are remarkable for developing countries such as China, where phi test is still not included in the national medical insurance reimbursement list.
The present study focused on only the cost-effectiveness at the diagnostic stage (in which men with elevated tPSA levels would undergo prostate biopsy). We did not take the subsequent treatments into account due to the homogeneous treatment patterns for PCa in mainland China. Zhao et al. (23) reported that only 2.33% of low-risk PCa patients received active surveillance (AS) or observation in China, even though AS is widely recommended by the guidelines for low-risk patients (24, 25). In other words, most indolent PCa patients in China actually receive curative treatment, and more than 80% of them undergo radical prostatectomy rather than radiation therapy (23). Several factors might explain the above differences. For example, the cultural background makes it hard to tolerate a malignancy without a dissection; the relatively low accessibility of persistent health care and follow-up service is another critical driven factor for the high rate of curative treatments (26). Therefore, the overtreatment effects caused by phi testing in the present study would be very low (natural overtreatment due to the disease management). For the same reason, we considered PCa rather than csPCa as the main outcome in the current study, as it was more suitable for our biopsy cohort in the Chinese population.
There were several limitations of this study. First, we evaluated the medical expenditures associated with phi test by using a hypothetical reimbursement percentage. However, the serum test and biopsy are both classified into the same reimbursement list in China, and the reimbursement percentage varies by age and socioeconomic status rather than medical usage. Thus, the hypothetical percentage might be reliable in our analysis. Second, the health utilities used in our model were from published articles of Western populations, due to a lack of related research in China. However, prostate biopsy is a short-term procedure that might vary little between Western and Asian populations. Third, the WTP threshold based on the GDP was used to evaluate the cost-effectiveness in each situation; however, the WTP was uncertain and lacked specificity (27, 28). We added the reimbursement percentage into our decision model to adjust for the budget impact. However, further analyses are needed to assess the long-term impact of phi test. Fourth, all medical centers participating in the present study were located in Shanghai, a large city in East China, which may cause selection bias. However, individuals all over the country seek the services of these tertiary hospitals.
In conclusion, phi test was cost-effective in decision making for initial prostate biopsy in the Chinese population, especially patients with tPSA values between 2–10 ng/ml. The phi cutoff of 27 was cost-effective regardless of the tPSA ranges, with limited missing positive cases, and should be recommended from a health-economic perspective.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by The institutional review board of Ruijin Hospital, Huashan Hospital, Cancer Center, Xinhua Hospital, Huadong Hospital, Ninth People’s Hospital, and Changhai Hospital in Shanghai. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
RN and DX conceived and designed the study. DH, XY, YW, XL, and JX contributed materials and collected the data. DH and XY analyzed the data. DH, XY, RN, and DX wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 81772741 and No. 81972645), Shanghai Rising-Star Program (Grant No. 18QA1402800), the “Chen Guang” project from Shanghai Municipal Education Commission and Shanghai Education Development Foundation (Grant No. 17CG09), Shanghai Jiao Tong University School of Medicine Gaofeng-Clinical Medicine Grant Support (Grant No. 20181701), Shanghai Municipal Human Resources and Social Security Bureau (Grant No. 2018052), and Shanghai Jiao Tong University SMC-Chenxing Scholar Project to RN. All the funders had no role in the study design, data collection, data analysis, interpretation, and writing of the report.
Conflict of Interest
In the present study, we declare that Beckman Coulter, Inc. provided the tests for tPSA, fPSA, and p2PSA. All the sample collection, data analyses, and manuscript writing were performed by the researchers, independent from Beckman Coulter, Inc.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all subjects included in this study. We would like to thank for the contribution of Shanghai Cancer Center, Shanghai Xinhua Hospital, Shanghai Huadong Hospital, Shanghai Ninth People’s Hospital, and Shanghai Changhai Hospital to this study. We thank Beckman Coulter, Inc. for measurements of tPSA, fPSA, and p2PSA.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.565382/full#supplementary-material
References
- 1. Welch HG, Schwartz LM, Woloshin S. Prostate-specific antigen levels in the United States: implications of various definitions for abnormal. J Natl Cancer Inst (2005) 97:1132–37. 10.1093/jnci/dji205 [DOI] [PubMed] [Google Scholar]
- 2. Schroder FH, Hugosson J, Roobol MJ, Tammela TLJ, Zappa M, Nelen V, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet (2014) 384:2027–35. 10.1016/S0140-6736(14)60525-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McNaughton-Collins M, Fowler FJ, Caubet J-F, Bates DW, Lee JM, Hauser A, et al. Psychological effects of a suspicious prostate cancer screening test followed by a benign biopsy result. Am J Med (2004) 117:719–25. 10.1016/j.amjmed.2004.06.036 [DOI] [PubMed] [Google Scholar]
- 4. Guazzoni G, Nava L, Lazzeri M, Scattoni V, Lughezzani G, Maccagnano C, et al. Prostate-specific antigen (PSA) isoform p2PSA significantly improves the prediction of prostate cancer at initial extended prostate biopsies in patients with total PSA between 2.0 and 10 ng/ml: results of a prospective study in a clinical setting. Eur Urol (2011) 60:214–22. 10.1016/j.eururo.2011.03.052 [DOI] [PubMed] [Google Scholar]
- 5. Lazzeri M, Haese A, de la Taille A, Palou Redorta J, McNicholas T, Lughezzani G, et al. Serum isoform [-2]proPSA derivatives significantly improve prediction of prostate cancer at initial biopsy in a total PSA range of 2-10 ng/ml: a multicentric European study. Eur Urol (2013) 63:986–94. 10.1016/j.eururo.2013.01.011 [DOI] [PubMed] [Google Scholar]
- 6. Tosoian JJ, Druskin SC, Andreas D, Mullane P, Chappidi M, Joo S, et al. Use of the Prostate Health Index for detection of prostate cancer: results from a large academic practice. Prostate Cancer Prostatic Dis (2017) 20:228–33. 10.1038/pcan.2016.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Na R, Ye D, Qi J, Liu F, Helfand BT, Brendler CB, et al. Prostate health index significantly reduced unnecessary prostate biopsies in patients with PSA 2-10 ng/mL and PSA >10 ng/mL: Results from a Multicenter Study in China. Prostate (2017) 77:1221–29. 10.1002/pros.23382 [DOI] [PubMed] [Google Scholar]
- 8. Filella X, Giménez N. Evaluation of [-2] proPSA and Prostate Health Index (phi) for the detection of prostate cancer: a systematic review and meta-analysis. Clin Chem Lab Med (2013) 51:729–39. 10.1515/cclm-2012-0410 [DOI] [PubMed] [Google Scholar]
- 9. Ferro M, De Cobelli O, Lucarelli G, Porreca A, Busetto GM, Cantiello F, et al. Beyond PSA: The Role of Prostate Health Index (phi). Int J Mol Sci (2020) 21:1184. 10.3390/ijms21041184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nichol MB, Wu J, An JJ, Huang J, Denham D, Frencher S, et al. Budget impact analysis of a new prostate cancer risk index for prostate cancer detection. Prostate Cancer Prostatic Dis (2011) 14:253–61. 10.1038/pcan.2011.16 [DOI] [PubMed] [Google Scholar]
- 11. Heijnsdijk EAM, Denham D, de Koning HJ. The cost-effectiveness of prostate cancer detection with the use of prostate health index. Value Health (2016) 19:153–57. 10.1016/j.jval.2015.12.002 [DOI] [PubMed] [Google Scholar]
- 12. Narayan VM, Konety BR, Warlick C. Novel biomarkers for prostate cancer: An evidence-based review for use in clinical practice. Int J Urol (2017) 24:352–60. 10.1111/iju.13326 [DOI] [PubMed] [Google Scholar]
- 13. Mathieu R, Castelli C, Fardoun T, Peyronnet B, Shariat SF, Bensalah K, et al. Cost analysis of prostate cancer detection including the prostate health index (phi). World J Urol (2019) 37:481–87. 10.1007/s00345-018-2362-z [DOI] [PubMed] [Google Scholar]
- 14. Teoh JY-C, Leung C-H, Wang MH, Chiu PK-F, Yee C-H, Ng C-F, et al. The cost-effectiveness of prostate health index for prostate cancer detection in Chinese men. Prostate Cancer Prostatic Dis (2020) 23:615–21. 10.1038/s41391-020-0243-1 [DOI] [PubMed] [Google Scholar]
- 15. Sathianathen NJ, Kuntz KM, Alarid-Escudero F, Lawrentschuk NL, Bolton DM, Murphy DG, et al. Incorporating biomarkers into the primary prostate biopsy setting: A cost-effectiveness analysis. J Urol (2018) 200:1215–20. 10.1016/j.juro.2018.06.016 [DOI] [PubMed] [Google Scholar]
- 16. Nichol MB, Wu J, Huang J, Denham D, Frencher SK, Jacobsen SJ. Cost-effectiveness of Prostate Health Index for prostate cancer detection. BJU Int (2012) 110:353–62. 10.1111/j.1464-410X.2011.10751.x [DOI] [PubMed] [Google Scholar]
- 17. National Bureau of Statistics of China National Economic and Social Development Statistical Communique (2018). Available at: http://www.stats.gov.cn/tjsj/zxfb/201902/t20190228_1651265.html (Accessed Feb 28, 2020).
- 18. Heijnsdijk EAM, Wever EM, Auvinen A, Hugosson J, Ciatto S, Nelen V, et al. Quality-of-life effects of prostate-specific antigen screening. N Engl J Med (2012) 367:595–605. 10.1056/NEJMoa1201637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Haes J, de Koning HJ, van Oortmarssen GJ, van Agt HME, de Bruyn AE, van der Maas PJ. The impact of a breast cancer screening programme on quality-adjusted life-years. Int J Cancer (1991) 49:538–44. 10.1002/ijc.2910490411 [DOI] [PubMed] [Google Scholar]
- 20. National Bureau of Statistics of China Annual National Economic Data. Available at: http://data.stats.gov.cn/ (Accessed Feb 16, 2020).
- 21. Edejer TTT, Baltussen R, Adam T, Hutubessy R, Acharya A, Evans DB, et al. Making choices in health: WHO guide to cost effectiveness analysis. Geneva: World Health Organization; (2003). [Google Scholar]
- 22. Catalona WJ, Partin AW, Sanda MG, Wei JT, Klee GG, Bangma CH, et al. A multicenter study of [-2] pro-prostate specific antigen combined with prostate specific antigen and free prostate specific antigen for prostate cancer detection in the 2.0 to 10.0 ng/ml prostate specific antigen range. J Urol (2011) 185:1650–55. 10.1016/j.juro.2010.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao F, Shen J, Yuan Z, Yu X, Jiang P, Zhong B, et al. Trends in Treatment for Prostate Cancer in China: Preliminary Patterns of Care Study in a Single Institution. J Cancer (2018) 9:1797–803. 10.7150/jca.25113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer (Version 4.2019). Available at: http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (Accessed May 15, 2020).
- 25. Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol (2017) 71:618–29. 10.1016/j.eururo.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 26. Wei Y, Liu L, Li X, Song W, Zhong D, Cao X, et al. Current Treatment for Low-Risk Prostate Cancer in China: A National Network Survey. J Cancer (2019) 10:1496–502. 10.7150/jca.29595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bertram MY, Lauer JA, De Joncheere K, Edejer T, Hutubessy R, Kieny M-P, et al. Cost-effectiveness thresholds: pros and cons. Bull World Health Organ (2016) 94:925–30. 10.2471/BLT.15.164418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marseille E, Larson B, Kazi DS, Kahn JG, Rosen S. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Organ (2015) 93:118–24. 10.2471/BLT.14.138206 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.