Abstract
Intermediate-stage Hepatocellular Carcinoma (HCC) represents a wide range of disease burden. Patients with different levels of liver function, tumor size, and number of lesions may all have intermediate-stage disease according to the Barcelona Clinic Liver Cancer (BCLC) staging system. Several minimally invasive image-guided locoregional therapies are available for the treatment of intermediate-stage HCC, including conventional transarterial chemoembolization (cTACE), drug-eluting bead TACE (DEB-TACE), yttrium-90 radioembolization (Y-90 RE), thermal ablation, bland embolization, and combination therapy. Available clinical evidence points to cTACE as the current gold standard for the locoregional treatment of intermediate-stage HCC. DEB-TACE is at best non-inferior to cTACE in terms of survival benefit. Y-90 RE is a maturing therapy, and some institutions have adopted it as first-line therapy for intermediate-stage HCC. Thermal ablation combined with TACE may be used in select patients, while bland embolization has only limited evidence for its use. The combination of locoregional therapy with VEGF inhibitors or immune checkpoint inhibitors has also been explored. This article will examine in detail the clinical evidence supporting available locoregional treatment options for intermediate-stage HCC.
Keywords: transarterial chemoembolization, radioembolization, ablation, hepatocellular carcinoma, Barcelona Clinic Liver Cancer
Intermediate-stage hepatocellular carcinoma (HCC) is the broadest class of the Barcelona Clinic Liver Cancer (BCLC) staging system in terms of variability of liver function, tumor size, and number of lesions. With respect to liver function, patients with both Child–Pugh (CP) classes A and B can be defined as BCLC stage B. 1 With respect to the number and size of lesions, patients with either three or more tumors regardless of size, two to three tumors greater than 3 cm in diameter, or a single unresectable tumor greater than 5 cm in diameter may qualify for BCLC stage B 1 2 as long as no macrovascular invasion is noted and clinical performance status is not impacted by the disease.
Despite being one of the most commonly accepted staging systems for HCC, the BCLC criteria are often criticized for lack of specificity of therapeutic recommendations, especially in BCLC stage B. 1 3 4 5 BCLC has also been critiqued for its development using primarily western patient cohorts with nonalcoholic steatohepatitis and hepatitis C as primary disease etiologies, 6 whereas most of HCC's global burden is seen in regions where hepatitis B is the predominant cause (mostly in eastern and southern Asia). 7 Alternative staging systems, such as the Hong Kong Liver Cancer (HKLC) staging system, have been proposed to offer better substratification of patients with what is commonly described as intermediate-stage HCC. 1 8 Nevertheless, the current BCLC system remains popular and is well understood by physicians worldwide.
With this context in mind, conventional (oil-based) transarterial chemoembolization (cTACE) is currently the only therapy with level 1A evidence demonstrating survival benefit over best supportive care in patients with BCLC stage B. 9 10 11 12 Beyond cTACE as the current gold standard in this group of patients, there are several different minimally invasive image-guided locoregional therapies available including drug-eluting bead TACE (DEB-TACE), Yttrium-90 radioembolization (Y-90 RE), thermal ablation, bland embolization, and combination therapy with systemic molecular targeted therapy with tyrosine kinase inhibitors (TKIs) or immune checkpoint inhibitors.
This review will provide an overview of currently available therapy options for intermediate-stage HCC and discuss supporting evidence. An overview of TACE in general will be given first, followed by a comparison of cTACE with DEB-TACE. Newer locoregional therapies, such as Y-90 RE, will also be explored within this context. This review will also discuss various combination therapies, including TACE plus thermal ablation, TACE plus systemic therapy with TKIs such as sorafenib, and TACE plus targeted immune checkpoint inhibitors, for the treatment of intermediate-stage HCC.
Transarterial Chemoembolization
Transarterial chemoembolization is the most well-established treatment for patients with BCLC stage B HCC, and it is recommended for this group of patients by both the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD), the latter specifically mentioning cTACE as the standard of care. 2 13 Globally, TACE is the most common first-line treatment for patients with BCLC stage B HCC. The BRIDGE study by Park et al analyzed a large international cohort involving over 18,000 HCC patients at 42 sites worldwide to better understand the real-world management of HCC. 14 The BRIDGE study investigators found that among patients with BCLC stage B HCC, approximately 60% underwent TACE as first-line therapy. While surgical resection can theoretically be offered to select patients with intermediate-stage HCC and multifocal disease, 15 concerns over dramatic reduction in functional liver volume and liver failure in patients that have compensated liver cirrhosis at baseline mostly preclude this approach for the vast majority of candidates, especially those with underlying HCV cirrhosis. Surgical resection is more commonly offered for intermediate-stage HCC in Asian countries, and the HKLC staging system demonstrated some benefits of a more aggressive surgical approach. 15 In fact, the Asian Pacific Association for the Study of the Liver (APASL), 16 Korean Liver Cancer Study Group (KLCSG), 17 and Japan Society of Hepatology (JSH) 18 recommend this treatment in specific circumstances when sufficient hepatic reserve is expected postoperatively. Therefore, resection accounts for slightly less than 20% of first-line therapy for BCLC stage B HCC worldwide. 14
From a technical perspective, TACE is a catheter-based, image-guided, minimally invasive therapy. Angiographic imaging allows physicians to localize HCC by visualizing a tumor blush, as contrast will accumulate in the hypervascular lesion. Cone-beam computed tomography (CBCT) is a cross-sectional enhancement to traditional angiographic imaging which can acquire three-dimensional CT images via the use of a flat-panel detector mounted on a c-arm. 19 CBCT offers a high spatial resolution and detailed mapping of vascular anatomy and arterial tumor supply, allowing for the interventional radiologist to identify tumors, detect feeding arteries, navigate complex variants of anatomy, plan treatment delivery, and assess therapy outcome with a high degree of precision. CBCT can provide diagnostic information which is comparable to conventional pretreatment CT or magnetic resonance images, 20 21 22 and it is superior to digital subtraction angiography (DSA) in the detection of liver tumors. 23 24 25 26 Intraprocedural CBCT can also be used to predict tumor response to the TACE procedure, 27 potentially optimizing follow-up treatment plans for patients. Studies have also shown improved survival rates in patients who undergo TACE using systems that are able to provide three-dimensional vascular imaging, 28 29 making it a necessary and indispensable standard of care to achieve higher response rates and better outcomes ( Fig. 1 ).
Fig. 1.
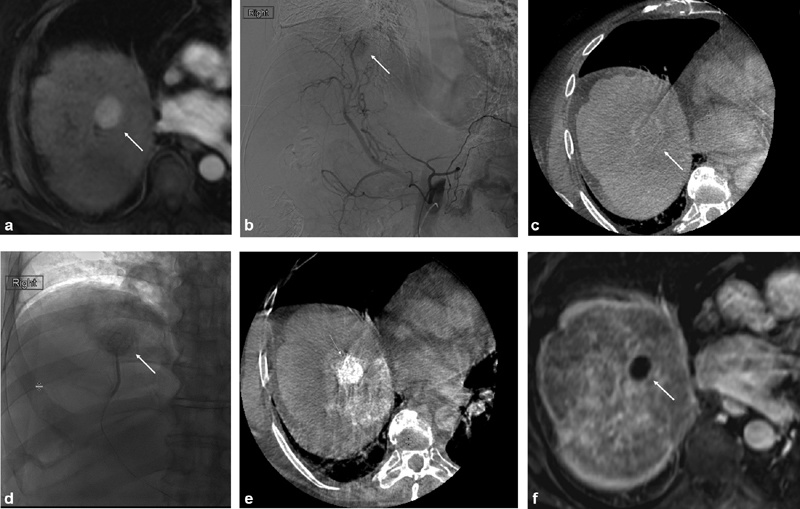
Imaging of conventional transarterial chemoembolization treatment with intraprocedural cone-beam computed tomography (CBCT) guidance. Pretreatment magnetic resonance imaging (MRI) reveals an arterially hypervascular tumor in segment 8 of the liver (white arrow) ( a ). Angiographyrevealed tumor blush in the expected location (white arrow) ( b ). Intra procedural CBCT was performed, revealing washout on the delayed imaging phase (white arrow) ( c ). The feeding vessel to the tumor was selected and embolized using doxorubicin emulsified with Lipiodol followed by polyvinyl alcohol particles. Preferential Lipiodol uptake by the tumor can be noted on both angiography (white arrow) ( d ) and CBCT ( e ). Follow-up MRI 1 month after the procedure demonstrates complete response with absence of any residual contrast enhancement (white arrow) ( f ).
Conventional Transarterial Chemoembolization
Conventional TACE is the most well-established TACE modality, with level 1A evidence supporting its use. 11 12 In cTACE, Lipiodol (Guerbet, Villepinte, France) is mixed with a chemotherapeutic agent such as doxorubicin, mitomycin, or cisplatin. Lipiodol is composed of di-iodinated ethyl esters of fatty acids derived from poppy seeds, 30 allowing for emulsion with the chosen chemotherapeutic agent, ideally in a 1:2 ratio, resulting in a water-in-oil emulsion. Lipiodol is both radiopaque and tumor seeking, which facilitates tumor visualization during cTACE, as the lesion will preferentially uptake and retain Lipiodol when compared with surrounding liver parenchyma. 31 Although the precise biological mechanisms underlying these observations have not yet been elucidated, possible explanations include lack of Kupffer cells and absent lysosomal function within tumors preventing Lipiodol removal. 31 32 33 34 Therefore, the degree of lipiodol uptake seen post-cTACE can be understood as a prognostic indicator for the severity of disease, as well as a potentially theranostic imaging biomarker for successful tumor targeting. 31 35 36 In animal models, zones of lipiodol deposition on post-cTACE CT have been shown to correlate well with areas of tumor necrosis on histologic examination. 31
An additional embolization step is traditionally performed after lipiodol delivery to stop blood flow to the tumor. A wide variety of embolic agents can be used for this purpose, although the superiority of any given material has not been firmly established. Embolic agents available for use with cTACE include Gelfoam (Pfizer, New York, NY), polyvinyl alcohol (PVA) particles, Embozene beads (Boston Scientific, Marlborough, MA), Embosphere beads (Merit Medical, South Jordan, UT), and degradable starch microspheres (DSMs). Gelfoam sponge is well-established in its use, as it was the embolic agent used in the earlier randomized controlled trials (RCTs) demonstrating the efficacy of TACE. 9 10 An analysis by Brown et al comparing the use of PVA and Gelfoam powder showed no difference in overall survival between the two groups, although treatment with PVA required fewer procedures to achieve response. 37 Lee et al provided some retrospective evidence that Embozene achieves greater reduction in tumor size (56.37 ± 25.91 mm vs. 43.44 ± 37.89 mm, p = 0.001) and a lower minor complication rate (12.9 vs. 28.9%, p = 0.04) compared with Embosphere. 38 Only limited data exist for the use of DSMs for intermediate-stage HCC. A single-arm study by Schicho et al evaluated the use of DSMs in 50 patients with intermediate-stage HCC and found an objective response rate of 44% and an adverse event rate of 52%. 39 Table 1 summarizes the physical properties of these various embolic agents.
Table 1. Physical properties of available embolic agents for use with cTACE.
| Embolic agent | Material | Size availability |
|---|---|---|
| Gelfoam | Porcine-derived gelatin | Varies based on preparation. Sheet form will typically produce particles ranging from 500 to 2,000 μm. Powdered form can reach sizes as low as 40 μm 82 |
| Contour PVA particles | PVA | 45–150, 150–250, 250–355, 355–500, 500–710, 710–100, and 1,000–1,180 μm. Manufacturing process may result in inconsistent sizing between individual particles 82 |
| Embozene | Hydrogel core with poly(bis[trifluoroethoxy]phosphazene) nanocoat | 30–50, 60–90, 75–125, 200–300, 350–450, 480–580, 650–750, 825–975, 1,025–1,175, and 1,225–1,375 μm |
| Embosphere | Acrylic smooth polymer matrix with bovine gelatin overcoat | 50–100, 40–120, 100–300, 300–500, 500–700, 700–900, and 900–1,200 μm |
| Bead Block | PVA hydrogel | 100–300, 300–500, 500–700, 700–900, and 900–1,200 μm |
| Degradable starch microsphere | Starch | Approximately 50 μm |
Abbreviations: cTACE, conventional transarterial chemoembolization; PVA, polyvinyl alcohol.
cTACE offers clear survival benefits to patients with intermediate-stage HCC when compared with best supportive care. Seminal RCTs by Llovet et al and Lo et al comparing cTACE versus conservative treatment for unresectable HCC found the relative risks of death to be 0.47 (95% confidence interval [CI]: 0.25–0.91) and 0.49 (95% CI: 0.29–0.81) respectively. 9 10 Llovet et al noted objective response according to WHO criteria was sustained for at least 6 months in 35% of cases. Not all early trials were positive, as Pelletier et al compared cTACE plus tamoxifen to tamoxifen alone and found that cTACE did not confer any difference in overall survival ( p = 0.77), although the investigators did note that cTACE afforded improved tumor response in this group. 40 A more recent retrospective analysis by Lewandowski et al contained 73 patients with BCLC stage B HCC who underwent cTACE. 41 For this specific subset of the study cohort, 55% had partial response according to EASL criteria. Median time to tumor progression (TTP) was 9.4 months, and median survival was 17.4 months.
Despite some heterogeneity in early trials, subsequent meta-analyses confirmed the beneficial effect of cTACE. Llovet and Bruix examined seven different RCTs including 545 total patients with unresectable HCC, and found the treatment reduced 2-year mortality with an odds ratio (OR) of 0.53 (95% CI: 0.32–0.89, p = 0.017). 11 Similarly, a meta-analysis by Cammà et al including 18 RCTs demonstrated that cTACE improved 2-year mortality compared with nonactive treatment with an OR of 0.54 (95% CI: 0.33–0.89, p = 0.015). 12 A recent systematic review of 101 articles by Lencioni et al examined 10,108 patients undergoing cTACE and found an objective response rate of 52.5% (multiple response criteria) and an overall survival at 1, 2, 3, and 5 years of 70.3, 51.8, 40.4, and 32.4%, respectively. 42 Overall, it must be noted that technological improvements such as the use of advanced microcatheter technology, superselective tumor targeting, modern image guidance systems (including CBCT), as well as better imaging follow-up routines with earlier retreatment of initial nonresponders allowed for dramatic survival improvements in more recent studies. This wealth of data and nearly 30 years of experience allowed for the development of expert guidelines recommending the cTACE as the standard of care. 43
Drug-Eluting Bead Transarterial Chemoembolization
Fifteen years ago, DEB technology emerged as an alternative to the oil-based technique of chemoembolotherapy. DEB-TACE is similar in principle to cTACE, as it involves arterial delivery of chemotherapy to a tumor facilitated by microvascular embolization. However, in contrast to cTACE, DEB-TACE uses solid particles designed to simultaneously achieve both the embolic effect and the locally confined delivery of chemotherapy to the tumor.
DEB-TACE was initially developed to address some of the limitations of cTACE by standardizing drug delivery, streamlining the TACE procedure, and reducing the passage of chemotherapy into systemic circulation. Specifically, uncontrolled separation of the drug-Lipiodol emulsion was thought to cause some drug leakage into systemic circulation, with various toxicities being attributed to this mechanistic flaw. Thus, DEBs were designed to allow for controlled doxorubicin release via the mechanism of ion exchange and gradual drug elution. 44 The use of DEBs also streamlined the TACE procedure, allowing for drug delivery and embolization in the same step. Initial in vitro studies with DEB-TACE supported the promise of the new technology, demonstrating higher intratumoral and lower systemic drug concentrations compared with cTACE with lobar injections. 45 46 47 These data raised expectations that higher tumor response rates and even better survival outcomes could be achieved using this more targeted drug delivery platform. However, after a decade of clinical trials, studies comparing both techniques failed to deliver unequivocal evidence favoring DEB-TACE in line with early expectations. While DEBs showed certain advantages over cTACE, particularly in terms of systemic drug concentration and in some cases tumor response, no definitive survival benefit has yet been demonstrated. DEB-TACE is therefore at best noninferior to cTACE. Lammer at al in the PRECISION V trial found that DEB-TACE may offer a slightly greater rate of tumor response compared with cTACE, but only in patients with bilobar disease or CP class B liver function. 48 However, the study's primary endpoint of objective tumor response at 6 months revealed no statistically significant difference between DEB-TACE and cTACE. Golfieri et al randomized 177 patients with HCC to receive either DEB-TACE or cTACE and did not find any differences between treatments with respect to tumor response, TTP, or survival rates at 1 and 2 years. 49 The results of this trial, however, should be extrapolated to intermediate-stage patients with caution. The study included patients with BCLC stages A, B, and C, and survival analysis was performed on the entire study cohort. With regard to systemic toxicity related to chemotherapy, the PRECISION V trial did find a significant reduction in doxorubicin-related side effects when DEB-TACE was used. However, while systemic toxicities may be less prevalent with DEB-TACE compared with cTACE, the tradeoff is that local complications may occur at a greater rate, which is especially true for cytotoxic biliary and ischemic injury. In retrospective analysis, various investigators found a higher incidence of complications such as biloma and liver infarction with DEB-TACE as compared with cTACE. 50 51 The results of available randomized clinical trials comparing DEB-TACE and cTACE are summarized in Table 2 .
Table 2. Summary of randomized control trials comparing DEB-TACE to cTACE in the treatment of HCC.
| Study | Patient cohort | No. of BCLC B | Results |
|---|---|---|---|
| Lammer et al 48 | 201 patients, 93 DEB-TACE and 108 cTACE | 69 | OR rate: 51.6 vs. 43.5% for DEB-TACE vs. cTACE ( p = 0.11) |
| Golfieri et al 49 | 177 patients, 89 DEB-TACE and 88 cTACE | 26 | Median TTP of 9 mo in both groups. 1- and 2-y survival rates equivalent between DEB-TACE and cTACE (86.2 and 56.8% vs. 83.5 and 55.4%, p = 0.949) |
| Sacco et al 83 | 67 patients, 33 DEB-TACE and 34 cTACE | 11 | 24-mo overall survival equivalent between DEB-TACE and cTACE (86.8 vs. 83.6%, p = 0.96) |
| van Malenstein et al 84 | 30 patients, 16 DEB-TACE and 14 cTACE | 19 | Rates of stable disease not significantly different between treatment DEB-TACE and cTACE (77 vs. 92%, p = 0.54) |
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; cTACE, conventional transarterial chemoembolization; DEB-TACE, drug-eluting bead TACE; HCC, hepatocellular carcinoma; TTP, time to tumor progression.
Note: The number of BCLC stage B patients included in each randomized controlled trial is noted.
Meta-analyses comparing DEB-TACE and cTACE have also yielded conflicting results. Facciorusso et al examined 12 studies including 1,449 total patients and did not find differences between the two treatments in terms of overall survival, complete response, or complication rates. 52 On the other hand, Zhou et al examined nine studies including 866 patients and concluded that DEB-TACE was superior in terms of complete response and overall survival. 53 A meta-analysis by Zhou et al was able to find evidence that DEB-TACE offers survival benefit to patients with HCC, but the authors cautioned that these results could only be inferred from non-RCTs, and not from prospective RCTs. 54 Given the inconclusive evidence, EASL guidelines do not offer a specific recommendation favoring either DEB-TACE or cTACE and defer to physician preference. 2 However, cTACE is the currently recommended therapy for intermediate-stage HCC as endorsed by the AASLD guidelines. 13 Whether cTACE or DEB-TACE should be performed may be dependent on the patient's overall liver function and tolerance to the side effects of systemic chemotherapy. The clearly documented function of Lipiodol as an imaging biomarker afforded by the radiopaque character of Lipiodol should also be considered, although newer generations of radiopaque DEBs have recently become available. 55 Several different products are available for DEB-TACE, including the DC/LC Bead (Boston Scientific, Marlborough, MA), Hepasphere (Merit Medical, South Jordan, UT), TANDEM (Varian, Palo Alto, CA), LifePearl (Terumo, Tokyo, Japan), and LUMI Bead (Boston Scientific). The physical properties of available DEBs are summarized in Table 3 .
Table 3. Physical properties of beads for use with DEB-TACE.
| DEB | Material | Size availability | Comments |
|---|---|---|---|
| DC/LC Bead | PVA microspheres with sulfonyl group attached | 100–300, 300–500, and 700–900 μm | Most well-established DEB, used in the PRECISION V and PRECISION ITALIA studies 48 49 |
| Hepasphere | Sodium acrylate with vinyl alcohol copolymer coat | 120–240, 200–400, 400–600, and 600–800 μm | Objective response rate of 67.5% reported according to mRECIST criteria. 85 The cited study includes patients with BCLC A, B, and C HCC |
| TANDEM | Negatively charged hydrogel core with perfluorinated polymer coating 86 | 30–50, 60–90, and 75–125 μm | Objective response rate of 63.8% reported according to mRECIST criteria. 87 The cited study did not report patient BCLC stages |
| LifePearl | Polyethylene glycol | 75–125, 150–250, and 350–450 μm | Objective response rate of 85.5% reported according to mRECIST criteria. 88 The cited study includes many patients with BCLC A HCC, so results should be extrapolated to intermediate stage with caution |
| LUMI Bead | PVA hydrogel with covalently bound iodine moiety. Radiopaque | 40–90 and 70–150 μm | Data supporting the use of LUMI Bead in routine clinical practice is currently limited. A small single-arm pilot study (NCT03474354) is currently ongoing 89 |
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; cTACE, conventional transarterial chemoembolization; DEB-TACE, drug-eluting bead TACE; HCC, hepatocellular carcinoma; PVA, polyvinyl alcohol; TTP, time to tumor progression.
In summary, despite initial promise, current evidence suggests that DEB-TACE is not superior to cTACE. There does not appear to be a discernable survival benefit when choosing DEB-TACE over cTACE, and concerns over significant systemic drug toxicity can be mitigated with superselective techniques and improved image guidance. Ultimately, cTACE continues to maintain the advantage over DEB-TACE primarily because of the wealth of established data and 30 years' worth of operator experience which support this technique as the standard of care.
Bland Embolization
In contrast to DEB-TACE, bland transarterial embolization (TAE) involves the use of embolic beads without any associated chemotherapy. Brown et al performed a randomized clinical trial with 101 patients allocated to treatment with either doxorubicin-loaded LC Beads or Bead Block (BB; Boston Scientific, Marlborough, MA). 56 This study found no differences in tumor response according to RECIST criteria (LC Bead 6.0% vs. BB 5.9%), median PFS (LC Bead 2.8 months vs. BB 6.2 months, p = 0.11), or overall survival (LC Bead 20.8 months vs. BB 19.6 months, p = 0.64) between DEB-TACE and TAE.
However, the results of this trial should be extrapolated to intermediate-stage HCC with caution, as only 55 patients in this single-institution study cohort had BCLC stage B HCC. Technical issues with the study design may also call into question some of the trial's conclusions. Stasis was chosen as the embolization endpoint for both study arms, which essentially precluded retreatment of the target lesions in the DEB-TACE arm, an otherwise clinically common scenario. DEB-TACE was therefore not performed according to the usual standard of care, with incomplete stasis being a formally agreed upon endpoint of the procedure. Instead, the authors used Bead Block particles to reach stasis in the DEB-TACE arm following the administration of doxorubicin-loaded LC Beads. This and other minor practical flaws may have inadvertently minimized differences between the two treatment arms. The results of this trial on its own are therefore insufficient to globally recommend the use of TAE in intermediate stage HCC.
Y-90 Radioembolization
Yttrium-90 radioembolization is an increasingly established treatment option for patients with intermediate-stage HCC. Overall, due to its mechanism of delivery, radioembolization is capable of delivering much higher doses of radiation to a tumor than would be possible using a technique such as external beam radiation. 57 Because Y-90 microspheres are generally smaller than embolic particles used during TACE, the embolic effect and resultant ischemic insult introduced by Y-90 RE is less pronounced than from TACE. 58
Early studies demonstrated the safety and efficacy of Y-90 RE. Kulik et al performed an open-label phase 2 study including 108 patients with unresectable HCC. 59 Out of this cohort, the investigators reported no significant complications or mortalities related to the technical performance of the procedure, and one death during the 6-month follow-up period that may have been related to the treatment. They did not report any instances of radiation pneumonitis, although one patient experienced radiation cholecystitis. A large percentage of patients (31%) experienced elevations in bilirubin, although the investigators argue that this high percentage may be due to disease progression instead of any treatment-related effect. Y-90 RE resulted in a response rate of 70% according to EASL criteria.
Y-90 RE shows similar survival benefit to TACE in patients with intermediate-stage HCC. Potential benefits of Y-90 RE for this cohort may include better quality of life for patients undergoing the procedure, 58 60 as well as superior local tumor control. 61 The PREMIERE trial, a randomized phase 2 clinical study performed by Salem et al, compared Y-90 RE with cTACE for 45 patients with unresectable BCLC stage A or B HCC. The PREMIERE trial found that Y-90 RE significantly improved median TTP (>26 vs. 6.8 months, p = 0.0012) compared with cTACE. 62 This trial also found that significantly fewer patients in the Y-90 RE group experienced diarrhea or hypoalbuminemia. However, median survival was similar between Y-90 RE and cTACE (18.6 vs. 17.7 months, p = 0.99). A randomized trial including 28 patients performed by Kolligs et al, however, did not show any differences in either health-related quality of life measures or PFS between patients receiving Y-90 RE or cTACE. 63 Salem et al examined data from 1,000 patients who underwent Y-90 RE for HCC, with 152 patients in the cohort having BCLC stage B disease. 64 After Y-90 RE, patients with BCLC stage B disease and CP A liver function had a median survival time of 25 months, while those with CP B liver function had median survival time of 15 months.
In summary, Y-90 RE shows great promise for patients with intermediate-stage disease and is especially valid for niche applications as mentioned earlier. Because of that, some institutions have proposed the adoption of this treatment as their institutional first line for patients with intermediate- or advanced-stage HCC. 64 As further data emerge, the use, levels of evidence, and grades of recommendation of Y-90 RE will be progressively reevaluated.
Transarterial Chemoembolization plus Thermal Ablation
The combination of TACE with thermal ablation (TACE + TA) has also been explored for the treatment of intermediate-stage HCC. There are a few reasons TACE + TA may be performed in theory. First, TACE + TA has the potential to induce a larger zone of ablation than TA alone because TACE reduces blood flow to a tumor, thereby attenuating the heat-sink effect present during subsequent TA. Similarly, TACE + TA may be useful if proximity to large vascular structures would make TA impractical due to the heat-sink effect. Sugimori et al demonstrated this effect in porcine models, with TACE + TA demonstrating a larger diameter of coagulation necrosis compared with TA alone. 65 Second, tumors near sensitive anatomic structures such as the heart, stomach, or gallbladder may require combination treatment to achieve complete response. Third, if a tumor is difficult to visualize on ultrasound or CT, the deposition of Lipiodol inside a tumor that occurs during cTACE may aid with visualization during the TA procedure.
The use of TACE + TA may be beneficial for select patients. Most notably for patients with tumors 3 to 5 cm in diameter, the combination of TACE + TA can reduce the number of treatments needed to achieve technical success as well as lower the rate of local tumor progression. 66 Several groups have found that combining TACE with RFA can offer survival benefit to patients with intermediate-stage HCC. A meta-analysis by Lu et al examining seven RCTs found that the combination of TACE and RFA could improve survival rates at 1, 3, and 5 years, but that tumors needed to be greater than 3 cm in size for this effect to be observed. 67 A meta-analysis by Ni found a similar result, with combination treatment offering improved survival at 1, 2, and 3 years for patients with intermediate- to large-sized HCC. 68 However, for patients with HCC under 3 cm, the combination of TACE with TA does not appear to offer survival benefit. 69
Transarterial Chemoembolization plus Sorafenib
The use of systemic therapy in combination with TACE has also been explored. Vascular endothelial growth factor (VEGF) inhibitor sorafenib is the most well-established systemic therapy for HCC, but recently bevacizumab in combination with atezolizumab has obtained Food and Drug Administration (FDA) approval. HCC tumor growth is driven largely by arterial supply, and VEGF levels are increased in HCC. 70 There is concern that TACE procedures may contribute to tumorigenesis due to increases in VEGF levels, as tumor cells may respond to a hypoxic environment by stimulating angiogenesis. 71 72 This explains the theoretical rationale for combining antiangiogenic therapy with TACE. Results from analysis of the GIDEON registry demonstrated the safety of sorafenib use in patients with CP class A and B liver function. 73 This registry contained data from over 3,000 patients with HCC treated with sorafenib. Adverse events leading to drug discontinuation occurred in 17% of patients with CP class A and 21% of patients with CP class B liver function.
The evidence in support of sorafenib in combination with TACE for intermediate-stage HCC is scarce. The TACE 2 trial by Meyer et al included 313 patients randomized to either TACE alone or TACE plus sorafenib and also found that the addition of sorafenib did not offer benefits in terms of progression-free survival, overall survival, or time to progression. 74 The SPACE trial by Lencioni et al included 307 patients with BCLC stage B HCC and randomized them to either TACE or TACE plus sorafenib treatment. 75 The SPACE trial did not find any reduction in time to progression when sorafenib was used in addition to TACE. More positive findings for the use of TACE with sorafenib can be found for patients who have advanced BCLC stage C HCC, but further discussion on this point is outside the scope of this review. The combination of TACE plus sorafenib has not yielded results for patients with intermediate-stage HCC, so use of this combination is not recommended in clinical practice.
Transarterial Chemoembolization plus Immune Checkpoint Inhibitors
Cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed death-ligand 1 (PD-L1) blockade in combination with TACE has the potential to enhance the native immune response against tumor cells, which may improve treatment response and survival. TACE is a therapy that induces tumor necrosis and releases various antigens into systemic circulation. TACE has been shown to alter the systemic cytokine environment, as well as circulating levels of type 1 helper T-cells and regulator T-cells. 76 77 A study by Liao et al found that increased levels of circulating Th17 cells were positively associated with both overall survival and TTP in patients with TNM stage III HCC. 78 Given TACE's potentially immunogenic profile, there is great interest in combining immune checkpoint inhibitor therapy with the procedure.
Immune checkpoint inhibitors have been shown to be safe in the treatment of HCC, although most existing data are for disease in the advanced stage. Nivolumab received FDA approval for second-line treatment of advanced stage HCC 2 based on results from the CheckMate 040 study, which showed a 20% objective response rate by mRECIST criteria in the dose-expansion phase as well as an acceptable safety profile. 79
For intermediate-stage HCC specifically, there are ongoing clinical trials investigating the use of TACE plus immune checkpoint inhibitors. The IMMUTACE trial (NCT03572582) is a phase II, single-arm, open label exploring the use of DEB-TACE plus nivolumab for patients with multinodular or large, solitary HCC not eligible for resection or ablation. 80 Another currently ongoing, nonrandomized, open-label trial (NCT03638141) aims to combine durvalumab and tremelimumab at various dosage levels 2 weeks after completion of DEB-TACE for patients with intermediate-stage HCC. 81 The primary outcome measure for these studies is objective response rate using mRECIST criteria, and secondary outcome measures include progression-free survival, TTP, and overall survival. At the time of writing, the results for these trials are pending. The potential expansion of immune checkpoint inhibitors into the treatment armamentarium for intermediate-stage HCC represents an exciting new development, and one that should be followed with close interest.
Conclusion
This review article summarized many of the available locoregional therapy options for intermediate-stage HCC. Although this patient group is very heterogeneous, cTACE continues to be the best explored therapy option and is widely considered as the standard of care. DEB-TACE offers performance that is comparable to cTACE, although there should be awareness of the benefits and drawbacks to each approach. For DEB-TACE, there are a wide variety of usable materials each with their own advantages and disadvantages. Y-90 RE is a promising approach that can be used in certain patients in whom TACE is contraindicated or ineffective. At the same time, data on Y-90 RE have matured to the point where some institutions have adopted it as the primary treatment modality for intermediate-stage HCC. The combination of TACE + TA should only be considered in select patients. Advanced intraprocedural image guidance with CBCT represents a new standard of care and good clinical practice and should therefore be pursued for all the aforementioned modalities. TACE with sorafenib is safe for patients with intermediate-stage HCC, although conclusive clinical benefit was not demonstrated and the combination will likely be abandoned in clinical practice. Data are pending for the combination of TACE with immune checkpoint inhibitors but anticipated with great interest and cautious enthusiasm.
Footnotes
Conflict of Interest None declared.
References
- 1.Bolondi L, Burroughs A, Dufour J-F. Heterogeneity of patients with intermediate (BCLC B) hepatocellular carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32(04):348–359. doi: 10.1055/s-0032-1329906. [DOI] [PubMed] [Google Scholar]
- 2.European Association for the Study of the Liver. . EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(01):182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Jung Y K, Jung C H, Seo Y S. BCLC stage B is a better designation for single large hepatocellular carcinoma than BCLC stage A. J Gastroenterol Hepatol. 2016;31(02):467–474. doi: 10.1111/jgh.13152. [DOI] [PubMed] [Google Scholar]
- 4.Barman P M, Su G L. Limitations of the Barcelona Clinic Liver Cancer staging system with a focus on transarterial chemoembolization as a key modality for treatment of hepatocellular carcinoma. Clin Liver Dis (Hoboken) 2016;7(02):32–35. doi: 10.1002/cld.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biolato M, Gallusi G, Iavarone M. Prognostic ability of BCLC-B subclassification in patients with hepatocellular carcinoma undergoing transarterial chemoembolization. Ann Hepatol. 2018;17(01):110–118. doi: 10.5604/01.3001.0010.7542. [DOI] [PubMed] [Google Scholar]
- 6.Dhanasekaran R, Limaye A, Cabrera R. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis, and therapeutics. Hepat Med. 2012;4:19–37. doi: 10.2147/HMER.S16316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Serag H B, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go? Hepatology. 2014;60(05):1767–1775. doi: 10.1002/hep.27222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sohn J H, Duran R, Zhao Y. Validation of the Hong Kong Liver Cancer staging system in determining prognosis of the North American patients following intra-arterial therapy. Clin Gastroenterol Hepatol. 2017;15(05):746–7.55E6. doi: 10.1016/j.cgh.2016.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barcelona Liver Cancer Group Llovet J M, Real M I, Montaña X.Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial Lancet 2002359(9319):1734–1739. [DOI] [PubMed] [Google Scholar]
- 10.Lo C-M, Ngan H, Tso W-K. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35(05):1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 11.Llovet J M, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37(02):429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 12.Cammà C, Schepis F, Orlando A. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224(01):47–54. doi: 10.1148/radiol.2241011262. [DOI] [PubMed] [Google Scholar]
- 13.Marrero J A, Kulik L M, Sirlin C B. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(02):723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 14.Park J-W, Chen M, Colombo M. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35(09):2155–2166. doi: 10.1111/liv.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hyun M H, Lee Y S, Kim J H. Hepatic resection compared to chemoembolization in intermediate- to advanced-stage hepatocellular carcinoma: a meta-analysis of high-quality studies. Hepatology. 2018;68(03):977–993. doi: 10.1002/hep.29883. [DOI] [PubMed] [Google Scholar]
- 16.Omata M, Lesmana L A, Tateishi R. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4(02):439–474. doi: 10.1007/s12072-010-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korean Liver Cancer Study Group (KLCSG) ; National Cancer Center, Korea (NCC) . 2014 KLCSG-NCC Korea Practice Guideline for the Management of Hepatocellular Carcinoma. Gut Liver. 2015;9(03):267–317. doi: 10.5009/gnl14460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liver Cancer Study Group of Japan Kudo M, Matsui O, Izumi N.JSH Consensus-Based Clinical Practice Guidelines for the management of hepatocellular carcinoma: 2014 update by the Liver Cancer Study Group of Japan Liver Cancer 20143(3-4):458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tacher V, Radaelli A, Lin M, Geschwind J-F. How I do it: cone-beam CT during transarterial chemoembolization for liver cancer. Radiology. 2015;274(02):320–334. doi: 10.1148/radiol.14131925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyayama S, Yamashiro M, Hattori Y. Efficacy of cone-beam computed tomography during transcatheter arterial chemoembolization for hepatocellular carcinoma. Jpn J Radiol. 2011;29(06):371–377. doi: 10.1007/s11604-011-0568-8. [DOI] [PubMed] [Google Scholar]
- 21.Wallace M J. C-arm computed tomography for guiding hepatic vascular interventions. Tech Vasc Interv Radiol. 2007;10(01):79–86. doi: 10.1053/j.tvir.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Yu L, Vrieze T J, Bruesewitz M R. Dose and image quality evaluation of a dedicated cone-beam CT system for high-contrast neurologic applications. AJR Am J Roentgenol. 2010;194(02):W193–W201. doi: 10.2214/AJR.09.2951. [DOI] [PubMed] [Google Scholar]
- 23.Lin M, Loffroy R, Noordhoek N. Evaluating tumors in transcatheter arterial chemoembolization (TACE) using dual-phase cone-beam CT. Minim Invasive Ther Allied Technol. 2011;20(05):276–281. doi: 10.3109/13645706.2010.536243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ning R, Chen B, Yu R, Conover D, Tang X, Ning Y. Flat panel detector-based cone-beam volume CT angiography imaging: system evaluation. IEEE Trans Med Imaging. 2000;19(09):949–963. doi: 10.1109/42.887842. [DOI] [PubMed] [Google Scholar]
- 25.Miyayama S, Yamashiro M, Hashimoto M. Identification of small hepatocellular carcinoma and tumor-feeding branches with cone-beam CT guidance technology during transcatheter arterial chemoembolization. J Vasc Interv Radiol. 2013;24(04):501–508. doi: 10.1016/j.jvir.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 26.Iwazawa J, Ohue S, Mitani T. Identifying feeding arteries during TACE of hepatic tumors: comparison of C-arm CT and digital subtraction angiography. AJR Am J Roentgenol. 2009;192(04):1057–1063. doi: 10.2214/AJR.08.1285. [DOI] [PubMed] [Google Scholar]
- 27.Loffroy R, Lin M, Yenokyan G. Intraprocedural C-arm dual-phase cone-beam CT: can it be used to predict short-term response to TACE with drug-eluting beads in patients with hepatocellular carcinoma? Radiology. 2013;266(02):636–648. doi: 10.1148/radiol.12112316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toyoda H, Kumada T, Sone Y. Impact of a unified CT angiography system on outcome of patients with hepatocellular carcinoma. AJR Am J Roentgenol. 2009;192(03):766–774. doi: 10.2214/AJR.08.1368. [DOI] [PubMed] [Google Scholar]
- 29.Kakeda S, Korogi Y, Ohnari N. Usefulness of cone-beam volume CT with flat panel detectors in conjunction with catheter angiography for transcatheter arterial embolization. J Vasc Interv Radiol. 2007;18(12):1508–1516. doi: 10.1016/j.jvir.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Idée J-M, Guiu B. Use of Lipiodol as a drug-delivery system for transcatheter arterial chemoembolization of hepatocellular carcinoma: a review. Crit Rev Oncol Hematol. 2013;88(03):530–549. doi: 10.1016/j.critrevonc.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 31.van Breugel J MM, Geschwind J-F, Mirpour S. Theranostic application of lipiodol for transarterial chemoembolization in a VX2 rabbit liver tumor model. Theranostics. 2019;9(13):3674–3686. doi: 10.7150/thno.32943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kan Z, Sato M, Ivancev K. Distribution and effect of iodized poppy seed oil in the liver after hepatic artery embolization: experimental study in several animal species. Radiology. 1993;186(03):861–866. doi: 10.1148/radiology.186.3.8381552. [DOI] [PubMed] [Google Scholar]
- 33.Kan Z, McCuskey P A, Wright K C, Wallace S. Role of Kupffer cells in iodized oil embolization. Invest Radiol. 1994;29(11):990–993. doi: 10.1097/00004424-199411000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Okayasu I, Hatakeyama S, Yoshida T. Selective and persistent deposition and gradual drainage of iodized oil, Lipiodol in the hepatocellular carcinoma after injection into the feeding hepatic artery. Am J Clin Pathol. 1988;90(05):536–544. doi: 10.1093/ajcp/90.5.536. [DOI] [PubMed] [Google Scholar]
- 35.Mondazzi L, Bottelli R, Brambilla G. Transarterial oily chemoembolization for the treatment of hepatocellular carcinoma: a multivariate analysis of prognostic factors. Hepatology. 1994;19(05):1115–1123. [PubMed] [Google Scholar]
- 36.Dumortier J, Chapuis F, Borson O. Unresectable hepatocellular carcinoma: survival and prognostic factors after lipiodol chemoembolisation in 89 patients. Dig Liver Dis. 2006;38(02):125–133. doi: 10.1016/j.dld.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 37.Brown D B, Pilgram T K, Darcy M D. Hepatic arterial chemoembolization for hepatocellular carcinoma: comparison of survival rates with different embolic agents. J Vasc Interv Radiol. 2005;16(12):1661–1666. doi: 10.1097/01.RVI.0000182160.26798.A2. [DOI] [PubMed] [Google Scholar]
- 38.Lee S H, Lin C Y, Hsu Y C, Liu Y S, Chuang M T, Ou M C. Comparison of the efficacy of two microsphere embolic agents for transcatheter arterial chemoembolization in hepatocellular carcinoma patients. Cancer Res Treat. 2020;52(01):24–30. doi: 10.4143/crt.2019.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schicho A, Pereira P L, Haimerl M. Transarterial chemoembolization (TACE) with degradable starch microspheres (DSM) in hepatocellular carcinoma (HCC): multi-center results on safety and efficacy. Oncotarget. 2017;8(42):72613–72620. doi: 10.18632/oncotarget.19997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Groupe CHC . Pelletier G, Ducreux M, Gay F. Treatment of unresectable hepatocellular carcinoma with lipiodol chemoembolization: a multicenter randomized trial. J Hepatol. 1998;29(01):129–134. doi: 10.1016/s0168-8278(98)80187-6. [DOI] [PubMed] [Google Scholar]
- 41.Lewandowski R J, Mulcahy M F, Kulik L M. Chemoembolization for hepatocellular carcinoma: comprehensive imaging and survival analysis in a 172-patient cohort. Radiology. 2010;255(03):955–965. doi: 10.1148/radiol.10091473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lencioni R, de Baere T, Soulen M C, Rilling W S, Geschwind J F. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology. 2016;64(01):106–116. doi: 10.1002/hep.28453. [DOI] [PubMed] [Google Scholar]
- 43.de Baere T, Arai Y, Lencioni R. Treatment of liver tumors with lipiodol TACE: technical recommendations from experts opinion. Cardiovasc Intervent Radiol. 2016;39(03):334–343. doi: 10.1007/s00270-015-1208-y. [DOI] [PubMed] [Google Scholar]
- 44.Namur J, Wassef M, Millot J M, Lewis A L, Manfait M, Laurent A. Drug-eluting beads for liver embolization: concentration of doxorubicin in tissue and in beads in a pig model. J Vasc Interv Radiol. 2010;21(02):259–267. doi: 10.1016/j.jvir.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 45.Lewis A L, Gonzalez M V, Lloyd A W.DC bead: in vitro characterization of a drug-delivery device for transarterial chemoembolization J Vasc Interv Radiol 200617(2, Pt 1):335–342. [DOI] [PubMed] [Google Scholar]
- 46.Hong K, Khwaja A, Liapi E, Torbenson M S, Georgiades C S, Geschwind J F. New intra-arterial drug delivery system for the treatment of liver cancer: preclinical assessment in a rabbit model of liver cancer. Clin Cancer Res. 2006;12(08):2563–2567. doi: 10.1158/1078-0432.CCR-05-2225. [DOI] [PubMed] [Google Scholar]
- 47.Zhang S, Huang C, Li Z. Comparison of pharmacokinetics and drug release in tissues after transarterial chemoembolization with doxorubicin using diverse lipiodol emulsions and CalliSpheres Beads in rabbit livers. Drug Deliv. 2017;24(01):1011–1017. doi: 10.1080/10717544.2017.1344336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.PRECISION V Investigators . Lammer J, Malagari K, Vogl T. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33(01):41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.PRECISION ITALIA STUDY GROUP . Golfieri R, Giampalma E, Renzulli M. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014;111(02):255–264. doi: 10.1038/bjc.2014.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Monier A, Guiu B, Duran R. Liver and biliary damages following transarterial chemoembolization of hepatocellular carcinoma: comparison between drug-eluting beads and lipiodol emulsion. Eur Radiol. 2017;27(04):1431–1439. doi: 10.1007/s00330-016-4488-y. [DOI] [PubMed] [Google Scholar]
- 51.Guiu B, Deschamps F, Aho S. Liver/biliary injuries following chemoembolisation of endocrine tumours and hepatocellular carcinoma: lipiodol vs. drug-eluting beads. J Hepatol. 2012;56(03):609–617. doi: 10.1016/j.jhep.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 52.Facciorusso A, Di Maso M, Muscatiello N. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma: a meta-analysis. Dig Liver Dis. 2016;48(06):571–577. doi: 10.1016/j.dld.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 53.Zou J H, Zhang L, Ren Z G, Ye S L. Efficacy and safety of cTACE versus DEB-TACE in patients with hepatocellular carcinoma: a meta-analysis. J Dig Dis. 2016;17(08):510–517. doi: 10.1111/1751-2980.12380. [DOI] [PubMed] [Google Scholar]
- 54.Zhou X, Tang Z, Wang J. Doxorubicin-eluting beads versus conventional transarterial chemoembolization for the treatment of hepatocellular carcinoma: a meta-analysis. Int J Clin Exp Med. 2014;7(11):3892–3903. [PMC free article] [PubMed] [Google Scholar]
- 55.Ashrafi K, Tang Y, Britton H. Characterization of a novel intrinsically radiopaque drug-eluting bead for image-guided therapy: DC Bead LUMI™. J Control Release. 2017;250:36–47. doi: 10.1016/j.jconrel.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown K T, Do R K, Gonen M. Randomized trial of hepatic artery embolization for hepatocellular carcinoma using doxorubicin-eluting microspheres compared with embolization with microspheres alone. J Clin Oncol. 2016;34(17):2046–2053. doi: 10.1200/JCO.2015.64.0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dancey J E, Shepherd F A, Paul K. Treatment of nonresectable hepatocellular carcinoma with intrahepatic 90Y-microspheres. J Nucl Med. 2000;41(10):1673–1681. [PubMed] [Google Scholar]
- 58.Salem R, Miller F H, Yaghmai V, Lewandowski R J. Response assessment methodologies in hepatocellular carcinoma: complexities in the era of local and systemic treatments. J Hepatol. 2013;58(06):1260–1262. doi: 10.1016/j.jhep.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 59.Kulik L M, Carr B I, Mulcahy M F. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008;47(01):71–81. doi: 10.1002/hep.21980. [DOI] [PubMed] [Google Scholar]
- 60.Salem R, Gilbertsen M, Butt Z. Increased quality of life among hepatocellular carcinoma patients treated with radioembolization, compared with chemoembolization. Clin Gastroenterol Hepatol. 2013;11(10):1358–13650. doi: 10.1016/j.cgh.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 61.Salem R, Lewandowski R J, Kulik L. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140(02):497–50700. doi: 10.1053/j.gastro.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salem R, Gordon A C, Mouli S. Y90 radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2016;151(06):1155–116300. doi: 10.1053/j.gastro.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kolligs F T, Bilbao J I, Jakobs T. Pilot randomized trial of selective internal radiation therapy vs. chemoembolization in unresectable hepatocellular carcinoma. Liver Int. 2015;35(06):1715–1721. doi: 10.1111/liv.12750. [DOI] [PubMed] [Google Scholar]
- 64.Salem R, Gabr A, Riaz A. Institutional decision to adopt Y90 as primary treatment for hepatocellular carcinoma informed by a 1,000-patient 15-year experience. Hepatology. 2018;68(04):1429–1440. doi: 10.1002/hep.29691. [DOI] [PubMed] [Google Scholar]
- 65.Sugimori K, Nozawa A, Morimoto M. Extension of radiofrequency ablation of the liver by transcatheter arterial embolization with iodized oil and gelatin sponge: results in a pig model. J Vasc Interv Radiol. 2005;16(06):849–856. doi: 10.1097/01.RVI.0000157780.44868.78. [DOI] [PubMed] [Google Scholar]
- 66.Morimoto M, Numata K, Kondou M, Nozaki A, Morita S, Tanaka K. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer. 2010;116(23):5452–5460. doi: 10.1002/cncr.25314. [DOI] [PubMed] [Google Scholar]
- 67.Lu Z, Wen F, Guo Q, Liang H, Mao X, Sun H. Radiofrequency ablation plus chemoembolization versus radiofrequency ablation alone for hepatocellular carcinoma: a meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol. 2013;25(02):187–194. doi: 10.1097/MEG.0b013e32835a0a07. [DOI] [PubMed] [Google Scholar]
- 68.Ni J-Y, Liu S-S, Xu L-F, Sun H-L, Chen Y-T. Meta-analysis of radiofrequency ablation in combination with transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2013;19(24):3872–3882. doi: 10.3748/wjg.v19.i24.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shibata T, Isoda H, Hirokawa Y, Arizono S, Shimada K, Togashi K. Small hepatocellular carcinoma: is radiofrequency ablation combined with transcatheter arterial chemoembolization more effective than radiofrequency ablation alone for treatment? Radiology. 2009;252(03):905–913. doi: 10.1148/radiol.2523081676. [DOI] [PubMed] [Google Scholar]
- 70.Zhang L, Wang J-N, Tang J-M. VEGF is essential for the growth and migration of human hepatocellular carcinoma cells. Mol Biol Rep. 2012;39(05):5085–5093. doi: 10.1007/s11033-011-1304-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li X, Feng G-S, Zheng C-S, Zhuo C-K, Liu X. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol. 2004;10(19):2878–2882. doi: 10.3748/wjg.v10.i19.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shim J H, Park J W, Kim J H. Association between increment of serum VEGF level and prognosis after transcatheter arterial chemoembolization in hepatocellular carcinoma patients. Cancer Sci. 2008;99(10):2037–2044. doi: 10.1111/j.1349-7006.2008.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marrero J A, Kudo M, Venook A P. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: the GIDEON study. J Hepatol. 2016;65(06):1140–1147. doi: 10.1016/j.jhep.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 74.Meyer T, Fox R, Ma Y T. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2(08):565–575. doi: 10.1016/S2468-1253(17)30156-5. [DOI] [PubMed] [Google Scholar]
- 75.Lencioni R, Llovet J M, Han G. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol. 2016;64(05):1090–1098. doi: 10.1016/j.jhep.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 76.Kim M J, Jang J W, Oh B S. Change in inflammatory cytokine profiles after transarterial chemotherapy in patients with hepatocellular carcinoma. Cytokine. 2013;64(02):516–522. doi: 10.1016/j.cyto.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 77.Takaki H, Imai N, Contessa T T. Peripheral blood regulatory T-cell and type 1 helper T-cell population decrease after hepatic artery embolization. J Vasc Interv Radiol. 2016;27(10):1561–1568. doi: 10.1016/j.jvir.2016.01.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liao Y, Wang B, Huang Z-L. Increased circulating Th17 cells after transarterial chemoembolization correlate with improved survival in stage III hepatocellular carcinoma: a prospective study. PLoS One. 2013;8(04):e60444. doi: 10.1371/journal.pone.0060444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.El-Khoueiry A B, Sangro B, Yau T.Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial Lancet 2017389(10088):2492–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Transarterial Chemoembolization in Combination with Nivolumab Performed for Intermediate Stage Hepatocellular Carcinoma (IMMUTACE) ClinicalTrialsgov Accessed October 17, 2020 at:https://clinicaltrialsgov/ct2/show/NCT03572582 [PMC free article] [PubMed]
- 81.The Effect of CTLA-4/PD-L1 Blockade Following Drug-eluting Bead Transarterial Chemoembolization (DEB-TACE) in Patients With Intermediate Stage of HCC Using Durvalumab (MEDI4736) and Tremelimumab ClinicalTrialsgov Accessed October 17, 2020 at:https://clinicaltrialsgov/ct2/show/study/NCT03638141
- 82.Vaidya S, Tozer K R, Chen J. An overview of embolic agents. Semin Intervent Radiol. 2008;25(03):204–215. doi: 10.1055/s-0028-1085930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sacco R, Bargellini I, Bertini M. Conventional versus doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2011;22(11):1545–1552. doi: 10.1016/j.jvir.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 84.van Malenstein H, Maleux G, Vandecaveye V. A randomized phase II study of drug-eluting beads versus transarterial chemoembolization for unresectable hepatocellular carcinoma. Onkologie. 2011;34(07):368–376. doi: 10.1159/000329602. [DOI] [PubMed] [Google Scholar]
- 85.Dekervel J, van Malenstein H, Vandecaveye V. Transcatheter arterial chemoembolization with doxorubicin-eluting superabsorbent polymer microspheres in the treatment of hepatocellular carcinoma: midterm follow-up. J Vasc Interv Radiol. 2014;25(02):248–550. doi: 10.1016/j.jvir.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 86.Richter G, Radeleff B, Stroszczynski C. Safety and feasibility of chemoembolization with doxorubicin-loaded small calibrated microspheres in patients with hepatocellular carcinoma: results of the MIRACLE I prospective multicenter study. Cardiovasc Intervent Radiol. 2018;41(04):587–593. doi: 10.1007/s00270-017-1839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Malagari K, Kiakidis T, Pomoni M. Pharmacokinetics, safety, and efficacy of chemoembolization with doxorubicin-loaded tightly calibrated small microspheres in patients with hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2016;39(10):1379–1391. doi: 10.1007/s00270-016-1382-6. [DOI] [PubMed] [Google Scholar]
- 88.Reicher J, Mafeld S, Priona G. Early experience of trans-arterial chemo-embolisation for hepatocellular carcinoma with a novel radiopaque bead. Cardiovasc Intervent Radiol. 2019;42(11):1563–1570. doi: 10.1007/s00270-019-02317-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.DC Bead LUMI (TM) Loaded With Doxorubicin for Intermediate Hepatocellular Carcinoma (HCC) ClinicalTrialsgov Accessed October 17, 2020 at:https://clinicaltrialsgov/ct2/show/NCT03474354


