Abstract
Exomic rare variant polymorphisms (~300,000) were analyzed in the Scripps Venous Thrombosis (VTE) registry (subjects < 55 years old). Besides F5 SNPs, FAM134B (rs78314670, Arg127Cys) and MYH8 (rs111567318, Glu1838Ala) SNPs were associated with recurrent VTE (N=34 cases) (FDR p<0.05). FAM134B (rs78314670) was associated with low plasma levels of anticoagulant glucosylceramide. Analysis of 50 chr17p13.1 MYH rare SNPs (clustered skeletal myosin heavy chain genes) using collapsing methods was associated with recurrent VTE (p=2.70 x10−16). When injected i.v., skeletal muscle myosin was procoagulant in a hemophilia mouse tail bleeding model. Thus, FAM134B and MYH genetic variants are plausibly linked to VTE risk.
Keywords: myosin, FAM134B, MYH, venous thrombosis, glucosylceramide
Introduction
Venous thromboembolism (VTE) contributes to morbidity and mortality in many patients, but genetic or acquired biomarkers or risk factors have not been identified for a substantial percentage of VTE cases (Di Nisio, et al 2016, Tregouet and Morange 2018). Genomic-based research is a powerful tool for discovery and confirmation of factors linked to VTE, and genome wide association studies (GWAS) that typically interrogate common variants have added important insights (Morange, et al 2015, Tregouet and Morange 2018). Recent meta-analyses identified several variants associated with VTE (Klarin, et al 2017, Lindstrom, et al 2019a, Tang, et al 2020). Recently discovered variants linked to VTE lack functional associations with regulation of blood coagulation. Here we used exome genotyping arrays for low frequency or rare missense variants using a younger VTE population in order to discover currently unknown VTE genetic risk factors.
Design and methods
Materials and methods are provided in Supporting Information.
Patients
The study population consisted of 104 VTE cases and 211 controls under age 55 years old from the Scripps Venous Thrombosis Registry (Table S1) (Deguchi, et al 2017).
Exomic array analysis
Subjects were genotyped at the DNA Core Laboratory of the Scripps Research Institute using the Axiom® Exome Genotyping Array (Affymetrix).
Tail Clip Procedure
Tail clip mouse hemophilic model with anti-FVIII antibody was employed to test skeletal muscle myosin’s procoagulant effect in vivo. All animal protocols were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute, (see details in Supporting Information.)
Statistical Analysis
The method details used for statistical analyses are presented in Supplemental Materials.
Results
Exomic low abundance variants and VTE
Initial exome chip-based association of 104 adult VTE cases vs. 211 controls identified two F5 related SNPs (rs6025 (F5 Leiden) and rs6687813) as significantly associated with VTE with FDR-adjusted p < 0.05 (Table 1, p=3.46 x10−8). No other significant SNPs were identified.
Table 1. The association of any VTE or recurrent VTE with genotypes for F5, FAM134B, MYH8, and two MYH variant sets containing either 7 or 9 MYH SNPs.
The association of F5 rs6025, FAM134B rs78314670, MYH8 rs111567318, and two variant sets of MYH variants were made for all VTE cases (N=104) vs controls (N=211) and for adult recurrent VTE cases (N=32) vs. controls (N=211), as noted. See Table 4S listing for 7 rare MYH variants with MAF < 0.01 and 2 uncommon MYH variants with MAF > 0.01 and < 0.05 which were used to define sets of MYH variants that were collapsed into the two variant sets used for comparisons in this Table.
| Gene | SNP | MAF | p value | |||
|---|---|---|---|---|---|---|
| control (N=211) | VTE all cases (N=104) | VTE recurrence (N=32) | VTE all cases | VTE recurrence | ||
| F5 | rs6025 | 0.021 | 0.130 | 0.141 | 3.46 x 10−8 | 2.68 x 10−6 |
| FAM134B | rs78314670 | 0.002 | 0.037 | 0.078 | 3.10 x 10−4 | 3.37 x 10−7 |
| MYH8 | rs111567318 | 0 | 0.019 | 0.047 | 4.10 x 10−3 | 8.55 x 10−6 |
| MYH variant set | 7 rare SNPs (MYH1,2,8) | 0 | 0.063 | 0.156 | 2.11 x 10−7 | 2.70 x 10−16 |
| MYH variant set | 7 rare + 2 uncommon SNPs (MYH1,2,4,8) | 0.047 | 0.125 | 0.219 | 4.30 x 10−4 | 6.03 x 10−7 |
For subgroup analysis, recurrent VTE cases (under age 55 years old) who are likely enriched with genetic risk factors were compared to controls (32 recurrent VTE cases vs. 211 controls), and 28 SNPs, including SNPs for FAM134B (rs78314670), F5 (rs6025 (F5 Leiden) and rs6687813), and MYH8 (rs111567318), achieved significance (FDR p < 0.05) (Tables 1 and S2). 22 of these 28 SNPs were uncommon or rare SNPs with minor allele frequency (MAF) of ≤ 0.05 to 0.01 or < 0.01, respectively (Table S2). The gnomAD database agreed with the MAF here for controls (Table S2), supporting the significance of accumulation here of variants in VTE. Literature searching of candidate SNP genes (Table S2) for any association with thrombosis, blood coagulation, fibrinolysis, or lipid metabolism identified FAM134B and skeletal muscle myosin (MYH) as two interesting candidates meriting further investigations for plausibility for VTE causation (Table S3).
Association of the FAM134B rs78314670 SNP with low plasma levels of glucosylceramide
FAM134B is a cis-Golgi transmembrane protein (Islam, et al 2018) and increases the activity of acetyl-CoA carboxylase and fatty acid synthase (Yuan, et al 2014), leading to an increase in a precursor of sphingolipids, palmitoyl-CoA, suggesting that FAM134B may affect the metabolism of sphingolipids. Glucosylceramide (GlcCer) is an anticoagulant sphingolipid cofactor for activated protein C, and plasma GlcCer deficiency has been definitively associated with increased risk of VTE based on multiple case-control studies (Deguchi, et al 2017). When the FAM134B rs78314670 SNP was analyzed for association with low plasma GlcCer, 7 out of 8 FAM134B rs78314670 carriers had <10%-ile of control GlcCer plasma levels (p< 0.0001, Fisher’s exact test) (Figure 1A). Thus, FAM134B rs78314670 could be causal for low GlcCer plasma levels associated with VTE.
Figure 1. FAM134B SNP association with low GlcCer plasma level (A) and skeletal muscle myosin’s effect on blood loss during tail bleeding in hemophilia A mouse models (B).
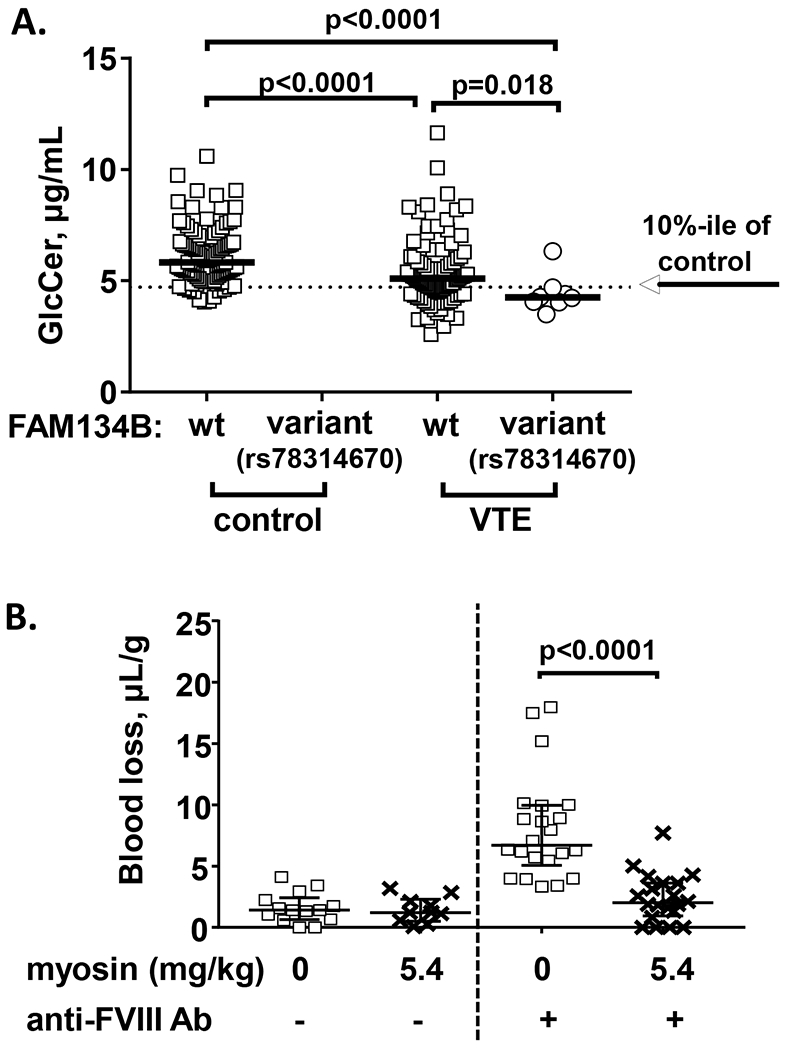
(A) The distribution of plasma GlcCer levels in VTE patients and controls with/without FAM134B rs78314670 mutation were shown. The plasma levels of GlcCer are shown as μg/mL. Solid thick lines indicate median values and the dotted line indicate the value for the 10th percentile of the control values. The difference of median values between VTE patients and controls was calculated by Mann-Whitney test and p-values were shown.
(B) Wild type C57BL/6J mice were injected with 0.25 mg/kg of anti-FVIII antibody (acquired hemophilia A model) (N=32) or vehicle (saline) (N=24) at 2 hours prior to tail cut. Then, mice were injected with vehicle (saline) or 5.4 mg/kg of rabbit skeletal muscle myosin at 15 min prior to tail cut. The distal portion of the tail was surgically removed via a scalpel blade at 1.5-mm tail diameter to induce a moderate bleeding effect. Tails were immersed in 50 mL of saline at 37°C and total blood loss per mouse weight (μL/g) was measured as the blood volume collected during 10 min following tail cut. Bars indicated median with interquartile range shown.
Association of MYH gene variants with recurrent VTE using collapsing analysis
Simple association tests for GWAS (e.g., univariate association tests) have poor power for analysis of rare variants. To enable testing the significance of low frequency missense variants, rare variant collapsing association tests can be employed (Auer and Lettre 2015, Bansal, et al 2010, Morange, et al 2015, Tregouet and Morange 2018). Rare variant sets can be defined in many ways, usually by proximity to individual genes, but also can be formed into multi-gene sets based on proximity to genes related by shared biological process or molecular function (Bansal, et al 2010).
The MYH8 gene and five highly homologous MYH genes MYH1, MYH2, MYH3, MYH13 and MYH4 are found at the chromosome 17p13.1 locus (Yoon, et al 1992). 356 SNPs in this region are interrogated by the Axiome Exome array. The rare allele was present in at least one VTE case or control at 78 of these 356 SNP sites, and univariate analysis of these SNPs for association with recurrent VTE identified 11 SNPs associated with recurrent VTE with unadjusted p<0.05 while two SNPs, MYH8 rs111567318 and MYH2 rs11658164, were each individually significant after FDR adjustment, suggestive of MYH8 and MYH2 genes being linked to VTE (Table S4).
For rare variant collapsing analysis of MYH genes, two variant sets were defined. One set comprised the 7 rare missense MYH variants (MAF < 0.01 in controls) and the other set comprised these 7 rare variants plus the 2 uncommon missense variants (MAF < 0.05, but > 0.01 in controls) (Table S4). A meta-SNP was defined for each individual by counting the total number of minor alleles across these variants. Genetic association was determined for all VTE cases (n=104) vs. controls and for recurrent VTE cases (n=32) vs. controls (n=211) (Table 1). For the MYH set of 7 rare missense variants, the cumulative frequency for all VTE cases vs. controls was 0.063 vs. 0 (p=2.11 x10−7) and for recurrent VTE cases vs. controls was 0.156 vs. 0 (p=2.70 x10−16). For the MYH set of 9 variants, the cumulative frequency for all VTE cases vs. controls was 0.125 vs. 0.047 (p=4.30 x10−4) and for recurrent VTE vs. controls was 0.219 vs. 0.047 (p=6.03 x10−7). The frequency of the F5 rs6025 (Leiden) variant for all VTE cases vs. controls was 0.130 vs. 0.021 (p=3.46 x10−8) and for recurrent VTE vs. controls was 0.141 vs. 0.021 (p=2.68 x10−6). Thus, like F5 Leiden in this VTE Registry, MYH genes are linked to VTE risk, and especially notable, the two chromosome 17p13.1 MYH rare variant sets appear linked to VTE recurrence.
Skeletal muscle myosin gene exhibits in vivo procoagulant activity
Skeletal muscle myosin exerts procoagulant activity in vitro (Deguchi, et al 2016), and here we provide in vivo proof of concept for skeletal muscle myosin’s effects on coagulation by showing that i.v. administration of this myosin prior to tail cutting reduced bleeding in hemophilic mice. Myosin administration reduced blood loss from 6.9 to 2.3 μL/gm (p<0.0001) (Figure 1B). This proof for skeletal muscle myosin’s in vivo procoagulant properties supports the hypothesis that MYH gene products may be very relevant for coagulation and thus, MYH mutations may reasonably be functionally related to risk for thrombosis.
Discussion
Rare variant exomics data here indicate that FAM134B (rs78314670, Arg127Cys) appears associated with VTE risk and with low plasma levels of anticoagulant GlcCer which have been linked to VTE risk in replicated studies (Deguchi, et al 2001, 2017). Data here also imply that some rare missense variants in MYH genes may be linked to VTE risk because skeletal muscle myosin has in vivo procoagulant activity. Thus, biologic plausibility for potential causality for VTE is clear for two genes, namely FAM134B and MYH8, based on new data provided above. Although some mutations are predicted to affect protein functions (PolyPhen-2 score >0.15 in Table S4), much more information is needed to demonstrate and understand the functional influences of these gene mutations.
FAM134B and MYH8 genes were not identified in previous GWAS VTE studies. Applying higher minimal allele frequency filters (MAF>0.01 (Lindstrom, et al 2019b) or >0.07 (Lee, et al 2017)) for some of the previous studies could be one reason. Certain recent metaanalysis studies used lower filter rates (Klarin, et al 2017, Lindstrom, et al 2019a, Tang, et al 2020), but most cohorts there consisted of older patients who become more affected by environmental factors related to age which then mask the influences of genetic factors. For the Scripps Registry, the selection of younger age VTE with recurrence, likely with more genetic influence, further reduced the number of patients with consequent diminution of statistical power such that the associations of variants with VTE reported here may be tenuous or even false. There is an obvious need for future extensive replication studies in other VTE cohorts to confirm that MYH gene variations and/or FAM134B rs78314670 can predict VTE risk. Clinical variables like lipids, body mass index and blood pressure could be a potential source of confounding factors. Replication studies with larger numbers of VTE patients are needed to enable the evaluation of associations of rare variants with these potential risk factors as an evaluation cannot be made with the small numbers of subjects in this current pilot study.
In summary, our exomic rare variant analyses led to the discovery of the procoagulant activity of skeletal muscle myosin and of potential gene SNPs associated with both VTE risk and low plasma GlcCer level.
Supplementary Material
Acknowledgments
This work was supported, in part, by the National Institutes of Health grants RO1 HL133728 (J.H.G.), and 8UL1 TR000109 plus 5 UL1 RR025774 (A.T. under PI: E. Topol).
Footnotes
Conflict-of-interest disclosure: The authors declare no conflicts of interest.
References
- Auer PL & Lettre G (2015) Rare variant association studies: considerations, challenges and opportunities. Genome Med, 7, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal V, Libiger O, Torkamani A & Schork NJ (2010) Statistical analysis strategies for association studies involving rare variants. Nat Rev Genet, 11, 773–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi H, Navarro S, Payne AB, Elias DJ, Dowling NF, Austin HD, Espana F, Medina P, Hooper WC & Griffin JH (2017) Low level of the plasma sphingolipid, glucosylceramide, is associated with thrombotic diseases. Res Pract Thromb Haemost, 1, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi H, Sinha RK, Marchese P, Ruggeri ZM, Zilberman-Rudenko J, McCarty OJ, Cohen MJ & Griffin JH (2016) Prothrombotic skeletal muscle myosin directly enhances prothrombin activation by binding factors Xa and Va. Blood, 128, 1870–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nisio M, van Es N & Buller HR (2016) Deep vein thrombosis and pulmonary embolism. Lancet, 388, 3060–3073. [DOI] [PubMed] [Google Scholar]
- Islam F, Gopalan V & Lam AK (2018) RETREG1 (FAM134B): A new player in human diseases: 15 years after the discovery in cancer. J Cell Physiol, 233, 4479–4489. [DOI] [PubMed] [Google Scholar]
- Klarin D, Emdin CA, Natarajan P, Conrad MF & Kathiresan S (2017) Genetic Analysis of Venous Thromboembolism in UK Biobank Identifies the ZFPM2 Locus and Implicates Obesity as a Causal Risk Factor. Circ Cardiovasc Genet, 10 e001643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Dykas DJ, Leavitt AD, Camire RM, Ebberink E, Garcia de Frutos P, Gnanasambandan K, Gu SX, Huntington JA, Lentz SR, Mertens K, Parish CR, Rezaie AR, Sayeski PP, Cromwell C, Bar N, Halene S, Neparidze N, Parker TL, Burns AJ, Dumont A, Yao X, Chaar CIO, Connors JM, Bale AE & Lee AI (2017) Whole-exome sequencing in evaluation of patients with venous thromboembolism. Blood Adv, 1, 1224–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom S, Brody JA, Turman C, Germain M, Bartz TM, Smith EN, Chen MH, Puurunen M, Chasman D, Hassler J, Pankratz N, Basu S, Guan W, Gyorgy B, Ibrahim M, Empana JP, Olaso R, Jackson R, Braekkan SK, McKnight B, Deleuze JF, O’Donnell CJ, Jouven X, Frazer KA, Psaty BM, Wiggins KL, Taylor K, Reiner AP, Heckbert SR, Kooperberg C, Ridker P, Hansen JB, Tang W, Johnson AD, Morange PE, Tregouet DA, Kraft P, Smith NL & Kabrhel C (2019a) A large-scale exome array analysis of venous thromboembolism. Genet Epidemiol, 43, 449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom S, Wang L, Smith EN, Gordon W, van Hylckama Vlieg A, de Andrade M, Brody JA, Pattee JW, Haessler J, Brumpton BM, Chasman DI, Suchon P, Chen MH, Turman C, Germain M, Wiggins KL, MacDonald J, Braekkan SK, Armasu SM, Pankratz N, Jackson RD, Nielsen JB, Giulianini F, Puurunen MK, Ibrahim M, Heckbert SR, Damrauer SM, Natarajan P, Klarin D, de Vries PS, Sabater-Lleal M, Huffman JE, Bammler TK, Frazer KA, McCauley BM, Taylor K, Pankow JS, Reiner AP, Gabrielsen ME, Deleuze JF, O’Donnell CJ, Kim J, McKnight B, Kraft P, Hansen JB, Rosendaal FR, Heit JA, Psaty BM, Tang W, Kooperberg C, Hveem K, Ridker PM, Morange PE, Johnson AD, Kabrhel C, Tregouet DA & Smith NL (2019b) Genomic and transcriptomic association studies identify 16 novel susceptibility loci for venous thromboembolism. Blood, 134, 1645–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morange PE, Suchon P & Tregouet DA (2015) Genetics of Venous Thrombosis: update in 2015. Thromb Haemost, 114, 910–919. [DOI] [PubMed] [Google Scholar]
- Tang W, Stimson MR, Basu S, Heckbert SR, Cushman M, Pankow JS, Folsom AR & Pankratz N (2020) Burden of rare exome sequence variants in PROC gene is associated with venous thromboembolism: a population-based study. J Thromb Haemost, 18, 445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregouet DA & Morange PE (2018) What is currently known about the genetics of venous thromboembolism at the dawn of next generation sequencing technologies. Br J Haematol, 180, 335–345. [DOI] [PubMed] [Google Scholar]
- Yoon SJ, Seiler SH, Kucherlapati R & Leinwand L (1992) Organization of the human skeletal myosin heavy chain gene cluster. Proc Natl Acad Sci U S A, 89, 12078–12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z, Song D & Wang Y (2014) The novel gene pFAM134B positively regulates fat deposition in the subcutaneous fat of Sus scrofa. Biochem Biophys Res Commun, 454, 554–559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


