Abstract
Curcumin (CUR), as a traditional Chinese medicine monomer extracted from the rhizomes of some plants in Ginkgo and Araceae, has shown a wide range of therapeutic and pharmacological activities such as anti-tumor, anti-inflammatory, anti-oxidation, anti-virus, anti-liver fibrosis, anti-atherosclerosis, and anti-Alzheimer’s disease. However, some issues significantly affect its biological activity, such as low aqueous solubility, physico-chemical instability, poor bioavailability, and low targeting efficacy. In order to further improve its curative effect, numerous efficient drug delivery systems have been carried out. Among them, physicochemical targeting preparations could improve the properties, targeting ability, and biological activity of CUR. Therefore, in this review, CUR carrier systems are discussed that are driven by physicochemical characteristics of the microenvironment (eg, pH variation of tumorous tissues), affected by external influences like magnetic fields and vehicles formulated with thermo-sensitive materials.
Keywords: curcumin, targeted delivery system, pH-sensitive, magnetic-response, thermo-sensitive, cancer
Introduction
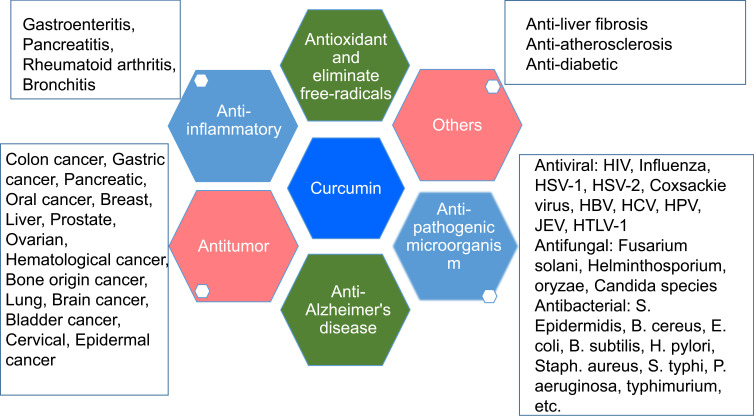
Chemotherapy is the most commonly used method for advanced cancer. However, the clinical usefulness of chemotherapy drugs are still restricted by severe toxic and side-effects such as myelosuppression, immunologic suppression, gastrointestinal reaction, and peripheral neuropathy.1–5 Some effective components of plants used in traditional medicine without the serious side-effects, as a supplementary treatment, have been widely studied.6,7 Among them, Curcumin (CUR), a traditional Chinese medicine monomer, has been extensively studied with broad pharmacological activities, as summarized in Figure 1.8–10 Its anti-tumor effect has been the most extensively studied and are related to regulating enzymes (COX-2, AMPK, MMPs, NADPH, and LOX), transcription factors (NF-κB, AP-1, β-catenin, and STAT-3) and protein kinases, growth factors (MAPK, AKT, JAK, VEGF, ERK, PKA, and Bcl-2), etc.11–13 However, it still needs to be improved in many fields, including low aqueous solubility and physico-chemical stability, low absorption rate, rapid metabolism, low bioavailability, and targeting efficacy14,15 These problems significantly affect its biological activity and application in the clinic.
Figure 1.
Pharmacological activities of Curcumin.
Abbreviations: PIV-3, parainfluenza virus type 3; FIPV, feline infectious peritonitis virus; VSV, vesicular stomatitis virus; HSV, herpes simplex virus; FHV, flock house virus; RSV, respiratory syncytial virus; HIV-1, type 1 human immunodeficiency virus; HSV-1, herpes simplex virus type 1; HBV hepatitis B virus; HCV hepatitis C virus; HPVs, high-risk human papillomaviruses; JEV, Japanese encephalitis virus; HTLV-1, human T-cell leukemia virus type 1.
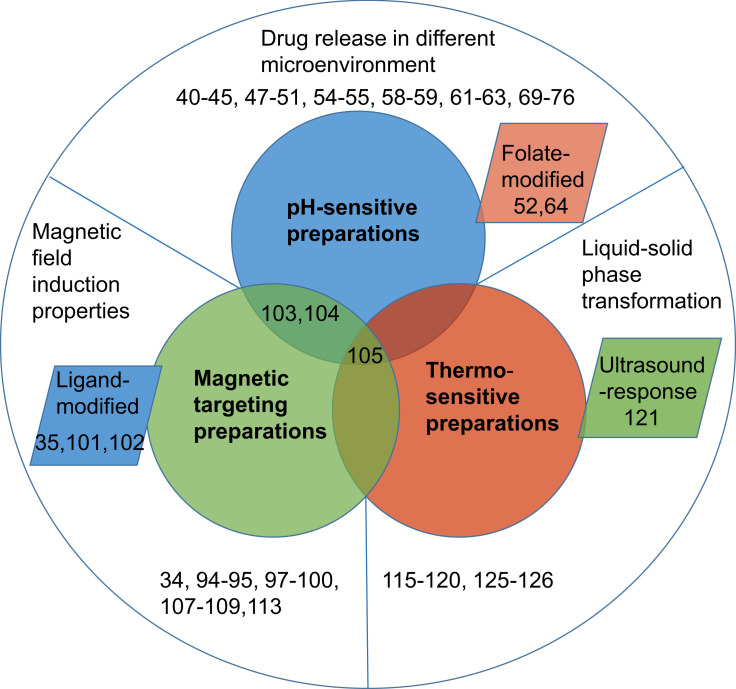
In recent years, some novel targeted drug delivery systems have been favored by researchers, such as passive targeting preparations, active targeting preparations, and physicochemical targeting preparations. Passive targeting preparations are enriched in target sites through normal physiological processes to improve the curative effect, such as liposomes, nanoparticles, emulsions, microcapsules, microspheres, and micelles.16–20 Although it was a potential method to solve the above problems of CUR to a certain degree,16,21–24 it is easy to be swallowed by macrophages in the body leading to drug accumulation in the liver, spleen, and kidney tissues.25 In a word, passive targeting preparations are non-intelligent and non-specific for drug delivery. Successful development of targeted therapy is dependent on the site-specific delivery of therapeutic agents partly. Active targeting preparations as “missiles” deliver drugs to target areas by modifying specific ligands or monoclonal antibodies on particle surfaces to avoid the uptake of the reticuloendothelial system and drug natural distribution.26 However, these active targeting preparations have several limitations such as complicated preparation procedures, non-specific tumor targeting, and single target properties that are only targeted to a certain receptor or ligand.27–29 Recent studies showed that physicochemical targeting preparations such as pH-, magnetic-, and heat-response proved to be promising strategies to obtain controllable and specific drug delivery (Figure 2). Among them, specific release behavior of pH sensitive preparations was triggered by the specific pH of the target site to increase target ability,30 especially in the acidic environment of tumor tissues.31 In consideration of tissue microenvironment, prodrug or carriers-modified design was reported for enhancing the antitumor effect by selectively transporting drugs to targets.32 Magnetic-response preparations could deliver drugs to specific target areas through blood vessels under the guidance of strong external magnetic fields.33 Magnetic-response drug delivery systems could improve serum bioavailability of CUR in mice up to 2.5-fold through delayed-metabolism and increased accumulation in vivo.34 When CUR-loaded magnetic nanoparticles combined with ligand/receptor, serving as novel platforms for multiple biomedical applications, they could enhance the targeting and cellular uptake ability, leading to an improved anticancer effect.35 Besides, this combination also achieved controllable and specific drug delivery through thermo-sensitive targeting preparations by temperature stimuli.36 Generally, these physicochemical targeting preparations could enhance the properties of CUR such as targeting ability, aqueous solubility, stability, bioavailability, and therapy effect (anti-tumor, anti-inflammatory, and antioxidant activity), as shown in Table 1. This review mainly focuses on different formulations containing CUR based on physicochemical properties of carriers and their pharmaceutical properties.
Figure 2.
Physicochemical targeting preparations of Curcumin.
Table 1.
The Influential Effect and Preparation Method of CUR-Loaded Physicochemical Targeting Preparations
| Preparations | Influential Effects | Preparation Method | Size | Ref. |
|---|---|---|---|---|
| pH-sensitive | ||||
| Liposome | Enhanced inhibitory effect on EC109 | Thin-film dispersion | 141 nm | 40 |
| Effectively transported to mouse (C57BL/6 mice bearing) tumor and significantly inhibited mouse tumor growth | Ultrasonic film hydration | 193 nm | 41 | |
| Superior cytotoxic activity and proapoptotic against both HL-60 and HL-60/CDDP cells | Lipid film hydration method and extrusion | 127 nm | 42 | |
| Anti-inflammatory and antioxidant activity in vitro | Micellar-vesicle transformation method and pH-driven method | <100 nm | 43 | |
| Nanoparticle | 1) Efficiently penetrate the blood–brain barrier and enhance brain delivery efficiency; 2) Increase mouse survival rate and reduce adverse reactions |
Single-emulsion solvent evaporation method |
100 nm | 35 |
| Colonic specific drug release | Solvent emulsion evaporation technique | 111 nm | 44 | |
| Enhanced permeation across Caco-2 cell and significantly decreased neutrophil infiltration and TNF-a secretion | Modified spontaneous emulsification solvent diffusion method | 116 nm | 46 | |
| Double inhibition of the cancerous cells | Solvent emulsion-evaporation technique | 97 nm | 47 | |
| Significantly released at pH 7.0 | Ionic-gelation method | 173 nm | 48 | |
| Enhanced significant cytotoxicity against MRC5 cells | Rotary evaporation and deposition coating | 234 nm | 50 | |
| Delivered effectively drug to tumor and improved targeting ability | Co-condensation and coated method | about 80 nm | 51 | |
| Micelle | Increased the tumor inhibition for HeLa, SiHa, and C33a cervical cell lines | Dialysis method | 95 nm | 57 |
| Extended the MRT and delayed the clearance of CUR | Solvent evaporation method | 143 nm | 58 | |
| Inhibited tumor growth and exert MDR | Synthesis and self-assemble | 228 nm | 60 | |
| Release rapidly CUR at the acidic environment | Synthesis and self-assemble | 222 nm | 61 | |
| Microsphere | 1) Prevented premature release and controlled release; 2) Significantly reduced severity of colonic damage |
Emulsion cross-linking method followed by coating | 77 nm | 66 |
| Molecular complexes | 1) Enhanced the aqueous solubility (>2 mg/mL); 2) Increased peak plasma concentration (6 times); 3) Improved bioavailability (about 20-fold) |
Nanoprecipitation method | - | 67 |
| Microcapsule | Suppressed degradation of CUR and controlled drug release | Synthesis and self-assemble | 1.05 um | 68 |
| Magnetic-response | ||||
| Nanoparticle | 1) Suppressed of PCNA, Bcl-xL, Mcl-1, MUC1, Collagen I and enhanced membrane β-catenin expression; 2) Improved serum bioavailability (2.5-fold) |
Water-dispersible multi-layer synthesis approach | 10.5±0.54 nm | 34 |
| Sustained-release | Modified co-precipitation | 100 nm | 87 | |
| Showed high stability, well-tolerated by mammalian cells and MRI relaxation properties | Ultrasonic dispersion and incubation chelation method | 13 ± 4 nm | 91 | |
| Displayed strong anticancer on MDA-MB-231 cancer cells and superior magnetic resonance imaging characteristics | Modified co-precipitation and injection method | 123 nm | 92 | |
| Functional particle | Significantly enhanced the cellular uptake and better antiproliferative effect on the C6 glioma cell and HeLa cells | Co-precipitation, dispersion and synthesis methods | 117 nm | 94 |
| 1) pH sensitive release, high loading capacity and hemocompatibility of CUR;2) Enhanced uptake and antiproliferation for MCF-7 breast cancer cell | Chemical co-precipitation method and molecular modified | 195 and 240 nm | 95 | |
| 1) improved the solubility (216 µg/mL), stability and bioavailability of CUR; 2) Suppressed the growth of Caco-2 cells (CC50=65 µg/mL) | Ice bath sonication and precipitation method | 10 µm | 97 | |
| Liposome | Magnetic heating controlled drug release by high-frequency magnetic field exposure; efficiently internalized into the cellular compartment and killed MCF-7 cells | Thin-film hydration method followed by extrusion techniques | 120–140 nm | 99 |
| Microsphere | Showed controllable particle size and possessed good magnetic mobility | Water-in-oil-in-water method | 16–207 µm | 100 |
| Induced HeLa cancer cells death through magnetocalorific effect | Solvent evaporation method | 75 nm | 101 | |
| Hydrogel | Cardioprotective effects against dox-induced cardiac toxicity in rat cardiomyocyte cell lines | Emulsion polymerization and synthesis method | 18–23 nm | 105 |
| Thermo-sensitive | ||||
| Polymer gel | Improved targeting and bioavailability in the brain | Solvent injection method | – | 108 |
| Temperature controlled release performance | Emulsion polymerization | 180 nm | 109 | |
| Significantly prolong retention time in solid tumors | Solvent injection method | - | 110 | |
| Liposomal-based gel | Showed better skin permeation in vitro and significant anti-inflammatory effect in auricle edemas mice | Emulsion evaporation-solidification at low temperature | 263.9 nm | 112 |
| Reduced toxicity of CUR and enhanced anti-tumor activity in tumor-bearing BALB/c mice | Thin-film rehydration method | 950 nm | 113 | |
| Micelle-based gel | 1) Inhibited tumor growth and metastasis, and prolonged survival of tumor-bearing mice; 2) Showed lower proliferation activity, more apoptotic cells, and fewer microvessels; 3) Showed higher AUC and longer t1/2. |
Dispersion method | 27.1 nm | 117 |
| Showed nano-scale size, low critical micelle concentration (0.0113–0.0144 mg/mL), high drug loading (20.4%) and stability (remain stable over 1 month) | Self-assembly and solvent evaporation/film hydration method |
47.5–88.2 nm | 118 | |
Abbreviation: CUR, curcumin.
pH-Sensitive Preparation
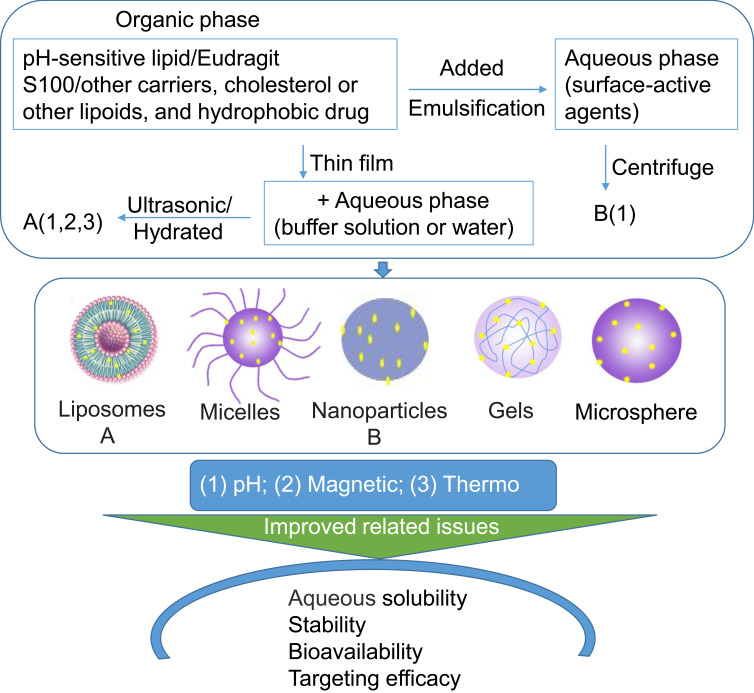
To increase the accumulation of drugs in the target tissues or cells, different trigger release mechanisms have been designed based on the body microenvironment, especially the tumor microenvironment. The different microenvironment of tumors from that of normal tissues is largely dictated by the abnormal tumor vasculature and heterogeneous microcirculation. Its hostile microenvironment, such as oxygen consumption, glucose, and energy deficiency, high lactate level and extracellular acidosis, causes the accumulation of acid metabolites resulting in the extracellular matrix of tumor tissue being acidic, and its pH (about 6.5–7.2) is lower than that of blood and normal tissue (7.4).37 A study demonstrated that pH-sensitive drug delivery systems could increase drug accumulation in the tumor, enhance the cellular uptake ability and improve antitumor activity in weakly acidic conditions.32 Due to the pH-response property, drugs were also delivered to the inflammatory regions and infected areas.30,31 In addition, in view of specific normal physiological microenvironments such as intestinal fluid (SIF, pH 6.8) and simulated gastric fluid (SGF, pH 1.2),38 drug delivery systems for the gastrointestinal tract have also been developed.39 Based on the different acidity/alkalinity microenvironment of the body for any situation, physiological or pathological, various CUR pH-sensitive preparations (liposomes, nanoparticle, micelles, microspheres) were developed to enhance the targeting ability and treatment effect of CUR (Figure 3).
Figure 3.
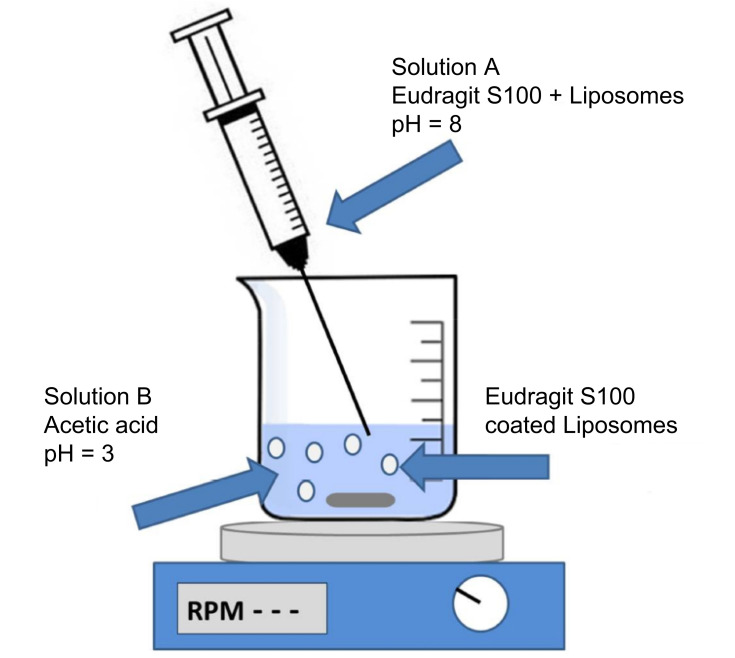
Schematic transition of CUR into physicochemical targeting preparations.
CUR pH-Sensitive Liposomes
Lipid carriers as pH-sensitive materials, responding to acidic condition of tumors or inflammation regions, are used to load CUR achieving intelligent release of drug, as shown in Table 2. This drug delivery platform could enhance the anti-tumor and anti-inflammatory effect of lipophilic CUR. For example, the pH-sensitive material of IM-Chol was synthesized through amidation reaction between the amino group of N-(3-Aminopropyl) imidazole and acyl chloride group of cholesteryl chloroformate in a weak base solution, which were used to prepare CUR pH-sensitive liposomes with phosphatidylcholine and cholesterol by thin-film dispersion method (Figure 3). The cumulative release of CUR (72.5%) after 24 hours at pH 5.0 were higher than that at pH 7.4 (32%), indicating that IM-Chol displayed a pH-response property. The cell toxicity result showed an enhanced inhibitory effect on EC109 cells with the cell viability of 30% compared with free CUR (60%).40 An endosomal pH-sensitive cationic lipid, consisting of glutamic acid backbone-based cationic amphiphiles that contain both endosomal pH-sensitive histidine and solubility enhancing guanidine moieties in their polar head-group regions, was used to encapsulate CUR for targeting therapy of the tumor. CUR release from the liposomes was most efficient at pH 5 or 6. And it showed a burst release in the first 1 hour followed by a controlled release over a period of 4 hours (85%). Importantly, the concentrations of CUR liposome in the tumor tissues were found to be remarkably higher than those in other tissues such as the lung, liver, kidney, spleen, and heart. The tumor volume of CUR liposome (about 800 mm3) and the vehicle control group (5% aqueous glucose) (about 4,200 mm3) revealed that CUR was effectively transported to mouse (C57BL/6 mice bearing) tumors and significantly contributed to inhibiting mouse tumor growth. The survival rate of tumor bearing mice with treatment of CUR liposome (about 45 days) was also improved compared to the control group (about 32 days).41 Besides, some non-lipid carriers as pH-sensitive materials have been reported to entrap CUR. For example, the pH-sensitive material of pI-pAA (2.5 mol%) and these carriers of dipalmitoylphosphathydilcholine/cholesterol (2:1 M ratio) and polyoxyethylated calix-4-arene (phospholipids/CUR, 1/0.1 M ratio) were used to obtain the CUR-loaded pH-sensitive liposomes by lipid film hydration method and extrusion. The liposomes showed a superior cytotoxic and apoptogenic activity against chemo-sensitive HL-60 and its resistant sublines HL-60/doxorubicin (Dox).42 Eudragit®S100, made of methacrylic acid and methacrylic acid methyl ester, was used to coat the surface of CUR-loaded liposomes for colon targeted therapy due to its pH-dependent solubility in the colon region. Vincenzo et al43 prepared CUR-loaded liposomes in a simple and organic solvent-free way by a micellar-vesicle transformation method and coated with Eudragit® S100 on its surface by a fast pH-driven method, as shown in Figure 4. The liposomes have small particle size (<100 nm), high encapsulation efficiency (98%), and high stability at both 4°C and 25°C. The cytotoxicity experiment of the liposomes showed safety on the Caco-2 human colon cell line in vitro. These drug delivery systems showed good characteristics such as intelligent drug release behavior and improved stability. However, the study of pharmacokinetics is important for the application of CUR in the clinic and needs to be carried out.
Table 2.
The Trigger/Targeting Carries and Preparation Method of CUR-Loaded Physicochemical Targeting Preparations
| Types | Trigger/Targeting Carries | Ref. |
|---|---|---|
| pH-sensitive | 1) N-(3-Aminopropyl)imidazole-cholesterol (IM-Chol) | 40 |
| 2) A new endosomal cationic lipid consisted of glutamic acid backbone-based cationic amphiphiles | 41 | |
| 3) Poly(isoprene-b-acrylic acid) copolymer (pI-pAA) | 42 | |
| 4) Eudragit®S100 | 43–47,66 | |
| 5) Chitosan and fucoidan | 48 | |
| 6) Functionalized dendritic mesoporous silica | 49 | |
| 7) Tannic acid-Fe(III) complex | 50 | |
| 8) Chitosan and folate | 51 | |
| 9) D-α-tocopheryl polyethylene glycol 1000-block-poly(β-amino ester) (TPGS-PAE copolymers) | 52 | |
| 10) PEG2000-DOX | 53 | |
| 11) Dox-oxidized sodium alginate (Dox-OSA) | 54 | |
| 12) Zn(II)-CUR | 55 | |
| 13) Amphiphilic N-benzyl-N,O-succinyl chitosan (BSCS) | 57 | |
| 14) Poly(3-caprolactone)-block-poly(diethylaminoethyl methacrylate)-block-poly(sulfobetaine methacrylate) (PCL-PDEA-PSBMA) | 58,59 | |
| 15) Poly(l-histidine)-poly(D,L-lactide-co-glycolide)-poly(ethylene glycol)-poly(D,L-lactide-co-glycolide)-poly(l-histidine) (PHis-PLA-PEG-PLA-Phis) and a folate targeting ligand | 60 | |
| 16) CUR-dextran | 61 | |
| 17) Poloxam 188-Cis-CUR conjugate | 62 | |
| 18) mPEG-poly(lactic acid)-CUR and mPEG-poly(lactic acid)-hydrazone-CUR (mPEG-PLA-CUR and mPEG-PLA-Hydr-CUR) | 63 | |
| 19) mPEG-PLA-tris(hydroxymethyl)aminomethane-CUR (mPEG-PLA-Tris-CUR) | 64 | |
| 20) mPEG-Chitosan-Ketal (PCK) | 65 | |
| 21) Poly(butyl-methacrylate-co-(2-dimethylaminoethyl) methacrylate-co-methyl-methacrylate) (Eudragit® EPO) | 67 | |
| 22) Silica particles and poly(L-lysine) | 68 | |
| 23) β-CD | 96 | |
| 24) Pectin maleate | 97 | |
| Magnetic | 1) Oleic acid-modified iron oxide nanoparticles and NH2-PEG3500-T7 | 35 |
| 2) Magnetic ferrite | 34,85–87,89–97,99–102,105 | |
| Thermo-sensitive | 1) N-isopropyl acrylamide | 97 |
| 2) Poloxamer 407 | 107,108,110–112 | |
| 3) Poly(N-vinylcaprolactam-co-hydroxyl ethyl ethacrylate) | 109 | |
| 4) N-cholesteryl hemisuccinate-O-sulfate chitosan | 113 | |
| (5) Poly(ethylene glycol)-poly(ε-caprolactone)-poly(ethylene glycol) copolymer | 117 | |
| 6) Poly(N-isopropylacrylamide-co-N,N-dimethylacrylamide)-b-poly(L-lactide)-b- poly(N-isopropylacrylamide-co-N,N-dimethylacrylamide) | 118 |
Abbreviations: CUR, curcumin; T7, human transferrin receptor-binding peptide T7.
Figure 4.
Schematic representation of liposomal coating with Eudragit@ S100 by the pH jump method.
Notes: Reprinted with permission from De LV, Milano F, Mancini E, et al. Encapsulation of curcumin-loaded liposomes for colonic drug delivery in a pH-responsive polymer cluster using a pH-driven and organic solvent-free process. Molecules. 2018;23(4):739. Copyright (2018) Multidisciplinary Digital Publishing Institute, Creative Common CC BY license.43
CUR pH-Sensitive Nanoparticles
Many CUR-loaded pH-sensitive nanoparticles were developed for oral colon-specific drug delivery systems (OCDDS), which could release drug under neutral conditions. This system has proved safety, good targeting property for colonic lesions, controlled/sustained release, and better therapeutic efficacy. As mentioned above, Eudragit®S100, as a good pH-sensitive carrier, could selectively release drug at the colon region rather than in the stomach and upper intestine due to the ionization of its carboxylic functional group at pH 7.0.44 Eudragit®S100 showed good security for oral administration. CUR-loaded polymeric nanoparticles with Eudragit®S100 showed no-toxicity evidenced by various toxicological evaluations including acute-toxicity study (at a dose equivalent to 2,000 mg/kg CUR), 28 days sub-acute-toxicity study (200 mg/kg CUR) and various genotoxicity studies (micronucleus assay, chromosomal aberration assay, and comet assay in vivo).45 In 1978, it was reported for the first time that after oral administration of CUR (1 g/kg) in Sprague-Dawley rats, negligible amounts of CUR were observed in blood plasma which could be its poor absorption from the gut.46 When CUR was encapsulated into pH-sensitive nanoparticles, it exhibited better permeation and selective release of drug in the inflamed colonic tissues. The CUR-loaded pH-sensitive nanoparticles (CUR-loaded NPs) consisting of PLGA (poly(lactic-co-glycolic) acid, 45 mg), Eudragit®S100 (45 mg), and PVA solution (polyvinyl alcohol, 0.5%, w/v) could enhance the permeation across Caco-2 cells monolayers. The apparent permeability (Papp) of the CUR-loaded NPs was about 2.6×10−6 cm/s, while that of CUR suspension was undetectable. Furthermore, CUR-loaded NPs showed a significant reduction on TNF-α secretion against J774 macrophage cell lines. The amount of TNF-α in the untreated group, CUR suspension and CUR-loaded NPs-treated cells was about 1,800, 1,400, and 510 pg/mL, respectively. CUR was not released in the medium at pH 1.2 or 4.5 (<15%), but was rapidly released at neutral pH values.47 Other CUR pH-sensitive nanoparticles were prepared by solvent emulsion-evaporation technique with CUR (10 mg), Eudragit®S100 (10 mg), TPGS (d-α-tocopheryl polyethylene glycol 1000 succinate, 0.05%, w/v) and PVP K-90/D (poly vinyl pyrrolidone, 0.075%, w/v) (Figure 3). It showed the superior cytotoxic action against HT-29 cells compared with free CUR (IC50, 5 vs 50 μM).48 However, its pharmacokinetics and therapy effect in vivo needs further study, which could be due to the extremely pronounced instability, and there are a number of prospective failures in determination of CUR content. Besides, Chitosan/Fucoidan was used to control release of CUR due to the ionization and deprotonation of the amino groups (-NH3+) on chitosan and the sulfate groups (-SO3-) on fucoidan for overcoming the barriers to the gastrointestinal (GI) tract. The chitosan/fucoidan nanoparticles, consisting of chitosan (5 mg), fucoidan (5 mg) and acetic acid (0.01%, v/v), showed representative pH-dependent sustained release behavior. The release of CUR was inhibited at pH 1.2 (<15 μg/mL, after 12 hours), but significantly rose at pH 7.0 (about 22.5 μg/mL).49
CUR pH-sensitive nanoparticles were developed for controlled drugs release in acidic or weak acidic conditions. Various nanostructured-mesoporous silica materials (MSMs) were modified with the functional group such as -NH2 and silanol groups for controlled release of CUR. Both MCM-41 (well-known 2D silica material) and KKC-1 (present unique new 3D fibrous-structured silica) were functionalized with aminopropyl groups to obtain MCM-NH2 (two dimensional) and KCC-NH2 (three dimensional). The nanoparticles containing MCM-NH2 or KCC-NH2 could achieve controlled, long-term and effective pH-stimulated release of CUR. The cumulative release rates of CUR from silica materials during 100 hours were about 14% in the case of MCM-NH2 and about 19% in the case of KCC-NH2 at pH 2.5, while they were only about 5.5% and 14% under neutral pH conditions (pH 7.5), respectively. As shown in the study of Abouaitah et al,50 the CUR-loaded functionalized dendritic mesoporous silica nanoparticles under low acidic condition (pH 2–5) exhibited positive charge, while under neutral pH condition (~7.5) was negative. It indicated that the enhanced cumulative release is contributed to the protonation of the -NH2 groups on the surface of silica nanoparticles under low pH condition. Mesoporous silica nanoparticles (MSNs) with tannic acid (TA)-Fe(III) complex deposited on its surface showed pH- and glutathione-responsive drug release. The CUR release from tannic acid-Fe(III) coated MCM-41 was significantly decreased compared to non-coated MCM-41. At pH values lower than 2, only the mono-complex TA can be formed, while at pH values higher than 7, three moieties of tannic acid are strongly bonded to Fe(III). The zeta potential values increase as pH value decreases from 7.4 to 4.0, because of the protonation of silanol groups of the silica surface. Thus, on the one hand, the rapid release of CUR was triggered by weak acid condition (pH 6.0 or 4.5), but sustainable drug release was shown under physiological conditions (pH 7.4). On the other hand, the glutathione concentrations of some cancer tissues could be 4-fold higher than normal tissues, and its concentration interior of the cells is significantly higher than the extracellular. Thus, the drug release could be controlled for the level of glutathione as a good ligand for Fe(III) ions which accelerated the decomposition of the TA-Fe(III) complex. The resulting dual-responsive drug delivery system could significantly enhance cytotoxicity against MRC5 cells. The IC50 of free CUR and the dual-responsive system were >150 µM and 20.2±3.5 µM, respectively.51 Therefore, this dual sensitive drug delivery system has great potential for tumor targeted therapy. Other MSNs were functionalized with (3-aminopropyl) triethoxysilane to obtain amine functionalized MSNs, then conjugated with succinic anhydride to obtain carboxylic acid functionalized MSNs (MSN-COOH) for encapsulating CUR, and lastly coated with chitosan-folate via electrostatic interaction on its surface. The CUR loading efficiency (CLE=31.2%) and loading capacity (CLC=4.6%) of MSNs with mesitylene as the swelling agent (LMSN-COOH-CUR) is 2-fold higher than that without mesitylene. It could be attributed to the fact that the decrease in the pore size of MSNs led to a drop in CLE and CLC. The CUR-loaded chitosan-folate coated mesoporous silica nanoparticles (LMSN-COOH-CUR@CS-FA) showed pH-sensitive drug release behavior. At pH 5.5, the release is significantly faster than pH 7.4. It could be due to the high permeability and swelling ability of chitosan at acidic environments. Cellular uptake studies showed that free CUR were more effective in penetrating both cell lines (HeLa and NIH-3T3) than CUR loaded MSNs.52 It could be attributed to the hydrophobic nature of CUR as a small molecule material which can easily diffuse through phospholipid cell membrane.53 The higher cellular uptake of LMSN-COOH-CUR@CS (more positive charge density) in NIH-3T3 than that of LMSNCOOH-CUR@CS-FA indicated that the interaction of positively charged MSNs with the negatively charged cell membrane (adsorptive mediated endocytosis) can be the most probable mechanism of the cellular uptake. In addition, the antiproliferative effect of CUR with chitosan-folate coated was more sensitive for HeLa cells than NIH-3T3 cells (folate receptor negative).52 Besides, the amphiphilic TPGS-PAE copolymer (D-α-tocopheryl polyethylene glycol 1,000-block-poly (β-amino ester)) as a pH-sensitive carrier was used to co-load Dox (a pro-apoptotic drug) and CUR (a potent drug for antiangiogenesis) by a one-step solvent evaporation method. The nanoparticles showed pH triggered obvious release behaviors in the endosomal environment of cancer cells. Over 90% of CUR were released at pH 5.8 after 192 hours, whereas 66.63% were released at pH 7.4. Its inhibition and apoptosis effect against SMMC 7721 cells were enhanced in a synergistic manner through decreased mitochondrial membrane potential. The induced total apoptosis rates of free (Dox and CUR) and (Dox and CUR) NPs were 38.3% and 76.2%, respectively. In addition, these co-loaded NPs also showed stronger anti-angiogenic effects including inhibition of HUVEC proliferation, migration, invasion, and tube formation mediated VEGF pathway modulation.54 It indicated that this co-delivery system of drugs with pro-apoptotic and anti-angiogenic activities is potentially effective in treating cancer.
The prodrug nano delivery system is also a good method for cancer treatment based on the weak acid environment of the tumor. In the study of Zhang et al,55 the PEG-Dox nanoparticles (PEG-Dox NPs) were prepared through by Schiff’s base reaction and self-assemble of Dox and PEG2000 in water at pH 7.4, and then encapsulate CUR into the core through hydrophobic interaction (PEG-Dox-CUR NPs). When PEG-Dox-CUR NPs were internalized into tumor cells, the Schiff’s base linker between PEG2000 and Dox would be broken down in the acidic environment, leading to CUR release into the cytoplasma, which could avoid significant drug leakage in the blood circulation. As shown in Figure 5, after 24 hours, the Dox fluorescence intensities in the main organs were very weak, but were still strong in tumor tissues for PEG-Dox-CUR NP-treated mice. Furthermore, a better anti-tumor effect was observed on BALB/c nude mice bearing HepG2 xenografts, the relative tumor volume of PBS, free Dox/CUR mixtures and PEG-Dox-CUR NPs was 1,000%, 500%, and 300%, respectively. However, some researchers have found that PEGylated nanoparticles are known to provoke certain immune responses after repeated dosing. When the PEGylated nanoparticles were repeatedly injected into the same animal (at intervals of several days), the long-term circulation characteristics were lost and the aggregation of PEG nanoparticles in the liver and spleen increased. Such immunogenicity of PEGylated nanoparticles present a barrier in the research of formulations and their use in the clinics.56,57 Based on the Schiff base linkage of Dox and oxidized sodium alginate (Dox-OSA), the novel co-delivery (CUR and Dox) pH-sensitive nanoparticles were prepared by the self-assembly of amphiphilic macromolecular Dox-prodrug. When the feeding ratio of CUR/Dox/OSA was 1.4/4/10 (w/w/w), these nanoparticles showed higher drug encapsulation efficiency (Dox, 80.45%; CUR, 44.6%) and the drug loading capacity (Dox, 20.14%; CUR, 4.2%). The nanoparticles could selectively release drug into tumor cells due to the Schiff base linkage and showed a remarkable cytotoxicity in MCF-7 cells.58 The prodrug delivery system with hydrophobic anticancer drugs deserves more extensive research. Besides, metal ion-CUR complexes could be a promising method to enhance the aqueous solubility of the hydrophobic drug and improve the anti-tumor effect. The Zn(II)-CUR nanoparticles (Zn(II)-CUR NPs) were prepared by solvent evaporation. The acid-labile coordination Zn(II)-O bond in Zn(II)-CUR NPs are broken, inducing the release of CUR responding to the tumor intracellular acidic environments. At pH 5, CUR was released about 90%, while only about 18% was released at pH 7.4 within 12 hours. Zn(II)-CUR NPs could effectively suppress the proliferation and migration growth on human bladder cancer cell lines HBC. As compared to the PBS control, the decreased tumor cell proliferation was observed for the Zn(II)-CUR NPs (79.54±5.09%).59
Figure 5.
Representative ex vivo images of tumor and main organs (heart, liver, spleen, lung, and kidney) after administration of DOX and PEG-Dox-CUR NPs.
Reprinted from Zhang Y, Yang C, Wang W, et al. Co-delivery of doxorubicin and curcumin by pH-sensitive prodrug nanoparticle for combinationtherapy of cancer. Sci Rep. 2016;6(1):21225. This work is licensed under a Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/). 55
CUR pH-Sensitive Micelles
Some representative pH sensitive chemical groups include polyacrylic acid, polymethacrylic acid, polyethyl acrylic acid, and other polybasic acids; sulfonamide group; polyamine, polyethyleneimine, and other basic groups; acid sensitive connection arm such as imine bond, hydrazide bond, hydrazone bond, cis-aconitamide, two methyl maleimide, ether bond, ortho ester, and polyacetal (ketone).60 Based on the pH-sensitivity of these groups, many micelles were developed for drug controlled and specific release to achieve targeted therapy for lesions. The drug controlled release profile was mainly induced by protonated/deprotonated process and pH-sensitive bond breaking. Among that, the design ways of pH-sensitive CUR-loaded micelles for controlled release mainly include pH-sensitive carriers and prodrugs.
Based on the protonated/deprotonated process, many pH-sensitive CUR-loaded micelles consisting of pH sensitive materials were developed for targeted therapy. The amphiphilic BSCS was selected to encapsulate CUR for oral CUR delivery. Its carbonyl groups (succinic acids) could be protonated at acidic pH (1.2), resulting in limited release, but be ionized at basic pH (5.5, 6.8, and 7.4) leading to dissociation of micelles. At pH 1.2 (gastric fluid), about 30% of CUR was released from the micelles after 24 hours, whereas at pH 5.5, 6.8, and 7.4, about 70% of CUR was released. The IC50 was 4.7-, 3.6-, and 12.2-fold lower than free CUR in HeLa, SiHa, and C33a cervical cell lines, respectively, and an increased early apoptosis (30–55%) was observed.61 Hence the BSCS micelles can be used for effective intestinal delivery of CUR. A new pH-sensitive tri-block copolymer of PCL-PDEA-PSBMA was used for the CUR-loaded micelles. At pH 5.0 and pH 7.4, CUR was released about 73.3% and 52.4% after 12 hours, and the cumulative amount reached about 91.6% and 67.4% after 60 hours, respectively. It is because the PDEA block could be protonation leading to faster drug release in an acidic environment. The MRT(0–∞) (the mean retention time) and CL/z (the clearance) of CUR solution and CUR-loaded micelles was 62.365 h and 87.199 h, 0.298 and 0.223 L/h/kg, respectively. CUR-loaded micelles provided a higher clearance half-life (T1/2a) (8.52-fold), distribution half-life (T1/2b) (4.39-fold), AUC(0-∞) (1.34-fold), and MRT(0-∞) (1.40-fold) compared to the CUR solution.62 It indicated that the tri-block copolymer of PCL-PDEA-PSBMA material used for encapsulating CUR can contribute to pH-sensitive controlled release and long circulation in vivo. This drug delivery system can increase the stability of CUR and resist its rapid metabolism in vivo. Similarly, CUR-loaded micelles, composed of poly(3-caprolactone)-b-poly(N,N-diethylaminoethyl methacrylate)-r-poly(N-(3-sulfopropyl)-N-methacryloxyethy-N,N-diethylammoniumbetainecopolymers (4s-PCL-PDEASB), also decreased CL/z (0.583 L/h/kg) compared to CUR solution (1.208 L/h/kg) and improved CUR bioavailability in rats. The AUC(0–∞), Cmax, and t1/2 of the CUR-loaded micelles were increased by 2.07-fold, 3.14-fold, and 1.42-fold, respectively compared to CUR solution.63 An endosomal micellar delivery system (F-pHSM-L61/CUR/Dox) consisting of a folate targeting ligand, poloxamer 407 (F127), and the pH-sensitive copolymer of PHis-PLA-PEG-PLA-Phis was used for multidrug resistance (MDR) reversal. The pH-sensitive copolymer PHis-PLA-PEG-PLA-PHis, composed of poly(ethylene glycol) (PEG), poly(D,L-lactide-co-glycolide) (PLA) and poly(l-histidine) (PHis), could disrupt the micellar structure triggering the release of Pluronic L61 unimers and CUR to the cytosol to exert their synergistic MDR reversal effect. At acidic pH, the imidazole groups of PHis block began to protonate inducing micellar structure destabilization and accelerated drug release. The IC50 of F-pHSM-L61/CUR/DOX formulations against MCF-7/ADR cells was less than 2-fold to F-pHSM-L61/DOX formulations and less than 4-fold to F-pHSM/CUR/DOX formulation after 24-hour incubations.64 One of the reasons for inducing MDR is the overexpression of ATP-binding cassette transporters (eg, P-gp and MRPs), which increase the efflux of drugs from cancer cells.65,66 CUR and Pluronic unimers are capable of reversing MDR of tumors by regulating its expression and function.67,68 Mechanistically, CUR and the Pluronic L61 unimers co-formulated pH-sensitive micelles exhibited a synergistic MDR reversal effect by inhibiting the expression and function of P-gp.
Based on pH-sensitive bond breaking, some micelles were designed to specifically deliver CUR to the target site such as polymer–drug conjugates. It can be achieved by introducing acid cleavable linkage to drugs/carrier materials to form a microenvironment responsive vesicle. The covalent bonds of polymer–drug conjugates would be broken down in an acid environment, resulting in drugs releasing rapidly. In a study, CUR dextran conjugate was synthesized with 2 g dextran, 0.2 g CUR hemi-succinate, 0.09 g DCC (N,N1 dicyclohexylcarbodiimide), 0.05 g DMAP (4-dimethylaminopyridine), and 0.04 g TEA (triethylamine) in 20 mL DMSO. The succinic acid spacer of CUR-dextran conjugate could be degraded at acidic pH, which afforded the capability of self-triggered drug release only at the tumor site and limited release at physiological pH. The sustained release (30.2±5.5%) of CUR-dextran micelles was observed at pH 7.4 in 72 hours, whereas an accelerated release (97.4±3.6%) was achieved at pH 4.5. The micelles had better therapeutic efficacy evidenced from the viability of C6 glioma cells (less than 20%) compared to free CUR (60%) at 50 μM CUR concentrations.69 Moreover, the F68-CUR conjugates by covalently conjugating CUR to the hydrophilic terminals of Poloxam 188 (F68) chains via cis-aconitic anhydride linkers was used for a novel flexible acid-responsive micelle formulation. The covalent bonds would be broken down leading to the faster release of CUR in a mildly acidic environment (pH 6.4 and pH 5.0) compared to in physiological pH 7.4. After 96 hours, almost 60% of CUR was released at pH 6.4 and 5.0, whereas only 40% CUR was released at pH 7.4. The IC50 values of free CUR and F68-cis-CUR micelles were 43.78 and 18.46 μM in A2780 cells after 24 hours and were 22.92 and 10.15 μM for 48 hours, respectively. For SMMC 7721 cells, the IC50 values of free CUR and F68-Cis-CUR micelles were 28.2 and 16.35 μM for 24 hours and were 15.54 and 7.63 μM for 48 hours, respectively.70 These results proved prodrug delivery nano-systems is an effective way to improve the anticancer action. However, it often suffers from the slow drug release followed by delaying onset of pharmacological action. For example, the ester-linked conjugate micelles of mPEG-PLA-CUR, conjugated CUR to poly(lactic acid) (PLA) viagluraric anhydride), showed slower drug release. After 24 hours, the amount released was only about 22% at pH 5.0. The IC50 of the micelles and free CUR were 68.8 and 29 μM against HepG2 cells after 24 hours, respectively.71 Fortunately, through the hydrazone linked CUR with mPEG-PLA, the hydrazone-linked polymer-CUR conjugate micelles (mPEG-PLA-Hyd-CUR) could increase the accumulated release, reaching about 55% at pH 5.0, attributing to the breaking down of hydrazone bonds. And it showed a better inhibitory effect on HepG2 cells (IC50, 27.7±5.3 μM after 24 hours).71 In another study, mPEG-PLA was conjugated with CUR via tris(hydroxymethyl) aminomethane (Tris) linker to produce mPEG-PLA-Tris-CUR micelles by self-assembly. The drug loading capacity (18.5%, w/w)) has been greatly improved by the style of conjugations and drug encapsulation. The sustained release action was observed due to diffusion and hydrolysis of ester bonds. The accumulated release of pure CUR and the micelles was about 6% and 8%.72 In addition, the material of mPEG-Chitosan-Ketal (PCK) was used to encapsulate CUR for pH-sensitive CUR-loaded ketal-based chitosan micelles, which could be selectively degraded in the acidic environment of the tumor. The tumor volume of free saline and CUR-loaded micelles was about 1,300 and 600 mm3, respectively. This suggested that the micelles could efficiently suppresstumor growth in vivo.73
Other pH-Sensitive Preparation
There are some other kinds of pH-sensitive preparations, such as microspheres, molecular complexes, microcapsules. For example, pH triggered Eudragit-coated chitosan microspheres of CUR for treating ulcerative colitis were initially prepared by emulsion cross-linking method and then coated with Eudragit®S100 that could prevent premature release of CUR in the upper gastrointestinal tract (GIT). An in vivo organ biodistribution study showed a negligible amount of CUR in the stomach (1.12±0.64%) and small intestine (2.63±0.87%), but high concentrations (51.23±2.53%) in the colon after 12 hours. Furthermore, the microspheres were evaluated by acetic acid-induced ulcerative colitis in mice. The CUR-loaded microspheres treated group exhibited mild lesions and inflammation, while the free CUR treated group showed moderate lesions and inflammatory reactions (Figure 6).74 A study developed a molecular complexation of CUR with a pH-sensitive cationic copolymer of Eudragit® EPO by hydrogen bond formation and hydrophobic interactions using a nano precipitation method. It enhanced the aqueous solubility (>2 mg/mL), increased peak plasma concentration (6 times) and improved bioavailability (about 20 times). The complexes were amorphous in solid and soluble only in buffers with pH less than 5.0.75 Microcapsules containing CUR were prepared with poly(L-lysine), trisodium citrate and silica sol by self-assembly, which were also proved pH-sensitive drug release. The release of CUR was more triggered in an acidic environment than the basic (63% over acidic environment, pH 8.0) and neutral (35% over acidic environment, pH 7.0) condition. The more release of CUR would attribute to weakening of ionic interactions between the silica nanoparticles and poly(L-lysine) because of silica particles losing its negative charges in an acidic environment.76
Figure 6.
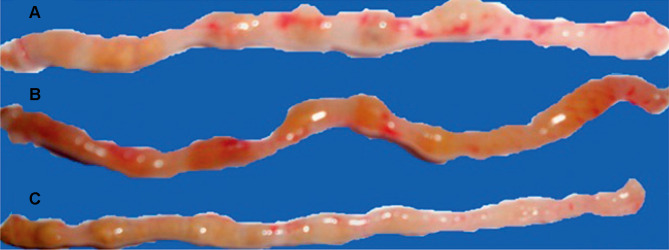
Evaluation of experimental colitis in the colon of mice in (A) control group (severe lesions and inflammation), (B) CUR-treated group (moderate lesions), and (C) CUR microsphere-treated group (mild lesions).
Reprinted with permission from Sareen R, Jain N, Rajkumari A, et al. pH triggered delivery of curcumin from Eudragit-coated chitosan microspheres for inflammatory bowel disease: characterization and pharmacodynamic evaluation. Drug Delivery. 2016;23(1):55–62. Copyright (2016) Taylor & Francis Ltd (http://www.tandfonline.com).74
Magnetic-Response Preparation
The basic principle of magnetic targeted drugs delivery system is that, in a magnetic field with a certain field strength, the magnetic carrier is gradually moved to the pathological area due to the flow properties and magnetic field induction properties of magnetic nanoparticles. Magnetic-response preparations are fabricated by three parts of magnetic core, skeleton material, and drug. Magnetic cores are usually prepared with Fe, Co, Ni, and ferrite materials (Fe2O3, Fe3O4, ferromanganese oxygen, zinc ferrite) by co-precipitation method, microemulsion method, sol-gel method, hydrothermal synthesis method, and polyol process method.77,78 The main magnetic core materials of ferrite are used for CUR-loaded magnetic-response preparations. At present, kinds of magnetic-response preparations of CUR are reported including magnetic nanoparticles, magnetic liposomes, magnetic hydrogel, magnetic microspheres, and nanospheres, etc.
Magnetic Nanoparticles
Magnetic nanoparticles had excellent performances, such as biocompatibility, large specific surface area, low toxicity, high saturation magnetization, and magnetism.79,80 They have been applied for magnetic resonance imaging,81 catalysts, magnetic recording materials, and biomedicine,82 especially anti-tumor therapy (eg, hepatoma cells and A549 cells).83–85 When CUR combined with magnetic nanoparticles (CUR-MNPs), the antitumor effect can be enhanced due to the increasing drug concentrations in target tissues by magnetic targeting and drug controlled/sustained release. In addition, CUR-MNPs were also served as magnetic resonance imaging (MRI) contrast agents to diagnose and to improve targeting ability.
Cancer is the second leading cause of human deaths and was responsible for nearly one in six deaths worldwide.86 Numerous chemotherapeutic drugs, even new drugs such as angiogenesis-targeted drugs and epidermal growth factor receptor-targeted drugs have been developed and used for the treatment of cancer.87–90 However, the curative effects and application are not very good due to low 5-year survival rate, drug induced-toxicities, and resistance, etc.10,91,92 There has been much interest in using magnetic targeted drug delivery system to treat tumors because of its unique physicochemical properties and good biocompatibility.79 Magnetic mediated CUR-MNPs could concentrate CUR on desired cells or organs by an external magnetic field. In a study, DXS/PLL was synthesized with positive polyelectrolyte poly-l-lysine (PLL) and negative polyelectrolyte (DXS) by alternating incubations and used as nanocarriers for layer-by-layer polymer-coated magnetic nanoparticles. (DXS/PLL)-coated iron oxide MNPs showed a better uptake profile of CUR in SKOV-3 cells in an extracorporeal magnetic field, as shown in Figure 7.93 CUR-loaded super-paramagnetic porous silica nanoparticles were developed for site-specific delivery of CUR under an external magnetic field. High loading of Fe3O4 nanoparticles (37% wt) and CUR (30% wt) were incorporated into the porous silica matrix under mild conditions at room temperature. Briefly, a mixture of H2O/CTABr/HCl/formamide was stirred at room temperature for 2 days, after which CUR (10 mg) and Fe3O4 (100 mg) nanoparticles were added. After a day, tetraethylorthosilicate was added. The solution was then kept under quiescent conditions for 3 days. The molar ratio of H2O/HCl/formamide/CTABr/tetraethylorthosilicate was 100:7.8:10.2:0.11:0.13.94 This new synthetic methodology for preparing composite materials is likely to be applicable to other biologically active materials. A study prepared CUR-loaded magnetic nanoparticles with 810/300 mg Fe3+/Fe2+ salts, 200 mg cyclodextrin (CD), and 250 mg F127, which could be efficiently internalized into human pancreatic cancer cells and improve mice survival. The amount of nanoparticle uptake was 54.06% for HPAF-II and 53.86% for Panc-1. The median survival was 40 days for MNPs and 24 days for Tween 20.34 CUR has poor absorption and stability, which is the leading reason for its rapid metabolism and low oral bioavailability.14 The CUR-loaded magnetic nanoparticles could improve the effective time and serum bioavailability of CUR in mice up to 2.5-fold through delayed-metabolism and increased accumulation in vivo. In addition, a considerable amount of CUR in MNP-CUR (1,792.19±644 ng/mL) was observed at 6 hours, while only 766.54±256 ng/mL of CUR in Tween 20 was present.34 This indicated that the stability of CUR was improved. CUR-MNPs can be used to enhance the therapeutic effect of CUR by increasing drug concentration and/or action time. In a study, CUR-loaded magnetic nanoparticles were prepared with epichlorohydrin-β-cyclodextrin copolymer (Epβ-CD) and oeoyldextran. When the formulation of CUR-loaded magnetic nanoparticles contained 2/1 proportion of Epβ-CD and oleoyldextran, it exhibited an improved drug release profile in extended manner up to 4 days (57.38%). In addition, the magnetization value of CUR-loaded magnetic nanoparticles was 58.23 emu/g, indicating paramagnetic behavior.95 Therefore, CUR-loaded MNPs should be used for cancer therapy with some properties in the future.
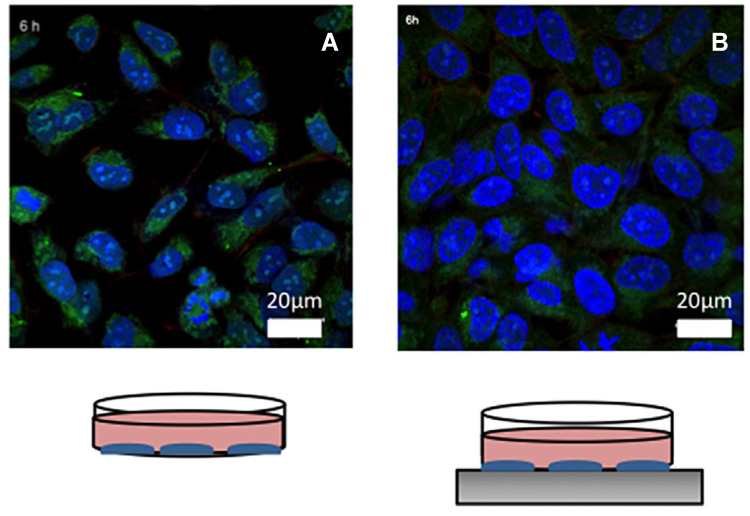
Figure 7.
Representative confocal images of (A) nano-Fe3O4-coated with CUR on SKOV-3, and (B) nano-Fe3O4-coated with CUR on SKOV-3 enhanced by magnetic field (Blue cells nuclei are labeled with DAPI, green nano-Fe3O4 are labeled with FITC (DXS-FITC), and red actin filaments are labeled with phalloidin-TRITC).
Notes: Reprinted with permission from Mancarella S, Greco V, Baldassarre F, et al. Polymer-coated magnetic nanoparticles for curcumin delivery to cancer cells. Macromol Biosci. 2015;15(10):1365–1374. © 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.93
Abbreviations: CUR, Curcumin; DAPI, 4',6-diamidino-2-phenylindole FITC (DXS-FITC), Fluorescein isothiocyanate-dextran.
MRI is produced by the radiation from the body material to the surrounding environment for helping disease diagnosis in the high frequency magnetic field. The two typical nanoparticles of quantum dots and magnetic nanoparticles have played important roles in the bioimaging field. Among them, magnetic nanoparticles serve as MRI contrast agents to improve the diagnostic sensitivity and specificity of MRI.96 As mentioned previously, CUR has been used for anti-Alzheimer’s disease. When combined with magnetic nanoparticles, it was used for visualized amyloid plaques diagnosis of Alzheimer’s disease by MRI. The CUR-MNPs were not cytotoxic to either Madin-Darby canine kidney (MDCK) or differentiated human neuroblastoma cells (SH-SY5Y) and demonstrated amyloid plaque detection ability on AD Tg2576 mice brain sections. CUR-MNPs were prepared by a multi-inlet vortex mixer (MIVM). A superparamagnetic iron oxide core was firstly coated by a layer of CUR through hydrogen-bonding and then was encapsulated by polyethylene glycol-polylactic acid (PEG-PLA) co-block polymer and polyvinylpyrrolidone (PVP) by a multi-inlet vortex mixer (MIVM).97,98 CUR, used as an anti-oxidant, has been shown to reduce ROS damage for tissues and organs. Due to the presence of under-coordinated Fe atoms (γ-Fe2O3), surface-active maghemite nanoparticles (SAMNs) were modified with CUR on the nanoparticle surface, which had good properties as a MRI contrast agent and preserved the redox properties of CUR.99 This CUR-modified SAMNs had a small size of 13±4 nm. Mouse fibroblasts test demonstrated that CUR-modified SAMNs were well-tolerated on mammalian cells. Compared with uncoated SAMNs, the CUR-modified SAMNs core-shell composite was able to yield nearly comparable relaxivity indexes, with a longitudinal relaxation rate (r1) of 0.266 mM−1 (Fe) s−1 and transverse relaxation rate (r2) of 28.05 mM−1 (Fe) s−1. The uncoated SAMNs system exhibited r1 of 0.419 mM−1 (Fe) s−1 and r2 of 44.79 mM−1 (Fe) s−1 at 293 K. The calculated r2 value rendered CUR-modified SAMNs an effective contrast agent material in MRI imaging (negative contrast). Besides, CUR-MNPs exhibited superior magnetic resonance imaging characteristics and significant tumor targeting capability. Yallapu et al100 developed a composite MNP formulation composed of an iron oxide nanoparticle core coated with CD (β-cyclodextrin) and pluronic polymer (F68). The prepared CUR-MNPs had an individual particle grain size of <9 nm and hydrodynamic average aggregative particle size of <123 nm. The r2 order detected was 7.040 s−1μg−1mL (CUR-MNPs) > 4.38 s−1μg−1mL (CUR-MNPs in cancer cells) > 3.40 s−1μg−1mL (MNP) > 2.38 s−1μg−1mL (MNP in cancer cells). The higher relaxivity values of CUR-MNPs were attributed to CUR and the nanoparticles induced greater inhomogeneity in the magnetic field than MNP. Moreover, CUR-MNPs were taken up in MDA-MB-231 cells through endocytosis. It displayed an antiproliferative effect with IC50 of 12.4 μM (free CUR, 17.2 μM). This concluded that combined CUR and MNPs with ultra-low particle size, high inherent magnetic properties, effective imaging and targeting ability can be extended to preclinical and clinical applications for cancer treatment and imaging in the future.
Functional Molecular Modified Magnetic Particles
In addition to described above about magnetic nanoparticles for targeted delivery, the drug is slowly selectively localized and released in a controlled manner (induced by targeting ligand/receptor or changes of physiological conditions such as pH), focusing on the target area to play a role. When CUR-loaded magnetic nanoparticles combined with ligand/receptor served as novel platforms for multiple biomedical applications, it could enhance the targeting and cellular uptake ability leading to an improved anticancer effect. For example, magnetite nanoaggregates were coated with poly(propylene glycol) bis(2-aminopropyl ether) (PPG-NH2) on its surface and then used to couple FA which served as a targeting ligand suggesting selective delivery towards MDA-MB-468 cells. The CUR-loaded folic acid-conjugated magnetic nanoaggregates exhibited 3.9-fold higher level of cellular uptake than non-FA magnetic nanoaggregates.101 In another study, magnetic nanoparticles were also modified with FA to obtain target specificity. N-[3(trimethoxysilyl) propyl]ethylenediamine was used to modify the MNPs to form a self-assembled monolayer and subsequently conjugated with folic acid and carboxymethylcyclodextrin through amidation between carboxy groups of folic acid/carboxymethylcyclodextrin and amine groups on the nanoparticle surface. Compared to non-folic acid magnetic targeted nanoparticles (23.4%), an enhanced cellular uptake was observed against C6 glioma cells (95.3%) by folic acid receptor mediated co-administration of paclitaxel and CUR.102 When the physiological conditions (pH) changed, CUR was selectively released at the target area that could improve target ability and enhance the anti-tumor effect. For example, multi-layer iron oxide magnetic nanoparticles (Fe@HAP-PEI-CD or Fe@HAP-CD), consisting of hydroxyapatite (HAP), β-CD, and/or polyethyleneimine (PEI), also showed pH-sensitive drug release. Firstly, superparamagnetic iron oxide nanoparticles as a magnetically responsive core were prepared by a co-precipitation method, then grafted with PEI as a hydrophilic polymer to increase drug release at acidic pH, and lastly HAP and CD were used for coating and modifying to enhance the loading capacity. The significantly different release of Fe@HAP-PEI-CD (about 52%) and Fe@PEI-CD (38.1%) was obtained after 5 days at pH 5.5. However, a significant diminution was observed at pH 7.4 at the same times (Fe@HAP-PEI-CD, 28%; Fe@PEI-CD, 18.4%). The CUR-loaded multi-layer iron oxide magnetic nanoparticles were more effective than free CUR in suppressing MCF-7 breast cancer cells proliferation evidenced by the IC50 values of 40 μM for free CUR, 22 μM for Fe@PEI-CD, and 13 μM for Fe@HAP-PEI-CD. In addition, the CLC and CLE for Fe@PEI-CD were 14.1% and 70.6%, respectively. Fe@HAP-PEI-CD showed more potential for CUR loading (CLC, 18% and CLE, 76.6%).103 In another study, sulfobutyl ether-β-CD and its derivatives functionalized MNPs (SBE-β-CD-MNPs) were used to load CUR. The SBE-β-CD-MNPs possessed excellent superparamagnetism with the remanence and coercivity of zero. The magnetization (58 emu/g) was enough for highly efficient magnetic manipulation when used as drug carriers in an external magnetic field. The loading efficiency of SBE-β-CD-MNPs for CUR was high, up to 39.4%. The CUR-loaded SBE-β-CD MNPs showed favorable drug release property under the weak acid condition. About 76% of CUR were released at pH 5.3, but only 25% were released at pH 7.4 due to the hydrogen-bonding interaction between -OH groups of cyclodextrin (β-CD) and CUR. The CUR-loaded SBE-β-CD MNPs showed an about 50% inhibition rate of HepG2 cells, but the SBE-β-CD MNPs had no significant toxicity when concentrations were as high as 40 μg/mL. Cell imaging demonstrated its successful internalization and distribution in the cell cytoplasm.104 Besides, dual pH- and thermo-responsive magnetic preparations were reported for colon-specific drug delivery. When the pH was close to the neutral condition and the temperature raised to the body temperature, it could increase the cumulative release. CUR-loaded dual pH- and thermo-responsive magnetic microgels were prepared with pectin maleate, N-isopropyl acrylamide (10%) and Fe3O4 nanoparticles (1%). In simulated intestinal fluid (SIF, pH 6.8) (25°C) the equilibrium was achieved after 35 hours (50%) without a magnetic field. However, the equilibrium was permitted at 80 hours (90%) under a magnetic field showing a slowly controlled and sustained CUR release. In simulated gastric fluid (SGF, pH 1.2) (25°C), the CUR release was not higher than 10% in the presence or absence of magnetic field. Moreover, in SGF (37°C), the released fraction of CUR was lower than 20%. However, in SIF (37°C), the amount of CUR released reached over 80% for all evaluated conditions. This enhanced release in SGF was mainly due to the galacturonic acid units over pectin being ionized, permitting repulsion among them, and hence the carrier was swelling, and diffusion to release drug in the colon site.105
Magnetic Liposomes
Liposome is composed of amphiphilic molecules with a hydrophilic polar head and hydrophobic tail. CUR-loaded liposomes were used to treat many types of cancer, such as lung, cervical, prostate, breast, and liver cancer.10 After loading magnetic nanoparticles, the liposomes have some magnetic-related characteristics such as heating generation and magnetic-targeted ability. It has been reported that temperatures in the range of 42–45°C are able to typically ablate cancer cells.106 Therefore, the appropriate temperature is particularly important for the therapeutic effect. One study reported that CUR-loaded PEGylated magnetic liposomes exhibited rapid release at 45°C compared with 37°C, and the rapid release behavior was enhanced by magnetic heating under an external high-frequency magnetic field (HFMF). Thus, the CUR-loaded PEGylated magnetic liposomes could increase the drug concentrations in the target site. The liposomes were prepared with 1.2-dipalmitoyl-sn-glycero-3-phosphocholine/cholesterol/1,2-distearoylsn-glycero-3-phosphoethanolamine N [carbonyl-methoxy(polyethylene glycol)-2000 lipid mixtures (80/20/5 mol%), oleic acid coated magnetic nanoparticles, and CUR in chloroform/methanol mixture (3/1, v/v) by a well-established thin-film hydration method (Figure 3). The CUR-loaded PEGylated magnetic liposomes could efficiently kill MCF-7 cells with IC50 of approximately 27.2 μM for 48 hours. In addition, PEGylated magnetic liposomes showed no significant toxicity for 48 hours when the concentrations of PEGylated magnetic liposomes were as high as 1 mg/mL.107 Therefore, the combination of magnetic targeting drug delivery systems and liposomes offer the potential application of CUR for cancer therapy through an inductive hyperthermia-triggering releasing system or magnetic heating therapy.
Magnetic Microspheres and Nanospheres
Microspheres are a kind of particle dispersion system formed by drug dispersion or adsorption in the polymer or polymer matrix. The size range of microspheres is from 1–500 µm, and microspheres with a size less than 500 nm are also called nanospheres. When combined with magnetic nanoparticles, microspheres have the properties of magnetic-response ability and magnetic fluid hyperthermia. A study developed CUR-loaded magnetic PLGA microspheres with a controllable particle size based on a composite emulsion. The microspheres containing hydrophilically modified Fe3O4 nanoparticles, gelatin aqueous solution (0.5 wt %), PLGA (0.25 g), and PVA aqueous solution (poly(vinyl alcohol), 1 wt%) were prepared by water-in-oil-in-water method. Its mean particle size could be controlled by the manipulation of the osmotic pressure difference between the internal (gelatin) and external aqueous phases (PVA) via changing the glucose concentration. The mean particle size of the microspheres was 16–207 µm with glucose concentrations from 0–20 wt%. It showed a rapid magnetic response, good superparamagnetism and a considerable magnetocaloric effect with a maximum magnetic entropy of 0.061 J/kg/K at 325 K. The magnetic microspheres also possessed good magnetic mobility, while the relatively low saturation magnetization (5.293 emu/g) was observed.108 Another study reported a poly(D,L-lactide-co-glycolide) (PLGA)-based controlled polymeric drug delivery system consisting of CUR and magnetic nanoparticles (MNPs). The PLGA nanospheres were prepared by the solvent evaporation method with polyvinyl alcohol as an emulsifying agent and acetone as a solvent phase. With an increase in PVA concentration from 1% to 7%, the size of the PLGA nanospheres decreased. After encapsulating MNPs, the average size of PLGA nanosphere decreased from >150 nm to 75 nm, which might be due to the surface charge stabilization with zeta potential value of −24 mV. The CUR-MNPs were served as a hyperthermic agent to induce cell death against HeLa cancer cells (90% higher than the control group) through magneto calorific effect (11.7 kA/m).109 Such alternative treatment is a thermal therapy combined nanotechnology and hyperthermia. Namely, the biocompatible magnetic nanoparticles were injected into the tumor producing an increase in heat with external alternating magnetic field, which resulted in tumor cell ablation.110 This indicated that the magnetic nanospheres can be useful as an agent for dual therapy in cancer treatment.
Magnetic Hydrogel
Functional hydrogel produced through the incorporation of magnetic nanoparticles are used in biomedical applications with the properties of biocompatibility and the response to an external field. Previously, the clinical application of Dox has been restricted by its specific toxicities to cardiac tissues.111 It has been reported that CUR has the activity of antimyocardial injury and preservation of cardiac function.112 After being loaded to the magnetic hydrogel composites, CUR was reported to play a role of cardio-protective effects against dox-induced cardiac toxicity. This magnetic hydrogel nanocomposite was synthesized by dissolving the hydrogel in NaOH solution in the presence of CUR and magnetic nanoparticles. The drug loading and entrapment efficiency for CUR were 21% and 91%, respectively. The prepared magnetic hydrogel nanocomposites were uniform spherical nanoparticles with a mean diameter of 18–23 nm. The expression of three heart failure markers (atrial natriuretic peptide, B type natriuretic peptide, and beta major histocompatibility complex) decreases with an increase in the concentration of CUR (P<0.05). This combination can be considered as a potent therapy for heart failure and hypertension and be a potential candidate to limit and mop-up free radical-mediated organ injury in the future.113
Thermo-Sensitive Preparation
Thermo-sensitive gel could transform from liquid to non-chemical cross-linked semisolid gel due to the thermo-sensitivity of materials to external temperature.114 Manifold thermo-sensitive polymers (such as collagen, chitosan, matrigel, agarose, pluronic, poly(N-isopropylacrylamide) and poly(ethylene glycol) block polymers) could be used as or modified to be thermo-sensitive hydrogels.36 As shown in Table 2, some polymers have been applied for temperature-mediated ‘on/off’ drug delivery of CUR and sustained drug delivery. In addition, a combination of other preparation delivery system with gel is also developed, such as liposomal-based/micelle-based thermo-sensitive gel. By this way, the properties of CUR were improved, involving control release, enhanced targeting, and therapy effect.
Polymer Gel
For local administration (such as transdermal and nasal agent), the better thermo-sensitive property is forming gel in the short time under body temperature to prevent the loss of liquid. The most common material of poloxamer 407 (F127) used for thermo-sensitive gel is liquid at room temperature and transforms semisolid gelatin in the skin or mucosa, which is a kind of tri-block copolymer composed of 70% poly(ethylene oxide) and 30% poly(propylene oxide). Thus, the lowest critical solution temperature (LCST) of the gel for local administration is preferably regulated to about 37°C. The CUR-loaded microemulsion thermo-sensitive hydrogel was prepared with Capryol 90, Transcutal HP, Solutol HS, and F127. When the ratio of F-127 solution (21%) to microemulsion concentrate is more than 2/1, the gelling temperature of the system is below 37°C. After gelling, the hydrogel could be gelation at 37°C within 1 minute to control release and prevent CUR loss, and the cumulative release of CUR in vitro was 93.76% in 24 hours.115 Additionally, a CUR-loaded temperature sensitive hydrogel was developed as local administration of the nasal cavity to improve the bioavailability of CUR in the brain. The gelation temperature of CUR-loaded temperature sensitive hydrogel (20% F-127, 2% F68, 0.02% Benza Australian gum, 0.9% uranium chloride, and 0.5% CUR) was 32±0.5°C, which was close to the physiological temperature of the nasal cavity (32–35°C). After local administration, the drug targeting efficiency (DTE) of the hydrogel in the brain, cerebellum, hippocampus, and foam was 1.82, 2.05, 2.07, and 1.51 times that of intravenous administration, respectively, indicating that in situ gel administration significantly enhanced the brain targeting of CUR.116 Another material of poly(N-vinylcaprolactam) (PNVC) was usually used to prepare the thermo-sensitive hydrogel because of its interesting LCST (varying between 32°C and 33°C). Poly(N-vinylcaprolactam-co-hydroxyl ethyl methacrylate) was obtained through free radical emulsion polymerization using methylene bis acrylamide as a cross-linker with N-vinylcaprolactam (NVCL) and hydroxyethyl methacrylate (HEMA). At 25°C (below LCST), the release of CUR from gels increased (about 88%), but at 37°C (above LCST) the cumulative release decreased (about 70%).117 Therefore, the topical administration of nanogels are potentially useful for targeted drug delivery.
CUR-loaded temperature sensitive hydrogels for injection were also developed. The CUR thermo-sensitive hydrogels (CTH) for injection were prepared with 2 mg CUR, 20 g F127, 4 g F68, 8 mL PEG400, and 12 mL 1.2-propanediol for treatment of solid tumors (HCA-F, high lymphatic metastasis cells of mouse ascites hepatocellular carcinoma). It could prolong the retention time of 43.02 hours compared to CUR suspension for injection (10.89 hours).118 Furthermore, the effectively inhibition of HCA-F cells was observed in vitro. After 24, 48, and 72 hours, the IC50 was 2,430.6, 283.4, and 56.8 μM in the positive control group (fluorouracil injection), 685.8, 61.9, and 11.4 μM in the CTH group, and 1,017.1, 227.8, and 63.9 μM in the CUR suspension group, respectively.119 For treatment of solid tumors, this intratumoral injection can increase the local drug concentration and retention through a thermo-sensitive drug delivery system.
Liposomal-Based Thermo-Sensitive Gel
Liposomal-based thermo-sensitive gel is a pharmaceutical composite by combing of the liposome delivery system and thermo-gels technology. In this system, CUR is encapsulated with lipid carriers firstly and then dispersed in thermo-sensitive carriers. The liposomal-based thermo-sensitive gel had small size and high entrapment efficiency properties and effective anti-tumor and anti-inflammatory effects. In a study, CUR was loaded in nanostructured lipid carriers (CUR-NLCs) by the emulsion evaporation–solidification method at low temperature and was dispersed in F127 to form a thermo-sensitive in situ gel (CUR-NLCs-Gel) for dermal delivery. CUR-NLCs and CUR-NLCs-Gel have similar results relating to parameters of uniform nano-sized spherical shape, mean size, entrapment efficiency, and drug loading of 263.9 nm, 91.76% and 2.19%, respectively. When the gels were applied to the skin topically, it could change from liquid to solid to decrease CUR loss and keep sustained skin drug delivery. The transdermal permeation rate of CUR could be significantly improved, and the cumulative amounts of CUR-NLCs, CUR-NLCs-Gel, and CUR propylene glycol solution were 2.453, 11.964, and 0.812 μg·cm−2h−1, respectively. Although the inhibition rates of CUR-NLCs-Gel (31.1%) were lower than indomethacin gel (65.7%) to auricle edemas induced by xylene in mice, CUR-NLCs-Gel showed a significant anti-inflammatory effect compared with the blank control group.120 Another study reported a novel chitosan derivative for temperature and ultrasound dual-sensitive liposomal microbubble gel. Firstly, CUR was encapsulated with S100PC, N-cholesteryl hemisuccinate-O-sulfate chitosan, and cholesterol (90:3:10) to obtain a Cur-loaded liposome microbubble (CUR-LM) (Figure 3), and then was dispersed in chitosan/glycerol phosphate (CS/GP) solution to obtain a dual-sensitive liposomal microbubble gel (CUR-LM-G). The biocompatible chitosan/glycerol phosphate (CS/GP) gel as a carrier for liposomal microbubble was liquid at room temperature, but gels at 37°C under pH 7.4. The N-cholesteryl hemisuccinate-O-sulfate chitosan was carried to modified liposomal microbubble to enhance the property and blood-contacting stability of CUR. The mean particle size, mean zeta potential, and entrapment efficiency of Cur-LM was about 950 nm, −28.7±3.8 mV, and above 90%, respectively. The release of CUR was increased (85%), applying ultrasound on the CUR-LM-G compared with without applying ultrasound (32%). The tumor growth inhibition of Cur-LM-G in BALB/c mice with the ultrasound was higher than non-ultrasound evidenced by the tumor volume (about 480 mm3 vs 600 mm3), followed by the saline control group (about 1,200 mm3).121
Micelle-Based Thermo-Sensitive Gel
Micelles have been widely investigated with excellent properties such as improved therapeutic efficacy, solubility, selective targeting, reduced side-effects, and prolonged blood circulation for hydrophobic anticancer drugs delivery.122–124 Polymer micelles are core-shell structures formed by self-assembly of amphiphilic block copolymers, which could load different types of bioactive compounds inside the hydrophobic core. Micelle-based thermo-sensitive gels could retain the excellent properties of micelles and give them temperature response characteristics. After drug loading, the in vitro and in vivo properties of CUR were improved. These gels were mainly prepared by two types of dispersion and self-assembly method. One is to disperse CUR micelles in the thermo-sensitive hydrogel at sol state. For example, Zhang et al125 prepared CUR-loaded polymeric micelles (CUR-M) by a solid dispersion method with monomethyl poly(ethylene glycol)-poly(ε-caprolactone) copolymer (MPEG-PCL) and mixing it with poly(ethylene glycol)-poly(ε-caprolactone)-poly(ethylene glycol) copolymer to obtain thermo-sensitive hydrogel (CUR-H) to treat colorectal cancer. The drug loading, encapsulation efficiency, and particle size of CUR-M were 14.82±0.07%, 98.83±0.45%, and 27.1±1.3 nm with a polydisperse index of 0.149±0.017, respectively. The CUR-H serving as a drug depot was a sol at ambient temperature and converted into gel at body temperature. CUR concentrations of the free CUR group in the abdominal cavity were much lower than those in the CUR-H group. The AUC0–24 and T1/2 values (2968.33 µg·h/mL and 3.692 h) of the CUR-H group in the abdominal cavity were 23.47-times and 6.51-times higher than those in the free CUR group. At day 22, the number and weight of tumor nodules (CT26 cells) in the CUR-H group was 49.0±15.4 and 0.88±0.30 g, vs 92.5±20.7 (P<0.01) and 1.57±0.24 g (P<0.01) in the CUR-M group and 136.8±14.3 (P<0.001) and 2.19±0.27 g (P<0.001) in the free CUR group. Therefore, the CUR-H could improve bioavailability and therapeutic effects of CUR through increasing the concentration and prolonging residence time in the abdominal cavity. Another method is the self-assembly process to form gel by encapsulating CUR directly in thermo-sensitive materials. For example, amphiphilic triblock copolymers containing a poly(L-lactide) (PLLA) central block and two poly(N-isopropylacrylamide-co-N,N-dimethylacrylamide) (P(NIPAAm-co-DMAAm)) lateral blocks were used to load CUR by self-assembly utilizing solvent evaporation/film hydration method. High drug loading (up to 20%) with high loading efficiency (>94%) were observed, and the LCST of drug loaded micelles was from 37.5 to 38.0°C, with drug loading increasing from 6.0 to 20%. The size of micelles ranged from 47.5–88.2 nm, remaining stable over 1 month with a zeta potential from −12.4 to −18.7 mV. The initial burst release is observed and followed by a slower release with a higher release rate at 40°C (above the LCST) than 37°C due to thermo-responsive release. In addition, MTT assay and cell morphology results showed that P(NIPAAm-co-DMAAm)-b-PLLA-b-P(NIPAAm-co-DMAAm) copolymers present excellent cytocompatibility.126 In general, micelle-based thermo-sensitive gel could be used for biomedical applications with outstanding properties, such as thermo-sensitive and sustained release, nano-scale size, high drug loading, and stability and good biocompatibility.
Conclusion
Some physicochemical targeting preparations for enhancing the pharmacokinetics and pharmacodynamics properties of CUR were summarized, such as pH-sensitive preparations, magnetic target preparations, and thermo-sensitive preparations. Due to the microenvironment of tumors, inflammation regions and gastrointestinal tract, pH-sensitive preparation could increase the accumulation of CUR in specific sites due to its property of pH-dependent drug release. Through this local controlled release of drugs, the stability of CUR could be improved leading to an enhanced therapy effect of CUR. By applying the external magnetic field, magnetic nanoparticles could deliver CUR to a specific site and increase the amount of the drug in the target area. The magnetic nanoparticles in magnetic hyperthermia for cancers treatment and served as MRI contrast agents used in diagnosis have also already been carried out based on the magnetocaloric effect. Thermo-sensitive preparation could respond to temperature variation to increase the concentration in the target area. It was an effective method to retain drugs by intratumoral injection. In addition, the combination of pH-, magnetic, temperature-response system and ligand could be a promising strategy in clinical practice to treat cancers.
Acknowledgments
This study was supported by the basic research fund of the science and technology department of Sichuan province of China (No. 2020YJ0336, 2020YJ0373), the Joint Fund of Luzhou City and Southwest Medical University (No. 2017LZXNYD-T02, 2019LZXNYDZ07), the Science and Technology Fund of Luzhou science and technology and Human Resources Bureau (No. 2019-SYF-35), Science and Technology Innovation Team from Jiucheng Science and Technology Talent Cultivation Plan in Luzhou City (No. 2019-1), the Scientific Research Foundation of the Education Department of Sichuan Province (No. 17ZA0439, 18ZB0646), the research grant from Traditional Chinese Medicine Administration in Sichuan Province (No. 2018QN069).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Di Maio M, Basch E, Bryce J, Perrone F. Patient-reported outcomes in the evaluation of toxicity of anticancer treatments. Nat Rev Clin Oncol. 2016;13(5):951–955. doi: 10.1038/nrclinonc.2015.222 [DOI] [PubMed] [Google Scholar]
- 2.Cinci L, Mannelli LD, Maidecchi A, et al. Effects of Hypericum perforatum extract on oxaliplatin-induced neurotoxicity: in vitro evaluations. Z Naturforsch C. 2017;72(5–6):219–226. doi: 10.1515/znc-2016-0194 [DOI] [PubMed] [Google Scholar]
- 3.Malekinejad H, Ahsan S, Delkhoshkasmaie F, et al. Cardioprotective effect of royal jelly on paclitaxel-induced cardio-toxicity in rats. Iran J Basic Med Sci. 2016;19(2):221–227. [PMC free article] [PubMed] [Google Scholar]
- 4.Mullally WJ, O’Súilleabháin CB, Brady C, et al. Vinorelbine induced perforation of a metastatic gastric lesion. Irish J Med Sci. 2017;186(3):571–575. doi: 10.1007/s11845-016-1536-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ninan PJ, Prasath D, James M. Toxicity and tolerability of paclitaxel and carboplatin regime in patients with epithelial ovarian cancer at a tertiary care hospital. SSRG Int J Med Sci. 2018;5(1):2393–9117. [Google Scholar]
- 6.Zhao SJ, Pi C, Ye Y, Zhao L, Wei YM. Recent advances of analogues of curcumin for treatment of cancer. Eur J Med Chem. 2019;180:524–535. doi: 10.1016/j.ejmech.2019.07.034 [DOI] [PubMed] [Google Scholar]
- 7.Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of Curcumin: lessons learned from clinical trials. AAPS J. 2013;15(1):195–218. doi: 10.1208/s12248-012-9432-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moghadamtousi SZ, Kadir HA, Hassandarvish P, et al. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed Res Int. 2014;2014(1):186864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marchiani A, Rozzo C, Fadda A, et al. Curcumin and curcumin-like molecules: from spice to drugs. Curr Med Chem. 2014;21(2):204–222. doi: 10.2174/092986732102131206115810 [DOI] [PubMed] [Google Scholar]
- 10.Shen L, Liu L, Ji HF. Regulative effects of curcumin spice administration on gut microbiota and its pharmacological implications. Food Nutr Res. 2017;61(1):1361780. doi: 10.1080/16546628.2017.1361780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng T, Wei Y, Lee RJ, et al. Liposomal curcumin and its application in cancer. Int J Nanomed. 2017;12:6027–6044. doi: 10.2147/IJN.S132434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramasamy TS, Ayob AZ, Myint HHL, et al. Targeting colorectal cancer stem cells using curcumin and curcumin analogues: insights into the mechanism of the therapeutic efficacy. Cancer Cell Int. 2015;15(1):96. doi: 10.1186/s12935-015-0241-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vishvakarma NK. Novel antitumor mechanisms of curcumin: implication of altered tumor metabolism, reconstituted tumor microenvironment and augmented myelopoiesis. Phytochem Rev. 2014;13(3):717–724. doi: 10.1007/s11101-014-9364-2 [DOI] [Google Scholar]
- 14.Prasad S, Tyagi AK, Aggarwal BB. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res Treat. 2014;46(1):2–18. doi: 10.4143/crt.2014.46.1.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yallapu MM, Nagesh PKB, Jaggi M, Chauhan SC. Therapeutic applications of curcumin nanoformulations. AAPS J. 2015;17(6):1341–1356. doi: 10.1208/s12248-015-9811-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev. 2013;65(1):71–79. doi: 10.1016/j.addr.2012.10.002 [DOI] [PubMed] [Google Scholar]
- 17.Bertrand N, Wu J, Xu XY, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev. 2014;66(1):2–25. doi: 10.1016/j.addr.2013.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gera M, Sharma N, Ghosh M, et al. Nanoformulations of curcumin: an emerging paradigm for improved remedial application. Oncotarget. 2017;8(39):66680–66698. doi: 10.18632/oncotarget.19164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naksuriya O, Okonogi S, Schiffelers RM, et al. Curcumin nanoformulations: a review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials. 2014;35(10):3365–3383. doi: 10.1016/j.biomaterials.2013.12.090 [DOI] [PubMed] [Google Scholar]
- 20.Tajbakhsh A, Hasanzadeh M, Rezaee M, et al. Therapeutic potential of novel formulated forms of curcumin in the treatment of breast cancer by the targeting of cellular and physiological dysregulated pathways. J Cell Physiol. 2017;233:3. [DOI] [PubMed] [Google Scholar]
- 21.Wei D, Hui L. Research progress in new formulation of curcumin for anti-tumor. Acad J Second Military Med Univ. 2015;36(7):771–775. [Google Scholar]
- 22.Lee WH, Loo CY, Young PM, et al. Recent advances in curcumin nanoformulation for cancer therapy. Expert Opin Drug Deliv. 2014;11(8):1183–1201. doi: 10.1517/17425247.2014.916686 [DOI] [PubMed] [Google Scholar]
- 23.Dagar P, Dahiya P, Bhambi M. Recent advances in curcumin nanoformulations. Nano Sci Nano Technol an Indian J. 2014;8(12):458–474. [Google Scholar]
- 24.Hashemi M, Ebrahimian M. Recent advances in nanoformulations for co-delivery of curcumin and chemotherapeutic drugs. Int J Nanomed. 2017;4(1):1–7. [Google Scholar]
- 25.Wang M, Thanou M. Targeting nanoparticles to cancer. Pharmacol Res. 2010;62(2):90–99. doi: 10.1016/j.phrs.2010.03.005 [DOI] [PubMed] [Google Scholar]
- 26.Wang JJ, Huang SW. Research progress on novel carrier-modified methods and evaluation of active targeting antitumor preparation. Chin Herb Med. 2014;6(1):22–28. doi: 10.1016/S1674-6384(14)60002-2 [DOI] [Google Scholar]
- 27.Sonekar S, Mishra M, Patel A, et al. Formulation and evaluation of folic acid conjugated gliadin nanoparticles of curcumin for targeting colon cancer cells. J Appl Pharm Sci. 2016;6(10):068–074. doi: 10.7324/JAPS.2016.601009 [DOI] [Google Scholar]
- 28.Wen X, Cheng X, Hu D, et al. Combination of curcumin with an anti-transferrin receptor antibody suppressed the growth of malignant gliomas in vitro. Turk Neurosurg. 2016;26(2):209–214. [DOI] [PubMed] [Google Scholar]
- 29.Mulik RS, Mönkkönen J, Juvonen RO, et al. Transferrin mediated solid lipid nanoparticles containing curcumin: enhanced in vitro anticancer activity by induction of apoptosis. Int J Pharm. 2010;398(1):190–203. doi: 10.1016/j.ijpharm.2010.07.021 [DOI] [PubMed] [Google Scholar]
- 30.Felber AE, Dufresne MH, Leroux JC. pH-sensitive vesicles, polymeric micelles, and nanospheres prepared with polycarboxylates. Adv Drug Deliv Rev. 2012;64(11):979–992. doi: 10.1016/j.addr.2011.09.006 [DOI] [PubMed] [Google Scholar]
- 31.Tila D, Yazdaniarazi SN, Ghanbarzadeh S, et al. pH-sensitive, polymer modified, plasma stable niosomes: promising carriers for anti-cancer drugs. Excli Journal. 2015;14(4):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu FQ, Zhang YY, You J, et al. pH triggered doxorubicin delivery of PEGylated glycolipid conjugate micelles for tumor targeting therapy. Mol Pharm. 2012;9(9):2469–2478. doi: 10.1021/mp300002v [DOI] [PubMed] [Google Scholar]
- 33.Wu T, Hua MY, Chen JP, et al. Effects of external magnetic field on biodistribution of nanoparticles: A histological study. J Magn Magn Mater. 2007;311(1):372–375. doi: 10.1016/j.jmmm.2006.10.1202 [DOI] [Google Scholar]
- 34.Yallapu MM, Ebeling MC, Khan S, et al. Novel curcumin-loaded magnetic nanoparticles for pancreatic cancer treatment. Mol Cancer Ther. 2013;12(8):1471–1480. doi: 10.1158/1535-7163.MCT-12-1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cui Y, Zhang M, Zeng F, Jin HY, Xu Q, Huang YZ. Dual-targeting magnetic PLGA nanoparticles for codelivery of paclitaxel and curcumin for brain tumor therapy. Acs Appl Mater Inter. 2016;8(47):32159–32169. doi: 10.1021/acsami.6b10175 [DOI] [PubMed] [Google Scholar]
- 36.Li ZQ, Guan JJ. Thermosensitive hydrogels for drug delivery. Expert Opin Drug Deliv. 2011;8(8):991–1007. doi: 10.1517/17425247.2011.581656 [DOI] [PubMed] [Google Scholar]
- 37.Vaupel P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin Radiat Oncol. 2004;14(3):198–206. doi: 10.1016/j.semradonc.2004.04.008 [DOI] [PubMed] [Google Scholar]
- 38.Bueno PVA, Souza PR, Follmann HDM, et al. N,N-Dimethyl chitosan/heparin polyelectrolyte complex vehicle for efficient heparin delivery. Int J Biol Macromol. 2015;75:186–191. doi: 10.1016/j.ijbiomac.2015.01.030 [DOI] [PubMed] [Google Scholar]
- 39.Talaie F, Atyabi F, Azjdarzadeh M, et al. Overcoming therapeutic obstacles in inflammatory bowel diseases: A comprehensive review on novel drug delivery strategies. Eur J Pharm Sci. 2013;49(4):712–722. doi: 10.1016/j.ejps.2013.04.031 [DOI] [PubMed] [Google Scholar]
- 40.Ju L, Cailin F, Wenlan W, et al. Preparation and properties evaluation of a novel pH-sensitive liposomes based on imidazole-modified cholesterol derivatives. Int J Pharm. 2016;518(1–2):213–219. doi: 10.1016/j.ijpharm.2016.11.044 [DOI] [PubMed] [Google Scholar]
- 41.Moku G, Gulla SK, Nimmu NV, et al. Delivering anti-cancer drugs with endosomal pH-sensitive anti-cancer liposomes. Biomater Sci. 2016;4(4):627–638. doi: 10.1039/C5BM00479A [DOI] [PubMed] [Google Scholar]
- 42.Jelezova I, Drakalska E, Momekova D, et al. Curcumin loaded pH-sensitive hybrid lipid/block copolymer nanosized drug delivery systems. Eur J Pharm Sci. 2015;78:67–78. doi: 10.1016/j.ejps.2015.07.005 [DOI] [PubMed] [Google Scholar]
- 43.De LV, Milano F, Mancini E, et al. Encapsulation of curcumin-loaded liposomes for colonic drug delivery in a pH-responsive polymer cluster using a pH-driven and organic solvent-free process. Molecules. 2018;23(4):739. doi: 10.3390/molecules23040739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gugulothu D, Kulkarni A, Patravale V, et al. pH-sensitive nanoparticles of curcumin–celecoxib combination: evaluating drug synergy in ulcerative colitis model. J Pharm Sci. 2014;103(2):687–696. doi: 10.1002/jps.23828 [DOI] [PubMed] [Google Scholar]
- 45.Dandekar P, Dhumal R, Jain R, et al. Toxicological evaluation of pH-sensitive nanoparticles of curcumin: acute, sub-acute and genotoxicity studies. Food Chem Toxicol. 2010;48(8):2073–2089. doi: 10.1016/j.fct.2010.05.008 [DOI] [PubMed] [Google Scholar]
- 46.Wahlstrom B, Blennow G. A study on the fate of curcumin in the rat. Acta Pharmacol Toxicol. 1978;43(2):86–92. doi: 10.1111/j.1600-0773.1978.tb02240.x [DOI] [PubMed] [Google Scholar]
- 47.Beloqui A, Coco R, Memvanga PB, et al. pH-sensitive nanoparticles for colonic delivery of curcumin in inflammatory bowel disease. Int J Pharm. 2014;473(1–2):203–212. [DOI] [PubMed] [Google Scholar]
- 48.Prajakta D, Ratnesh J, Chandan K, et al. Curcumin loaded pH-sensitive nanoparticles for the treatment of colon cancer. J Biomed Nanotechnol. 2009;5(5):445–455. doi: 10.1166/jbn.2009.1038 [DOI] [PubMed] [Google Scholar]
- 49.Huang YC, Lam UI. Chitosan/fucoidan pH sensitive nanoparticles for oral delivery system. J Chin Chem Soc. 2011;58(6):779–785. doi: 10.1002/jccs.201190121 [DOI] [Google Scholar]
- 50.Abouaitah KE, Farghali AA, Swiderska-Sroda A, et al. pH-controlled release system for curcumin based on functionalized dendritic mesoporous silica nanoparticles. J Nanomed Nanotechnol. 2016;7(1):351. [Google Scholar]
- 51.Kim S, Philippot S, Fontanay S, et al. pH- and glutathione-responsive release of curcumin from mesoporous silica nanoparticles coated using tannic acid-Fe(III) complex. RSC Adv. 2015;5(110):90550–90558. doi: 10.1039/C5RA16004A [DOI] [Google Scholar]
- 52.Daryasari MP, Akhgar MR, Mamashli F, et al. Chitosan-folate coated mesoporous silica nanoparticles as a smart and pH-sensitive system for curcumin delivery. RSC Adv. 2016;6(107):105578–105588. doi: 10.1039/C6RA23182A [DOI] [Google Scholar]
- 53.Gao Y, Li Y, Li Y, et al. PSMA-mediated endosome escape-accelerating polymeric micelles for targeted therapy of prostate cancer and the real time tracing of their intracellular trafficking. Nanoscale. 2014;7(2):597–612. doi: 10.1039/C4NR05738D [DOI] [PubMed] [Google Scholar]
- 54.Zhang J, Li J, Shi Z, et al. pH-sensitive polymeric nanoparticles for co-delivery of doxorubicin and curcumin to treat cancer via enhanced pro-apoptotic and anti-angiogenic activities. Acta Biomater. 2017;58:349–364. doi: 10.1016/j.actbio.2017.04.029 [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y, Yang C, Wang W, et al. Co-delivery of doxorubicin and curcumin by pH-sensitive prodrug nanoparticle for combination therapy of cancer. Sci Rep. 2016;6(1):21225. doi: 10.1038/srep21225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu W, Wan J, She ZJ, Jiang XG. Brain delivery property and accelerated blood clearance of cationic albumin conjugated pegylated nanoparticle. J Control Release. 2007;118(1):38–53. doi: 10.1016/j.jconrel.2006.11.015 [DOI] [PubMed] [Google Scholar]
- 57.Ishihara T, Takeda M, Sakamoto H, et al. Accelerated blood clearance phenomenon upon repeated injection of PEG-modified PLA-nanoparticles. Pharm Res. 2009;26(10):2270–2279. doi: 10.1007/s11095-009-9943-x [DOI] [PubMed] [Google Scholar]
- 58.Gao C, Tang F, Gong G, et al. pH-Responsive prodrug nanoparticles based on a sodium alginate derivative for selective co-release of doxorubicin and curcumin into tumor cells. Nanoscale. 2017;9(34):12533. doi: 10.1039/C7NR03611F [DOI] [PubMed] [Google Scholar]
- 59.Xing ZH, Wei JH, Cheang TY, et al. Bifunctional pH-sensitive Zn(II)-curcumin nanoparticles/siRNA effectively inhibit growth of human bladder cancer cells. J Mater Chem B. 2014;2(18):2714–2724. doi: 10.1039/c3tb21625j [DOI] [PubMed] [Google Scholar]
- 60.Wang DS, Zhou YX, Li CW, et al. Recent progress in research of pH-sensitive polymeric micelles as drug delivery system. Chin J New Drugs. 2016;25(19):2218–2224. [Google Scholar]
- 61.Sajomsang W, Gonil P, Saesoo S, et al. Synthesis and anticervical cancer activity of novel pH responsive micelles for oral curcumin delivery. Int J Pharm. 2014;477(1–2):261–272. doi: 10.1016/j.ijpharm.2014.10.042 [DOI] [PubMed] [Google Scholar]
- 62.Zhai S, Ma Y, Chen Y, et al. Synthesis of an amphiphilic block copolymer containing zwitterionic sulfobetaine as a novel pH-sensitive drug carrier. Polym Chem. 2014;5(4):1285–1297. [Google Scholar]
- 63.Wu Z, Cai M, Xie X, et al. The effect of architecture/composition on the pH sensitive micelle properties and in vivo study of curcuminin-loaded micelles containing sulfobetaines. RSC Adv. 2015;5(129):106989–107000. doi: 10.1039/C5RA20847E [DOI] [Google Scholar]
- 64.Wei H, Shi H, Qiao M, et al. pH-sensitive micelles for the intracellular co-delivery of curcumin and Pluronic L61 unimers for synergistic reversal effect of multidrug resistance. Sci Rep. 2017;7:42465. doi: 10.1038/srep42465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Plasschaert SLA, de Bont ESJM, Boezen M, et al. Expression of multidrug resistance-associated proteins predicts prognosis in childhood and adult acute lymphoblastic leukemia. Clin Cancer Res. 2005;11(24 Pt 1):8661–8668. doi: 10.1158/1078-0432.CCR-05-1096 [DOI] [PubMed] [Google Scholar]
- 66.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5(3):219–234. [DOI] [PubMed] [Google Scholar]
- 67.Alvarez AI, Real R, Pérez MG, Prieto JG, Merino G. Modulation of the activity of ABC transporters (P-glycoprotein, MRP2, BCRP) by flavonoids and drug response. J Pharm Sci. 2010;99(2):598–617. doi: 10.1002/jps.21851 [DOI] [PubMed] [Google Scholar]
- 68.Evers R, Kool M, Smith AJ, van Deemter L, de Haas M, Borst P. Inhibitory effect of the reversal agents V-104, GF120918 and Pluronic L61 on MDR1 Pgp-, MRP1- and MRP2-mediated transport. Br J Cancer. 2000;83(3):366–374. doi: 10.1054/bjoc.2000.1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raveendran R, Bhuvaneshwar GS, Sharma CP. Hemocompatible curcumin-dextran micelles as pH sensitive pro-drugs for enhanced therapeutic efficacy in cancer cells. Carbohyd Polym. 2016;137:497–507. doi: 10.1016/j.carbpol.2015.11.017 [DOI] [PubMed] [Google Scholar]
- 70.Fang XB, Zhang JM, Xie X, et al. pH-sensitive micelles based on acid-labile pluronic F68-curcumin conjugates for improved tumor intracellular drug delivery. Int J Pharm. 2016;502(1–2):28–37. doi: 10.1016/j.ijpharm.2016.01.029 [DOI] [PubMed] [Google Scholar]
- 71.Li H, Li M, Chen C, et al. On-demand combinational delivery of curcumin and doxorubicin via a pH-labile micellar nanocarrier. Int J Pharm. 2015;495(1):572–578. doi: 10.1016/j.ijpharm.2015.09.022 [DOI] [PubMed] [Google Scholar]
- 72.Yang RL, Zhang SA, Kong DL, et al. Biodegradable polymer-curcumin conjugate micelles enhance the loading and delivery of low-potency curcumin. Pharm Res. 2012;29(12):3512–3525. doi: 10.1007/s11095-012-0848-8 [DOI] [PubMed] [Google Scholar]
- 73.Yu H, Mu H, Xiu L, et al. A novel ketal-based chitosan as nano-vehicles for potential pH-sensitive nanomedicine delivery. Nanosci Nanotech Let. 2013;5(9):1007–1011. doi: 10.1166/nnl.2013.1660 [DOI] [Google Scholar]
- 74.Sareen R, Jain N, Rajkumari A, et al. pH triggered delivery of curcumin from Eudragit-coated chitosan microspheres for inflammatory bowel disease: characterization and pharmacodynamic evaluation. Drug Deliv. 2016;23(1):55–62. [DOI] [PubMed] [Google Scholar]
- 75.Kumar S, KesharwaniHimanshi Mathur SS, Tyagi M, et al. Molecular complexation of curcumin with pH sensitive cationic copolymer enhances the aqueous solubility, stability and bioavailability of curcumin. Eur J Pharm Sci. 2016;82:86–96. doi: 10.1016/j.ejps.2015.11.010 [DOI] [PubMed] [Google Scholar]
- 76.Patra D, Sleem F. A new method for pH triggered curcumin release by applying poly(L-lysine) mediated nanoparticle-congregation. Anal Chim Acta. 2013;795(18):60–68. doi: 10.1016/j.aca.2013.07.063 [DOI] [PubMed] [Google Scholar]
- 77.Lin YP, Zhang K, Zhang RH, et al. Magnetic nanoparticles applied in targeted therapy and magnetic resonance imaging: crucial preparation parameters, indispensable pre-treatments, updated research advancements and future perspectives. J Mater Chem B. 2020;8(28):5973–5991. doi: 10.1039/D0TB00552E [DOI] [PubMed] [Google Scholar]
- 78.Asnaashari Eivari H, Rahdar A, Arabi H. Preparation of super paramagnetic iron oxide nanoparticles and investigation their magnetic properties. Int J Eng Sci Invest. 2012;1(3):2251–8843. [Google Scholar]
- 79.Gao YL, Zhu GM, Ma TT. Progress in Fe3O4 magnetic nanoparticles and its application in biomedical fields. Chem Ind Eng Prog. 2017;36(3):973–980. [Google Scholar]
- 80.Pankhurst QA, Connolly JS, Jones SK, Dobso J. Application of magnetic nanoparticles in biomedicine. J Phys D Appl Phys. 2003;36(13):R167–R181. doi: 10.1088/0022-3727/36/13/201 [DOI] [Google Scholar]
- 81.Janjic JM, Shao P, Zhang S, et al. Perfluorocarbon nanoemulsions with fluorescent, colloidal and magnetic properties. Biomaterials. 2014;35(18):4958–4968. doi: 10.1016/j.biomaterials.2014.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yan H, Zhang JC, You CX, Song ZW, Yu BW, Shen Y. Surface modification of Fe3O4 nanoparticles and their magnetic properties. Int J Min Met Mater. 2009;16(002):226–229. doi: 10.1016/S1674-4799(09)60038-8 [DOI] [Google Scholar]
- 83.Jain TK, Richey J, Strand M, Leslie-Pelecky DL, Flask CA, Labhasetwar V. Magnetic nanoparticles with dual functional properties: drug delivery and magnetic resonance imaging. Biomaterials. 2008;29(29):4012–4021. doi: 10.1016/j.biomaterials.2008.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pilapong C, Sitthichai S, Thongtem S, Thongtem T. Smart magnetic nanoparticle-aptamer probe for targeted imaging and treatment of hepatocellular carcinoma. Int J Pharm. 2014;473(1–2):469–474. doi: 10.1016/j.ijpharm.2014.07.036 [DOI] [PubMed] [Google Scholar]
- 85.Kasai H, Kawai K, Shiraishi T, et al. Effects of Fe3O4 Magnetic Nanoparticles on A549 Cells. Int J Mol Sci. 2013;14(8):15546–15560. doi: 10.3390/ijms140815546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Polo E, Pino PD, Pardo A, et al. Magnetic nanoparticles for cancer therapy and bioimaging. Nanooncology. 2018;239–279. [Google Scholar]
- 87.Sideris S, Aoun F, Zanaty M, et al. Efficacy of weekly paclitaxel treatment as a single agent chemotherapy following first-line cisplatin treatment in urothelial bladder cancer. Mol Clin Oncol. 2016;4(6):1063–1067. doi: 10.3892/mco.2016.821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bae YJ, Yoon YI, Yoon TJ, et al. Ultrasound-guided delivery of siRNA and a chemotherapeutic drug by using microbubble complexes: in vitro and in vivo evaluations in a prostate cancer model. Korean J Radiol. 2016;17(4):497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meador CB, Jin H, De SE, et al. Optimizing the sequence of anti-EGFR targeted therapy in EGFR-mutant lung cancer. Mol Cancer Ther. 2015;14(2):542–552. doi: 10.1158/1535-7163.MCT-14-0723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yonesaka K, Hirotani K, Kawakami H, et al. Anti-HER3 monoclonal antibody patritumab sensitizes refractory non-small cell lung cancer to the epidermal growth factor receptor inhibitor erlotinib. Oncogene. 2015;35(7):878–886. doi: 10.1038/onc.2015.142 [DOI] [PubMed] [Google Scholar]
- 91.Zhou Q, Ye M, Lu Y, et al. Curcumin improves the tumoricidal effect of mitomycin C by suppressing ABCG2 expression in stem cell-like breast cancer cells. PLoS One. 2015;10(8):e0136694. doi: 10.1371/journal.pone.0136694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Talekar M, Ouyang Q, Goldberg MS, et al. Cosilencing of PKM-2 and MDR-1 sensitizes multidrug-resistant ovarian cancer cells to paclitaxel in a murine model of ovarian cancer. Mol Cancer Ther. 2015;14(7):1521–1531. doi: 10.1158/1535-7163.MCT-15-0100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mancarella S, Greco V, Baldassarre F, et al. Polymer-coated magnetic nanoparticles for curcumin delivery to cancer cells. Macromol Biosci. 2015;15(10):1365–1374. doi: 10.1002/mabi.201500142 [DOI] [PubMed] [Google Scholar]
- 94.Chin SF, Iyer KS, Saunders M, et al. Encapsulation and sustained release of curcumin using superparamagnetic silica reservoirs. Chem-Eur J. 2010;15(23):5661–5665. doi: 10.1002/chem.200802747 [DOI] [PubMed] [Google Scholar]
- 95.Silambarasi T, Latha S. Formulation and evaluation of curcumin loaded magnetic nanoparticles for cancer therapy. Int J Pharm Sci & Res. 2012;3(5):1–16. [Google Scholar]
- 96.Latorre M, Rinaldi C. Applications of magnetic nanoparticles in medicine: magnetic fluid hyperthermia. P R Health Sci J. 2009;28(3):227–238. [PubMed] [Google Scholar]
- 97.Cheng KK, Wang YX, Chow AHL, et al. Amyloid plaques binding curcumin conjugated magnetic nanoparticles for diagnosis in Alzheimer’s disease TG2576 mice. Alzheimers Dement. 2014;10(4):152–153. doi: 10.1016/j.jalz.2014.04.12223954029 [DOI] [Google Scholar]
- 98.Cheng KK, Chan PS, Fan S, et al. Curcumin-conjugated magnetic nanoparticles for detecting amyloid plaques in Alzheimer’s disease mice using magnetic resonance imaging (MRI). Biomaterials. 2015;44(44):155–172. doi: 10.1016/j.biomaterials.2014.12.005 [DOI] [PubMed] [Google Scholar]
- 99.Magro M, Campos R, Baratella D, et al. A magnetically drivable nanovehicle for curcumin with antioxidant capacity and MRI relaxation properties. Chemistry. 2015;20(37):11913–11920. doi: 10.1002/chem.201402820 [DOI] [PubMed] [Google Scholar]
- 100.Yallapu MM, Othman SF, Curtis ET, et al. Curcumin-loaded magnetic nanoparticles for breast cancer therapeutics and imaging applications. Int J Nanomed. 2012;7(1):1761–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Salem M. Curcumin-loaded magnetic nanoaggregates conjugated with folic acid for targeted cancer treatment. Electronic Thesis Dissertation Repository. 2014;2206. [Google Scholar]
- 102.Manju S, Sharma CP, Sreenivasan K. Targeted coadministration of sparingly soluble paclitaxel and curcumin into cancer cells by surface engineered magnetic nanoparticles. J Mater Chem. 2011;21(39):15708–15717. doi: 10.1039/c1jm12528a [DOI] [Google Scholar]
- 103.Akrami M, Khoobi M, Khalilvand-Sedagheh M, et al. Evaluation of multilayer coated magnetic nanoparticles as biocompatible curcumin delivery platforms for breast cancer treatment. RSC Adv. 2015;5(107):88096–88107. doi: 10.1039/C5RA13838H [DOI] [Google Scholar]
- 104.Zhou Y, Wang C, Wang F, et al. β-Cyclodextrin and its derivatives functionalized magnetic nanoparticles for targeting delivery of curcumin and cell imaging. Chin J Chem. 2016;34(6):599–608. doi: 10.1002/cjoc.201500756 [DOI] [Google Scholar]
- 105.Almeida EAMS, Bellettini IC, Garcia FP, et al. Curcumin-loaded dual pH- and thermo-responsive magnetic microcarriers based on pectin maleate for drug delivery. Carbohyd Polym. 2017;171:259–266. doi: 10.1016/j.carbpol.2017.05.034 [DOI] [PubMed] [Google Scholar]
- 106.Tang YD, Jin T, Flesch RCC. Numerical temperature analysis of magnetic hyperthermia considering nanoparticle clustering and blood vessels. IEEE T Magn. 2017;53(10):1–6. doi: 10.1109/TMAG.2017.2722425 [DOI] [Google Scholar]
- 107.Hardiansyah A, Yang MC, Liu TY, et al. Hydrophobic drug-loaded PEGylated magnetic liposomes for drug-controlled release. Nanoscale Res Lett. 2017;12(1):355. doi: 10.1186/s11671-017-2119-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hu L, Huang M, Wang J, et al. Preparation of magnetic poly(lactic-co-glycolic acid) microspheres with a controllable particle size based on a composite emulsion and their release properties for curcumin loading. J Appl Polym Sci. 2016;133(16):43317. doi: 10.1002/app.43317 [DOI] [Google Scholar]
- 109.Kini S, Bahadur D, Panda D. Magnetic PLGA nanospheres: a dual therapy for cancer. Ieee T Magn. 2011;47(10):2882–2886. doi: 10.1109/TMAG.2011.2158403 [DOI] [Google Scholar]
- 110.Hu RL, Ke XF, Jiang H, et al. Effect of interleukin-2 treatment combined with magnetic fluid hyperthermiaon lewis lung cancer-bearing mice. Biomed Rep. 2016;50(1):59–62. doi: 10.3892/br.2015.540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhou S, Palmeira CM, Wallace KB. Doxorubicin-induced persistent oxidative stress to cardiac myocytes. Toxicol Lett. 2001;121(3):151–157. doi: 10.1016/S0378-4274(01)00329-0 [DOI] [PubMed] [Google Scholar]
- 112.Srivastava G, Mehta JL. Currying the heart: curcumin and cardioprotection. J Cardiovasc Pharmacol Ther. 2009;14(1):22–27. doi: 10.1177/1074248408329608 [DOI] [PubMed] [Google Scholar]
- 113.Namdari M, Eatemadi A. Cardioprotective effects of curcumin-loaded magnetic hydrogel nanocomposite (nanocurcumin) against doxorubicin-induced cardiac toxicity in rat cardiomyocyte cell lines. Artif Cell Nanomed B. 2017;45(4):731–739. doi: 10.1080/21691401.2016.1261033 [DOI] [PubMed] [Google Scholar]
- 114.Rahman CV, Kuhn G, White LJ, et al. PLGA/PEG-hydrogel composite scaffolds with controllable mechanical properties. J Biomed Mater Res B. 2013;101B(4):648–655. doi: 10.1002/jbm.b.32867 [DOI] [PubMed] [Google Scholar]
- 115.Li XY, Dou JF, Wang S, Zhang L, Zhai GX. Preparation and characteristics of curcumin loaded microemulsion based in situ thermosensitive gelling system. Pharm Biotechno. 2013;020(003):225–228. [Google Scholar]
- 116.Chen X, Zhi F, Jia X, et al. Enhanced brain targeting of curcumin by intranasal administration of a thermosensitive poloxamer hydrogel. J Pharm Pharmacol. 2013;65(6):807–816. doi: 10.1111/jphp.12043 [DOI] [PubMed] [Google Scholar]
- 117.Sudhakar K, Rao KM, Subha MCS, et al. Temperature-responsive poly(-vinylcaprolactam-co-hydroxyethyl methacrylate) nanogels for controlled release studies of curcumin. Des Monomers Polym. 2015;18(8):705–713. doi: 10.1080/15685551.2015.1070497 [DOI] [Google Scholar]
- 118.Gao M, Bao X, Lei AN, et al. Retention time of curcumin thermosensitive hydrogels for injection in solid tumor. China Pharm. 2012;23(3):206–208. [Google Scholar]
- 119.Gao M, Xu H, Zhang C, et al. Preparation and characterization of curcumin thermosensitive hydrogels for intratumoral injection treatment. Drug Dev Ind Pharm. 2014;40(11):1557–1564. doi: 10.3109/03639045.2013.838579 [DOI] [PubMed] [Google Scholar]
- 120.Chen P, Zhang H, Cheng S, et al. Development of curcumin loaded nanostructured lipid carrier based thermosensitive in situ gel for dermal delivery. Colloids Surfaces A. 2016;506:356–362. doi: 10.1016/j.colsurfa.2016.06.054 [DOI] [Google Scholar]
- 121.Chen D, Yu H, Mu H, et al. Novel chitosan derivative for temperature and ultrasound dual-sensitive liposomal microbubble gel. Carbohydr Polym. 2013;94(1):17–23. doi: 10.1016/j.carbpol.2012.12.069 [DOI] [PubMed] [Google Scholar]
- 122.Cagel M, Tesan FC, Bernabeu E, et al. Polymeric mixed micelles as nanomedicines: achievements and perspectives. Eur J Pharm Biopharm. 2017;113:211–228. doi: 10.1016/j.ejpb.2016.12.019 [DOI] [PubMed] [Google Scholar]
- 123.Biswas S, Kumari P, Lakhani PM, et al. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur J Pharm Sci. 2016;83:184–202. doi: 10.1016/j.ejps.2015.12.031 [DOI] [PubMed] [Google Scholar]
- 124.Yi Y, Lin G, Chen SY, et al. Polyester micelles for drug delivery and cancer theranostics: current achievements, progresses and future perspectives. Mater Sci Eng C. 2017;83(2):218–232. doi: 10.1016/j.msec.2017.10.004 [DOI] [PubMed] [Google Scholar]
- 125.Zhang W, Cui T, Liu L, et al. Improving anti-tumor activity of Curcumin by polymeric micelles in thermosensitive hydrogel system in colorectal peritoneal carcinomatosis model. J Biomed Nanotechnol. 2015;11(7):1173–1182. doi: 10.1166/jbn.2015.2073 [DOI] [PubMed] [Google Scholar]
- 126.Hu YF, Darcos V, Monge S, et al. Thermo-responsive release of curcumin from micelles prepared by self-assembly of amphiphilic P(NIPAAm-co-DMAAm)-b-PLLA-b-P(NIPAAm-co-DMAAm) triblock copolymers. Int J Pharm. 2014;476(1–2):31–40. doi: 10.1016/j.ijpharm.2014.09.029 [DOI] [PubMed] [Google Scholar]