Abstract
Breast cancer is the most common cancer in women and breast cancer liver metastasis may be associated with poor outcomes. Emerging locoregional therapies can be given in outpatient settings or with short hospital stays, to provide local control, support quality of life, preserve liver function, and potentially prolong survival. This review discusses retrospective studies suggesting potential benefits of locoregional treatment of breast cancer liver metastasis. Future prospective studies are needed to demonstrate efficacy and optimize patient selection.
Keywords: breast cancer, locoregional, hepatic metastasis, ablation, chemoembolization, radioembolization
Breast cancer is the most common cancer in women and the fifth cause of death among men and women overall. 1 In the United States, the lifetime female incidence of breast cancer is 12%. 2 Metastasis develops in approximately half of breast cancer patients, with liver metastasis occurring in at least two-thirds of these patients. 3 Liver metastasis is associated with relatively poor outcomes and may be the cause of 20% or more of the deaths in breast cancer. 2 4
The goal of systemic therapy in metastatic breast cancer is to support quality of life and extend survival. Systemic therapies include cytotoxic, hormonal, and immunotherapeutic agents. 5 Hormone-based endocrine therapy is designed to target HER2, resulting in metastatic site reduction and long-term stabilization. In hormone receptor-negative breast cancer, treatment entails single-agent chemotherapy or immunotherapy agents. 6 Triple-negative breast cancer is particularly challenging, with a relative paucity of systemic options. 7 When patients develop resistance to systemic therapies, or when few systemic options are available, locoregional therapies may be offered as refractory options. Most commonly, locoregional therapies are offered in the setting of hepatic metastasis, as hepatic lesions are thought to limit survival and may cause symptoms such as pain. 2 3
This article reviews emerging locoregional therapies for hepatic metastasis due to breast cancer and categorizes approaches based on the extent of hepatic disease. We review these studies and suggest potential areas for future development.
Limited Hepatic Metastases
Oligometastatic breast cancer (OMBC) is defined as five or fewer metastatic sites that may be treated locally with a goal of long-term remission. 8 9 OMBC treatment is typically palliative in nature, involving systemic chemotherapy and hormonal therapies. For patients with OMBC confined to the liver, systemic treatments extend survival to 27 months and achieve a partial response rates of 50 to 60% in retrospective studies. 8 10 Over the past several decades, advances such as surgical resection, external radiation, and percutaneous ablation have emerged ( Table 1 ). Combined with systemic therapies, locoregional interventions may further extend survival or delay the need to transition to a new line of systemic therapy by controlling local hepatic tumor growth. 11
Table 1. Locoregional therapies for oligometastatic breast cancer liver tumors.
| Reference | Year | n | Treatment modality | OS | Other survival outcomes | AE rate |
|---|---|---|---|---|---|---|
| Vlastos et al 47 | 2004 | 31 | Resection | 63 mo | 2-y OS: 86% 5-y OS: 61% |
|
| Adam et al 4 | 2006 | 85 | Resection | 32 mo | 5-y OS: 37% | 22% any AE |
| Caralt et al 13 | 2008 | 12 | Resection | 1-y OS: 100% 3-y OS: 79% 5-y OS: 33% |
17% any AE | |
| van Walsum et al 48 | 2012 | 32 | Resection | 55 mo | 5-y OS: 37% | 34% any AE |
| Abbott et al 49 | 2012 | 86 | Resection | 57 mo | PFS: 14.2 mo | 21% any AE |
| Mariani et al 50 | 2013 | 51 | Resection | 3-y OS: 74% 5-y OS: 50% |
20% any AE | |
| Kim et al 14 | 2014 | 13 | Resection | 1-y OS: 83% 3-y OS: 49% |
||
| Margonis et al 51 | 2016 | 131 | Resection | 53 mo | 1-y OS: 99% 3-y OS: 75% |
23% any AE |
| Ruiz et al 12 | 2018 | 139 | Resection | 7-y OS: 76% 10-y OS: 36% |
25% any AE | |
| Ruiz et al 52 | 2018 | 662 | Resection | 82 mo | 3-y OS: 81% 5-y OS: 69% |
|
| Wieners et al 53 | 2011 | 41 | SBRT | 6-mo OS: 97% 12-mo OS: 79% 18-mo OS: 60% |
<2% grade 3 and higher AE | |
| Milano et al 16 | 2012 | 121 | SBRT | 2-y OS: 50% 2-y PFS: 26% 5-y OS: 28% 5-y PFS: 20% |
<1% grade 3 and higher AE | |
| Palma et al 17 | 2018 | 159 | SBRT | 20 mo | PFS: 15 mo | 30% grade 2 and higher AE |
| Milano et al 16 | 2018 | 48 | SBRT | 5-y OS: 31% 10-y OS: 17% |
||
| Mahadevan et al 54 | 2018 | 42 | SBRT | 21 mo | 1-y OS: 66% | |
| Onal et al 55 | 2018 | 22 | SBRT | 1-y OS: 85% 2-y OS: 57% |
||
| Sofocleous et al 21 | 2007 | 12 | RFA | 60 mo | 3-y OS: 70% 5-y OS: 30% |
|
| Jakobs et al 33 | 2008 | 43 | RFA | 59 mo | 7% major AE | |
| Meloni et al 23 | 2009 | 52 | RFA | 42 mo | 5-y OS: 32% | 4% minor AE |
| Veltri et al 56 | 2014 | 45 | RFA | 1-y OS: 90% 2-y OS: 58% 3-y OS: 44% |
10% any AE | |
| Kümler et al 57 | 2015 | 32 | RFA | 34 mo | 3% grade 3 and higher AE | |
| Barral et al 28 | 2016 | 50 | RFA/MWA/ Cryoablation | 1-y OS: 98% 2-y OS: 96% |
||
| Bai et al 58 | 2018 | 69 | RFA | 26 mo | 1-y OS: 82% 2-y OS: 50% 3-y OS: 25% 5-y OS: 11% |
1% grade 3 and higher AE |
| Ridouani et al 24 | 2020 | 35 | RFA/MWA/ Cryoablation | 70 mo | Time to progression: 11 mo | 8% any AE |
Abbreviations: AE, adverse event; MWA, microwave ablation; OS, overall survival; PFS, progression-free survival; RFA, radiofrequency ablation; SBRT, stereotactic body radiation therapy.
Notes: Selected studies are presented. Survival reported as median value from time of treatment; n indicates number of breast cancer patients studied.
Hepatectomy
Surgical hepatectomy involves resection of compromised hepatic segments or lobes. When there are three or fewer sites, a minor resection may be performed; when there are more than three sites, a major or radical resection may be performed. 12 13 Hepatectomies for OMBC are associated with 20 to 40% rates of any adverse events, and hospital admissions of several following surgery ( Table 1 ). Roughly 60% of patients develop recurrence ( Fig. 1 ) and median overall survival ranges from 30 to 80 months ( Table 1 ). 12 13 14 Findings suggest that locoregional treatment with hepatectomy in combination with systemic therapy was associated with a potential 14% cure rate, 12 though prospective studies have not been performed. Compared with less invasive treatment, posthepatectomy care entails hospitalization and extensive follow-up. 14
Fig. 1.
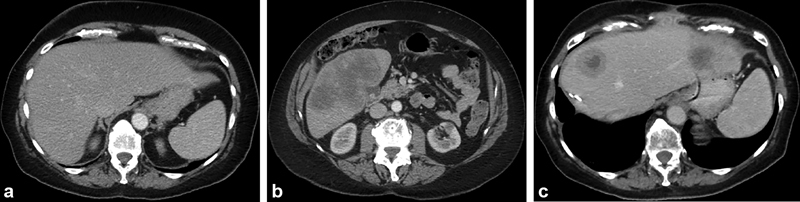
Liver resection with subsequent progression. Axial images from a contrast-enhanced CT in a 68-year-old woman with triple negative breast cancer demonstrate no tumors in the left lobe ( a ) and a solitary large multilobulated tumor in the right lobe ( b ) that is too large to eradicate with percutaneous ablation. One month later, the right lobe was surgically resected. Six months later, follow-up contrast-enhanced CT shows new tumors in the left hepatic lobe ( c ).
External Radiation
Stereotactic body radiation therapy (SBRT) is a localized external radiation treatment for hepatic OMBC that uses imaging guidance to create a three-dimensional model allowing for specific targeting and delivery of high fractional doses of radiation. 15 This is usually done in a varying dosage delivered every 2 days over 2 weeks, to minimize toxicity to healthy tissue. 16 Overall median survival after SBRT has been reported as just over 20 months ( Table 1 ), with 5- and 10-year survival rates of 30 and 17%, respectively. 17 18 One prospective phase 2 study which combined patients with several oligometastatic cancer types including breast cancer, colorectal cancer, and lung cancer demonstrated a trend for prolonged survival in patients who received SBRT to all metastatic sites compared with patients who received chemotherapy alone. 19 However, the patients who received radiation had significantly a higher major adverse event rate compared with the control group (29 vs. 9%), with a nearly 5% rate of SBRT-related deaths. Thus, though it is possible that SBRT may extend survival, nearly a third may experience clinically significant adverse events.
Percutaneous Thermal Ablation
Liver lesions are most commonly treated with heat-based modalities including radiofrequency ablation (RFA) and microwave ablation (MWA) and performed with imaging guidance, usually under general anesthesia. Patient candidates include those with three or fewer lesions, each less than 3 cm in diameter and located away from a critical vessel or other organ such that an adequate margin can be achieved. 20
Survival outcomes are similar compared with surgical resection, with reported median overall survival ranging from 30 to 70 months ( Table 1 ), though ablation can often be performed on an outpatient basis, or with significantly shorter hospital stays. 21 Ablation is associated with a technical success rate of 95%. 21 22 23 Most adverse events are mild, 21 and the grade 3–4 adverse event rate is less than 10% ( Table 1 ). 24 One large retrospective case–control study compared patients who underwent thermal ablation or hepatectomy with patients with liver metastasis who did not undergo locoregional therapy, and found no survival benefit, though locoregional treatment rendered patients without any evidence of disease for long periods. 25 Roughly 15% of patients had no evidence of disease for more than 5 years following locoregional therapy, and over half of patients could refrain from systemic therapy for 2 years. Prospective randomized studies would be necessary to determine whether thermal ablation impacts survival, and future clinical studies could highlight benefits regarding management of systemic therapies as potential important outcome measures.
Local tumor progression after ablation may occur in more than 10% of cases, but can be avoided when margins exceed 5 mm. 24 Given the importance of margin assessment in precluding residual disease and recurrence, advanced imaging techniques are helpful to estimate the ablation margin. Breast cancer liver metastases are typically hypodense, hypoenhancing, and fluorodeoxyglucose- (FDG)-avid. 26 Because ablation zones are also hypodense and hypoenhancing, PET/CT imaging may be helpful to more accurately estimate the ablation zone and margin and assure eradication of tumor ( Fig. 2 ). 27
Fig. 2.
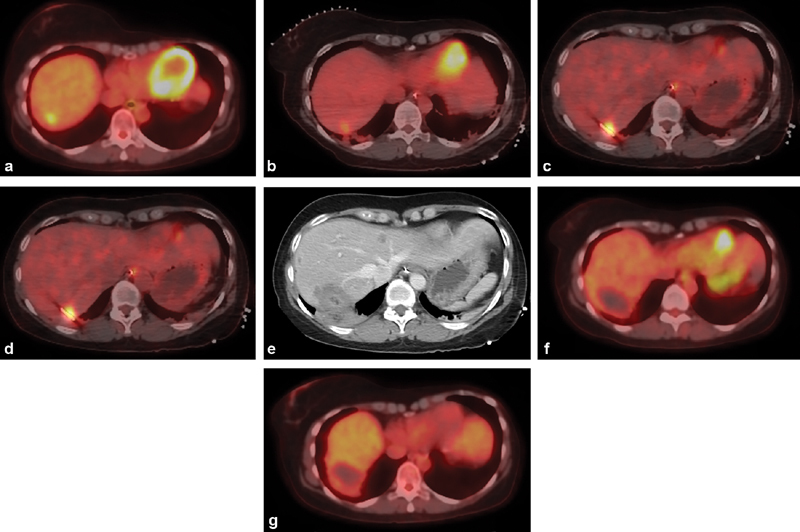
Percutaneous PET/CT-guided microwave ablation. ( a ) Axial image from a PET/CT in a 48-year-old woman with locally advanced hormone receptor-positive breast cancer shows a solitary FDG-avid liver metastasis in the hepatic dome, which developed 10 years after tamoxifen therapy. ( b ) Intraprocedure preablation PET/CT is obtained after administration of 4 mCi of FDG. ( c ) Once the ablation probe is placed, a repeat PET/CT is obtained to demonstrate positioning of the probe within the tumor. ( d ) Once ablation is complete, an additional 8-mCi FDG is administered and a final intraprocedure postablation PET/CT is obtained to demonstrate eradication of FDG-avid tumor. This imaging is performed 30 minutes after FDG administration; during that period, a contrast-enhanced CT is obtained to demonstrate the ablation zone ( e ). Follow-up PET/CT performed 6 weeks ( f ) and 6 months ( g ) later demonstrate no residual, recurrent, or new hepatic metastasis.
Eventually, over half of patients will develop new hepatic lesions despite eradication of targeted tumor. 24 25 These patients may be eligible for additional thermal ablation, or for embolotherapies described later in the “Multifocal Metastases” section.
Radiation Segmentectomy
Radiation segmentectomy, which entails the intra-arterial delivery of radiation doses of 200 Gy or greater to one or two hepatic segments, is an alternative local therapy when percutaneous thermal ablation is not feasible, such as when the lesion exceeds 3 cm or abuts critical structures ( Fig. 3 ). 28 While there are numerous publications regarding the efficacy of radiation segmentectomy for hepatocellular cancer, less is published regarding the use of this approach in metastatic hepatic disease. One study in a small number of patients including one patient with breast cancer suggested low complication rates and high objective response rates on early follow-up imaging. 24 Further research is necessary to delineate the expected outcomes following radiation segmentectomy of breast cancer liver metastasis.
Fig. 3.
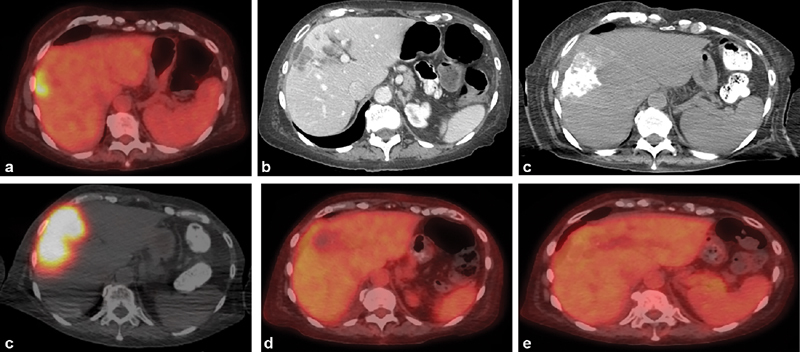
Radiation segmentectomy for solitary liver metastasis. ( a ) Axial image from PET/CT in a 69-year-old woman with hormone receptor-positive invasive breast cancer shows a solitary liver metastasis. ( b ) Axial image from a contrast-enhanced CT 2 months later demonstrates that tumor grew over 2 months despite systemic therapy. Liver biopsy demonstrated that the liver metastasis was HER2-negative, in contrast to the primary cancer. ( c ) Intraprocedural CTA identified the segmental arteries supplying the tumor, allowing for a segmental treatment. ( d ) Postadministration SPECT/CT demonstrates dense distribution of yttrium-90 within the tumor. Axial PET/CT images at 1 month ( e ) and 4 months ( f ) demonstrate complete response with no residual disease.
Comparing Local Therapies for Oligometastatic Disease
In summary, OMBC confined to the liver may be treated with locoregional therapies including surgical resection, external radiation, thermal ablation, and potentially radiation segmentectomy. No prospective studies have been performed to directly compare modalities. Surgery and ablation are performed in one visit, but surgery is associated with a significantly longer hospitalization. External radiation is delivered over multiple sessions on an outpatient basis over 2 weeks. Morbidity may be similar between approaches. Survival is difficult to compare between approaches, as different studies have reported different survival statistics. For all three treatments, smaller tumor size is associated with better outcomes. 29 The decision to proceed with one or another approach is at this point patient-specific, determined by location and size of tumor, candidacy for invasive therapies, and availability of equipment and advanced techniques.
Multifocal Metastases
Multifocal disease is defined as more than five well-defined metastatic sites that have the potential for extrahepatic spread and are unresectable. 30 Similar to OMBC, treatments for multifocal disease are palliative in nature, and systemic treatments alone may not provide adequate local control, leading to disease progression. Emerging locoregional treatment modalities, including yttrium-90 ( 90 Y) transarterial radioembolization (TARE) and transarterial chemoembolization (TACE), may be offered in the setting of liver-dominant progression to potentially support quality of life, preserve liver function, and prolong survival ( Table 2 ). 31
Table 2. Embolotherapies for multifocal breast cancer liver metastasis.
| Reference | Year | n | Treatment modality | OS | Other survival outcomes | Response criteria | Response rate | AE rate |
|---|---|---|---|---|---|---|---|---|
| Maes et al 59 | 2008 | 30 | Drug-eluting bead TACE | 7 mo | PFS: 3 mo | PERCIST | 3-mo DC: 44% | 20% grade 3 and higher AE |
| Vogl et al 43 | 2010 | 208 | Conventional TACE | 25 mo | RECIST | 3-mo DC: 64% | 0% major AE | |
| Cho et al 60 | 2010 | 10 | Drug-eluting bead TACE | 26 mo | RECIST | 1-mo DC: 50% | 70% any AE | |
| Duan et al 61 | 2011 | 44 | Drug-eluting bead TACE | 1-y OS: 63% 2-y OS: 48% 3-y OS: 28% |
RECIST | 1–3-mo DC: 84% | 77% any AE | |
| Martin et al 42 | 2012 | 40 | Drug-eluting bead TACE | PFS: 17 mo | RECIST | 1-mo DC: 90% | 17% any AE | |
| Nielsen et al 62 | 2012 | 16 | Drug-eluting bead TACE | 25 mo | PFS: 8 mo | RECIST 1.1 | 1-mo DC: 50% | 13% grade 3 and higher AE |
| Eichler et al 63 | 2013 | 43 | Drug-eluting bead TACE | 12 mo | RECIST | 3-mo DC: 44% | 20% any AE | |
| Damian et al 64 | 2013 | 14 | Drug-eluting bead TACE | 26 mo | RECIST | |||
| Lin et al 65 | 2017 | 23 | Drug-eluting bead TACE | 17 mo | RECIST 1.1 | 3-mo DC: 83% | 35% major AE | |
| Li et al 45 | 2005 | 28 | Drug-eluting bead TACE | 28 mo | WHO | 3-mo DC: 71% | 0% grade 3 and higher AE | |
| Chang et al 44 | 2018 | 17 | Drug-eluting bead TACE | 5 mo | mRECIST | 3-mo DC: 29% | 9% grade 3 and higher, 71% any AE | |
| Haug et al 39 | 2012 | 58 | Resin TARE | 11 mo | RECIST 1.1 | 3-mo DC: 66% | ||
| Cianni et al 35 | 2013 | 77 | Resin TARE | 11 mo | RECIST | 2-mo DC: 80% | 3% grade 3, 7% grade 1–2 AE | |
| Saxena et al 32 | 2014 | 40 | Resin TARE | 11 mo | RECIST | 3-mo DC: 70% | 40% grade 1–2 AE | |
| Gordon et al 66 | 2014 | 75 | Resin TARE | 7 mo | RECIST | 1-mo DC: 98% | 8% grade 3 and higher AE | |
| Pieper et al 36 | 2016 | 44 | Resin TARE | 6 mo | RECIST 1.1 | 3-mo DC: 82% | 2% grade 3 and higher AE | |
| Bangash et al 67 | 2007 | 27 | Glass TARE | 6 mo | WHO | 3-month DC 91% | 11% grade 3 and higher AE | |
| Fendler et al 34 | 2016 | 81 | Glass and resin TARE | 8 mo | PERCIST | 3-mo OR: 52% | <10% grade 3 and higher AE | |
| Deipolyi et al 38 | 2018 | 31 | Glass and resin TARE | 11 mo | PERCIST | 3–5-mo OR: 69% | 12% major AE | |
| Chang et al 44 | 2018 | 30 | Glass and resin TARE | 13 mo | mRECIST | 3-mo DC: 47% | 0% grade 3 and higher, 44% any AE | |
| Deipolyi et al 41 | 2019 | 49 | Glass and resin TARE | 11 mo | PERCIST | 3–5-mo OR: 75% | 8% grade 3 and higher AE |
Abbreviations: AE, adverse event; DC, disease control including stable disease, partial response, and complete response; mRECIST, modified RECIST; OR, objective response, including partial and complete response; OS, overall survival; PERCIST, Positron Emission Tomography Response Criteria In Solid Tumors; PFS, progression-free survival; RECIST, Response Evaluation Criteria in Solid Tumors; TACE, transarterial chemoembolization; TARE, transarterial radioembolization; WHO, World Health Organization.
Notes: Selected studies are presented. Survival reported as median value from the time of treatment; n indicates number of breast cancer patients studied.
Radioembolization
TARE may involve lobar or segmental treatment depending on size, number, and locations of metastases ( Fig. 4 ). 32 33 34 When performed for breast cancer metastasis, TARE is associated with a less than 15% grade 3 and higher adverse events, including nontarget embolization causing gastroduodenal ulcers and liver failure. 32 33 By RECIST criteria, disease control rates at 1 to 3 months after treatment range from 80 to 100% ( Table 2 ). Overall survival ranges between 6 and 14 months following treatment. 32 34 35 36 Systemic therapies are most commonly continued before and after TARE, except for bevacizumab which can potentiate vascular complications such as dissection and is held at minimum for 2 weeks, and optimally longer for 4 to 6 weeks. 37
Fig. 4.
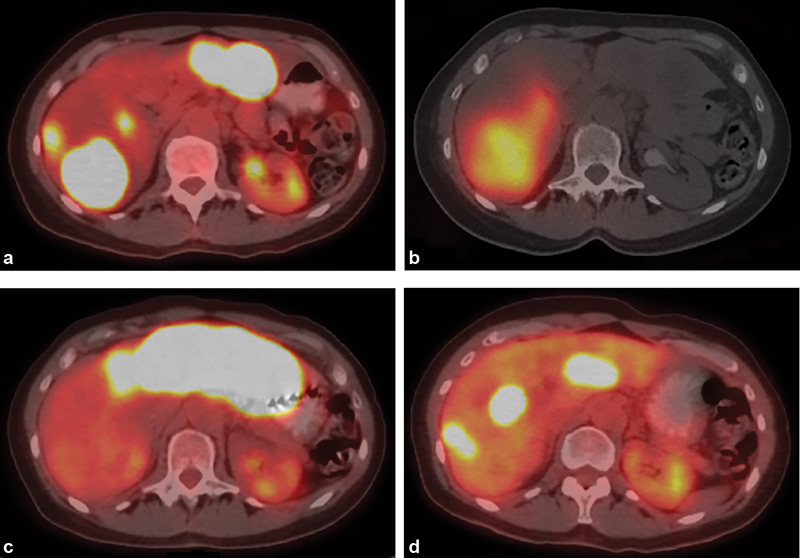
Radioembolization of multifocal liver metastasis. (a) Axial image from PET/CT in a 57-year-old woman with hormone receptor-positive breast cancer shows multifocal bilobar liver metastasis progressing despite several lines of systemic therapy. ( b ) Axial SPECT/CT image obtained immediately following right lobar radioembolization demonstrates distribution of yttrium-90 within right hepatic metastases. ( c ) Axial image from PET/CT 2 months later demonstrates complete response in right hepatic metastases, but progression in left lobar metastases, with subsequent left lobar radioembolization. ( d ) Axial image from PET/CT 2 months after left lobar radioembolization demonstrates partial response in left hepatic metastases, with interval regrowth in the right lobe.
Optimal imaging follow-up after TARE for breast cancer liver metastasis has not been established, but may entail metabolic imaging. Several studies have demonstrated that imaging response on early follow-up PET/CT 2 to 4 months after TARE is associated with longer survival. 38 39 Furthermore, breast cancer is hypoenhancing, 26 suggesting that response criteria based on enhancement will not be helpful. For other hypovascular tumors such as colorectal cancer metastasis, anatomic/size-based imaging criteria do not reflect posttreatment pathological response. 40 Taken together, findings suggest that PET/CT response assessments may better predict survival and reflect pathologic response compared with size- or enhancement-based strategies, and may therefore be the optimal follow-up imaging strategy. After TARE administered for multifocal breast cancer liver metastasis, complete or partial response is often noted by 1 to 3 months on PET/CT, and endures until approximately 6 months after treatment. 41
Transarterial Chemoembolization
TACE involves the transarterial administration of chemotherapy drugs, often in a lobar distribution. 42 Chemotherapy is administered with ethiodized oil in the context of conventional TACE, or loaded onto drug-eluting beads containing doxorubicin or other common chemotherapy drugs. 43 TACE may involve a postprocedural hospitalization of 1 or more days. 43 Possible complications include leukopenia (11%), hypochromia (11%), thrombocytopenia (7%), gastrointestinal system reaction (4%), and renal dysfunction (18%). 42 Reported major adverse events rates range from 0 to 35%, and by RECIST criteria, disease control rates at 1 to 3 months range from 40 to 90% ( Table 2 ). 42 44 45 Most retrospective studies evaluating tumor response after TACE for breast cancer liver metastasis have used RECIST; data regarding newer response criteria based on metabolic imaging are limited.
Comparing Embolotherapies
TACE and TARE have similar reported survival outcomes given that they are palliative treatments for patients who typically have refractory disease. 46 A retrospective literature search comparing the two treatment modalities by RECIST imaging response criteria show better disease control rates for TARE (78%) compared with TACE (59%). 46 While survival outcomes are similar and wide ranging in various retrospective studies, one single-center retrospective study comparing both modalities demonstrated a trend for longer survival after TARE compared with TACE. 44
TARE is associated with fewer adverse events and potentially better quality of life compared with TACE. The single-center retrospective study comparing TACE and TARE reported significantly higher adverse event rates for TACE compared with TARE. 44 In the context of hepatocellular carcinoma, TARE is associated with improved quality-of-life scores, whereas TACE is associated with worsened quality-of-life scores after treatment. 31 It is not clear what drives this finding, though TACE often entails hospitalization for one or more nights, 34 whereas TARE is performed on an outpatient basis. Similar studies have not yet been conducted in the context of breast cancer.
Conclusion
Several locoregional therapies have emerged in the treatment of liver-dominant metastatic breast cancer. In the setting of oligometastatic disease, locoregional therapies including hepatectomy, SBRT, percutaneous thermal ablation, and radiation segmentectomy could potentially prolong survival, particularly when performed in conjunction with systemic therapies. However, prospective studies demonstrating survival or quality-of-life benefits, or comparing treatment modalities have not been performed.
For multifocal hepatic metastasis, both TACE and TARE have been applied. Retrospective data suggest that compared with TACE, TARE offers improved imaging outcomes, reduced adverse events, improved quality of life, and potentially longer survival. Variation in follow-up imaging strategies between studies hampers direct comparison between modalities, and prospective comparative studies are needed. Retrospective evidence suggests that metabolic imaging with PET/CT may be the most helpful imaging strategy, given the strong relationship between PET/CT imaging outcomes and survival after TARE.
Additionally, though overall survival and progress-free survival have been traditional outcomes in clinical studies, locoregional therapies may provide other benefits such as delaying changes in systemic therapy or allowing patients to refrain from systemic therapy. These other outcomes warrant future investigation and validation. Finally, there is wide variation between patients in outcomes following liver-directed therapy, and the variables determining these outcomes are not currently known. Delineating factors that predict outcomes after locoregional therapies would enable a more personalized approach to treating breast cancer liver metastasis, excluding patients who are unlikely to benefit and avoiding the potential unnecessary risks of invasive procedures. The efficacy of locoregional therapies may also be improved by combination strategies, such as using immunotherapy to generate abscopal effects or radiosensitizers to enhance response to radiotherapies. Much work is needed to justify and expand the application of liver-directed therapy in metastatic breast cancer.
Funding Statement
Funding This research was funded through the NIH/NCI Cancer Center Support Grant P30 CA008748. A.R.D. reports personal fees from BTG, Inc. and from Dova Pharmaceuticals, outside the submitted work.
Footnotes
Conflict of Interest None declared.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(05):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Hagemeister F B, Jr, Buzdar A U, Luna M A, Blumenschein G R. Causes of death in breast cancer: a clinicopathologic study. Cancer. 1980;46(01):162–167. doi: 10.1002/1097-0142(19800701)46:1<162::aid-cncr2820460127>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 3.Kenny L M, Orsi F, Adam A. Interventional radiology in breast cancer. Breast. 2017;35:98–103. doi: 10.1016/j.breast.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 4.Adam R, Aloia T, Krissat J.Is liver resection justified for patients with hepatic metastases from breast cancer? Ann Surg 200624406897–907., discussion 907–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DESTINY-Breast01 Investigators . Modi S, Saura C, Yamashita T. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382(07):610–621. doi: 10.1056/NEJMoa1914510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waks A G, Winer E P. Breast cancer treatment: a review. JAMA. 2019;321(03):288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 7.Bianchini G, Balko J M, Mayer I A, Sanders M E, Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13(11):674–690. doi: 10.1038/nrclinonc.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.EORTC Breast Cancer and Early Clinical Studies Groups . Atalay G, Biganzoli L, Renard F. Clinical outcome of breast cancer patients with liver metastases alone in the anthracycline-taxane era: a retrospective analysis of two prospective, randomised metastatic breast cancer trials. Eur J Cancer. 2003;39(17):2439–2449. doi: 10.1016/s0959-8049(03)00601-4. [DOI] [PubMed] [Google Scholar]
- 9.Cardoso F, Costa A, Senkus E. 3rd ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 3) Ann Oncol. 2017;28(12):3111–3111. doi: 10.1093/annonc/mdx036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi T, Ichiba T, Sakuyama T. Possible clinical cure of metastatic breast cancer: lessons from our 30-year experience with oligometastatic breast cancer patients and literature review. Breast Cancer. 2012;19(03):218–237. doi: 10.1007/s12282-012-0347-0. [DOI] [PubMed] [Google Scholar]
- 11.Seidensticker M, Garlipp B, Scholz S. Locally ablative treatment of breast cancer liver metastases: identification of factors influencing survival (the Mammary Cancer Microtherapy and Interventional Approaches (MAMMA MIA) study) BMC Cancer. 2015;15:517. doi: 10.1186/s12885-015-1499-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruiz A, Sebagh M, Wicherts D A. Long-term survival and cure model following liver resection for breast cancer metastases. Breast Cancer Res Treat. 2018;170(01):89–100. doi: 10.1007/s10549-018-4714-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caralt M, Bilbao I, Cortés J. Hepatic resection for liver metastases as part of the “oncosurgical” treatment of metastatic breast cancer. Ann Surg Oncol. 2008;15(10):2804–2810. doi: 10.1245/s10434-008-0072-2. [DOI] [PubMed] [Google Scholar]
- 14.Kim J Y, Park J S, Lee S A. Does liver resection provide long-term survival benefits for breast cancer patients with liver metastasis? A single hospital experience. Yonsei Med J. 2014;55(03):558–562. doi: 10.3349/ymj.2014.55.3.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polistina F, Costantin G, Febbraro A, Robusto E, Ambrosino G. Aggressive treatment for hepatic metastases from breast cancer: results from a single center. World J Surg. 2013;37(06):1322–1332. doi: 10.1007/s00268-013-1986-9. [DOI] [PubMed] [Google Scholar]
- 16.Milano M T, Katz A W, Zhang H, Okunieff P. Oligometastases treated with stereotactic body radiotherapy: long-term follow-up of prospective study. Int J Radiat Oncol Biol Phys. 2012;83(03):878–886. doi: 10.1016/j.ijrobp.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 17.Palma D A, Olson R A, Harrow S. Stereotactic ablative radiation therapy for the comprehensive treatment of oligometastatic tumors (SABR-COMET): results of a randomized trial. Int J Radiat Oncol Biol Phys. 2018;102(03):S3–S4. [Google Scholar]
- 18.Chmura S J, Winter K, Salama J K. Phase I trial of stereotactic body radiation therapy (SBRT) to multiple metastatic sites: a NRG oncology study. Int J Radiat Oncol. 2018;102(03):S68–S69. [Google Scholar]
- 19.Palma D A, Olson R, Harrow S.Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial Lancet 2019393(10185):2051–2058. [DOI] [PubMed] [Google Scholar]
- 20.Kucharczyk M J, Parpia S, Walker-Dilks C, Banfield L, Swaminath A. Ablative therapies in metastatic breast cancer: a systematic review. Breast Cancer Res Treat. 2017;164(01):13–25. doi: 10.1007/s10549-017-4228-2. [DOI] [PubMed] [Google Scholar]
- 21.Sofocleous C T, Nascimento R G, Gonen M. Radiofrequency ablation in the management of liver metastases from breast cancer. AJR Am J Roentgenol. 2007;189(04):883–889. doi: 10.2214/AJR.07.2198. [DOI] [PubMed] [Google Scholar]
- 22.Jakobs T F, Hoffmann R-T, Schrader A. CT-guided radiofrequency ablation in patients with hepatic metastases from breast cancer. Cardiovasc Intervent Radiol. 2009;32(01):38–46. doi: 10.1007/s00270-008-9384-7. [DOI] [PubMed] [Google Scholar]
- 23.Meloni M F, Andreano A, Laeseke P F, Livraghi T, Sironi S, Lee F T., Jr Breast cancer liver metastases: US-guided percutaneous radiofrequency ablation--intermediate and long-term survival rates. Radiology. 2009;253(03):861–869. doi: 10.1148/radiol.2533081968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ridouani F, Solomon S B, Bryce Y, Bromberg J F, Sofocleous C T, Deipolyi A R. Predictors of progression-free survival and local tumor control after percutaneous thermal ablation of oligometastatic breast cancer: retrospective study. J Vasc Interv Radiol. 2020;31(08):1201–1209. doi: 10.1016/j.jvir.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 25.Sadot E, Lee S Y, Sofocleous C T. Hepatic resection or ablation for isolated breast cancer liver metastasis: a case-control study with comparison to medically treated patients. Ann Surg. 2016;264(01):147–154. doi: 10.1097/SLA.0000000000001371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niikura N, Costelloe C M, Madewell J E. FDG-PET/CT compared with conventional imaging in the detection of distant metastases of primary breast cancer. Oncologist. 2011;16(08):1111–1119. doi: 10.1634/theoncologist.2011-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryan E R, Sofocleous C T, Schöder H. Split-dose technique for FDG PET/CT-guided percutaneous ablation: a method to facilitate lesion targeting and to provide immediate assessment of treatment effectiveness. Radiology. 2013;268(01):288–295. doi: 10.1148/radiol.13121462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barral M, Auperin A, Hakime A. Percutaneous thermal ablation of breast cancer metastases in oligometastatic patients. Cardiovasc Intervent Radiol. 2016;39(06):885–893. doi: 10.1007/s00270-016-1301-x. [DOI] [PubMed] [Google Scholar]
- 29.Bale R, Putzer D, Schullian P. Local treatment of breast cancer liver metastasis. Cancers (Basel) 2019;11(09):1341. doi: 10.3390/cancers11091341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pekar G, Hofmeyer S, Tabár L. Multifocal breast cancer documented in large-format histology sections: long-term follow-up results by molecular phenotypes. Cancer. 2013;119(06):1132–1139. doi: 10.1002/cncr.27877. [DOI] [PubMed] [Google Scholar]
- 31.Salem R, Gilbertsen M, Butt Z. Increased quality of life among hepatocellular carcinoma patients treated with radioembolization, compared with chemoembolization. Clin Gastroenterol Hepatol. 2013;11(10):1358–13650. doi: 10.1016/j.cgh.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 32.Saxena A, Kapoor J, Meteling B, Morris D L, Bester L. Yttrium-90 radioembolization for unresectable, chemoresistant breast cancer liver metastases: a large single-center experience of 40 patients. Ann Surg Oncol. 2014;21(04):1296–1303. doi: 10.1245/s10434-013-3436-1. [DOI] [PubMed] [Google Scholar]
- 33.Jakobs T F, Hoffmann R-T, Fischer T. Radioembolization in patients with hepatic metastases from breast cancer. J Vasc Interv Radiol. 2008;19(05):683–690. doi: 10.1016/j.jvir.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 34.Fendler W P, Lechner H, Todica A. Safety, efficacy, and prognostic factors after radioembolization of hepatic metastases from breast cancer: a large single-center experience in 81 patients. J Nucl Med. 2016;57(04):517–523. doi: 10.2967/jnumed.115.165050. [DOI] [PubMed] [Google Scholar]
- 35.Cianni R, Pelle G, Notarianni E. Radioembolisation with (90)Y-labelled resin microspheres in the treatment of liver metastasis from breast cancer. Eur Radiol. 2013;23(01):182–189. doi: 10.1007/s00330-012-2556-5. [DOI] [PubMed] [Google Scholar]
- 36.Pieper C C, Meyer C, Wilhelm K E. Yttrium-90 radioembolization of advanced, unresectable breast cancer liver metastases - a single-center experience. J Vasc Interv Radiol. 2016;27(09):1305–1315. doi: 10.1016/j.jvir.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 37.Bilbao J L, Iezzi R, Goldberg S N. The ten commandments of hepatic radioembolization: expert discussion and report from Mediterranean Interventional Oncology (MIOLive) congress 2017. Eur Rev Med Pharmacol Sci. 2017;21(18):4014–4021. [PubMed] [Google Scholar]
- 38.Deipolyi A R, Riedl C C, Bromberg J. Association of PI3K pathway mutations with early positron-emission tomography/CT imaging response after radioembolization for breast cancer liver metastases: results of a single-center retrospective pilot study. J Vasc Interv Radiol. 2018;29(09):1226–1235. doi: 10.1016/j.jvir.2018.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haug A R, Tiega Donfack B P, Trumm C. 18F-FDG PET/CT predicts survival after radioembolization of hepatic metastases from breast cancer. J Nucl Med. 2012;53(03):371–377. doi: 10.2967/jnumed.111.096230. [DOI] [PubMed] [Google Scholar]
- 40.Chalabi M, Fanchi L F, Dijkstra K K. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med. 2020;26(04):566–576. doi: 10.1038/s41591-020-0805-8. [DOI] [PubMed] [Google Scholar]
- 41.Deipolyi A R, England R W, Ridouani F. PET/CT imaging characteristics after radioembolization of hepatic metastasis from breast cancer. Cardiovasc Intervent Radiol. 2020;43(03):488–494. doi: 10.1007/s00270-019-02375-7. [DOI] [PubMed] [Google Scholar]
- 42.Martin R C, Robbins K, Fagés J F. Optimal outcomes for liver-dominant metastatic breast cancer with transarterial chemoembolization with drug-eluting beads loaded with doxorubicin. Breast Cancer Res Treat. 2012;132(02):753–763. doi: 10.1007/s10549-011-1926-z. [DOI] [PubMed] [Google Scholar]
- 43.Vogl T J, Naguib N N, Nour-Eldin N-E, Eichler K, Zangos S, Gruber-Rouh T. Transarterial chemoembolization (TACE) with mitomycin C and gemcitabine for liver metastases in breast cancer. Eur Radiol. 2010;20(01):173–180. doi: 10.1007/s00330-009-1525-0. [DOI] [PubMed] [Google Scholar]
- 44.Chang J, Charalel R, Noda C. Liver-dominant breast cancer metastasis: a comparative outcomes study of chemoembolization versus radioembolization . Anticancer Res. 2018;38(05):3063–3068. doi: 10.21873/anticanres.12563. [DOI] [PubMed] [Google Scholar]
- 45.Li X P, Meng Z-Q, Guo W-J, Li J. Treatment for liver metastases from breast cancer: results and prognostic factors. World J Gastroenterol. 2005;11(24):3782–3787. doi: 10.3748/wjg.v11.i24.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mouli S K, Gupta R, Sheth N, Gordon A C, Lewandowski R J. Locoregional therapies for the treatment of hepatic metastases from breast and gynecologic cancers. Semin Intervent Radiol. 2018;35(01):29–34. doi: 10.1055/s-0038-1636518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vlastos G, Smith D L, Singletary S E. Long-term survival after an aggressive surgical approach in patients with breast cancer hepatic metastases. Ann Surg Oncol. 2004;11(09):869–874. doi: 10.1245/ASO.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 48.Dutch Liver Surgeons Group . van Walsum G AM, de Ridder J AM, Verhoef C. Resection of liver metastases in patients with breast cancer: survival and prognostic factors. Eur J Surg Oncol. 2012;38(10):910–917. doi: 10.1016/j.ejso.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 49.Abbott D E, Brouquet A, Mittendorf E A. Resection of liver metastases from breast cancer: estrogen receptor status and response to chemotherapy before metastasectomy define outcome. Surgery. 2012;151(05):710–716. doi: 10.1016/j.surg.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mariani P, Servois V, De Rycke Y. Liver metastases from breast cancer: Surgical resection or not? A case-matched control study in highly selected patients. Eur J Surg Oncol. 2013;39(12):1377–1383. doi: 10.1016/j.ejso.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 51.Margonis G A, Buettner S, Sasaki K. The role of liver-directed surgery in patients with hepatic metastasis from primary breast cancer: a multi-institutional analysis. HPB (Oxford) 2016;18(08):700–705. doi: 10.1016/j.hpb.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruiz A, van Hillegersberg R, Siesling S. Surgical resection versus systemic therapy for breast cancer liver metastases: Results of a European case matched comparison. Eur J Cancer. 2018;95:1–10. doi: 10.1016/j.ejca.2018.02.024. [DOI] [PubMed] [Google Scholar]
- 53.Wieners G, Mohnike K, Peters N. Treatment of hepatic metastases of breast cancer with CT-guided interstitial brachytherapy - a phase II-study. Radiother Oncol. 2011;100(02):314–319. doi: 10.1016/j.radonc.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 54.Mahadevan A, Blanck O, Lanciano R. Stereotactic body radiotherapy (SBRT) for liver metastasis - clinical outcomes from the international multi-institutional RSSearch® Patient Registry. Radiat Oncol. 2018;13(01):26. doi: 10.1186/s13014-018-0969-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Onal C, Guler O C, Yildirim B A. Treatment outcomes of breast cancer liver metastasis treated with stereotactic body radiotherapy. Breast. 2018;42:150–156. doi: 10.1016/j.breast.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 56.Veltri A, Gazzera C, Barrera M. Radiofrequency thermal ablation (RFA) of hepatic metastases (METS) from breast cancer (BC): an adjunctive tool in the multimodal treatment of advanced disease. Radiol Med (Torino) 2014;119(05):327–333. doi: 10.1007/s11547-013-0354-z. [DOI] [PubMed] [Google Scholar]
- 57.Kümler I, Parner V K, Tuxen M K. Clinical outcome of percutaneous RF-ablation of non-operable patients with liver metastasis from breast cancer. Radiol Med (Torino) 2015;120(06):536–541. doi: 10.1007/s11547-014-0489-6. [DOI] [PubMed] [Google Scholar]
- 58.Bai X-M, Yang W, Zhang Z-Y. Long-term outcomes and prognostic analysis of percutaneous radiofrequency ablation in liver metastasis from breast cancer. Int J Hyperthermia. 2019;35(01):183–193. doi: 10.1080/02656736.2018.1488279. [DOI] [PubMed] [Google Scholar]
- 59.Maes T, Wildiers H, Heye S. Intra-hepatic mitomycin C bolus infusion in the treatment of extensive liver metastases of breast cancer. Breast Cancer Res Treat. 2008;110(01):135–142. doi: 10.1007/s10549-007-9707-4. [DOI] [PubMed] [Google Scholar]
- 60.Cho S W, Kitisin K, Buck D. Transcatheter arterial chemoembolization is a feasible palliative locoregional therapy for breast cancer liver metastases. Int J Surg Oncol. 2010;2010:251621. doi: 10.1155/2010/251621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duan X F, Dong N N, Zhang T, Li Q. Treatment outcome of patients with liver-only metastases from breast cancer after mastectomy: a retrospective analysis. J Cancer Res Clin Oncol. 2011;137(09):1363–1370. doi: 10.1007/s00432-011-1008-y. [DOI] [PubMed] [Google Scholar]
- 62.Nielsen D L, Nørgaard H, Vestermark L W. Intrahepatic and systemic therapy with oxaliplatin combined with capecitabine in patients with hepatic metastases from breast cancer. Breast. 2012;21(04):556–561. doi: 10.1016/j.breast.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 63.Eichler K, Jakobi S, Gruber-Rouh T, Hammerstingl R, Vogl T J, Zangos S. Transarterial chemoembolisation (TACE) with gemcitabine: phase II study in patients with liver metastases of breast cancer. Eur J Radiol. 2013;82(12):e816–e822. doi: 10.1016/j.ejrad.2013.08.046. [DOI] [PubMed] [Google Scholar]
- 64.Damian S, Tessari A, Capri G.Fondazione IRCCS Istituto Nazionale dei Tumori M, Fondazione IRCCS Istituto Nazionale dei Tumori M. Hepatic trans-arterial chemoembolization (TACE) in metastatic breast cancerAccessed July 29, 2020 at:https://meetinglibrary.asco.org/record/86304/abstract
- 65.Lin Y-T, Médioni J, Amouyal G, Déan C, Sapoval M, Pellerin O. Doxorubicin-loaded 70-150 μm microspheres for liver-dominant metastatic breast cancer: results and outcomes of a pilot study. Cardiovasc Intervent Radiol. 2017;40(01):81–89. doi: 10.1007/s00270-016-1465-4. [DOI] [PubMed] [Google Scholar]
- 66.Gordon A C, Gradishar W J, Kaklamani V G.Yttrium-90 radioembolization stops progression of targeted breast cancer liver metastases after failed chemotherapy J Vasc Interv Radiol 201425101523–1532., 1532.e1–1532.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bangash A K, Atassi B, Kaklamani V. 90Y radioembolization of metastatic breast cancer to the liver: toxicity, imaging response, survival. J Vasc Interv Radiol. 2007;18(05):621–628. doi: 10.1016/j.jvir.2007.02.019. [DOI] [PubMed] [Google Scholar]


