Abstract
Previous research suggests that sending non-participants a reminder letter, 1 year after their initial invitation, can improve coverage for bowel scope screening (BSS), also known as flexible sigmoidoscopy screening. We hypothesised that adding a general practitioner's (GPs) endorsement to the reminder letter could improve coverage even further. We conducted a randomised controlled trial in North West London, UK. Participants were screening-eligible men and women who had not responded to their initial BSS invitation at least 12 months prior to the trial period. Eligible adults were randomised in a 1:1 ratio to receive either a GP-endorsed reminder letter, or a standard reminder letter from June to August 2019. Logistic regression models were used to test the effect of the GP endorsement on attendance at BSS, adjusting for sex, clinical commissioning group, and local area socioeconomic deprivation. In total, 1200 participants were enrolled into the study and randomised to either the control (n = 600) or the intervention (n = 600) group. Those who received the GP-endorsed reminder letter were only slightly more likely to attend BSS than those who received the standard reminder letter (4% vs. 3%); this difference was not statistically significant (Adjusted OR = 1.30; 95% CI: 0.69, 2.43). Adding a GP-endorsement to the annual reminder letter did not have an effect on attendance at BSS. One possible explanation for this is that the endorsement used was not personalised enough. Future research should examine stronger GP-endorsements or other methods to promote uptake.
Keywords: Early detection of cancer, Early diagnosis of cancer, Cancer screening, Colon cancer
Highlights
-
•
GP endorsements and reminder letters can increase attendance of cancer screening.
-
•
First study to investigate GP-endorsed reminder letters for bowel scope screening.
-
•
Time since first invitation is important for effectiveness of reminder letters.
-
•
Strength of endorsements should be researched in the future.
1. Introduction
Colorectal cancer (CRC) is the 4th most common cancer in the UK, accounting for 11% of all cancers (Cancer Research UK, 2020). CRC can be prevented by the use of Flexible sigmoidoscopy (FS) screening, which works by detecting and removing polyps before they can develop into cancer. Randomised controlled trials (RCTs) have found that FS screening is associated with reduced CRC incidence and mortality when offered as a one-off test to men and women aged 55–64 years (Atkin et al., 2010; Hoff et al., 2009; Segnan et al., 2011).
In England, FS screening, also known as ‘Bowel scope’ screening (BSS), was introduced in March 2013 by the National Health Service (NHS) (Koo et al., 2017). Men and women receive an invitation at age 55 and, if they do not attend, they can self-refer up until the age of 60. Achieving a high uptake of BSS is essential for the overall success of programme (Geurts et al., 2015). However, since BSS was implemented the uptake has been low, with only 43% of those eligible attending an appointment which is considerably lower compared to the other screening programmes offered in the England for breast and cervical screening (71% and 72% respectively) (Public Health England, 2019; McGregor et al., 2016). Uptake is particularly low in areas with hard-to-reach populations e.g., in areas with high levels of deprivation, large immigrant populations, and among those who have English as an additional language.
Previous research investigating barriers to BSS has identified a range of practical and emotional barriers such as inconvenient appointment times, difficulties attending appointments, and worry about pain or discomfort arising from FS (Hall et al., 2016; von Wagner et al., 2019; von Wagner et al., 2018a). Reminder letters for those who do not attend screening are commonly used in the screening programme and have been shown to be a cost-effective way to facilitate uptake in up to 22% of previous non-participants (Vernon, 1997; Senore et al., 2015; Kerrison et al., 2017; Kerrison et al., 2016; Kerrison et al., 2018). There still remains considerable scope for modification and refinement of the reminder letters. For example, several studies have shown that primary care can have a positive impact on CRC screening participation through the use of GP endorsements (Zajac et al., 2016; Raine et al., 2016; Hewitson et al., 2011; Goodwin et al., 2019). To date, no studies have tested the effectiveness of a GP-endorsed reminder on BSS which could offer a low-cost approach to improving uptake.
The primary aim of this study was to test whether adding a GP endorsement to the ‘non-participant’ reminder letter improves the self-referral of BSS among previous non-responders, over and above a non-participant reminder letter without a GP endorsement.
2. Methods
A detailed protocol for this trial has been previously published (von Wagner et al., 2018b). The following provides a brief summary of the trial methods following the CONSORT (Consolidated Standards of Reporting Trial) guidelines for reporting (Schulz et al., 2010).
2.1. Design
This study was a non-clinical randomised controlled trial (RCT) with two parallel arms. The intervention group received a GP-endorsed reminder letter (online Supplementary Appendix A) by post, the control group received the same reminder letter by post, without the GP endorsement (online Supplementary Appendix B).
2.2. Participants
Prior to the commencement of the trial, invitation letters were sent to all GP practices served by London North West University Healthcare (LNWH) NHS Trust within the Clinical Commissioning Groups (CCGs) of the London Boroughs of Brent, Harrow and Hillingdon. LNWH was an ideal setting for this study as uptake of BSS is below the national average (40.5% vs. 43.1%), and they serve areas with large immigrant populations and high levels of deprivation (McGregor et al., 2016). Recruitment to the trial was achieved by calling GP practices, sending emails, and opportunistically attending a training event for GP practices. We aimed to recruit 50% of practices to take part in the study and received consent from 67% (93/138) to use their practice name in the GP-endorsed reminder letters.
Adults were eligible to take part in the study if they were: (1) aged 56 years at the time of enrolment (2) registered with a consenting GP practice in London North West University Healthcare NHS Trust, (3) had been offered, but not responded to, a routine BSS appointment at least 12 months previously, (4) met the clinical eligibility criteria for BSS and (5) had not opted out from sharing their personal data for purposes beyond direct care (referred to as type 2 objectors).
Our aim was to send reminders 12 months after the first invitation for screening, however, due to delays to the study start date after participants were first identified,reminders were sent between 14 and 25 months (mean and median = 19 months; standard deviation = 2 months) after the first invitation. This was an unintended consequence of the method we used to identify participants and was adjusted for in the analysis.
2.3. Procedure
Participants were identified by NHS Digital (formerly known as the Health and Social Care Information Centre) via the Bowel Cancer Screening System on the 1st of April 2019. This system provides an up-to-date electronic record of uptake data for individuals enrolled in the national bowel cancer screening programme. A total of 1200 men and women were identified, excluding type 2 objectors, and randomised in a 1:1 ratio to receive either the GP-endorsed reminder letter or the standard reminder letter using simple pseudorandom allocation methods. The intervention group names, addresses, and GP practice of participants were then shared by NHS Digital with a third party mailing company (CFH Docmail Limited, an NHS Information Governance toolkit accredited mailing company), who printed and mailed 150 reminder letters (75 in each group) for each of the 8 weeks starting on the 17th June 2019 and finishing on the 5th of August 2019. Due to the nature of the intervention, it was not possible to blind participants to their group allocation. As participants were not aware of a comparison group, it is unlikely that this biased participation.
We allowed 6 months from the date the final letters were sent (i.e. until early February 2020) for individuals to respond to the self-referral reminder, after which NHS Digital used NHS numbers to query the screening episode status and the Index of Multiple Deprivation (IMD) scores of each person included in the study (McLennan et al., 2019). An anonymised dataset was then shared with the research team at University College London (UCL) for analysis, after which all datasets containing identifiable data were destroyed. The follow-up period was extended from the 8-week period stated in the protocol to 6 months. This decision was made based on guidance from the screening centre that it can take several months for participants to move through the patient pathway.
2.4. Intervention
The standard reminder letter was the same reminder letter that has been used in previous trials (Kerrison et al., 2017; Kerrison et al., 2016; Kerrison et al., 2018). It was a personally addressed letter from BSS St Mark's Bowel Cancer Screening Centre at the North West London Hospitals Trust that invited recipients to make an appointment by returning an ‘appointment-request slip’ or calling the Freephone number at the screening centre. As with previous trials, the reminder also gave recipients the option to express a preference for the day and time of their appointment, and the sex of the practitioner performing the test.
The GP-endorsed reminder letter was the same as the standard reminder letter, except that it contained an additional statement of GP endorsement. This was in the form of a banner at the top of the letter which stated:
Your GP practice, [name of practice] supports the NHS Bowel Scope Screening Programme
2.5. Primary outcome
The primary outcome of this study was the proportion of individuals attending a BSS appointment within each group. Attendance was determined by checking the episode status of each individual included in the study 6 months after the distribution of the final reminder letter. Attenders constituted only those who made and attended an appointment. Non-attenders included individuals who did not respond to the reminder, those who made an appointment but did not attend, those who declined screening and those who had made an appointment but had not yet attended by the end of the study.
2.6. Secondary outcomes
A secondary outcome of the study was the proportion of individuals responding to the invitation for BSS. Responders were those who contacted the screening centre to make an appointment (whether attended or not) or to decline screening.
Socio-demographic variables included sex, IMD, CCG (Brent, Harrow, and Hillingdon). For analysis of IMD, participants were divided into five equally sized groups. Time between the first invitation to screening and reminder letter was measured in days. This measure was converted to months by dividing by 30 then grouped into three categories: “14–17 months”, “18–21 months” and “22+ months”.
2.7. Sample size calculation
As described in the protocol, the study was designed to detect a five-percentage point increase in uptake between the GP-endorsed and standard reminder group (von Wagner et al., 2018b). We estimated that with 80% power at the 5% alpha level, with two-sided testing, 600 participants per trial arm were required, giving a total sample size of 1200. This was based on the effect size of using GP endorsement to promote uptake of screening for CRC using alternative tests, such as the Faecal Occult Blood Test (FOBt) (Zajac et al., 2016; Raine et al., 2016; Hewitson et al., 2011).
2.8. Statistical analysis
We presented descriptive statistics for participant characteristics by trial arm. Differences in attendance and response were assessed using logistic regression models adjusted for sex, IMD quintiles, CCG and time between the first invitation to screening and reminder letter. We also tested if there were any interactions between the intervention and socio-demographic variables.
3. Results
3.1. Participants
Table 1. shows the participant characteristics by trial arm. 1200 participants were randomised (600 participants in each condition) in April 2019. Of the 1200 participants included in the trial, 52% (622) were male and 48% (578) were female.
Table 1.
Participant characteristics.
| Standard reminder letter (n = 600) | GP-endorsed reminder letter (n = 600) | P-value | |
|---|---|---|---|
| Sex (n and %) | |||
| Male | 314 (52.3) | 308 (51.3) | 0.729 |
| Female | 286 (47.7) | 292 (48.7) | |
| IMD (mean and SD) | 21.3 (11.4) | 22.2 (11.9) | 0.184 |
| IMD quintile (mean and SD) | |||
| 1 (low deprivation) | 7.6 (2.4); n = 125 | 7.2 (2.5); n = 116 | 0.182 |
| 2 | 14.3 (1.9); n = 127 | 14.5 (1.9); n = 112 | 0.609 |
| 3 | 20.3 (1.4); n = 117 | 20.3 (1.5); n = 123 | 0.994 |
| 4 | 27.2 (2.1); n = 123 | 27.2 (2.3); n = 122 | 0.970 |
| 5 (high deprivation) | 39.8 (7.1); n = 108 | 39.9 (7.7); n = 127 | 0.952 |
| Clinical commissioning group (n and %) | |||
| Brent | 249 (41.5) | 261 (43.5) | 0.521 |
| Harrow | 138 (23.0) | 122 (20.3) | |
| Hillingdon | 213 (35.0) | 217 (36.2) | |
| Time from first invitation to reminder letter (n and %) | |||
| 14–17 months | 160 (26.7) | 197 (32.8) | 0.061 |
| 18–21 months | 318 (53.0) | 296 (49.3) | |
| 22+ months | 122 (20.3) | 107 (17.8) | |
IMD = index of multiple deprivation; SD = standard deviation. P-value refers to χ2 test for proportions and t-test for means and SD.
3.2. Attendance at BSS
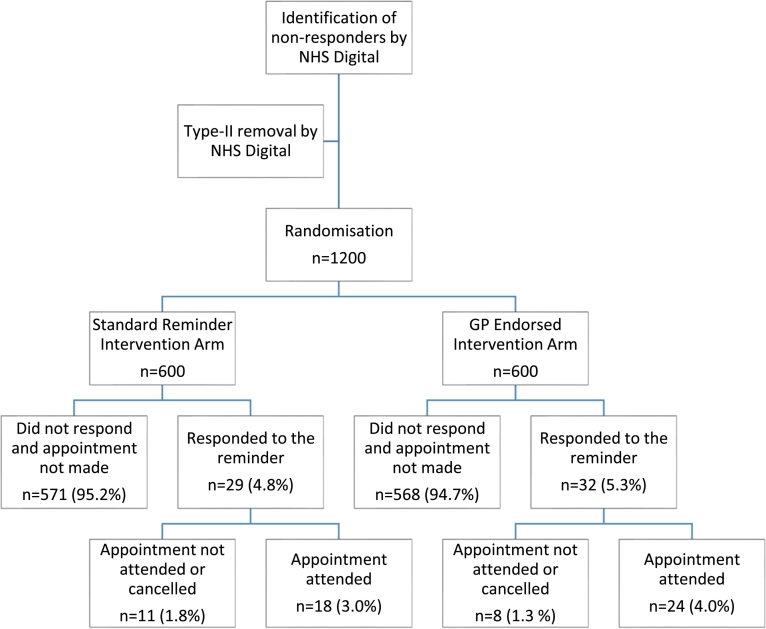
Fig. 1 shows that the proportion of people attending BSS was slightly higher in the GP-endorsed reminder group compared to the standard reminder group (4.0% vs. 3.0%). The logistic regression model showed that this difference was not statistically significant (Crude OR = 1.35; 95% CI:0.72, 2.51), even after adjusting for sex, IMD and time since first invitation (Adjusted OR = 1.30; 95% CI: 0.69, 2.43;
Fig. 1.
CONSORT (Consolidated Standards of Reporting Trial) flow diagram.
Table 2). Those in IMD quintile 4 were less likely to attend compared to those in quintile 1 (OR = 0.23; 95% CI: 0.07, 0.78). No interactions were found between socio-demographic variables and the intervention on attendance at BSS (See Supplementary Appendix C).
Table 2.
Effect of GP-endorsed reminder on attendance for bowel scope screening and response to reminder.
| Attendance at BSS |
Response to reminder |
|||
|---|---|---|---|---|
| Crude OR (95% CI) | Adjusted OR (95% CI) | Crude OR (95% CI) | Adjusted OR (95% CI) | |
| Group | ||||
| Standard letter | 1.00 | 1.00 | 1.00 | 1.00 |
| GP-endorsed letter | 1.35 (0.72, 2.51) | 1.30 (0.69, 2.43) | 1.11 (0.66, 1.86) | 1.07 (0.63, 1.79) |
| Sex | ||||
| Male | 1.00 | 1.00 | ||
| Female | 1.25 (0.67, 2.33) | 1.43 (0.85, 2.42) | ||
| IMD quintile | ||||
| 1 (low deprivation) | 1.00 | 1.00 | ||
| 2 | 0.49 (0.18, 1.30) | 0.61 (0.27, 1.35) | ||
| 3 | 0.50 (0.19, 1.35) | 0.58 (0.25, 1.36) | ||
| 4 | 0.23 (0.07, 0.78)* | 0.30 (0.11, 0.82)* | ||
| 5 (high deprivation) | 0.59 (0.21, 1.65) | 0.85 (0.36, 2.01) | ||
| CCG | ||||
| Brent | 1.00 | 1.00 | ||
| Harrow | 0.66 (0.24, 1.80) | 0.95 (0.42, 2.13) | ||
| Hillingdon | 0.91 (0.41, 2.01) | 0.95 (0.48, 1.87) | ||
| Time since first invitation | ||||
| 14–17 months | 1.00 | 1.00 | ||
| 18–21 months | 0.73 (0.32, 1.66) | 0.75 (0.43, 1.31) | ||
| 22+ months | 0.47 (0.12, 1.83) | 0.37 (0.15, 0.93)* | ||
OR = odds ratio; CI=confidence interval *P < 0.05; IMD = index of multiple deprivation; CCG = clinical commissioning group.
3.3. Response to reminder
The proportion of people responding to the reminder was slightly higher in the GP-endorsed reminder group compared with the standard reminder group (5.3% vs. 4.8%). The logistic regression model showed that this difference was not statistically significant (Adjusted OR = 1.07; 95% CI: 0.63, 1.79; Table 2). Those in IMD quintile 4 were less likely to respond compared with those in quintile 1 (OR = 0.30; 95% CI: 0.11, 0.82). Those invited more than 22 months after their initial invitation were less likely to respond to the reminder intervention than those invited 12–17 months after their initial invitation (OR = 0.37; 95% CI:0.15, 0.93). No interactions were found between socio-demographic variables and the intervention on response to the reminder (See Supplementary Appendix C).
4. Discussion
This study found that adding a GP-endorsement to a reminder letter for BSS did not significantly increase uptake or response. We found some evidence that higher levels of deprivation are associated with lower levels of response and attendance. We also found that as the time between the initial invitation and the reminder increased, response rates decreased. However, there were no interactions found between socio-demographic variables and the intervention on attendance at BSS or response to the reminder.
Our findings are in contrast to previous research, which found that GP-endorsed reminders improve uptake of colorectal cancer screening (Hewitson et al., 2011). Possible explanations for this difference is that the similarity between the GP-endorsed letter and the standard reminder letter resulted in the GP-endorsement not being strong enough to have an effect on participation. For example, Hewitson et al. tested a range of reminders, including a GP-endorsed letter using the practice letterhead and the GPs signature. They found the GP-endorsed letter resulted in a 6% increase in participation compared to the usual invitation, and that personalised letters which included the signature of the GP resulted in a greater participation compared to those sent ‘on behalf of the practice’ (Hewitson et al., 2011; Benton et al., 2017). The mode of GP endorsement delivery used in this trail was identical to the one used in the RCT conducted by Raine et al. Indeed, our results are very similar too: they found the GP endorsement resulted in a 0.7% increase in uptake equating to an additional 61 diagnoses of cancer per year in England compared to a 1% increase found in this study (Raine et al., 2016). Although this study showed a similar percentage increase, the small sample size meant that it was not possible to test if the increase was statistically significant. Another reason for the difference in results may simply be because the decision to have BSS, which requires a hospital visit and a medical procedure, is perhaps not as easy to influence with a GP endorsement as it is to complete an FOBt, a relatively simple and low risk test which can be carried out at home.
The overall level of uptake of BSS was low (3.5%) compared to other studies which found that 15.5% attended screening after receiving reminder letters for BSS (Kerrison et al., 2017; Kerrison et al., 2016). A possible reason for this difference is that our study identified some participants who may have already received the standard 12-month reminder letter. Combined with the small delay to the start our study, this could have resulted in some participants receiving multiple reminder letters. Previous research in the USA has shown that screening participation declines with each subsequent reminder which may have had an adverse impact on the overall response to the reminder letters (Zhu et al., 2006). However, a planned analysis aiming to investigate the impact of this did not uncover any associations with response to the invitation or attendance at BSS. Another possible reason for the null finding in this study is that reminder letters were sent between 14 and 25 months after the first invitation, rather than the 12-months originally planned. We found that a later reminder was associated with non-response, it is possible that this results in an overall lower response than if reminders were sent 12 months after the initial invitation.
A strength of this study is the randomised design which was able to account for the effect of confounding variables. The outcome measures were derived from high quality clinical data sets and as participants were most likely unaware they were part of a trial, there is very little chance of systematic bias influencing the results. The study was conducted in North West London soit is not possible to generalise the findings to other regions of the UK. Although randomisation accounts for unknown confounding variables, we had limited information on participant demographics and were therefore unable to examine differences by factors such as ethnicity, comorbidities, and marital status and how they relate to non-participation.
5. Conclusions
This is the first study evaluating the effect of GP-endorsed reminder letters for BSS. We did not find any strong evidence that adding a GP-endorsement to the annual reminder letter for BSS had an effect on uptake of BSS. Future research should examine stronger GP-endorsements or other methods to promote uptake in hard-to-reach groups.
Ethics and consent to participate
The study was approved by the Yorkshire & Humber—Bradford Leeds Research Ethics Committee (16/YH/0298) and the Confidentiality Advisory Group (17/CAG/0162). Trial registration number: ISRCTN82867861.
Consent for publication
Not applicable.
Funding
This report presents independent research commissioned and funded by the National Institute for Health Research (NIHR) Policy Research Programme, UK, conducted through the Policy Research Unit in Cancer Awareness, Screening and Early Diagnosis, PR-PRU-1217-21601. The views expressed are those of the authors and not necessarily those of the NIHR, the Department of Health and Social Care or its arm's length bodies, or other Government Departments. Those listed in the acknowledgements did not provide financial support but administrive support in accessing data, recruting practices and posting letters.
Declaration of competing interest
The authors have declared that no competing interests exist.
Acknowledgments
Acknowledgments
We would like to acknowledge the support provided to this project by Penny Aitchison, Rachel Brannan, Rachel Crowther from Public Health England, Jason Lusty and Shanie Skelton from CFH Docmail, and the GP practices and CCGs London North West University Healthcare NHS Trust.
Authors' contributions
AK managed the trial, completed the analyses and wrote the paper. YH, RSK, UM, SD, DV, ST, SM and AP and CVW conceptualised the project, developed the protocol and contributed to reviewing and editing drafts.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ypmed.2020.106268.
Appendix A. Supplementary data
Supplementary material 1
Supplementary material 2
Supplementary material 3
References
- Atkin W.S., Edwards R., Kralj-Hans I. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375(9726):1624–1633. doi: 10.1016/S0140-6736(10)60551-X. [published Online First: 2010/05/01] [DOI] [PubMed] [Google Scholar]
- Benton S.C., Butler P., Allen K. GP participation in increasing uptake in a national bowel cancer screening programme: the PEARL project. Br. J. Cancer. 2017;116(12):1551–1557. doi: 10.1038/bjc.2017.129. [published Online First: 2017/05/20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Research UK Bowel Cancer Statistics. 2020. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer Available from: accessed 6th April 2020.
- Geurts S.M., Massat N.J., Duffy S.W. Likely effect of adding flexible sigmoidoscopy to the English NHS Bowel Cancer Screening Programme: impact on colorectal cancer cases and deaths. Br. J. Cancer. 2015;113(1):142–149. doi: 10.1038/bjc.2015.76. [published Online First: 2015/06/26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin B.C., Crawford-Williams F., Ireland M.J. General practitioner endorsement of mail-out colorectal cancer screening: the perspective of nonparticipants. Transl. Behav. Med. 2019;10(2):366–374. doi: 10.1093/tbm/ibz011. [DOI] [PubMed] [Google Scholar]
- Hall N., Birt L., Rees C.J. Concerns, perceived need and competing priorities: a qualitative exploration of decision-making and non-participation in a population-based flexible sigmoidoscopy screening programme to prevent colorectal cancer. BMJ Open. 2016;6(11) doi: 10.1136/bmjopen-2016-012304. e012304. [published Online First: 2016/11/12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitson P., Ward A.M., Heneghan C. Primary care endorsement letter and a patient leaflet to improve participation in colorectal cancer screening: results of a factorial randomised trial. Br. J. Cancer. 2011;105(4):475–480. doi: 10.1038/bjc.2011.255. [published Online First: 2011/08/11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff G., Grotmol T., Skovlund E. Risk of colorectal cancer seven years after flexible sigmoidoscopy screening: randomised controlled trial. BMJ. 2009;338 doi: 10.1136/bmj.b1846. b1846. [published Online First: 2009/06/02] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrison R.S., McGregor L.M., Marshall S. Use of a 12 months’ self-referral reminder to facilitate uptake of bowel scope (flexible sigmoidoscopy) screening in previous non-responders: a London-based feasibility study. Br. J. Cancer. 2016;114(7):751–758. doi: 10.1038/bjc.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrison R.S., McGregor L.M., Marshall S. Improving uptake of flexible sigmoidoscopy screening: a randomized trial of nonparticipant reminders in the English Screening Programme. Endoscopy. 2017;49(1):35–43. doi: 10.1055/s-0042-118452. [published Online First: 2016/12/21] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrison R.S., McGregor L.M., Counsell N. Use of two self-referral reminders and a theory-based leaflet to increase the uptake of flexible sigmoidoscopy in the English bowel scope screening program: results from a randomized controlled trial in London. Ann. Behav. Med. 2018;52(11):941–951. doi: 10.1093/abm/kax068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo S., Neilson L.J., Von Wagner C. The NHS Bowel Cancer Screening Program: current perspectives on strategies for improvement. Risk Manag. Healthc. Policy. 2017;10:177–187. doi: 10.2147/RMHP.S109116. [published Online First: 2017/12/23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor L.M., Bonello B., Kerrison R.S. Uptake of Bowel Scope (Flexible Sigmoidoscopy) Screening in the English National Programme: the first 14 months. J. Med. Screen. 2016;23(2):77–82. doi: 10.1177/0969141315604659. [published Online First: 2015/09/22] [DOI] [PubMed] [Google Scholar]
- McLennan D., Noble S., Noble M. 2019. The English Indices of Deprivation 2019: Technical Report. [Google Scholar]
- Public Health England Public Health Profiles, Cancer Services: Demographics, Screening and Diagnositcs. 2019. https://fingertips.phe.org.uk/profile/cancerservices Available from: accessed 28th April 2020.
- Raine R., Duffy S.W., Wardle J. Impact of general practice endorsement on the social gradient in uptake in bowel cancer screening. Br. J. Cancer. 2016;114(3):321–326. doi: 10.1038/bjc.2015.413. [published Online First: 2016/01/08] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz K.F., Altman D.G., Moher D. Research methods & reporting. BMJ. 2010;340:699. [Google Scholar]
- Segnan N., Armaroli P., Bonelli L. Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian Randomized Controlled Trial—SCORE. J. Natl. Cancer Inst. 2011;103(17):1310–1322. doi: 10.1093/jnci/djr284. [DOI] [PubMed] [Google Scholar]
- Senore C., Inadomi J., Segnan N. Optimising colorectal cancer screening acceptance: a review. Gut. 2015;64(7):1158–1177. doi: 10.1136/gutjnl-2014-308081. [published Online First: 2015/06/11] [DOI] [PubMed] [Google Scholar]
- Vernon S.W. Participation in colorectal cancer screening: a review. J. Natl. Cancer Inst. 1997;89(19):1406–1422. doi: 10.1093/jnci/89.19.1406. [published Online First: 1997/11/05] [DOI] [PubMed] [Google Scholar]
- von Wagner C., Bonello B., Stoffel S. Barriers to bowel scope (flexible sigmoidoscopy) screening: a comparison of non-responders, active decliners and non-attenders. BMC Public Health. 2018;18(1) doi: 10.1186/s12889-018-6071-8. 1161. [published Online First: 2018/10/07] [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wagner C., Hirst Y., Tookey S. Use of a GP-endorsed 12 months’ reminder letter to promote uptake of bowel scope screening: protocol for a randomised controlled trial in a hard-to-reach population. BMJ Open. 2018;8(5) doi: 10.1136/bmjopen-2018-022263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wagner C., Bonello B., Stoffel S.T. Predictors of intention translation in flexible sigmoidoscopy screening for colorectal cancer. Health Psychol. 2019;38(12):1083–1095. doi: 10.1037/hea0000793. [published Online First: 2019/09/27] [DOI] [PubMed] [Google Scholar]
- Zajac I.T., Duncan A.C., Flight I. Theory-based modifications of an advanced notification letter improves screening for bowel cancer in men: a randomised controlled trial. Soc. Sci. Med. 2016;165:1–9. doi: 10.1016/j.socscimed.2016.06.036. [published Online First: 2016/08/04] [DOI] [PubMed] [Google Scholar]
- Zhu J., Davis J., Taira D.A. PEER REVIEWED: screening rates and characteristics of health plan members who respond to screening reminders. Prev. Chronic Dis. 2006;3(2) [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2
Supplementary material 3