Abstract
Patients with early stage hepatocellular carcinoma have good prognosis and are treated with curative intent. Although this cohort of patients is generally defined by limited tumor burden, good liver function, and preserved functional status, there remains utility in further stratification to optimize overall survival and limit post-operative morbidity and mortality. Transplant, resection, ablation, transarterial radioembolization, and transarterial chemoembolization, either as monotherapy or in combination, may play a crucial role in treating this cohort of patients depending on a multitude of factors. In this section, we review each treatment modality and provide general guidelines for patient selection.
Keywords: Early stage hepatocellular carcinoma, transarterial radioembolization, transarterial chemoembolization, radiation segmentectomy, radiation lobectomy
Patients with early-stage hepatocellular carcinoma (HCC), as defined by the Barcelona Clinic Liver Cancer (BCLC) staging, have a good performance status (Eastern Cooperative Oncology Group [ECOG] 0), compensated liver function (Child–Pugh A or B), and limited tumor burden (a single tumor or up to three tumors each not more than 3 cm in size). Patients meeting these criteria have demonstrated good prognosis and are typically treated with curative intent. 1 Traditional treatment options include liver transplantation (LT), hepatic resection, thermal ablation and transarterial chemoembolization (TACE). Transarterial radioembolization (TARE), traditionally used for intermediate- and advanced-stage disease, has been increasingly used in early-stage HCC with curative intent in patients who are not candidates for surgery or ablation. Ablation, TACE, and TARE can be used to bridge patients to LT while TARE and portal vein embolization (PVE) can be used to bridge patients with limited future liver remnant (FLR) to resection.
In this section, we will review each treatment modality with emphasis on patient selection and expected outcomes.
Liver Transplantation
Liver transplantation is a curative surgical option for patients with early-stage HCC who are not resection candidates. Transplant not only treats the initial cancer but also has the added benefit of removing the underlying cirrhotic liver, which may lead to future de novo HCC formation. However, in 1996 after a period of unrestricted transplantation, it was determined that the 5-year survival of transplanted patients with HCC was less than 50%. The Milan criteria were introduced to improve posttransplant outcomes. 2 A systematic review by Mazzaferro et al in 2011 determined that patients within Milan criteria have 5-year survival comparable to non-HCC liver transplant patients, with less than 10% tumor recurrence. 3 While expanded criteria to improve access to transplant—such as the UCSF model—have been validated, 4 5 Milan criteria remains the gold standard and is the most commonly used. Limited organ supply and long waitlist times place these patients at risk of falling outside of the Milan criteria due to disease progression, no longer qualifying for transplant. Under the current policy, patients are initially listed with their natural MELD (model for end-stage liver disease) score and awarded a MELD exception score of 28 points after a 6‐month waiting period. The points increase every 3 months to a maximum score of 34 points. This change was made to ensure a more equitable allocation of donor organs between HCC and non‐HCC patients. 6 7 As this has in effect created a mandatory wait time of 6 months for early-stage HCC patients, “bridging” therapy has become an integral part of the pretransplant algorithm. Several studies and meta-analyses of local regional therapy (LRT) for bridging have demonstrated a significantly lower dropout rate due to tumor progression. 8 9 Mehta et al found that a subgroup of patients having a single tumor between 2 and 3 cm in size with complete response after the first LRT, and an AFP level ≤ 20 ng/mL after the first LRT, had 1‐ and 2‐year probabilities of dropout of 1.3 and 1.6%, respectively, whereas the probabilities were 21.6 and 26.5% for all other patients ( p = 0.004). 8
A retrospective study by Oligane et al comparing the use of bridging with TACE and/or radiofrequency ablation (RFA) to no bridging found that in the unmatched cohort, as well as in a propensity score–matched subgroup, bridging reduced the rate of posttransplant recurrence and prolonged overall survival (OS). 10 More recently, Gabr et al published a study looking at long-term outcomes in patients who received TARE as bridging or downstaging to transplant. Three-, 5-, and 10‐year OS rates were 84, 77, and 60%, respectively. Twenty-four patients developed recurrence, with median recurrence-free survival of 120 months. Disease-specific mortality rates at 3, 5, and 10 years were 6, 11, and 16%, respectively. 11 Good response to bridging and downstaging therapy has also been shown to be associated with favorable histology such as absence of microvascular invasion and low-grade tumor pathology, and, as such, has become an important prognostic indicator of underlying pathology. 9 In effect, LRT provides a biologic test of time allowing identification of patients with unfavorable tumor biology that cannot be delineated by imaging alone. Given limited organ availability, the use of LRT has become an important tool in the selection of transplant patients which is reflected in the 2018 EASL guidelines: bridging a patient to transplant with the use of LRT is strongly recommended with wait times of 6 months or longer. 12
Bridging techniques for early-stage HCC vary by institution and include TACE, ablation, combination of TACE and ablation, and TARE. The method of LRT for bridging is not specifically dictated by the EASL guidelines. At present, the most commonly used bridging technique around the world is TACE, 13 although this is institutionally dependent and both ablation and TARE are utilized at many centers.
Liver Resection
Liver resection is a curative surgical option for patients with early-stage hepatocellular carcinoma (HCC). It is ideally performed in patients with preserved liver function and limited comorbidities. Expected 5-year OS ranges widely from 40 to 70% and is dependent on a combination of factors including underlying liver disease, extent of resection, tumor size/number, vascular involvement, AFP level, and operative factors such as whether R0 resection was achieved and complications such as intra/postoperative bleeding. 14 15 16 To avoid postoperative liver failure and mortality, it is crucial to weigh liver function with the necessary amount of liver to be resected. There is an abundance of data that supports resecting the minimum amount of liver to achieve adequate margins. Yip et al showed that patients who underwent right posterior sectionectomy had significantly lower incidence of postoperative liver failure compared with patients who underwent right hepatectomy (2 vs. 9.4%, p = 0.005) with similar 5-year OS (83 vs. 76%) and disease-free survival (52%). 14 16 A similar study comparing mesohepatectomy to extended hepatectomy resulted in comparable findings. 17 The exact FLR volume required for safe resection is still debated in the literature and depends on liver function, but in general, 20 to 30% FLR is considered adequate for healthy livers, whereas >40% is considered safe for cirrhotic livers. 18 19
Several clinical findings, laboratory values, and scoring systems have been used to assess liver function prior to resection. Clinical findings to suggest portal hypertension such as platelets <100,000 cells/mm 3 , splenomegaly, and varices are widely used as a contraindication to resection. This practice is somewhat controversial, as there are several retrospective studies showing that resection can be safely performed in patients with clinical findings of portal hypertension but without confirmed hepatic venous pressure gradient ≥ 10 mm Hg. Boleslawski et al showed that patients with elevated HVPG ≥ 10 mm Hg were more likely to have postoperative liver dysfunction and mortality and 90-day mortality, whereas esophageal varices, splenomegaly, and thrombocytopenia were not predictive of outcome. 20 Despite this, it is common practice to exclude patients with clinical findings suggestive of portal hypertension from resection in order to optimize results.
The MELD, Child–Pugh score, and albumin–bilirubin (ALBI) grading system are scores routinely used to evaluate patients' liver function prior to resection. Ideal resection candidates have a MELD score of less than 9, CP score of 5, and ALBI grade 1. In a retrospective review of 82 patients undergoing liver resection, perioperative mortality was 29% for patients with MELD > 8 and 0% for patients with MELD <9. 21 In a different study of 229 patients, there were zero instances of liver failure/death and an 8.1% complication rate in patients with MELD < 9 compared with 37.5% liver failure and 83.3% complication rate in patients with elevated MELD scores. 22
Patients who have suboptimal liver function and/or require resections that will leave them with inadequate FLR benefit from either PVE or Y90 radioembolization to hypertrophy the FLR prior to surgery. There is strong evidence that PVE prior to resection does not negatively impact postsurgical outcomes in FLR-matched cohorts and helps patients who would not otherwise be surgical candidates. 23 24 PVE and Y90 have their respective advantages and disadvantages. For example, PVE results in rapid and exuberant FLR hypertrophy, often peaking in 4 to 8 weeks, but does not address the underlying malignancy which may progress if left untreated. Y90 radioembolization provides good tumor control but at the expense of less dramatic hypertrophy, which may take 3 to 9 months to peak. Marti et al showed that in their cohort of 82 patients who underwent PVE, despite 34.1% having radiographic progression of disease, 87.8% were able to undergo resection with similar disease-free survival and OS at 1 and 3 years. 25 A meta-analysis of PVE by Wajswol et al similarly demonstrated a relative FLR hypertrophy rate of 49.4% with 75.9% of patients proceeding to resection. 26
Data for using Y90 radioembolization to induce hypertrophy is less robust comprising retrospective series of patients treated with lobar Y90 not necessarily with the intent of optimizing for resection. In their retrospective matched pair analysis, Garlipp et al showed that PVE resulted in significantly greater hypertrophy than TARE (61.5 vs. 29%) within a shorter time frame (33 vs. 46 days). 27 The Y90 cohort had more prior intervention possibly explaining the difference. Vouche et al reported a post Y90 hypertrophy of 45% at 9 months but only 7% hypertrophy at 1 month. 28 Similarly, Teo et al reported 26 to 47% hypertrophy ranging from 44 days to 9 months following unilobar TARE with Y90. 29 In their systematic review, Birgin et al demonstrated that only 30% of patients receiving lobar Y90 underwent resection following Y90. 30 Given the available data, our practice is to perform PVE for patients who will undergo resection in the near future and Y90 for patients who may or may not undergo resection. Some institutions practice the concept of “test of time” prior to resection where patients at high risk of recurrence undergo watchful waiting or locoregional therapy prior to planned resection 3 to 6 months later. Those with aggressive tumor biology will progress and not undergo surgery, thereby avoiding the morbidity associated with surgery. At the center of this concept is the assumption that those who progress shortly following LRT would have progressed after early resection as well.
Given the functional reserve required to safely undergo resection, many early-stage HCC patients are not candidates for resection and are better served with parenchyma-preserving alternatives such as ablation or embolization.
Ablation
Ablation is a curative treatment option for patients with early-stage HCC who are not candidates for surgery. It is also an excellent choice as a bridge treatment for patients awaiting transplant. The ideal candidate is someone with tumors less than 3 cm located within liver parenchyma not in close proximity to large vascular or biliary structures. There is overwhelming evidence—including numerous randomized controlled trials (RCTs) and meta-analyses—supporting ablation, specifically RFA, as a curative option comparable to resection.
Multiple RCTs have demonstrated the benefit of RFA. Chen et al randomized 161 early-stage CP-A HCC patients with tumors less than 5 cm and ICG-R15 less than 30% to RFA or resection. 31 The ablation cohort required more treatments and had higher recurrence rates; however, the 4-year OS was not significantly different between the cohorts (65.9% for RFA and 64% for resection). Interestingly, 34 of the 71 patients randomized to ablation had tumors greater than 3 cm and would be considered suboptimal ablation candidates. Complete response was present in 62% of patients after initial ablation and 91.5% after repeat ablations. Similar results with comparable OS between surgical resection and ablation were discovered in numerous other RCTs. 31 32 33 34 35 The sole RCT showing statistically significant survival benefit of resection over ablation was performed by Huang et al. They randomized 230 patients with HCC within Milan criteria to RFA or resection. 32 In this study, 5-year OS was significantly better for resection (55% for ablation and 76% for resection, p < 0.01). In the surgical cohort, 16 additional lesions were identified and treated at the time of resection. Twenty-seven of 84 patients who underwent ablation had tumors larger than 3 cm, and tumors in suboptimal locations such as near large vessels, bowel, or diaphragm requiring artificial pleural effusion were not excluded. While critical structures such as bowel may be successfully hydrodissected away, the effect of heat sink is difficult to mitigate. Consensus from these trials is that the OS is likely not significantly different between ablation and resection for HCCs less than 3 cm, but ablation shows a higher recurrence rate. The higher local recurrence rate following ablation is postulated to be a result of satellite nodules or the presence of microvascular portal vein invasion not conspicuous on preoperative imaging. For this reason, at least a 5-mm ablation margin around the tumor is recommended. Because RFA relies on conduction of heat through ionic tissue, it is plagued by decreasing efficacy in tumors larger than 3 cm. Because the likelihood of complete ablation decreases with increasing tumor size and complete ablation correlates with survival, RFA has been supplanted by microwave ablation at many centers due to several theoretical advantages microwave has over RFA. 36 37 38
In contrast to RFA, microwave ablation results in volumetric heating and is thereby not as restricted by high impedance of charred tissue. Other benefits include less susceptibility to heat sink and higher intertumoral temperatures. Poggi et al demonstrated 100% CR for HCC < 3 cm, 90% CR for HCC 3 to 5 cm, and 69% CR for HCC > 5 cm. 39 Despite these theoretical advantages, clinical outcomes have been similar to RFA. 40 Liang et al demonstrated 5-year OS of 51% in a series of 288 patients. 41 The only RCT to compare microwave ablation to hepatic resection as well as a meta-analysis by Zang et al demonstrated no difference in OS at 3 years. 42 43 The most recent meta-analysis with long-term outcomes comparing microwave to resection demonstrated a significantly better OS with resection but is biased by retrospective studies with sicker patients in the ablation arms. The microwave treatment arm also included tumors that were unresectable because of comorbidities or poor liver function. 43 Predictive factors effecting long-term survival following microwave ablation include size of tumor, number of tumors, and underlying liver function. Unlike resection, patients with ALBI grade 2 or CP-B scores can be safely ablated without significant risk of postoperative liver failure and death. 44 45 46
Ablation is avoided as a bridge to transplant at some centers due to concern for tract seeding and aggressive tumor recurrence. The original article on the matter published by Llovet et al reported a 12.5% tract seeding rate, but this was attributed to a combination of technique and a large portion of their patients having subcapsular lesions. 47 Numerous studies have since shown that the rate is closer to 0 to 2.5% and more common in subcapsular lesions that have been previously biopsied. The incidence of tract seeding can be minimized by avoiding direct puncture of the lesion, utilizing the no-touch ablation technique, and by ablating the tract. 48 49 50 51 Aggressive tumor recurrence is less well understood and may be a sequela of ablation or represent the natural progression of an aggressive tumor biology. Reported incidence ranges from 0.7 to 8% and is associated with larger tumors, tumors with infiltrative margins, increased AFP, and location near portal veins. Techniques to minimize the incidence of aggressive tumor recurrence have not been validated, but we prefer to pretreat with TACE and include a generous ablation margin when possible ( Fig. 1 ). Other suggested steps are utilizing the no-touch technique and ablating for longer period of time at a lower wattage. 52 Combining TACE and ablation has advantages in both small and larger tumors. In HCCs less than 3 cm in size that are not well seen by ultrasound or noncontrast CT, conventional TACE (c-TACE) can be used to stain the tumor increasing its conspicuity during the ablation. For larger tumors, TACE is believed to help increase the area of coagulative necrosis and help treat concurrent satellite tumors. A meta-analysis by Ni et al showed that combination of TACE and RFA improved OS in patients with intermediate (between 3 and 5 cm) and large (>5 cm) tumors. 53
Fig. 1.
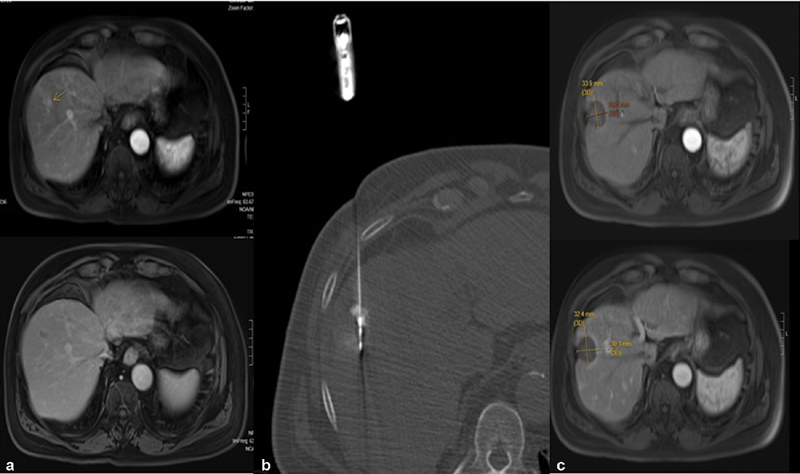
A 76-year-old male with hepatitis B, Child–Pugh score 6, MELD 10, ECOG 0 with history of prior transarterial chemoembolization/ablations presented with new 17-mm LI-RADS 5 lesion in segment 8 of the liver. ( a ) Pretreatment arterial phase imaging shows enhancing lesions. ( b ) Delayed phase imaging shows washout. ( c ) CT-guided microwave ablation with needle traversing the center of the tumor which has been stained with lipiodol. One-year post–microwave ablation, arterial phase imaging ( d ) and venous ( e ) phase imaging demonstrate no viable tumor.
Other ablation modalities including cryoablation, ethanol ablation, and irreversible electroporation are less commonly used except in niche situations and have been supplanted at many U.S. institutions by Y90 radiation segmentectomy.
Transarterial Radioembolization
While Y90 has traditionally been used as salvage therapy in patients for whom other therapies have failed, there is a growing body of evidence to support the use of Y90 as first-line at multiple stages of HCC and potentially curative therapy in early-stage disease when radiation segmentectomy doses are applied. In 2014, an article by Vouche et al demonstrated that in treatment-naive patients with a solitary unresectable tumor of 5 cm or less not amenable to RFA, the median time to progression was 33.1 months with complete, partial, and stable disease responses achieved in 47, 39, and 12% of patients by mRECIST criteria. 54 Pathologic correlation of patients who went on to transplant demonstrated >90% pathologic tumor necrosis. Subanalysis of the data revealed that complete necrosis was significantly more likely when the radiation dose to the intended segment exceeded 190 Gy. A phase II trial from the Milan group showing a higher rate of CR and PR when tumor dosing exceeds 500 Gy supports the concept of greater response when “hyper” or segmental dosing to a specific segment is achieved. 55 Padia et al found that complete response was achieved in 95% of patients when segmentectomy dosing was applied with minimal toxicity (no grade 3 or 4 toxicities were reported). 56 Padia et al went on to demonstrate superior complete response rate of Y90 segmentectomy dosing when compared with TACE using mRECIST criteria. In that study, CR of 84 and 58% for TARE and TACE, respectively, was reported. 57 A propensity score matched retrospective study by Biederman et al in 2017 demonstrated that for treatment-naive patients with tumors less than 3 cm in diameter there was no difference in overall CR rate, TTP, and OS. 58 A retrospective study by Lewandowski et al in 2018 of 70 early-stage unresectable HCC patients who underwent radiation segmentectomy demonstrated a median OS of 6.7 years with survival probability of 98, 66, and 57% at 1, 3, and 5 years, respectively. Furthermore, OS probability rates at 1, 3, and 5 years were 100, 82, and 75%, respectively, in patients with baseline tumors less than 3 cm in size. 59 These data are comparable to other curative intent therapies.
As a result of these data, many centers have adopted radioembolization as part of the primary treatment algorithm for patients with early-stage HCC defined by the Barcelona Clinic Liver Cancer (BCLC) and have led to its adoption as first-line therapy in unresectable early-stage HCC ( Fig. 2 ).
Fig. 2.
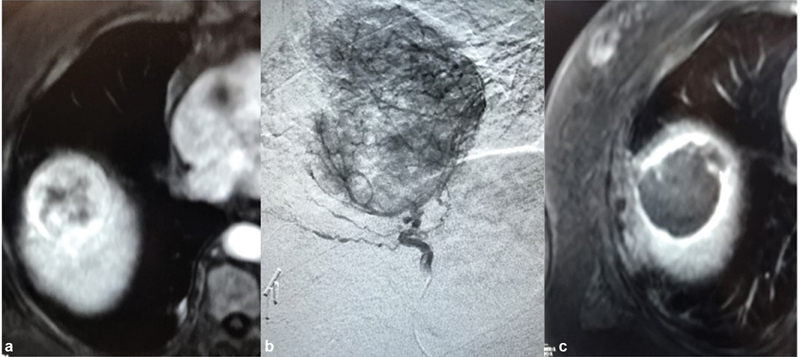
Transarterial radioembolization of early-stage hepatocellular carcinoma. ( a ) Arterial phase contrast-enhanced MRI of the liver shows an enhancing mass in segment 8 measuring 5.5 cm, abutting the diaphragm, and surrounded by lung parenchyma in a 75-year-old man with alcoholic cirrhosis. ( b ) The same lesion demonstrated on angiography with single feeding arterial vessel. ( c ) Arterial phase imaging from a contrast-enhanced MRI 6 months posttreatment without any evidence of residual enhancement to suggest viable tumor.
Transarterial Chemoembolization
Transarterial chemoembolization is a well-established treatment for intermediate-stage HCC and is generally considered the first-line noncurative therapy for many patients being bridged to transplant. Two widely accepted techniques for TACE are available. c-TACE is the administration of an emulsion of chemotherapeutic agent with lipiodol followed by an embolic agent to obtain a synergistic effect of drug cytotoxic activity and ischemia. With drug-eluting bead TACE (DEB-TACE), the cytotoxic agent is loaded directly onto embolic agent resulting in an increased local concentration of the chemotherapeutic agent 60 61 62 while simultaneously reducing systemic side effects. In 2009, the PRECISION V study compared c-TACE to DEB-TACE but failed to show statistical superiority of DEB-TACE to c-TACE in all categories including complete response, objective response, and disease control. The short follow-up period prevented assessment of OS and time to progression. 63 The PRECISION ITALIA trial, another RCT also comparing c-TACE to DEB-TACE, was prematurely stopped, as no significant difference in median TTP nor in 2-year survival rate could be demonstrated. 64 Multiple meta-analyses have been published on the topic with mixed conclusions and the topic continues to be debated and both techniques are regularly used. Based on this data, we prefer c-TACE for tumors less than 3 cm and for combination treatment with ablation due to enhanced visibility from the lipiodol staining and DEB-TACE for larger tumors (>3 cm) to limit systemic side effects.
As this section is focused on the treatment of early-stage disease, it is important to discuss the concept of treatment stage migration strategy which, in essence, states that all stages of HCC require individually tailored treatment. Therefore, when LT, resection, and ablation are not possible in the BCLC stage A population, TACE becomes a viable treatment option. This concept is supported by data from Burrel et al demonstrating a median OS of 54.2 months in BCLC stage A patients treated with subselective DEB-TACE. 65 TACE has also been proven to be a beneficial adjunctive for ablative candidates, where combination TACE–ablation therapy demonstrates decreased long-term local tumor progression rates for BCLC stage A patients versus TACE alone or RFA alone, which was discussed previously in the “Ablation” section.
Summary
Locoregional therapy plays a critical role as both adjunctive and primary therapy and the modality of treatment should be tailored to the individual patient to optimize outcomes. Our algorithm favors transplant for patients within Milan criteria and decompensated liver disease. These patients are bridged with TACE, ablation, or radioembolization depending on number, distribution, size, and location of the lesions. Given the data supporting longer time to progression for ablation and radioembolization, we favor these techniques in patients with longer expected wait times. TACE is favored as a bridging technique in patients not suitable for ablation or with limited hepatic reserve (CP-B9 and CP-C), therefore unsuitable for TARE.
Resection is favored for patients within Milan criteria or with a single large lesion and compensated liver disease, provided there is adequate FLR. If adequate FLR is not present, PVE or radiation lobectomy is used to promote FLR hypertrophy. Radiation lobectomy is favored in patients when there is concern for aggressive disease to allow for local tumor control during a biological test of time prior to resection.
For patients who are neither resection nor transplant candidates, our algorithm is as follows. Ablation is reserved for patients with tumors less than 3 cm in size. If the tumor is subscapular or near a large vessel or critical structure, we prefer TARE using a segmentectomy technique. TACE is reserved for patients who are not candidates for any of the other treatment modalities mentioned.
As exemplified by our algorithm, the effective management of early-stage HCC is ideally managed through a multidisciplinary approach with interventional radiology, hepatology, transplant surgery, and surgical oncology ( Fig. 3 ).
Fig. 3.
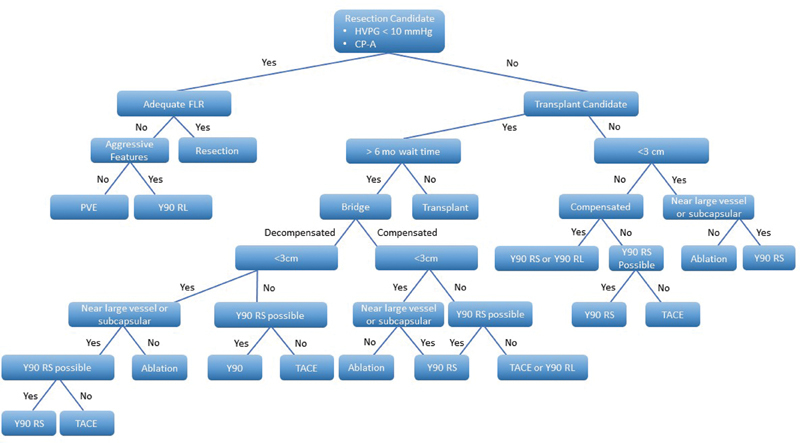
Sample treatment algorithm for the treatment of early-stage hepatocellular carcinoma. Aggressive features are defined as ill-defined margins and satellite nodules. Compensated liver disease: CPA-B8, ALBI 1. FLR, future liver remnant; PVE, portal vein embolization; RL, radiation lobectomy; RS, radiation segmentectomy; TACE, transarterial chemoembolization; TARE, transarterial radioembolization.
Footnotes
Conflict of Interest None declared.
References
- 1.Llovet J M, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19(03):329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 2.Mazzaferro V, Regalia E, Doci R. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 3.Mazzaferro V, Bhoori S, Sposito C. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011;17 02:S44–S57. doi: 10.1002/lt.22365. [DOI] [PubMed] [Google Scholar]
- 4.Yao F Y, Ferrell L, Bass N M. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33(06):1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 5.Yao F Y, Xiao L, Bass N M, Kerlan R, Ascher N L, Roberts J P. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7(11):2587–2596. doi: 10.1111/j.1600-6143.2007.01965.x. [DOI] [PubMed] [Google Scholar]
- 6.Parikh N D, Singal A G. Model for end-stage liver disease exception points for treatment-responsive hepatocellular carcinoma. Clin Liver Dis (Hoboken) 2016;7(05):97–100. doi: 10.1002/cld.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heimbach J K, Hirose R, Stock P G. Delayed hepatocellular carcinoma model for end-stage liver disease exception score improves disparity in access to liver transplant in the United States. Hepatology. 2015;61(05):1643–1650. doi: 10.1002/hep.27704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta N, Dodge J L, Goel A, Roberts J P, Hirose R, Yao F Y. Identification of liver transplant candidates with hepatocellular carcinoma and a very low dropout risk: implications for the current organ allocation policy. Liver Transpl. 2013;19(12):1343–1353. doi: 10.1002/lt.23753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao F Y, Mehta N, Flemming J. Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology. 2015;61(06):1968–1977. doi: 10.1002/hep.27752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oligane H C, Xing M, Kim H S. Effect of bridging local-regional therapy on recurrence of hepatocellular carcinoma and survival after orthotopic liver transplantation. Radiology. 2017;282(03):869–879. doi: 10.1148/radiol.2016160288. [DOI] [PubMed] [Google Scholar]
- 11.Gabr A, Kulik L, Mouli S.2020), Liver Transplantation Following Yttrium‐90 Radioembolization: 15‐year Experience in 207‐Patient Cohort. Hepatology. Accepted Author Manuscript. [DOI] [PubMed] [Google Scholar]
- 12.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu ; European Association for the Study of the Liver . EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(01):182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Park J W, Chen M, Colombo M. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35(09):2155–2166. doi: 10.1111/liv.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang M D, Li C, Li J. Long-term survival outcomes after liver resection for binodular hepatocellular carcinoma: a multicenter cohort study. Oncologist. 2019;24(08):e730–e739. doi: 10.1634/theoncologist.2018-0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsilimigras D I, Bagante F, Moris D. Defining the chance of cure after resection for hepatocellular carcinoma within and beyond the Barcelona Clinic Liver Cancer guidelines: a multi-institutional analysis of 1,010 patients. Surgery. 2019;166(06):967–974. doi: 10.1016/j.surg.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Yip V S, Poon R T, Chok K S. Comparison of survival outcomes between right posterior sectionectomy and right hepatectomy for hepatocellular carcinoma in cirrhotic liver: a single-centre experience. World J Surg. 2015;39(11):2764–2770. doi: 10.1007/s00268-015-3146-x. [DOI] [PubMed] [Google Scholar]
- 17.Lee S Y, Sadot E, Chou J F. Central hepatectomy versus extended hepatectomy for liver malignancy: a matched cohort comparison. HPB (Oxford) 2015;17(11):1025–1032. doi: 10.1111/hpb.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kishi Y, Abdalla E K, Chun Y S. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg. 2009;250(04):540–548. doi: 10.1097/SLA.0b013e3181b674df. [DOI] [PubMed] [Google Scholar]
- 19.Shoup M, Gonen M, D'Angelica M. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7(03):325–330. doi: 10.1016/s1091-255x(02)00370-0. [DOI] [PubMed] [Google Scholar]
- 20.Boleslawski E, Petrovai G, Truant S. Hepatic venous pressure gradient in the assessment of portal hypertension before liver resection in patients with cirrhosis. Br J Surg. 2012;99(06):855–863. doi: 10.1002/bjs.8753. [DOI] [PubMed] [Google Scholar]
- 21.Teh S H, Christein J, Donohue J.Hepatic resection of hepatocellular carcinoma in patients with cirrhosis: model of end-stage liver disease (MELD) score predicts perioperative mortality J Gastrointest Surg 20059091207–1215., discussion 1215 [DOI] [PubMed] [Google Scholar]
- 22.Cucchetti A, Ercolani G, Vivarelli M. Impact of model for end-stage liver disease (MELD) score on prognosis after hepatectomy for hepatocellular carcinoma on cirrhosis. Liver Transpl. 2006;12(06):966–971. doi: 10.1002/lt.20761. [DOI] [PubMed] [Google Scholar]
- 23.Abulkhir A, Limongelli P, Healey A J. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247(01):49–57. doi: 10.1097/SLA.0b013e31815f6e5b. [DOI] [PubMed] [Google Scholar]
- 24.Palavecino M, Chun Y S, Madoff D C. Major hepatic resection for hepatocellular carcinoma with or without portal vein embolization: perioperative outcome and survival. Surgery. 2009;145(04):399–405. doi: 10.1016/j.surg.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Marti J, Giacca M, Alshebeeb K. Analysis of preoperative portal vein embolization outcomes in patients with hepatocellular carcinoma: a single-center experience. J Vasc Interv Radiol. 2018;29(07):920–926. doi: 10.1016/j.jvir.2018.01.780. [DOI] [PubMed] [Google Scholar]
- 26.Wajswol E, Jazmati T, Contractor S, Kumar A. Portal vein embolization utilizing N-Butyl cyanoacrylate for contralateral lobe hypertrophy prior to liver resection: a systematic review and meta-analysis. Cardiovasc Intervent Radiol. 2018;41(09):1302–1312. doi: 10.1007/s00270-018-1964-6. [DOI] [PubMed] [Google Scholar]
- 27.Garlipp B, de Baere T, Damm R. Left-liver hypertrophy after therapeutic right-liver radioembolization is substantial but less than after portal vein embolization. Hepatology. 2014;59(05):1864–1873. doi: 10.1002/hep.26947. [DOI] [PubMed] [Google Scholar]
- 28.Vouche M, Lewandowski R J, Atassi R. Radiation lobectomy: time-dependent analysis of future liver remnant volume in unresectable liver cancer as a bridge to resection. J Hepatol. 2013;59(05):1029–1036. doi: 10.1016/j.jhep.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teo J Y, Allen J C, Jr, Ng D C. A systematic review of contralateral liver lobe hypertrophy after unilobar selective internal radiation therapy with Y90. HPB (Oxford) 2016;18(01):7–12. doi: 10.1016/j.hpb.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Birgin E, Rasbach E, Seyfried S. Contralateral liver hypertrophy and oncological outcome following radioembolization with 90 Y-microspheres: a systematic review . Cancers (Basel) 2020;12(02):294. doi: 10.3390/cancers12020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen M S, Li J Q, Zheng Y. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243(03):321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang J, Yan L, Cheng Z. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252(06):903–912. doi: 10.1097/SLA.0b013e3181efc656. [DOI] [PubMed] [Google Scholar]
- 33.Feng K, Yan J, Li X. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57(04):794–802. doi: 10.1016/j.jhep.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Fang Y, Chen W, Liang X. Comparison of long-term effectiveness and complications of radiofrequency ablation with hepatectomy for small hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29(01):193–200. doi: 10.1111/jgh.12441. [DOI] [PubMed] [Google Scholar]
- 35.Lee H W, Lee J M, Yoon J H. A prospective randomized study comparing radiofrequency ablation and hepatic resection for hepatocellular carcinoma. Ann Surg Treat Res. 2018;94(02):74–82. doi: 10.4174/astr.2018.94.2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Livraghi T, Goldberg S N, Lazzaroni S. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000;214(03):761–768. doi: 10.1148/radiology.214.3.r00mr02761. [DOI] [PubMed] [Google Scholar]
- 37.Xu H X, Lu M D, Xie X Y. Prognostic factors for long-term outcome after percutaneous thermal ablation for hepatocellular carcinoma: a survival analysis of 137 consecutive patients. Clin Radiol. 2005;60(09):1018–1025. doi: 10.1016/j.crad.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Unità Interdipartimentale Neoplasie Epatiche (U.I.N.E) Group . Cammà C, Di Marco V, Orlando A. Treatment of hepatocellular carcinoma in compensated cirrhosis with radio-frequency thermal ablation (RFTA): a prospective study. J Hepatol. 2005;42(04):535–540. doi: 10.1016/j.jhep.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 39.Poggi G, Montagna B, DI Cesare P. Microwave ablation of hepatocellular carcinoma using a new percutaneous device: preliminary results. Anticancer Res. 2013;33(03):1221–1227. [PubMed] [Google Scholar]
- 40.Huo Y R, Eslick G D. Microwave ablation compared to radiofrequency ablation for hepatic lesions: a meta-analysis. J Vasc Interv Radiol. 2015;26(08):1139–114600. doi: 10.1016/j.jvir.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 41.Liang P, Dong B, Yu X. Prognostic factors for survival in patients with hepatocellular carcinoma after percutaneous microwave ablation. Radiology. 2005;235(01):299–307. doi: 10.1148/radiol.2351031944. [DOI] [PubMed] [Google Scholar]
- 42.Xu J, Zhao Y. Comparison of percutaneous microwave ablation and laparoscopic resection in the prognosis of liver cancer. Int J Clin Exp Pathol. 2015;8(09):11665–11669. [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang M, Ma H, Zhang J, He L, Ye X, Li X. Comparison of microwave ablation and hepatic resection for hepatocellular carcinoma: a meta-analysis. OncoTargets Ther. 2017;10:4829–4839. doi: 10.2147/OTT.S141968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glassberg M B, Ghosh S, Clymer J W, Wright G WJ, Ferko N, Amaral J F. Microwave ablation compared with hepatic resection for the treatment of hepatocellular carcinoma and liver metastases: a systematic review and meta-analysis. World J Surg Oncol. 2019;17(01):98. doi: 10.1186/s12957-019-1632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chong C CN, Lee K F, Chu C M. Microwave ablation provides better survival than liver resection for hepatocellular carcinoma in patients with borderline liver function: application of ALBI score to patient selection. HPB (Oxford) 2018;20(06):546–554. doi: 10.1016/j.hpb.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 46.Oh I S, Sinn D H, Kang T W. Liver function assessment using albumin-bilirubin grade for patients with very early-stage hepatocellular carcinoma treated with radiofrequency ablation. Dig Dis Sci. 2017;62(11):3235–3242. doi: 10.1007/s10620-017-4775-8. [DOI] [PubMed] [Google Scholar]
- 47.Wakuta A, Nouso K, Kariyama K. Radiofrequency ablation for the treatment of hepatocellular carcinoma with decompensated cirrhosis. Oncology. 2011;81(01):39–44. doi: 10.1159/000331411. [DOI] [PubMed] [Google Scholar]
- 48.Barcelona Clínic Liver Cancer (BCLC) Group . Llovet J M, Vilana R, Brú C. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001;33(05):1124–1129. doi: 10.1053/jhep.2001.24233. [DOI] [PubMed] [Google Scholar]
- 49.Jaskolka J D, Asch M R, Kachura J R. Needle tract seeding after radiofrequency ablation of hepatic tumors. J Vasc Interv Radiol. 2005;16(04):485–491. doi: 10.1097/01.RVI.0000151141.09597.5F. [DOI] [PubMed] [Google Scholar]
- 50.Yu J, Liang P, Yu X L, Cheng Z G, Han Z Y, Dong B W. Needle track seeding after percutaneous microwave ablation of malignant liver tumors under ultrasound guidance: analysis of 14-year experience with 1462 patients at a single center. Eur J Radiol. 2012;81(10):2495–2499. doi: 10.1016/j.ejrad.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 51.Stigliano R, Marelli L, Yu D, Davies N, Patch D, Burroughs A K. Seeding following percutaneous diagnostic and therapeutic approaches for hepatocellular carcinoma. What is the risk and the outcome? Seeding risk for percutaneous approach of HCC. Cancer Treat Rev. 2007;33(05):437–447. doi: 10.1016/j.ctrv.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 52.Kei S K, Rhim H, Choi D, Lee W J, Lim H K, Kim Y S. Local tumor progression after radiofrequency ablation of liver tumors: analysis of morphologic pattern and site of recurrence. AJR Am J Roentgenol. 2008;190(06):1544–1551. doi: 10.2214/AJR.07.2798. [DOI] [PubMed] [Google Scholar]
- 53.Ni J Y, Liu S S, Xu L F, Sun H L, Chen Y T. Meta-analysis of radiofrequency ablation in combination with transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2013;19(24):3872–3882. doi: 10.3748/wjg.v19.i24.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vouche M, Habib A, Ward T J. Unresectable solitary hepatocellular carcinoma not amenable to radiofrequency ablation: multicenter radiology-pathology correlation and survival of radiation segmentectomy. Hepatology. 2014;60(01):192–201. doi: 10.1002/hep.27057. [DOI] [PubMed] [Google Scholar]
- 55.Mazzaferro V, Sposito C, Bhoori S. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology. 2013;57(05):1826–1837. doi: 10.1002/hep.26014. [DOI] [PubMed] [Google Scholar]
- 56.Padia S A, Kwan S W, Roudsari B, Monsky W L, Coveler A, Harris W P. Superselective yttrium-90 radioembolization for hepatocellular carcinoma yields high response rates with minimal toxicity. J Vasc Interv Radiol. 2014;25(07):1067–1073. doi: 10.1016/j.jvir.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 57.Padia S A, Johnson G E, Horton K J. Segmental yttrium-90 radioembolization versus segmental chemoembolization for localized hepatocellular carcinoma: results of a single-center, retrospective, propensity score-matched study. J Vasc Interv Radiol. 2017;28(06):777–7850. doi: 10.1016/j.jvir.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 58.Biederman D M, Titano J J, Bishay V L. Radiation segmentectomy versus TACE combined with microwave ablation for unresectable solitary hepatocellular carcinoma up to 3 cm: a propensity score matching study. Radiology. 2017;283(03):895–905. doi: 10.1148/radiol.2016160718. [DOI] [PubMed] [Google Scholar]
- 59.Lewandowski R J, Gabr A, Abouchaleh N. Radiation segmentectomy: potential curative therapy for early hepatocellular carcinoma. Radiology. 2018;287(03):1050–1058. doi: 10.1148/radiol.2018171768. [DOI] [PubMed] [Google Scholar]
- 60.Varela M, Real M I, Burrel M. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46(03):474–481. doi: 10.1016/j.jhep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 61.Hong K, Khwaja A, Liapi E, Torbenson M S, Georgiades C S, Geschwind J F. New intra-arterial drug delivery system for the treatment of liver cancer: preclinical assessment in a rabbit model of liver cancer. Clin Cancer Res. 2006;12(08):2563–2567. doi: 10.1158/1078-0432.CCR-05-2225. [DOI] [PubMed] [Google Scholar]
- 62.Lewis A L, Taylor R R, Hall B, Gonzalez M V, Willis S L, Stratford P W. Pharmacokinetic and safety study of doxorubicin-eluting beads in a porcine model of hepatic arterial embolization. J Vasc Interv Radiol. 2006;17(08):1335–1343. doi: 10.1097/01.RVI.0000228416.21560.7F. [DOI] [PubMed] [Google Scholar]
- 63.PRECISION V Investigators . Lammer J, Malagari K, Vogl T. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33(01):41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.PRECISION ITALIA Study Group . Golfieri R, Giampalma E, Renzulli M. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014;111(02):255–264. doi: 10.1038/bjc.2014.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burrel M, Reig M, Forner A. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using drug eluting beads. Implications for clinical practice and trial design. J Hepatol. 2012;56(06):1330–1335. doi: 10.1016/j.jhep.2012.01.008. [DOI] [PubMed] [Google Scholar]


